Abstract
In 1957 Sakai and Kitagawa in Japan reported the clinical and biochemical findings in a patient with tyrosinemia, tyrosyluria, liver cirrhosis, and renal rickets. Subsequently, reports were published from various countries of other patients with hepatorenal tyrosinemia (HRT). 4-Hydroxyphenylpyruvate dioxygenase deficiency was originally proposed as the cause of HRT. However, in 1977 Lindblad et al. found that succinylacetone, which accumulates in the serum and urine from patients with HRT, inhibits delta-aminolevulinic acid (ALA) dehydratase in vitro. They suggested that the primary enzyme deficiency in patients with HRT was fumarylacetoacetate hydrolase, and this was soon confirmed. Thus, the elucidation of the pathogenesis of this disease has led to the possibility that, if a reliable newborn screening method could be developed, the prognosis of these patients would be improved. Early treatment would require a diet low in phenylalanine and tyrosine, administration of 2-(2-nitoro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC), and liver transplantation.
Keywords: hepatorenal tyrosinemia (HRT), tyrosinemia type 1, fumarylacetoacetate hydrolase deficiency (FAHD), 4-hydroxyphenylpyruvate dioxygenase (4-HPPD), liver cirrhosis, Fanconi type renal rickets
Introduction
Hepatorenal tyrosinemia (HRT) is an autosomal recessive inborn error of metabolism which mainly affects the liver and kidneys. It is also known as tyrosinemia type 1, hereditary tyrosinemia, congenital tyrosinosis, and fumarylacetoacetate hydrolase (FAH) deficiency (FAHD), and is assigned OMIM 276700.1)
In 1932, more than 25 years before the discovery of the clinical entity HRT, Grace Medes2,3) in the US described the biochemical findings in a 49 year old man with myasthenia gravis, under the title of “A new error of tyrosine metabolism: Tyrosinosis. The intermediary metabolism of tyrosine and phenylalanine”. Medes found an unusual reducing substance in the urine of this patient which was identified as 4-hydroxyphenylpyruvate (4-HPP). She proposed that the defect in tyrosinosis was a deficiency of 4-hydroxyphenylpyruvate dioxygenase (4-HPPD). However, the patient remains an enigma, as I will discuss later, and this condition has been assigned1) a separate MIM number (76800).
In 1957 Sakai and Kitagawa4,5) reported the case of a two-year-old boy with marked hepatosplenomegaly, failure to thrive, and tyrosyluria. His urine contained a large amount of 4-hydroxyphenyllactate (4-HPL) and a small amount of 4-HPP, 4-hydroxyphenylacetate (4-HPA) and tyrosine. The authors proposed, on the basis of the physical findings and biochemical observations, that the patient might have another type of inborn error of tyrosine metabolism, different from that in Medes’s case. In three reports4–6) between 1957 and 1959 we described the clinical, biochemical and pathological findings of this patient under the heading “An atypical case of tyrosinosis” with the subtitle “l-para-hydroxyphenyllactic aciduria”.
In this review the present author summarizes advances in the study of HRT, focusing on the clinical aspects.
Recognition of hepatorenal tyrosinemia
1). Clinical features.4,5)
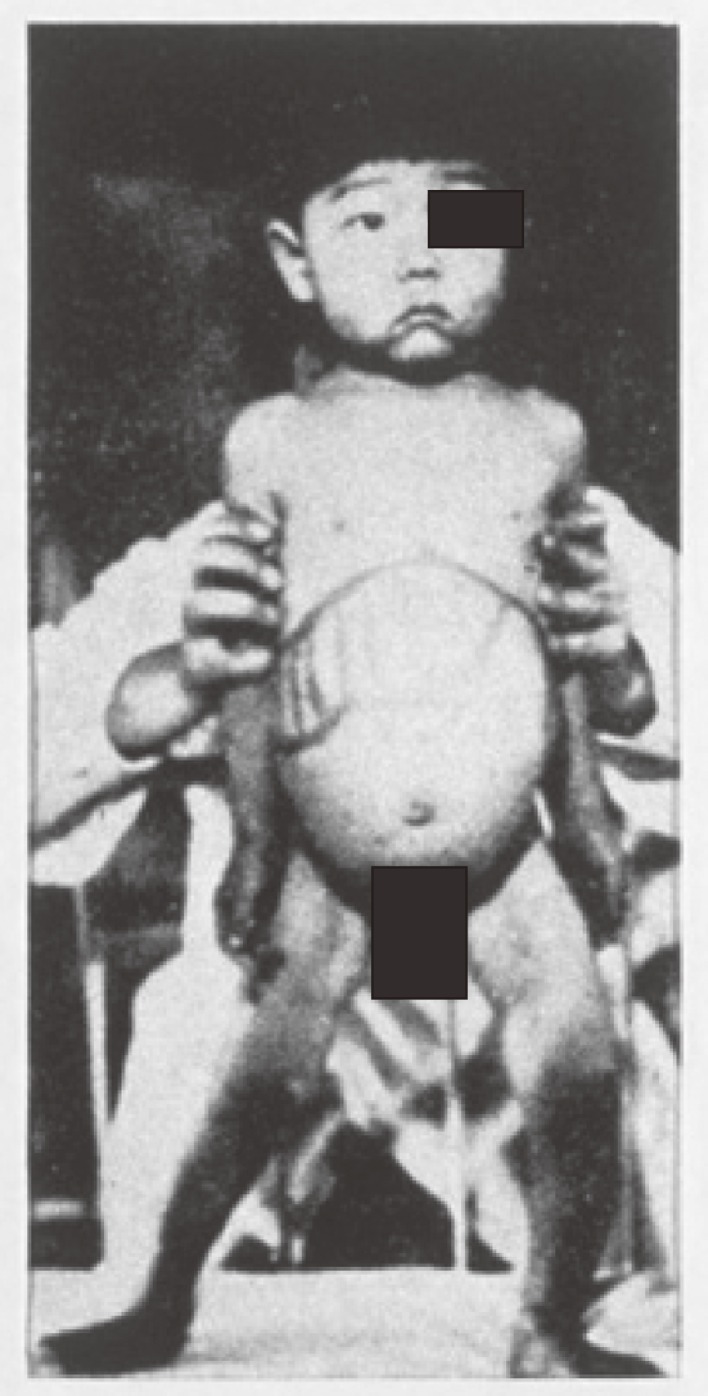
In 1955, when I was a junior staff member at the Department of Pediatrics, Jikei University School of Medicine, Tokyo (Chairman: Professor Kiyoshi Sakai) a two year-old boy (K.O.) was admitted with marked hepatosplenomegaly and failure to thrive (Fig. 1). He was the second child of a consanguineous marriage and was born normally at term on October 9, 1952, weighing 3,900 g. His parents lived in Oshima island, 120 km from Tokyo. Their first child (male) had died of unknown cause on the 15th day of life.
Figure 1.
Case K. O.
During infancy the patient showed normal growth and development. In his second year, however, the parents noticed loss of appetite and gradually increasing abdominal distension. There was no history of neuromuscular abnormality. On admission, physical examination revealed the liver and spleen were enlarged to 5 cm and 4 cm, respectively, below the costal margin. No abnormalities were found in the heart, lungs, skin, or eyes.
2). Laboratory investigations and diagnosis.4,5)
All routine laboratory tests were normal, except for a low serum inorganic phosphorus and slightly elevated values of liver function tests. The urinary Millon’s reaction consistently showed a dark red color. The urine also showed a positive Benedict’s test and phosphomolibdic acid reaction. Two-dimensional paper chromatography of the urinary Millon positive compounds revealed four spots: A, B, C, and D. The B and D spots were positive for Millon’s reaction only. The A spot was positive for both Millon’s and the Ninhydrin reaction. The C spot was positive for both Millon’s and the phosphomolibdic acid reaction.
The authors tried to isolate and identify the Millon positive compounds. Two liters of the urine in cold storage were concentrated under vacuum at 40℃ and the residue extracted with ethyl alcohol. The alcohol insoluble materials were removed by centrifugation.
The supernatant solution was re-concentrated and the residue re-extracted with alcohol. After repeating these procedures several times the final residue was dissolved in water and treated with an ion exchange resin, then with activated carbon powder, and filtered. The filtrate was concentrated at 40℃ under vacuum. Eventually, one of the Millon positive compounds was isolated in long needle crystals.4)
This compound was strongly positive for Millon’s reaction, and negative for the Ninhydrin and phosphomolibdic acid reactions. Elemental analysis showed it was nearly identical to 4-HPL.
In 1955 in Japan, only l-tyrosine was commercially available, so we had to synthesize other 4-hydroxyphenyl compounds, such as 4-HPL, 4-HPP and 4-HPA, in our laboratory.4) The physical and chemical properties of the compound isolated from the urine were compared with those of the three synthetic specimens and found to be identical to 4-HPL (the B spot on paper chromatography). The other three compound spots, A, C, and D, were subsequently identified as tyrosine, 4-HPP, and 4-HPA, respectively, by paper chromatography.4)
Quantitative analysis of the Millon positive compounds in the urine of our patient while on a hospital diet revealed that the daily outputs of 4-HPL, tyrosine, 4-HPP, and 4-HPA were approximately 1000 mg, 350 mg, 250 mg, and 150 mg, respectively. Following the oral ingestion of 1 g of 4-HPP, the urinary output of 4-HPL rose markedly and that of 4-HPA increased slightly. After the administration of 1 g of tyrosine, the output of tyrosine and 4-HPL increased moderately while that of 4-HPP and 4-HPA increased slightly.5)
These findings suggested that the metabolic defect in our patient was a deficiency of 4-HPPD. Although the mechanism of increased formation of 4-HPL in our patient was not known, it was considered to be due to increased enzymatic reduction of 4-HPP to 4-HPL.
3). Clinical course and outcome.6)
From April 1956 he regularly suffered from diarrhea and often caught colds. His weight gradually decreased and rachitic changes, including genu valgum, became evident. Rickets was confirmed by X-ray examination. On the basis of clinical observations and persistent hypophosphatemia, it was suspected that he suffered from Fanconi type renal rickets, such as cystinosis.
In December 1956 we observed that his hepatosplenomegaly was increasing and the liver felt irregular and hard.
He contracted measles in March 1957, after which his appetite became further reduced, vomiting sometimes occurred, and he gradually grew weaker. The edge of the liver and spleen were both palpable about 8 cm below the costal margin. He was hospitalized again November 1957. Finally, he fell into hepatic coma and he died in November.
At autopsy, the liver showed portal cirrhosis with malignant hepatoma. The spleen showed fibroadeny, and tubular nephrosis was found.
With the parents’ permission, a liver biopsy was performed one hour after death. The tyrosine oxidation activity of the liver homogenate was found to be only 22.4% of normal.6) From these observations, it seemed that the low activity of 4-HPPD caused a large accumulation of 4-hydroxyphenyl compounds in the tissues, and these were excreted in the urine.
4). Differences between Medes’s case and our patient.
As shown in Table 1, while a deficiency of 4-HPPD activity was proposed in both Medes’s3) and our case,5,6) there were significant differences between the clinical features and biochemical abnormalities in the two patients. In Medes’s patient only 4-HPP was excreted (except on a high tyrosine diet), whereas in our patient4,5) 4-HPL was the major tyrosyl product in the urine. Kretchmer et al.7,8) reported that 4-HPPD is relatively deficient in premature infants, but if they are fed extra tyrosine they will excrete several times as much 4-HPL as 4-HPP. Scorbutic guinea pigs9) and children10) also excrete appreciable quantities of the lactic derivative of the 4-hydroxyphenyl compound. Therefore, it was difficult to understand why only 4-HPP was excreted in Medes’s case. One would expect that if 4-HPP was present in the tissues, reduction of a considerable amount of keto acid to 4-HPL by lactic acid dehydrogenase11) and/or aromatic α-keto acid reductase12,13) would take place.
Table 1.
A comparison of the clinical and biochemical features between Medes’s and our patients
| Medes’s case | Our case | ||
|---|---|---|---|
| Clinical features | Age at diagnosis | 49 years old | 2 years old |
| Liver cirrhosis | (−) | (++) | |
| Renal tubular defect | (−) | (+) | |
| Renal rickets | (−) | (+) | |
| Myasthenia gravis | (+) | (−) | |
| Urinary excretion of 4-hydroxyphenyl compounds (mg/day)* | Tyrosine | 380 | 350 |
| 4-HPP | 2800 | 250 | |
| 4-HPL | (−) | 1000 | |
| 4-HPA | (−) | 150 | |
| l-Dopa | (+) | (−) | |
| Proposed enzyme defect | 4-HPPD | 4-HPPD | |
*:4-hydroxyphenyl compounds in urine from the both patients on mixed hospital diet were measured.
According to the description of Tyrosinosis (Medes) and Tyrosinosis (Sakai) by Auerbach VII and DiGeorge AM in Nelson Textbook of Pediatrics 1975,14) although 4-HPPD deficiency was proposed in both cases, the clinical differences between the two cases were as follows.
Tyrosinosis (Medes): In 1932, Medes reported a study of an adult male with a defect of tyrosine metabolism. No symptoms could be related to the metabolic defect. He excreted more than 1 g/day of 4-HPP in the urine. Medes proposed that the tyrosyluria was due to an almost complete absence of 4-HPPD.
Tyrosinosis (Sakai): Another clinical disorder associated with a defect in 4-HPPD in infants and children was first described by Sakai. The onset was usually between 1 and 6 months of age. The most frequent clinical manifestations were failure to thrive, irritability, fever, and hepatomegaly, with anorexia, vomiting, diarrhea, and abdominal distension being commonly found.
5). Review of the literature (1932–1976).
Several studies of infants15,16) and adults17) with liver cirrhosis and/or hepatospenomegaly together with Fanconi type renal rickets appeared before our original report was published in 1957. However, among these patients there was no suggestion of abnormal tyrosine metabolism.
After reading Medes’s pioneering 1932 study,3) we were inspired to publish our report as an atypical case of tyrosinosis. As no other cases resembling Medes’s have emerged as far as we are aware, our case appears to be the first report of a typical HRT patient.
In the mid-1960s a Scandinavian group18–23) and a Canadian group24) published papers on the clinical features and laboratory findings in patients with HRT in which they also referred to our original reports and suggested that all these patients belonged to the same clinical entity. Also, a Scandinavian group23) and a Canadian group24) found that hypermethioninemia occasionally occurred in patients with HRT, especially in the acute stage.
In June 1965, L.R. Gjessing and S. Halvorsen in Norway organized a symposium on tyrosinosis in honour of Dr. Grace Medes. The meeting was attended by Dr. Medes and Dr. Asbjorn Fölling, Professor Emeritus, University of Oslo, who described the first case of phenylketonuria in 1934. The proceedings of the symposium were published in 1966.25) Sixteen participants attended the meeting and nine scientific lectures, including one by Medes, were given. However, no additional information was presented by Medes.
Another symposium on tyrosinemia, under the title of “Conference on hereditary tyrosinemia”, was held at the Hospital for Sick Children, Toronto, Canada, in March, 1966.26)
Twenty six scientists attended the conference. Three came from the United States and 23 were from Canada. The lectures and case reports presented at the conference appeared in Canadian Medical Association J., vol. 97, 1967.
In six of these case reports, the clinical manifestations and laboratory findings of 48 patients with HRT were presented. One paper, by Scriver, C.R. et al.,27) presented the clinical manifestations of four patients with HRT whose onset was between two and 32 months. Larochelle, J. et al.28) reported the clinical observations of 37 infants with HRT. The most common clinical features in those patients were hepatomegaly, failure to thrive, fever, edema, vomiting, diarrhea, irritability, lethargy, abdominal distension, a hemorrhagic tendency, and a peculiar odor. It is noteworthy that all these features were found in our patient, except for the odor. It is recognised that the odor in patients with HRT is associated with hypermethioninemia, and this, together with aforementioned symptoms and signs, are seen frequently in acute type HRT. It is interesting that two of the four cases reported by Scriver et al.27) and all three cases described by Sass-Kortsak, A. et al.29) showed Fanconi syndrome, as in our case.
At the conference, Dr. Scriver gave a lecture entitled “The phenotypic manifestation of hereditary tyrosinemia and tyrosyluria: a hypothesis”. He kindly acknowledged our original work as follows:30) “I would like to draw attention to an excellent study published quietly a decade ago in a university medical journal in Japan. Sakai, Kitagawa and Yoshioka investigated a single Japanese patient with “tyrosinosis” and described practically everything we have to say today. They in fact laid the foundation for this conference.”
Laberge, C. in 196931) and Bergeron, P. et al. in 197432) reported that the incidence of HRT in the province of Quebec, Canada was as high as one in 800 births, and DeBraekeleer et al.33) in 1990 reported that the prevalence at birth was estimated at one in 1846 live born in the same region of Quebec, while Kvittingen, E.A.34) described an incidence of one in 50,000 births in Scandinavia in 1986. In Quebec, the acute form of HRT is usually diagnosed in the first few months of life and if untreated these patients die from liver failure within their first year of life.
In the chronic form of HRT, as described in Scandinavia, the patients show the gradual development of liver cirrhosis as well as Fanconi type renal rickets, and often develop hepatocellular carcinoma in their teens.35)
Our patient was diagnosed at the age of 3 years and died at 5 years one month of age, without treatment. Thus, our patient may be classified as the subchronic type of HRT.
Discovery of the pathogenesis of HRT
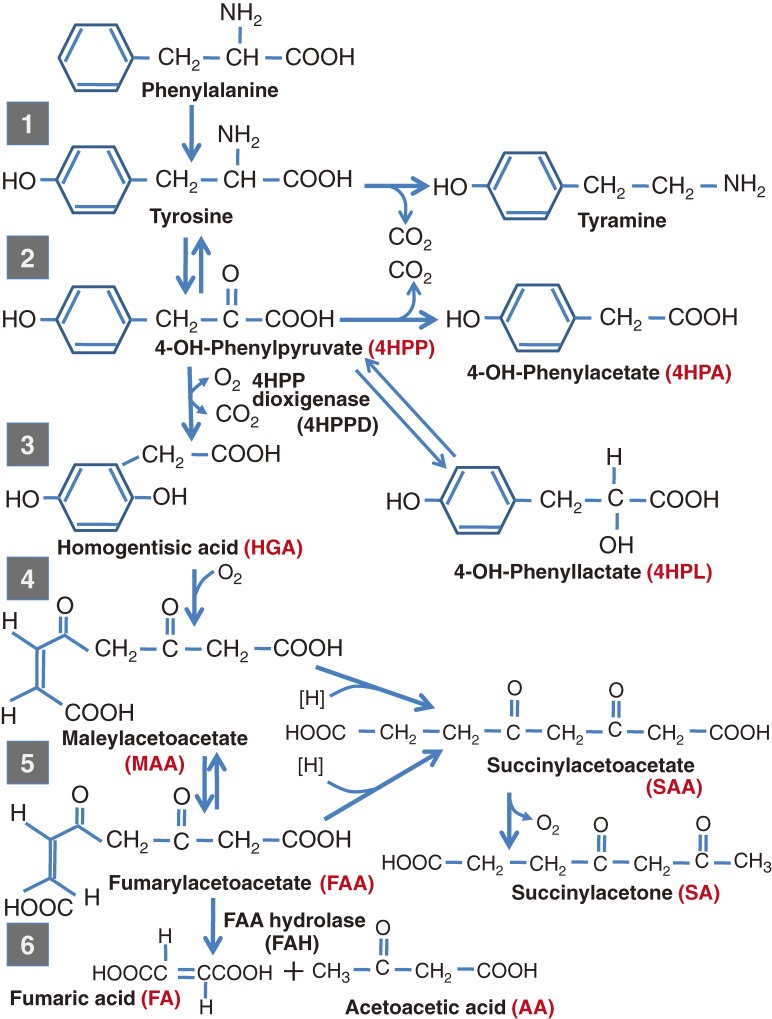
As described previously, 4-HPPD deficiency was originally proposed as the cause of HRT6,21,22,36) (Fig. 2) based on enzymatic studies of liver tissue obtained at biopsy or autopsy. However, since 1965 there was increasing doubt that this hypothesis was correct, and suggestions were made that the primary metabolic defect in HRT is something other than a lack of 4-HPPD. As mentioned before, Scandinavian and Canadian groups19,23,24) reported that hypermethioninemia is occasionally found in patients with acute HRT. In 1968 and 1970, Gaull et al.37,38) described reduced activity of methionine activating enzyme and cystathionine synthetase in these patients, which they suggested were secondary manifestations of an unknown hereditary metabolic disease. It has also been reported that several patients with HRT showed signs of polyneuropathy which resembled crises in acute intermittent porphyria. In 1969, Gentz, J. et al.39) in a Swedish group measured the excretion of delta-aminolevulinic acid (ALA), porphobilinogen and porphyrins in patients with hepatorenal tyrosinemia who had attacks of polyneuropathy. They found that the urinary ALA was about 100 times the normal level, but urinary porphobilinogen and porphyrin were within the normal ranges or slightly elevated.
Figure 2.
Metabolic pathway of phenylalanine and tyrosine.
Lindblad, B., Lindstedt, S., and Steen, G.40) in the same group in 1977 found that ALA dehydratase activity in erythrocytes and liver from patients with HRT was less than 5% and 1% deficient, respectively, compared with controls. They conjectured that the high excretion of ALA in the urine of some patients with HRT was due to low activity of ALA dehydratase. Subsequently, when the serum and urine from these patients, but not from controls, was added to an assay for ALA dehydratase activity, they found the enzyme activity markedly decreased. Eventually, by gas-liquid chromatography-mass-spectrometry, they identified succinylacetone and succinylacetoacetate in the urine from these patients as the enzyme inhibitors.
From these results, they concluded that the severe liver and kidney damage found in HRT was due to the accumulation of these tyrosine metabolites, and that the primary enzyme defect in HRT was decreased activity of FAH. These observations have been confirmed by work done between 197941) and 198142,43) by direct enzyme assays. Once the primary enzyme defect was identified as FAH, the characteristic pattern of increased urinary 4-hydroxyphenyl compounds with absence of homogeneity, could be attributed to secondary 4-HPPD deficiency.
As also mentioned above, two major clinical forms of HRT have been reported: acute and chronic. Tanguay, R.M. et al.44) demonstrated that the basic defect in the acute form is due to the absence of enzymatically and immunologically measurable activity of FAH in the liver and kidneys.
It has been reported that residual FAH immunoreactivity in liver extracts of patients with chronic HRT correlates with their enzyme activity measured by hydrolysis of fumarylacetoacetate. These observations suggest that the two forms of HRT are the result of different molecular defects.
It is interesting that soon after the primary enzyme defect in HRT was confirmed, two patients with a new form of hereditary tyrosinemia with primary 4-HPPD defect were identified in 1983 independently in the US45) and Japan.46) This disease was assigned MIM 276710 and called tyrosinemia type III.
Tyrosinemia and tyrosyluria are two of the important indicators in the biochemical phenotype of patients with HRT. However, an accumulation of tyrosine and its early metabolites, such as 4-hydroxyphenyl compounds, are unlikely to cause the hepatorenal symptoms. In contrast, it has been known that FAH deficiency may cause accumulation of maleylacetoacetate (MAA) and fumarylacetoacetate (FAA) and their derivatives, such as succinylacetoacetic acid (SAA) and succinylacetone (SA), and these may cause the hepatorenal features.
Laboratory findings, diagnosis and treatment
1). Laboratory findings and diagnosis.
Hypertyrosinemia and tyrosyluria are the most common findings, but hypermethioneima and increased excretion of ALA in the urine are occasionally detected, especially in the acute stage. Another important feature is renal rickets associated with hyperphosphaturia and hypophosphatemia due to reduced tubular reabsorption of phosphorus. Other signs of renal dysfunction are glucosuria, proteinuria, and hyperaminoaciduria (renal Fanconi syndrome).
Coagulation factors produced in the liver are reduced, and there is a bleeding tendency and moderate anemia. In the acute stage, patients show hypoproteinemia, hyperbilirubinemia, deterioration of liver function and occasionally polyneuropathic pain. Leukopenia and thrombocytopenia are usually present in the chronic phase.
The α-fetoprotein level is raised in the acute and in chronic stages, especially with the development of hepatocellular carcinoma, and the serum transaminases are increased, particularly in acute hepatic episodes.
The plasma tyrosine level has less diagnostic value than the elevated concentration of succinylacetone in the blood or urine and the reduced level of fumarylacetoacetase in the blood. In neonatal screening by measuring the blood tyrosine level, only a minority of patients with HRT are identified. A more reliable way to identify these patients would be to measure fumarylacetoacetase using ELISA47) or to determine succinylacetone using UPLC-MS/MS in dried blood spots from newborn infants.48)
If it became practicable to measure succinylacetone or fumarylacetoacetase as part of a newborn screening program, this might lead to an improved prognosis of patients with HRT.
2). Treatment.
A low phenylalanine and tyrosine diet for HRT patients was introduced by Halvorsen and Gjessing in 196420) and for a long time was the only treatment available. It had a beneficial effect on the renal tubular defects, but did not cure the liver disease.
After the discovery of FAH deficiency as the primary defect in HRT, tratment with NTBC (2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cycloxanedione), which is a potent inhibitor of 4-HPPD and reduces the accumulation of harmful tyrosine metabolites, was introduced by Lindstedt, S. et al. in 1992.49) This treatment may prevent acute hepatic crises. Patients treated with NTBC are also prescribed a diet low in phenylalanine and tyrosine.
In 2010, Schlump, J.-U. et al.50) reported that a significant increase in succinylacetone was observed within the first 12 hours of life in a newborn baby with HRT without treatment. It may be necessary to make an early diagnosis and start the treatment. It appeared that NTBC slowed progression of the disease, but the liver damage was irreversible and patients must be followed for the development of hepatocellular carcinoma. Recent reports, however, indicate that the results of treatment with NTBC are considerably better than had been expected:51,52) succinylacetone may become undetectable in the urine and α-fetoprotein is maintained in the normal range during this treatment. However, long term follow-up is needed to confirm these results.
In an overview of HRT by Kvittingen in 1986,34) the case of a patient with HRT is described who was treated in 1978 with a liver transplant performed by Fisch et al. The patient died three months later but the biochemical derangements apparently improved. Subsequently, the use of liver transplants in HRT cases has increased and the benefits appear to be confirmed. Paradis et al.53) reported in 1990 that six out of seven patients who received liver transplants survived with normal liver function and growth on a normal diet. Thus, liver transplantation seems to be an effective treatment and it may reduce the risk of hepatocellular carcinoma.54) However, long term follow-up of the patients with HRT treated with liver trasplantation may be neccessary to confirm the results.
Conclusion
Following our first report of a typical patient with HRT in 1957 and the identification in 1977 by Lindblad et al. of the primary enzyme defect, effective treatment is now a possibility if the condition is detected early. It would require the combination of NTBC therapy, a low phenylalanine and tyrosine diet, and liver transplantation.
Acknowledgement
The author very much appreciated to Prof. Kiyoshi Sakai, the Department of Pediatrics, the Tokyo Jikei University School of Medicine for his helpful guidance in the clinical work of this study and also to Prof. Katashi Makino, the Department of Biochemistry, the Tokyo Jikei University School of Medicine for his great teaching in the biochemical work and Prof. Tamio Yamakawa, the Department of Biochemistry, the Tokyo University School of Medicine for his helpful advice and stimulating discussions in the biochemical work of this study.
Profile
Teruo Kitagawa was born in Tokyo in 1926. He graduated from Tokyo Jikei University School of Medicine in 1950. From 1952 to 1957, he received postgraduate training at the Department of Pediatrics, Tokyo Jikei University School of Medicine and he obtained a Ph.D. in 1957. During his training he had the opportunity to see patients with various inherited metabolic disorders such as congenital erythropoietic porphyria, hepatorenal tyrosinemia and others. Thus, he became interested in the study of inherited metabolic disorders. In 1959, he was appointed to Assistant Professor of Pediatrics at Tokyo Jikei University. From 1960–61, he was a post-doctoral fellow at the Oncology Research Institute of Wisconsin University where he studied tryptophan metabolism, and from 1961–62, he was a post-doctoral fellow at the Thannhauser Memorial Institute at Tafts University School of Medicine where he studied inborn errors of lipid metabolism. In 1966, he was appointed to Associate Professor of Pediatrics at Tokyo Jikei University. In 1971, he was promoted to Professor of Pediatrics at Nihon University School of Medicine. In 1974, Teruo Kitagawa developed a mass-screening system for inborn errors of metabolism in the Tokyo Metropolitan Area in collaboration with the Tokyo Obstetrician Association and Tokyo Health Service Association. From 1977 to 1993, he was the Chairman of the Committee of Newborn Screening for Inborn Errors of Metabolism in the Tokyo Metropolitan Area conducted by the Department of Maternal and Child Health of the Tokyo Metropolitan Government.
From 1980 to the present, he has been the Chairman of the Committee on the Study and Development of Special Dietary Products for the Treatment of Inborn Errors of Metabolism. The main activity of the committee is the development of new products and the keeping of a stable supply of formula for the treatment of inborn errors of metabolism. These activities have contributed to the prevention of mental retardation as well as other disabilities which are caused by inherited metabolic disorders.
In 1986, Teruo Kitagawa received the Memorial Award from the Japanese Society for Human Genetics and was appointed to Chairman and Professor of Pediatrics, Nihon University School of Medicine. In 1992, Teruo Kitagawa received the Medal with Purple Ribbon from the Japanese Government for excellence in pediatric research and he was promoted to Professor of Institute of Medical Sciences, Nihon University School of Medicine. In 1994, he was appointed to Vice President of Kokusai Gakuin Saitama Junior College, and Emeritus Professor of Nihon University. Since 1997, Teruo Kitagawa has been the Chairman of the Board of Directors, Tokyo Health Service Association.
References
- 1).Mitchell, G.A., Grompe, M., Lambert, M. and Tanguay, R.M. (2007) Chapter 79 Hypertyrosinemia. Part 8 Amino acids In Scriver’s The Online Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill, New York, pp 1–51. [Google Scholar]
- 2).Medes G., Berglund H., Lohmann A. (1927) An unknown reducing urinary substance in myasthenia gravis. Proc. Soc. Exp. Biol. Med. 25, 210–211 [Google Scholar]
- 3).Medes G. (1932) A new error of tyrosine metabolism, tyrosinosis. The intermediary metabolism of tyrosine and phenylalanine. Biochem. J. 26, 917–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Sakai K., Kitagawa T. (1957) An atypical case of tyrosinosis (l-para-hydroxyphenyllactic aciduria) Part 1. Clinical and laboratory findings. Jikeikai Med. J. 4, 1–10 [Google Scholar]
- 5).Sakai K., Kitagawa T. (1957) An atypical case of tyrosinosis (l-para-hydroxyphenyllactic aciduria) Part 2. A research on the metabolic block. Jikeikai Med. J. 4, 11–15 [Google Scholar]
- 6).Sakai K., Kitagawa T., Yoshikawa K. (1959) An atypical case of tyrosinosis (l-para-hydroxyphenyllactic aciduria) III. The outcome of the patients. Pathological and biochemical observations on the organ tissues. Jikeikai Med. J. 6, 15–24 [Google Scholar]
- 7).Kretchmer N., Levine S.Z., McNamara H., Barnett H.L. (1956) Certain aspects of tyrosine metabolism in the young. I. The development of the tyrosine oxidizing system in human liver. J. Clin. Invest. 35, 236–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Kretchmer N., Levine S.Z., McNamara H. (1957) The in vitro metabolism of tyrosine and its intermediates in the liver of the premature infant. Am. J. Dis. Child. 93, 19–20 [Google Scholar]
- 9).Sealock R.R., Silberstein H.E. (1940) The excretion of homogentisic acid and other tyrosine metabolites by the vitamin C-deficient guinea pig. J. Biol. Chem. 135, 251–258 [Google Scholar]
- 10).Huisman T.H.J., Jonxis J.H.P. (1957) Some investigations on the metabolism of phenylalanine and tyrosine in children with vitamin C deficiency. Arch. Dis. Child. 32, 77–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Meister A. (1950) Reduction of α,γ-diketo and α-keto acids catalyzed by muscle preparations and by crystalline lactic dehydrogenase. J. Biol. Chem. 184, 117–129 [PubMed] [Google Scholar]
- 12).Zannoni V.G., Weber W.W. (1966) Isolation and properties of aromatic α-keto acids reductase. J. Biol. Chem. 241, 1340–1344 [PubMed] [Google Scholar]
- 13).Weber W.W., Zannoni V.G. (1966) Reduction of phenylpyruvic acids to phenyllactic acids in mammalian tissues. J. Biol. Chem. 241, 1345–1349 [PubMed] [Google Scholar]
- 14).Auerbach, V.H. and DiGeorge, A.M. (1975) Defects in metabolism of amino acids. In Nelson Textbook of Pediatrics, 10th (eds. Vaughan, V.C., Mackey, R.J. and Nelson, W.E.). WB Saunders, Philadelphia, pp 412–432. [Google Scholar]
- 15).Baber M.D. (1956) A case of congenital cirrhosis of the liver with renal tubular defects akin to those in the Fanconi syndrome. Arch. Dis. Child. 31, 335–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Guild H.G., Pierce J.A., Lilienthal J.L. (1937) An unfamiliar rachitic syndrome. Am. J. Dis. Child. 54, 1186–1188 [Google Scholar]
- 17).Stowers J.M., Dent C.E. (1947) Studies on the mechanism of the Fanconi syndrome. Q. J. Med. 16, 275–292 [PubMed] [Google Scholar]
- 18).Zetterström R. (1963) Tyrosinosis. Ann. N. Y. Acad. Sci. 111, 220–226 [DOI] [PubMed] [Google Scholar]
- 19).Fritzell S., Jagenburg R., Schnurer L.B. (1964) Familial cirrhosis of the liver, renal tubular defects with rickets and impaired tyrosine metabolism. Acta Paediatr. Scand. 53, 18–32 [DOI] [PubMed] [Google Scholar]
- 20).Halvorsen S., Gjessing L.R. (1964) Studies on tyrosinosis. 1. Effect of low-tyrosine and low-phenylalanine diet. Br. Med. J. 2, 1171–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Gentz J., Jagenburg M., Zetterström R. (1965) Tyrosinemia: an inborn error of tyrosine metabolism with cirrhosis of the liver and multiple renal tubular defects (de Toni-Debre-Fanconi Syndrome). J. Pediatr. 66, 670–696 [DOI] [PubMed] [Google Scholar]
- 22).Taniguchi K., Gjessing L.R. (1965) Studies on tyrosinosis: 2, activity of the transaminase, parahydroxy-phenylpyruvate oxidase, and homogentisic-acid oxidase. Br. Med. J. 1, 968–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Gjessing L.R., Halvorsen S. (1965) Hypermethioninemia in acute tyrosinosis. Lancet II, 1132–1133 [Google Scholar]
- 24).Scriver C.R., Clow C.L., Siverberg M. (1966) Hypermethioninemia in acute tyrosinosis. Lancet 1, 153–154 [Google Scholar]
- 25).Medes, G. and Gjessing, L.G. (1966) Symposium on Tyrosinosis, in honour of Dr. Grace Medes, 2–3 June, 1965. Universitetsforlaget, Oslo. [Google Scholar]
- 26).Scriver C.R., Partington M., Sass-Kortsak A. (1967) Conference on Hereditary Tyrosinemia. Can. Med. Assoc. J. 97, 1045–1101 [Google Scholar]
- 27).Scriver C.R., Silverberg M., Clow C.L. (1967) Hereditary tyrosinemia and tyrosyluria: clinical report of four patients. Can. Med. Assoc. J. 97, 1047–1050 [PMC free article] [PubMed] [Google Scholar]
- 28).Larochelle J., Mortezai A., Belanger M., Tremblay M., Claveau J.C. (1967) Experience with 37 infants with tyrosinemia. Can. Med. Assoc. J. 97, 1051–1054 [PMC free article] [PubMed] [Google Scholar]
- 29).Sass-Kortsak A., Ficici S., Paunier L., Kooh S.W., Fraser D., Jackson S.H. (1967) Clinical and biochemical study of three patients with tyrosyluria. Can. Med. Assoc. J. 97, 1056–1058 [PMC free article] [PubMed] [Google Scholar]
- 30).Scriver C.R. (1967) The phenotypic manifestations of hereditary tyrosinemia and tyrosyluria: a hypothesis. Can. Med. Assoc. J. 97, 1073–1075 [PMC free article] [PubMed] [Google Scholar]
- 31).Laberge C. (1969) Hereditary tyrosinemia in a French Canadian isolate. Am. J. Hum. Genet. 21, 36–46 [PMC free article] [PubMed] [Google Scholar]
- 32).Bergeron P., Laberge C., Grenier A. (1974) Hereditary tyrosinemia in the province of Quebec: prevalence at birth and geographic distribution. Clin. Genet. 5, 157–162 [DOI] [PubMed] [Google Scholar]
- 33).DeBraekeleer M., Larochelle J. (1990) Genetic epidemiology hereditary tyrosinemia in Quebec and in Saguenay-Lac-St-Jean. Am. J. Hum. Genet. 47, 302–307 [PMC free article] [PubMed] [Google Scholar]
- 34).Kvittingen E.A. (1986) Hereditary tyrosinemia type 1—an overview. Scand. J. Clin. Invest. 46 (suppl 184), 27–34 [PubMed] [Google Scholar]
- 35).Winberg A.G., Mize C.E., Worthen H.G. (1976) The occurrence of the hepatoma in the chronic form of hereditary tyrosinemia. J. Pediatr. 88, 434–438 [DOI] [PubMed] [Google Scholar]
- 36).La Du B.N. (1967) The enzymatic deficiency in tyrosinemia. Am. J. Dis. Child. 113, 54–57 [DOI] [PubMed] [Google Scholar]
- 37).Gaull G.E., Rassin D.K., Sturman J.A. (1968) Significance of hypermethioninaemia in acute tyrosinosis. Lancet 1, 1318–1319 [DOI] [PubMed] [Google Scholar]
- 38).Gaull G.E., Rassin D.K., Solomon G.E., Harris R.C., Sturman J.A. (1970) Biochemical observations on so-called hereditary tyrosinemia. Pediatr. Res. 4, 337–344 [DOI] [PubMed] [Google Scholar]
- 39).Gentz J., Johansson S., Lindblad B., Lindstedt S., Zetterström R. (1969) Excretion of δ-aminolevulinic acid in hereditary tyrosinemia. Clin. Chim. Acta 23, 257–263 [DOI] [PubMed] [Google Scholar]
- 40).Lindblad B., Lindstedt S., Steen G. (1977) On the enzyme defects in hereditary tyrosinemia. Proc. Natl. Acad. Sci. U.S.A. 74, 4641–4645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Fällström S.-P., Lindblad B., Lindstedt S., Steen G. (1979) Hereditary tyrosinemia-fumarylacetoacetase deficiency. Pediatr. Res. 13, 78 (abstr.) [Google Scholar]
- 42).Berger R., Smit G.P.A., Stoker-de Vaies S., Duran M., Ketting D., Wadman S.K. (1981) Deficiency of fumarylacetoacetase in a patients with hereditary tyrosinemia. Clin. Chim. Acta 114, 37–47 [DOI] [PubMed] [Google Scholar]
- 43).Kvittingen E.A., Jellum E., Stokke O. (1981) Assay of fumarylacetoacetate fumarylhydrolase in human liver-deficient activity in case of hereditary tyrosinemia. Clin. Chim. Acta 115, 311–319 [DOI] [PubMed] [Google Scholar]
- 44).Tanguay R.M., Vaet J.P., Lescaul A., Duband J.L., Laberge C., Lettre F., Plante M. (1990) Different molecular basis for fumarylacetoacetate hydrolase deficiency in the two clinical forms of hereditary tyrosinemia. Am. J. Hum. Genet. 47, 308–316 [PMC free article] [PubMed] [Google Scholar]
- 45).Giardini O., Cantani A., Kennaway N.G., D’Eufemia P. (1983) Chronic tyrosinemia associated with 4-hydroxyphenylpyruvate dioxgenase deficiency with acute intermittent ataxia and without visceral and bone involvement. Pediatr. Res. 27, 25–29 [DOI] [PubMed] [Google Scholar]
- 46).Endo F., Kitano A., Uehara I., Nagata N., Matsuda I., Shinka T., Kuhara T., Matsumoto I. (1983) Four-hydroxyphenylpyruvic acid oxidase deficiency with normal fumarylacetoacetase: A new variant form of hereditary hypertyrosinemia. Pediatr. Res. 17, 92–96 [DOI] [PubMed] [Google Scholar]
- 47).Laberge C., Grenier A., Valet J.P., Morissette J. (1990) Fumarylacetoacetase measurement as a mass-screening procedure for hereditary tyrosinemia type 1. Am. J. Hum. Genet. 47, 325–328 [PMC free article] [PubMed] [Google Scholar]
- 48).Al-Dirbashi O.Y., Rashed M.S., Jacob M., Al-Ahaideb L.Y., Al-Amoudi M., Rahbeeni Z., Al-Sayed M.M., Al-Hassnan Z., Al-Owain M., Al-Zeidan H. (2008) Improved method to determine succinylacetone in dried blood spots for diagnosis if tyrosinemia type 1 using UPLC-MS/MS. Biomed. Chromatogr. 22, 1181–1185 [DOI] [PubMed] [Google Scholar]
- 49).Lindstedt S., Holme E., Lock E.A., Hjalmarson O., Strandvik B. (1992) Treatment of hereditary tyrosinemia type 1 by inhibition of 4-hydroxphenylpyruvate dioxygenase. Lancet 340, 813–817 [DOI] [PubMed] [Google Scholar]
- 50).Schlump J.-U., Mayatepek E., Spiekerkoetter U. (2010) Significant increase of succinylacetone within the first 12 h of life in hereditary tyrosinemia type 1. Eur. J. Pediatr. 169, 569–572 [DOI] [PubMed] [Google Scholar]
- 51).El-Karaksy H., Rashed M., El-Sayed R., El-Raziky M., El-Koofy N., El-Hawary M., Al-Dirbashi O. (2010) Clinical practice NTBC therapy for tyrosinemia type 1: how much is enough. Eur. J. Pediatr. 169, 689–693 [DOI] [PubMed] [Google Scholar]
- 52).Ito M., Matsuda S., Shinahara K. (2005) A single case report of tyrosinemia type 1 who shows an excellent clinical status with long term administration of NTBC. Bulletin on Special Formula 41, 27–30 [Google Scholar]
- 53).Paradis K., Weber A., Seidman E.G., Larochelle J., Garel L., Lanaerts C., Roy C.C. (1990) Liver transplantation for hereditary tyrosinemia Quebec experience. Am. J. Hum. Genet. 47, 338–342 [PMC free article] [PubMed] [Google Scholar]
- 54).Ueda A., Ito T., Okubo Y., Yokoi A., Tokari H. (2005) A case of tyrosinemia type 1 treated with administration of NTBC followed by liver transplantation. Bulletin on Special Formula 41, 23–26 [Google Scholar]