Abstract
Objective
To investigate whether short-term treatment with pivmecillinam was more effective than sulfamethizole in patients with acute uncomplicated urinary tract infection (UTI).
Design
Randomized controlled trial.
Setting
General practice, Denmark.
Subjects
Patients (n = 167) with uncomplicated UTI confirmed by positive urine phase-contrast microscopy.
Main outcome measures
Drug efficacy based on clinical and bacteriological cure.
Results
Urinary symptoms disappeared first in patients treated with pivmecillinam, but after five days there was no significant difference in clinical cure rate between the two antibiotics. At the follow-up visit 7–10 days after initiation of treatment, 95.4% of patients treated with pivmecillinam and 92.6% of patients treated with sulfamethizole had no persistent cystitis symptoms (difference 2.8%, CI −4.5%; 10.0%). Bacteriological cure was observed in 68.8% of patients randomized to pivmecillinam and in 77.9% randomized to sulfamethizole (difference −9.2%, CI −24.7%; 6.3%). Some 26.8% of patients randomized to pivmecillinam experienced a new UTI within 6 months after treatment compared with 18.4% of patients randomized to sulfamethizole (difference 8.4%, CI −4.5%;21.4%). No patients developed septicaemia with urinary pathogens within one year after initial treatment.
Conclusion
Patients treated with a three-day regime of pivmecillinam experienced faster relief of symptoms compared with patients treated with a three-day regime of sulfamethizole. Five days after initiation of treatment there was no significant difference in clinical and bacteriological cure between the two antibiotic regimes.
Keywords: Family practice, general practice, pivmecillinam, sulfamethizole, treatment, urinary tract infection
In Denmark a considerable number of patients with uncomplicated UTI are prescribed short-term treatment with sulfamethizole. Due to an increasing sulfa-resistance in Escherichia coli the rationale of this has been questioned and it has been debated whether empiric treatment should be changed to pivmecillinam, to which resistance is negligible.
This randomized trial compared a three-day regime of pivmecillinam with a three-day regime of sulfamethizole and showed that both antibiotic regimes were followed by a rapid reduction of symptoms, but patients treated with pivmecillinam experienced faster relief of symptoms compared with patients treated with sulfamethizole.
Five days after initiation of treatment there was no significant difference in clinical and bacteriological cure between the two antibiotic regimes.
A major proportion of women with uncomplicated urinary tract infection (UTI) are treated in general practice, and it is one of the most frequent reasons for which women consult their GP [1]. In Denmark, most patients are prescribed a short-term (three days) treatment with sulfamethizole or pivmecillinam [2]. About 80% of uncomplicated UTIs are caused by Escherichia coli. The rationale of sulfamethizole has been questioned due to increasing rates of in vitro resistance to sulfamethizole. Today, up to 30% of E. coli strains isolated at bacteriological laboratories are resistant to sulfamethizole [3–5]. It has been debated whether sulfamethizole should still be recommended for empiric treatment of uncomplicated UTI or whether it should be changed to pivmecillinam to which resistance is negligible. According to Danish recommendations, both pivmecillinam and sulfamethizole may be used for patients with uncomplicated UTI [6], [7]. The efficacy of sulfamethizole and pivmecillinam has been compared in epidemiological studies based on prescriptions from more than 57 000 patients with UTI, and no significant difference in the rate of treatment failures was found [8], [9]. Only a few trials have compared the effect of pivmecillinam and sulfamethizole, and most of them are more than 20 years old [10], [11]. We need data from recent studies to explore whether the empiric treatment of uncomplicated UTI should be modified.
We hypothesized that in women with acute uncomplicated UTI short-term treatment with pivmecillinam was more effective than sulfamethizole. The aim of this study was to test this hypothesis among patients in general practice.
Material and methods
Study population
The study was conducted in the period 1 January 2003 to 31 December 2004 as a multi-practice study including 20 general practices in the County of Funen, Denmark. Two of the authors (LB and PG) visited all practices and instructed the staff in carrying out the trial. Patients assessed for inclusion were women who contacted general practice due to symptoms of uncomplicated UTI. A complete history was obtained at the study enrolment, and urinary symptoms were considered to be due to an uncomplicated UTI if the patient was a non-pregnant, previously healthy woman between 18 and 65 years, who had no episodes of UTI within the last three months, had not been treated with antibiotics within the last two weeks, and had no known functional or anatomical abnormalities of the genitourinary tract. Patients were excluded from enrolment if they were pregnant, had symptoms suggestive of upper urinary tract infection or revealed a history of allergy to sulfa or mecillinam. Patients were also excluded if they were immunoincompetent, had diabetes or other chronic illness requiring medical treatment and supervision, or if they did not want to participate.
Patient assessment and follow-up
At the assessment a midstream urine specimen was collected for urine phase contrast microscopy. Only patients with a positive phase contrast microscopy were included in the study. Positive microscopy was defined by ≥1 rods per field of vision at 400 times magnification in uncentrifuged urine [12].
After informed consent the participants were randomized to pivmecillinam (400 mg x 3 daily) or sulfamethizole (1 gram x 2 daily) each for three days. We used a blocked randomization and allocated one block with 20 treatments (10 courses of sulfamethizole cures and 10 courses of pivmecillinam cures) to each practice. Assignments were placed in sealed, sequentially numbered envelopes, which were opened at the time of enrolment. The severity of urinary symptoms (dysuria and pollakisuria) was monitored at baseline on a scale from 0 (no symptoms) to 3 (severe symptoms). At the follow-up visit after 7–10 days patients were asked for the effect of treatment and the number of days until the symptoms disappeared. A midstream urine specimen for culture was obtained at baseline and follow-up visit. Urine cultures were forwarded to the Department of Clinical Microbiology, Odense University Hospital, where standard methods were used for organism isolation, quantification, and identification. Antimicrobial susceptibility testing was done using an agar diffusion method.
Outcome measures
The primary study outcome was drug efficacy based on clinical cure. Patients were considered to be cured when the urinary symptoms (dysuria and pollakisuria) had subsided and no persistent symptoms were present at the follow-up visit. Patients were also requested to record any adverse effects during the follow-up period. The secondary outcome was drug efficacy based on bacteriological cure. Bacteriological cure was defined as eradication of the causative uropathogens with sterile urine culture (< 103 colony forming units (CFU)/mL) at follow-up. In order to investigate potential relapse or complications all GPs received a questionnaire six months after the treatment. We also explored the bacteriological laboratory database covering all the patients included, in order to identify any episodes of bacteraemia up to one year after enrolment in the study.
Based on an expected effect of sulfamethizole of 85%, a minimal relevant effect difference of 10%, a type 1 error of 5%, and a power of 80%, we calculated to include 320 patients, 160 in each group. All analyses were performed according to the intention to treat principle, and calculations were performed by means of the statistical programme STATA, version 9.0 [13]. We used 95% confidence intervals.
Results
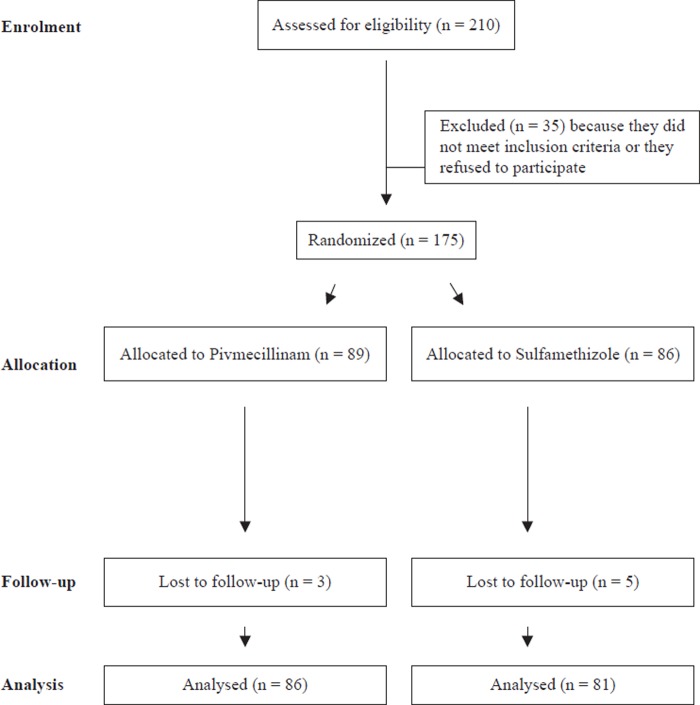
A total of 210 women were assessed for eligibility (Figure 1). A total of 35 patients were excluded because they did not meet the inclusion criteria or they refused to participate; 175 patients were randomized to either pivmecillinam (n = 89) or sulfamethizole (n = 86). Eight patients (three randomized to pivmecillinam and five to sulfamethizole) did not turn up to the follow-up visit, leaving us with 167 patients (86 randomized to pivmecillinam and 81 randomized to sulfamethizole) for the analysis of clinical cure. Some 44 patients were excluded from the analysis of bacteriological cure because they had a negative urine culture at baseline (n = 33) or no urine sample at follow-up (n = 11), leaving us with 123 patients for the analysis of bacteriological cure.
Figure 1.
Derivation of the study population.
The two treatment groups were similar in terms of age, intensity, and duration of symptoms and there were no significant differences found by urine microscopy (Table I). In patients with a positive urine culture, the most frequent microorganism was E. coli, which was isolated in 80% of patients randomized to pivmecillinam and in 85% to sulfamethizole. In both groups, about 4/5 of positive cultures contained >105 CFU/mL.
Table I.
Baseline patient demographic and clinical and microscopic characteristics (95% confidence intervals) by treatment groups: pivmecillinam (n = 86) and sulfamethizole (n = 81).
| Characteristics | Pivmecillinam treated | Sulfamethizole treated |
| Age, mean (range) | 32 (18–64) | 34 (17–61) |
| Symptoms: | ||
| Pollakisuria | 89.3% (80.6; 95.0) | 90% (81.4; 95.6) |
| Dysuria | 81.2% (71.2; 88.8) | 87% (78.5; 93.9) |
| Intensity of symptoms: | ||
| Moderate to severe pollakisuria | 69.1% ( 57.9; 78.9) | 65% (53.0; 75.0) |
| Moderate to severe dysuria | 57.3% (45.9; 68.1) | 54.4% (42.8; 65.7) |
| Duration of symptoms>3 days: | ||
| Pollakisuria | 28.6% (19.2; 39.5) | 35.8% (25.4; 47.2) |
| Dysuria | 22.3% (14.0; 32.7) | 29.6% (19.9; 49.8) |
| No. of bacteria per field of vision: | ||
| 1–10 | 48.8% (37.9; 59.9) | 45.0% (33.8; 56.5) |
| ≥10 | 51.1% (40.1; 62.1) | 55.0% (43.5; 66.2) |
| No. of leucocytes per field of vision: | ||
| 1–10 | 81.4% (71.5; 90.0) | 80.0% (69.6; 88.1) |
| ≥10 | 18.6% (11.0; 28,4) | 20.0% (11.9; 30.4) |
The symptoms disappeared first in patients treated with pivmecillinam (Figure 2). Two days after initiation of treatment 46.5% of patients treated with pivmecillinam still had persistent dysuria compared with 66.3% treated with sulfamethizole (difference 19.7%, CI 5.0%; 34.5%). For pollakisuria the corresponding figures were 47.1% for pivmecillinam and 58.8% for sulfamethizole, respectively (difference 11.7%, CI 3.4%; 26.8%). After five days there was no significant difference in the cure rate between the two antibiotics (Figure 2). At the follow-up visit 7–10 days after initiation of treatment, more than 90% of all patients were clinically cured without any persistent symptoms irrespective of the type of antibiotic used (Table II). In patients with UTI caused by sulfa-resistant coli the cure rate was slightly higher for pivmecillinam than for sulfa, and in patients with UTI caused by coli strains susceptible to sulfamethizole the cure rates were similar for the two antibiotics. Bacteriological cure rates were slightly higher in patients treated with sulfamethizole than in patients treated with pivmecillinam, but the differences were not significant (Table II).
Figure 2.
Percentage of patients with persistent dysuria and pollakisuria after treatment with sulfamethizole or pivmecillinam for uncomplicated urinary tract infection.
Table II.
Clinical and microbiological outcomes at follow-up visit 7–10 days after start of treatment.
| Pivmecillinam | Sulfamethizole | Difference (95% CI) | |
| Clinical outcomes: | |||
| Clinical cure (no persistent urinary symptoms) | |||
| UTI, all infections (n = 167) | 95.4% | 92.6% | 2.8% (−4.5%; 10.0%) |
| UTI caused by E. coli (n = 109) | 96.4% | 96.3% | 0.1% (−7.0%; 7.1%) |
| UTI caused by sulfa resistant E. coli (n = 36) | 95.5% | 92.9% | 2.6% (−13.5%; 18.6%) |
| UTI caused by sulfa susceptible E. coli (n = 73) | 97.0% | 97.5% | −0.5% (−8.1%; 7.1%) |
| UTI not caused by E. coli (n = 25) | 93.3% | 90.0% | 3.3% (−19.16%; 25.8%) |
| UTI culture negative (n = 33) | 93.8% | 82.4% | 11.4% (−10.2%; 33.1%) |
| Bacteriological outcomes: | |||
| Microbiological cure (< 103 CFU per ml) | |||
| UVI caused by all bacteria (n = 123) | 68.8% | 77.9% | −9.2% (−24.7%; 6.3%) |
| UVI caused by E. coli (n = 101) | 72.6% | 80.0% | −7.5% (−24.0%; 9.1%) |
| UTI caused by sulfa resistant E. coli (n = 34) | 65.0% | 85.7% | −20.7% (−48.5%; 7.1%) |
| UTI caused by sulfa susceptible E. coli (n = 67) | 77.4% | 77.8% | −0.3% (−20.4%; 19.7%) |
| UVI not caused by E. coli (n = 22) | 53.8% | 66.7% | −12.8% (−53.8%; 28.2%) |
Both antibiotic drugs were well tolerated, but minor adverse effects were reported by 14.1% of patients treated with pivmecillinam and 12.8% of patients treated with sulfamethizole (difference 1.3%, CI −9.5%; 11.8%). Most adverse reactions were from the gastrointestinal tract with nausea and diarrhoea as the most frequent symptoms.
Data on potential relapse or complications up to six months after treatment of the UTI were obtained from 158 patients corresponding to a response rate of 95% in the pivmecillinam group and 94% in the sulfa group. A total of 26.8% of patients randomized to pivmecillinam experienced a new UTI within six months after treatment compared with 18.4% of patients randomized to sulfamethizole (difference 8.4%, CI −4.5%; 21.4%). Only one of the patients included developed septicaemia within one year after initial treatment. This patient was operated on for a dermoid cyst and the causative agent was a Bacteroides fragilis.
Discussion
This trial compared a three-day regime of pivmecillinam with a three-day regime of sulfamethizole in patients with uncomplicated UTI. Both antibiotic regimes were followed by a rapid reduction of symptoms. However, patients treated with pivmecillinam experienced a faster relief of symptoms compared with patients treated with sulfamethizole. Five days after initiation of treatment there was no significant difference in clinical and bacteriological cure between the two antibiotic regimes. At follow-up after 7–10 days more than 90% of patients were clinically cured in both groups, and there was no significant difference between the effects of the two antibiotics. The clinical effect of sulfamethizole was only slightly reduced in patients with UTI caused by sulfa-resistant E. coli compared with UTI caused by sulfa-susceptible E. coli. When focusing on bacteriological cure rates and the rate of relapse within six months after treatment, we found a slightly better effect of sulfamethizole compared with pivmecillinam, but the differences were not significant. No patients developed septicaemia with urinary pathogens within one year after the initial treatment.
It is a limitation that we used different regimes for pivmecillinam and sulfamethizole. For some patients it may have been difficult to adhere to a regime of three doses a day and some treatment failures in the pivmecillinam group might be due to lower compliance.
Another limitation is the restricted number of patients included. Uncomplicated UTI is a frequent reason for encounter in general practice [1], [14], and we expected to include more than 300 patients. However, in spite of repeated reminders, the GPs were not able to include the expected number of patients within the study period. The GPs participated on a voluntary basis, and they were not paid for the extra work related to the project. For some GPs, it might have been difficult to dedicate sufficient time, and they may not have included all patients with uncomplicated UTI. We may have committed a type 2 error, i.e. overlooked a real difference in cure rate between pivmecillinam and sulfamethizole. However, our data do not support a potential superiority of pivmecillinam in excess of 10% (95% confidence). A potential selection bias might have occurred if some patients fulfilling the criteria for inclusion were not invited to participate. However, due to the randomization, we do not think that such a potential selection bias should have a major influence on the main results.
We only found one trial that compared the effect of sulfamethizole and pivmecillinam in patients with uncomplicated UTI. Bitsch et al. compared pivmecillinam (three days) with sulfamethizole (six days) in women with uncomplicated UTI and found similar clinical cure rates to those in our study (pivmecillinam: 95%; sulfamethizole 89%), while bacteriological cure rates were somewhat higher (pivmecillinam 96%; sulfamethizole 90%) [10]. In a randomized study from Copenhagen, patients with UTI were treated with sulfamethizole (three days) or pivmecillinam (three days) and bacteriological cure was found in 81% of sulfamethizole-treated and 74% of pivmecillinam-treated patients, but this trial only included children [11]. Bergan et al. examined the effect of sulfamethizole and sulfamethoxazole in a prospective, randomized study of patients with uncomplicated UTI and found, like us, that most symptoms disappeared after 4–5 days (cure rate 92–93%) [15]. Compared with our results, Mabeck et al. found a slightly lower bacteriological effect of sulfamethizole (cure rate 68.5%) while Keenan et al. found a higher effect (bacteriological cure rate 90%) [16], [17]. Nicolle et al. examined the bacteriological effect of pivmecillinam in patients with uncomplicated UTI and found a slightly higher cure rate (75%) than we did [18]. Similar cure rates for pivmecillinam have been found in other studies [19].
It was surprising that pivmecillinam in our study was associated with a lower bacteriological cure rate than sulfamethizole. However, the relation between laboratory resistance and clinical outcome is unpredictable, and susceptibility to antibiotics measured by in vitro techniques may be a poor predictor of clinical outcome [20]. Furthermore, a considerable number of patients with uncomplicated UTI are cured spontaneously [19]. Thus Ferry et al. found that about 25% of patients with uncomplicated UTI not treated with antibiotics were cured after one week [21], [22]. Mabeck found that chemotherapy was no better than placebo for immediate symptomatic effect in women with uncomplicated UTI [23].
The bacteriological effect of pivmecillinam and sulfamethizole is correlated with the period of time the antibiotic concentration exceeds the minimal inhibitory concentration (MIC) in the urine. Kerrn et al. found that pivmecillinam (400 mg x 3) inhibits sensitive coli strains all round the clock, while sulfamethizole (1 gm x 2) only exceeds the MIC of sensitive coli strains about two-thirds of the day [24]. However, sub-inhibitory levels of antibiotics may have an influence on the clinical cure by reducing the adherence of bacteria to the epithelial cells [19].
In summary, the group receiving pivmecillinam experienced faster relief of symptoms (clinical cure) compared with the group receiving sulfamethizole, but after five days there was no significant difference between the two groups.
Acknowledgements
The general practitioners are thanked for data collection.
Approval
The study was performed according to the Helsinki III declaration and approved by the Scientific Ethical Committee for the County of Vejle and Funen. The study was registered by the Danish Data Protection Agency and approved by the Danish Medicines Agency. Patients received oral and written information concerning the aim of the project, and signed informed consent before inclusion.
Funding
The study was supported by the Institute for Rational Pharmacotherapy, Denmark, and the County of Funen, Denmark.
References
- 1.Hummers-Pradier E, Kochen MM. Urinary tract infections in adult general practice patients. Br J Gen Pract. 2002;52:752–61. [PMC free article] [PubMed] [Google Scholar]
- 2.Lægemiddelstyrelsen. Lægemiddelstatistik. 2007. 1992–2006. http://www.laegemiddelstyrelsen.dk.
- 3.Bager F. DANMAP: Monitoring antimicrobial resistance in Denmark. Int J Antimicrob Agents. 2000;14:271–4. doi: 10.1016/s0924-8579(00)00135-7. [DOI] [PubMed] [Google Scholar]
- 4.Alos JI, Serrano MG, Gomez-Garces JL, Perianes J. Antibiotic resistance of Escherichia coli from community-acquired urinary tract infections in relation to demographic and clinical data. Clin Microbiol Infect. 2005;11:199–203. doi: 10.1111/j.1469-0691.2004.01057.x. [DOI] [PubMed] [Google Scholar]
- 5.Miller LG, Tang AW. Treatment of uncomplicated urinary tract infections in an era of increasing antimicrobial resistance. Mayo Clin Proc. 2004;79:1048–53. doi: 10.4065/79.8.1048. [DOI] [PubMed] [Google Scholar]
- 6.Hoiby N, Tvede M. [Estimated consequences of the Danish sulfonamide treatment of uncomplicated urinary tract infections] Ugeskr Laeger. 2002;164:2305–6. [PubMed] [Google Scholar]
- 7.Institute for Rational Pharmacotherapy. National List of Recommended Drugs. 2007. http://www.irf.dk/rekommandationsliste/national_rekommandationsliste.htm. Available at: www.irf.dk/rekommandationsliste/national_rekommandationsliste.htm.
- 8.Schonheyder HC, Thrane N, Sorensen HT. [Repeat antibiotic prescriptions following treatment with sulfonamide or pivmecillinam. A survey of prescriptions among women aged 15–50 years in the county of Nordjylland] Ugeskr Laeger. 2001;164:43–6. [PubMed] [Google Scholar]
- 9.Bjerrum L, Dessau RB, Hallas J. Treatment failures after antibiotic therapy of uncomplicated urinary tract infections: A prescription database study. Scand J Prim Health Care. 2002;20:97–101. [PubMed] [Google Scholar]
- 10.Bitsch M, Hansen PH, Pagh J. [Treatment of acute urinary infections. Comparison between pivmecillinam for 3 days and sulfamethizole therapy for 6 days] Ugeskr Laeger. 1985;147:1392–5. [PubMed] [Google Scholar]
- 11.Short-term treatment of acute urinary tract infection in girls. Copenhagen Study Group of Urinary Tract Infections in Children. Scand J Infect Dis. 1991;23:213–20. doi: 10.3109/00365549109023403. [DOI] [PubMed] [Google Scholar]
- 12.Bjerrum L, Grinsted P, Sogaard P. [Can we rely on the results of urine microscopy and culture when tests are performed in general practice?] Ugeskr Laeger. 2002;164:1927–30. [PubMed] [Google Scholar]
- 13.Stata Press. Texas:: Stata Statistica Software; 1999. Stata for Windows. [Google Scholar]
- 14.Teunissen D, Van Den BW, Van WC, Lagro-Janssen T. “It can always happen”: The impact of urinary incontinence on elderly men and women. Scand J Prim Health Care. 2006;24:166–73. doi: 10.1080/02813430600739371. [DOI] [PubMed] [Google Scholar]
- 15.Bergan T, Skjerven O. Double blind comparison of short and medium term sulfonamides, sulfamethizole and sulfamethoxazole, in uncomplicated acute urinary tract infections. Scand J Infect Dis. 1979;11:219–23. doi: 10.3109/inf.1979.11.issue-3.08. [DOI] [PubMed] [Google Scholar]
- 16.Mabeck CE, Vejlsgaard R. Treatment of urinary tract infections in general practice with sulfamethizole, trimethoprim or co-trimazine (sulphadiazine- trimethoprim) J Antimicrob Chemother. 1980;6:701–8. doi: 10.1093/jac/6.6.701. [DOI] [PubMed] [Google Scholar]
- 17.Keenan TD, Eliott JC, Bishop V, Peddie BA, Bailey RR. Comparison of trimethoprim alone with co-trimoxazole and sulphamethizole for treatment of urinary tract infections. N Z Med J. 1983;96:341–2. [PubMed] [Google Scholar]
- 18.Nicolle LE, Madsen KS, Debeeck GO, Blochlinger E, Borrild N, Bru JP, et al. Three days of pivmecillinam or norfloxacin for treatment of acute uncomplicated urinary infection in women. Scand J Infect Dis. 2002;34:487–92. doi: 10.1080/00365540110080728. [DOI] [PubMed] [Google Scholar]
- 19.Hooton TM, Stamm WE. Diagnosis and treatment of uncomplicated urinary tract infection. Infect Dis Clin North Am. 1997;11:551–81. doi: 10.1016/s0891-5520(05)70373-1. [DOI] [PubMed] [Google Scholar]
- 20.Davey P, Steinke D, MacDonald T, Phillips G, Sullivan F. Not so simple cystitis: How should prescribers be supported to make informed decisions about the increasing prevalence of infections caused by drug-resistant bacteria? Br J Gen Pract. 2000;50:143–6. [PMC free article] [PubMed] [Google Scholar]
- 21.Ferry SA, Holm SE, Stenlund H, Lundholm R, Monsen TJ. The natural course of uncomplicated lower urinary tract infection in women illustrated by a randomized placebo controlled study. Scand J Infect Dis. 2004;36:296–301. doi: 10.1080/00365540410019642. [DOI] [PubMed] [Google Scholar]
- 22.Ferry SA, Holm SE, Stenlund H, Lundholm R, Monsen TJ. Clinical and bacteriological outcome of different doses and duration of pivmecillinam compared with placebo therapy of uncomplicated lower urinary tract infection in women: The LUTIW project. Scand J Prim Health Care. 2007;25:49–57. doi: 10.1080/02813430601183074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mabeck CE. Treatment of uncomplicated urinary tract infection in non-pregnant women. Postgrad Med J. 1972;48:69–75. [PMC free article] [PubMed] [Google Scholar]
- 24.Kerrn MB, Frimodt-Moller N, Espersen F. Urinary concentrations and urine ex-vivo effect of mecillinam and sulphamethizole. Clin Microbiol Infect. 2004;10:54–61. doi: 10.1111/j.1469-0691.2004.00737.x. [DOI] [PubMed] [Google Scholar]