Abstract
Congenital cystic lesions of the lung in fetuses are rare. The most common malformations of the lower respiratory tract are congenital cystic adenomatoid malformation and bronchopulmonary sequestration. With the increased use of obstetric ultrasound, cystic lung lesions are detected more often antenatally, which allows for proper planning of peripartum and neonatal management. This article discusses a range of diagnostic and management options.
Key words: Congenital cystic adenomatoid malformation, Bronchopulmonary sequestration, Congenital pulmonary airway malformation, Pulmonary hypoplasia
Congenital cystic lesions of the lung are rare. The most common malformations of the lower respiratory tract are congenital cystic adenomatoid malformation (CCAM), also known as congenital pulmonary airway malformation, and bronchopulmonary sequestration (BPS). With the increased use of obstetric ultrasound, cystic lung lesions are detected more often, which allows for proper planning of peripartum and neonatal management.
Incidence and Pathogenesis
The reported incidence of CCAM ranges from 1 in 11,000 to 1 in 35,000 live births, with a higher incidence in the midtrimester due to spontaneous resolution.1,2 BPS is even more rare, with no published population incidence. CCAM and BPS represent abnormalities that occur during the branching and proliferation of the bronchial structures. CCAM is a hamartomatous lesion containing tissue from different pulmonary origins. BPS is made of extraneous and nonfunctioning lung tissue that has separated itself from the normal pulmonary structure. Both lesions have malignant potential.3 In addition, hybrid lesions exist that contain features of both.4,5 Thus, although the pathogenesis of these lesions is poorly understood, they may have a common origin.6 Theories of their pathogenesis include abnormal proliferation of tissues, airway obstruction, and dysplasia and metaplasia of normal tissues.7 In most cases, it seems that the insult occurs during the pseudoglandular phase of lung development, which spans 7 to 17 weeks of gestation.
Key Diagnostic Features
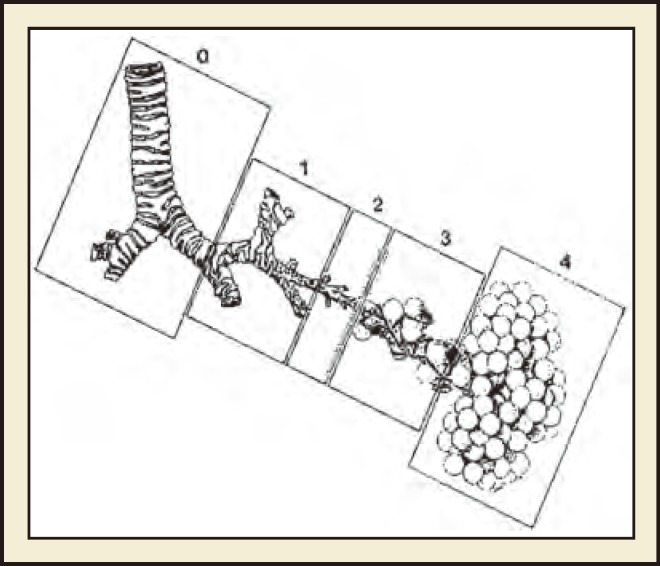
There is significant overlap in the findings and course of CCAM and BPS; however, a number of key features distinguish them from one another (Table 1). Classification schemes for CCAM have evolved, and there are currently five main types, which differ based on the embryologic level of origin and the histologic features (Figure 1).8,9
Table 1.
Differences Between CCAM and BPS
| CCAM | BPS |
||
| Intralobar | Extralobar | ||
| Incidence | 1/11,000–1/35,000 | Rare | |
| Vascular supply | Pulmonary | Systemic: lower thoracic or upper abdominal aorta | Systemic: thoracic aorta |
| Laterality | 80%–95% unilateral, either lobe | 60% left sided | 90% left sided |
| Sex | Male > Female | Male = Female | Male > Female |
| Tracheobronchial communication | Present, constricted/anomalous | None | None |
| Associated anomalies | Rare except for Type 2 (60%) | 17% | 40% |
Figure 1.
Congenital cystic adenomatoid malformation classification based on presumed site of development of the malformation. 0 = tracheobronchial, 1 = bronchial/bronchiolar, 2 = bronchiolar, 3 = bronchiolar/alveolar, 4 5 distal acinar. Stocker JT, Fetal Pediatr Pathol. 2009;28:155–184, copyright © 2009, Informa Healthcare. Reproduced with permission from Informa Healthcare.9
Type 0
Type 0 CCAM is the rarest form and arises from the trachea or bronchus. The presentation is severe and usually lethal.10 Cysts are small.
Type 1
Type 1 CCAM is the most common form, representing 50% to 70% of cases, and it arises from the distal bronchus or proximal bronchiole. There are usually a small number of large echolucent cysts, measuring 3 to 10 cm.10 A single dominant cyst may also be seen. Cyst walls are thin and are lined by ciliated pseudostratified epithelium, although other cell types such as cartilage may be found between the cysts. Because these CCAMs may be large, they may have significant mass effect, which can lead to hydrops.
Type 2
Type 2 CCAMs account for 15% to 30% of cases and arise from terminal bronchioles. They are composed of smaller cysts, measuring 0.5 to 2 cm, as well as solid areas that may be difficult to distinguish from surrounding tissue. These are lined by ciliated cuboidal or columnar epithelium, and elements of bronchioles or alveoli may be seen. Frequently the cysts are more evenly spaced than in Type 1 CCAMs. Type 2 CCAMs have the highest incidence of associated anomalies, up to 60%, and prognosis depends on these findings, which include most organ systems (Figures 2–7).10
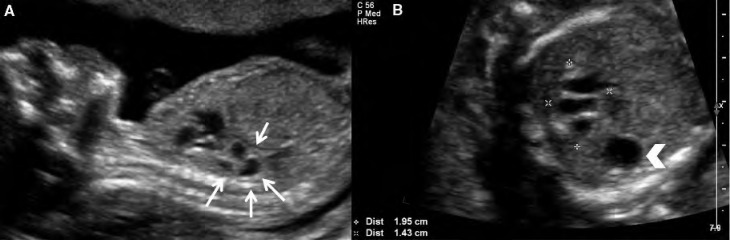
Figure 2.
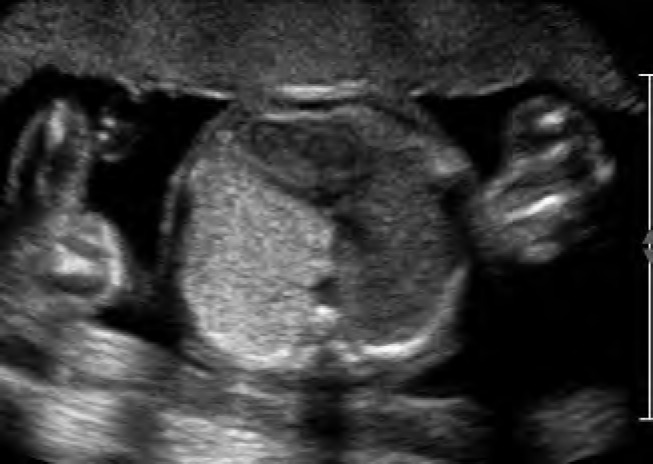
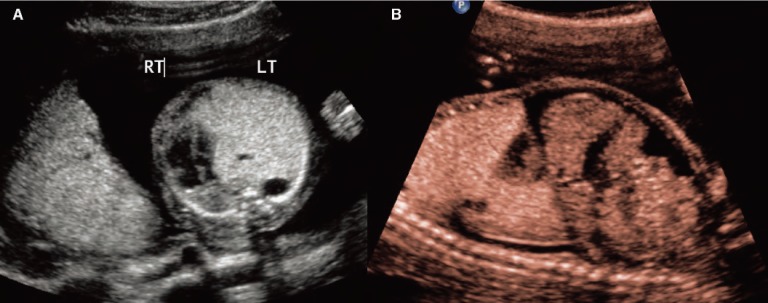
Case 1. Type 2 congenital cystic adenomatoid malformation (CCAM). A, Sagittal image of a fetus at 24 weeks with Type 2 CCAM located in the posterior chest (arrows). B, Transverse image with measurements showing the inferior extent of the lesion. The mass is multicystic and located inferior and posterior to the heart. Arrowhead indicates the stomach.
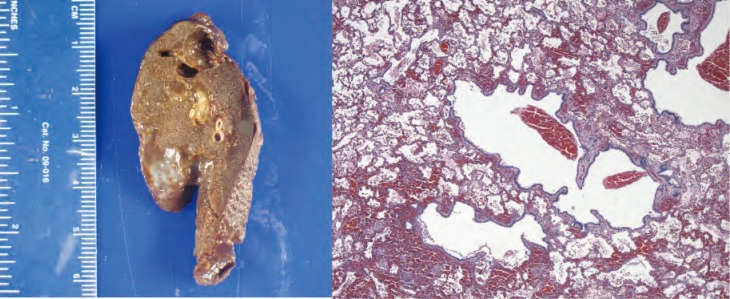
Figure 7.
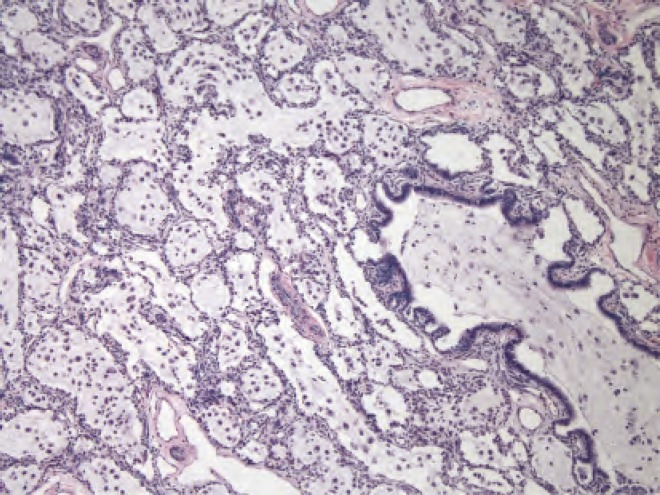
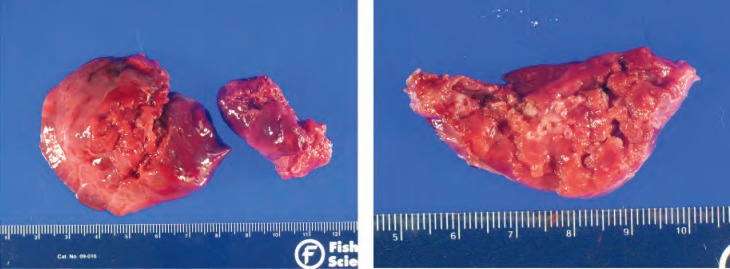
Gross and histologic specimens of the patient in Case 2; the patient underwent left upper lobe lobectomy at 4 months of life. Histology shows several small evenly spaced cystic structures of relatively uniform size are present in the area illustrated. The cysts are lined by ciliated cuboidal to columnar epithelium that overlies a fine fibromuscular layer, barely visible at this magnification. Image courtesy of A. Brian West, MD, FRCPath, Yale School of Medicine, New Haven, CT.
Type 3
Type 3 CCAMs account for 5% to 10% of cases and are thought to arise from acinar-like tissue. Type 3 CCAMs are composed of cysts that are so small the mass appears to be solid and highly echogenic on ultrasound (Figures 8 and 9). The tissue is acinar and shows adenomatoid elements consistent with distal airway. These masses may be large and may distort the thoracic contents; prognosis depends on the extent to which they do so.
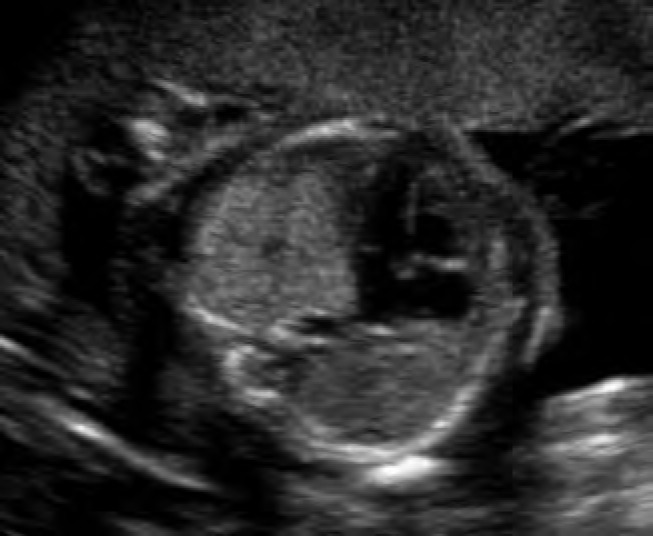
Figure 8.
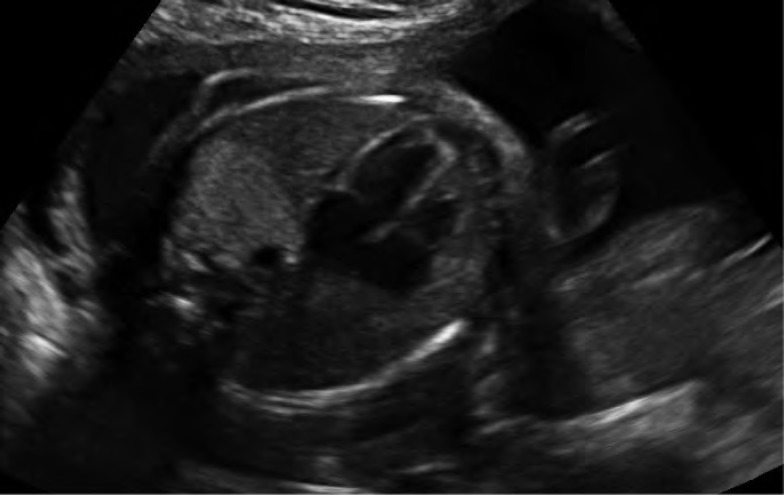
Case 3. Sagittal image of a fetus at 20 weeks with Type 3 congenital cystic adenomatoid malformation demonstrating its position posterior and to the left of the heart. The heart is displaced to the right.
Figure 9.
Case 3. Severe Type 3 congenital cystic adenomatoid malformation. The lesion has become large enough to compress the cardiac anatomy. This fetus is at risk for hydrops.
Type 4
Type 4 CCAMs account for 5% to 15% of cases. These CCAMs contain large cysts that may be as large as 10 cm and have been associated with malignancy, specifically pleuropulmonary blastoma.11 They are alveolar in origin.
Antenatally, CCAMs have been classified as microcystic (< 5 mm) versus macrocystic (> 5 mm). Microcystic lesions are frequently significantly larger than macrocystic lesions and as such have been associated with poorer prognosis.12 Types 1, 2, and 4 CCAMs are classified as macrocystic or both macrocystic and microcystic. Type 3 CCAMs are microcystic.
BPS can be either intralobar (ILS) or extralobar (ELS) depending on whether the mass is within or outside of a normal lung lobe.13 ILS are contained within the lung and do not have their own pleura, whereas EPS are completely covered with pleura. Although this distinction cannot usually be made on ultrasound, ELS is more common in the fetus and may even be extrathoracic, with 10% of lesions noted below the diaphragm, usually on the left side.14 Both ILS and ELS appear as solid, well-circumscribed and echogenic masses on ultrasound, similar to Type 3 CCAM (Figures 10–12). The major differences between ILS and ELS are summarized in Table 1.
Figure 10.
Case 4. Axial image of a fetus with bronchopulmonary sequestration demonstrating the fourchamber view and the proximity to the descending aorta. The fetus never developed hydrops and the mass was resected electively at age 3 months.
Figure 12.
Histology slide from the resection in Case 4. Dilated airways in this part of the specimen are filled with pale-staining mucin secondary to obstruction. Image courtesy of A. Brian West, MD, FRCPath, Yale School of Medicine, New Haven, CT.
On ultrasound, Types 1 and 2 CCAM are usually easier to distinguish from BPS because of their more macrocystic appearance. Type 3 CCAM and BPS have similar sonographic appearances and are therefore classically distinguished by their location (ie, BPS is intra-abdominal) and through their vascular supply using Doppler interrogation. CCAM are supplied and drained through the pulmonary circulation, whereas BPS has arterial flow directly from the aorta. However, as mentioned previously, CCAMs supplied through the systemic circulation have been reported,4 and ultimately histologic diagnosis cannot be made antenatally.5 In addition, the normal fetal lung becomes more echogenic through gestation, and therefore these lesions may become more difficult to visualize over time.
Depending on the size of the lesion, other possible findings include polyhydramnios, mediastinal shift, pleural effusions, and hydrops. Large lesions may compress residual tissue, thus increasing the risk of pulmonary hypoplasia, which cannot—at this time—be predicted by antenatal imaging.
Differential Diagnosis
The differential diagnosis of a thoracic mass is broad. Specific lesions and clues to refine their differential diagnoses are discussed next.
Differentiating Type 3 CCAM from intrathoracic BPS may be challenging. Both Type 3 CCAM and BPS are solid-appearing echogenic masses with well-defined borders. They are primarily distinguished through their blood supply, with BPS having direct systemic vascularization off the aorta. However, differentiation may be difficult, especially if the vascular connections cannot be visualized. Other diagnoses such as congenital diaphragmatic hernia (CDH), congenital lobar emphysema, and bronchogenic cyst should be considered.
In CDH, the lung mass is intestine, which may appear cystic and thus mimic CCAM and/or BPS. The presence of peristalsis suggests CDH. The stomach may also be intrathoracic, in which case it may fill and empty. Absence of an intra-abdominal stomach bubble also suggests CDH. Depending on the size of the CDH, the herniated organs may move from intrathoracic to intra-abdominal. CDH and both CCAM and BPS have also been reported in the same patient, further complicating the diagnosis.15
Mediastinal masses such as cystic hygroma and teratoma must be considered in the differential. Teratoma tend to be more vascular and may create more ultrasound shadowing.
A bronchogenic cyst is usually isolated and originates from the upper airway, with which a direct connection can sometimes be visualized. However, if the cyst is more removed from the airway, differentiating it from a macrocystic CCAM may be difficult.
ELS and ILS cannot usually be distinguished, but a pleural effusion would suggest the former.
Intra-abdominal ELS are usually located on the left and must be distinguished from adrenal and renal lesions such as neuroblastoma and mesoblastic nephroma. Another diagnosis to consider is enteric duplication cysts, which are also more common but have a more cystic appearance.
MRI may be useful in distinguishing these lesions; however, the technique has not been studied extensively.16,17
Associated Anomalies
CCAMs are usually isolated and sporadic, although they have been associated with other anomalies (most commonly cardiac and renal) in 15% to 20% of cases.18 An important exception is Type 2 CCAM, in which a majority of cases (∼ 60%) are associated with other findings, including cardiac anomalies, renal agenesis/dysgenesis, gastrointestinal atresia, and skeletal anomalies.10 Specific cardiac anomalies include truncus arteriosus and tetralogy of Fallot.
BPS is more commonly associated with other anomalies than CCAM. Abnormalities of the chest wall, lung, diaphragm, spine, intestine, and heart have been reported in 40% to 50% of ELS cases.19 ELS and Type 2 CCAM have been reported to occur together in 50% of ELS cases.20 The incidence of associated anomalies is much less with ILS, ranging around 15%.21 Connections to the gastrointestinal system (stomach, esophagus) are most common and may affect management due to risk of infection.19 There is no known association with chromosome abnormalities in either CCAM or BPS.19,22
Obstetrical Management
Suspicion of a lung mass should trigger referral to a center specializing in prenatal diagnosis. Initial evaluation should include detailed ultrasound to assess for associated anomalies. Fetal echocardiogram is important to fully assess cardiac anatomy and function. Amniocentesis for karyotype is not absolutely indicated, but is useful especially if it will help guide treatment decisions. Consultation should be arranged with the neonatology and pediatric surgery services in order to fully counsel patients on possible outcomes.
Depending on gestational age, termination of pregnancy should be discussed, especially in the setting of associated anomalies, abnormal karyotype, or early-onset circulatory compromise. Patients whose fetuses have small lesions, or those fetuses in whom lesions seem to regress, may likely deliver in their usual facility. Care should be transferred to a facility with expert neonatal services and a range of pediatric surgery options in cases of fetuses with large lesions, or those with evidence of fetal compromise. In these cases, significant respiratory support and even extracorporeal membrane oxygenation may be required.
Intervention during pregnancy is rarely required for the fetus with BPS, unless pleural effusions or hydrops develops. In that case, antenatal intervention is recommended because prognosis is poor. Possible interventions include thoracocentesis, thoracoamniotic shunt, and laser ablation or injection of a sclerosing agent into the feeding artery. Experience with these therapies is limited to case reports and case series. A systematic review that summarized this experience reported prenatal survival of 100% in published cases; neonatal survival was 92%.23
Hydrops develops more commonly in CCAM than in BPS, with reported rates up to 40%.24 Hydrops is more common in microcystic CCAM, CCAM with a dominant cyst, and CCAM with a higher volume as measured by the CCAM volume ratio.12,24 However, none of these markers is sensitive enough to allow accurate prediction of hydrops, and even CCAMs in the presence of hydrops have been reported to resolve; therefore, close follow-up of all fetuses with CCAM is suggested. Hydrops is unlikely to develop after 28 weeks, given the natural course of CCAM growth to plateau at 25 weeks. Mortality in the setting of hydrops is high, and fetal intervention for CCAM with hydrops is recommended depending on gestational age.3 Similar to BPS, options include thoracocentesis, thoracoamniotic shunts, and open fetal surgery with CCAM resection.25 Data regarding the effectiveness of these procedures are limited to observational studies. A systematic review of these studies found that survival was improved with drainage or resection in the setting of hydrops, but not in fetuses without hydrops.26 Both thoracocentesis and thoracoamniotic shunting allow for decompression of the cyst and/or the thoracic cavity with relief of both cardiac and pulmonary compression. However, cysts may accumulate fluid again rapidly and shunts may become dislodged, so repeat placement is often necessary. The more definitive option is open fetal surgery, which is associated with both fetal and maternal complications. Therefore, open surgery is reserved for cases with the poorest prognosis and to those prior to 32 to 34 weeks of gestation.25 After that point, the fetus should be delivered and treated accordingly. Laser ablation and injection of sclerosing agents have also been described in the treatment of microcystic CCAM, in which cysts are too small for decompression; however, these reports are limited to cases.23
Small series suggest that there may be a benefit to steroid therapy in the setting of hydrops CCAM and this should be considered if other fetal interventions are not available, or perhaps as a first-line agent prior to open surgery. 23,27 Cesarean delivery is the usual obstetric indication for both lesions.
Antenatal Monitoring
Serial ultrasound monitoring of congenital cystic lung lesions has demonstrated that a significant proportion of these lesions decrease in size and may regress spontaneously; therefore, antenatal treatment is not usually required.18 It appears that the natural course of CCAM is growth until 25 weeks of gestation, after which it may plateau in size or even regress.24 Given that the fetus continues to grow, it appears that the CCAM is resolving. However, although the lesions may seem to disappear antenatally, a significant proportion persist on postnatal imaging and therefore follow-up is suggested regardless of the prenatal ultrasound course.28
We monitor patients at 1- to 3-week intervals until stability of the lesion has been established, and then typically monthly thereafter. Antenatal testing with nonstress test or biophysical profile has not been studied prospectively. If there are signs of hydrops, more intensive monitoring, possibly in the inpatient setting, is indicated.
Neonatal Management
Treatment of CCAM and BPS depends on location and neonatal status. In the case of respiratory compromise, resection is indicated and is curative. Minimally invasive surgery is quickly becoming the standard of care for these patients.
At least half of patients diagnosed with CCAM antenatally are asymptomatic at birth. Because of the risk of infection and of malignant transformation, most authors recommend resection of all antenatally diagnosed CCAMs, although often the surgery can be deferred until several months after birth. All removed tissue should be examined histologically. In stable patients, the timing of elective surgery is controversial. A systematic review and meta-analysis of cases of congenital cystic lung lesions was performed in order to answer this question of timing.29 A total of 41 reports including 1070 patients were studied and it was found that elective surgery was associated with improved outcomes. Only 3.2% of patients became symptomatic in the period of follow-up, and this occurred within 10 months in the majority of cases. Expectant management could be considered but if surgery is elected it should be performed in the first 10 months of life. Data were not analyzed separately for CCAM and BPS.
Another argument for resection of all lesions, regardless of symptomatology, is the discordance in radiologic and histologic diagnosis, which may occur in a significant number of patients.30 Finally, early resection may allow for compensatory lung development in the remaining tissue.18
Surgical management of CCAM and BPS involves lobectomy or nonanatomical segmentectomy. Lobectomy is suggested for CCAM and ILS because of risks of incomplete resection, which occurs in 15% of cases.29 In both ILS and ELS, the vascular supply may be difficult to identify and bleeding is a risk. ILS has been associated with chronic infections due to connections with the gastrointestinal tract and many authors recommend resection regardless of the presence or absence of symptoms.18 ELS has not been associated with infection or malignant transformation and therefore many authors recommend expectant management with serial imaging in asymptomatic patients.
Prognosis
Prognosis of antenatally detected cystic lung lesions depends mainly on specific histology of the lesion, associated anomalies, presence or absence of hydrops or other signs of cardiovascular compromise, and risk of pulmonary hypoplasia based on degree of residual lung compression.
Survival to delivery is reported in > 95% of cases of CCAM and BPS.23,29 In fetuses who do not develop hydrops, postnatal survival has been reported at nearly 100%.23,25 In fetuses with hydrops who undergo prenatal intervention, survival has been reported at a mean of 80%, with rates up to 100% for those treated with thoracocentesis.23 Neonatal survival was 69%. Only two small studies have reported on long-term neurodevelopmental follow-up after fetal surgery for microcystic CCAM, and outcome was favorable in those 10 patients.25,31
Type 0 CCAM is considered lethal. Resection of Type 1 CCAM is considered to be curative and outcomes are excellent.18 Outcomes for Type 2 CCAM depend largely on the presence of associated anomalies, as just reviewed. The risk of pulmonary hypoplasia is highest with Type 3 CCAM, given its tendency for growth and mass effect. Pulmonary hypoplasia cannot, at this time, be predicted antenatally.
Similar to Type 3 CCAM, the prognosis for BPS depends on the degree of pulmonary hypoplasia. Intra-abdominal ELS seems to have improved outcomes over ILS because of decreased risk for pulmonary hypoplasia.
Main Points.
Congenital cystic lesions of the lung are rare. The most common malformations of the lower respiratory tract are congenital cystic adenomatoid malformation (CCAM) and bronchopulmonary sequestration (BPS). Although the pathogenesis of these lesions is poorly understood, they may have a common origin.
There is significant overlap in the findings and course of CCAM and BPS; however, a number of key features distinguish them from one another. CCAMs are currently classified into one of five main types.
Serial ultrasound monitoring of congenital cystic lung lesions has demonstrated that a significant proportion of these lesions decrease in size and may regress spontaneously; therefore, antenatal treatment is not usually required.
Treatment of CCAM and BPS depends on location and neonatal status. In the case of respiratory compromise, resection is indicated and is curative. Minimally invasive surgery is quickly becoming the standard of care for these patients.
Figure 3.
Computed tomography scan of the neonate in Case 1, performed on day of life 1. There is a 0.9 × 0.9 cm mass with several cysts within the medial segment of the right lower lobe. No systemic vessels can be seen supplying the mass. Findings are consistent with a Type 2 congenital cystic adenomatoid malformation.
Figure 4.
Gross image of the Type 2 congenital cystic adenomatoid malformation seen in Figure 1. The ascites resolved by 24 weeks and the fetus was stable until delivery. The neonate underwent right lower lobe lobectomy on day of life 2 due to persistent mediastinal shift.
Figure 5.
Case 2. A, Transverse image of a fetus at 22 weeks with Type 2 congenital cystic adenomatoid malformation (CCAM). The heart has been displaced into the right thorax due to the large CCAM. Note the two cysts within the echogenic mass of the CCAM. B, Sagittal image of the fetus demonstrating ascites. Note the liver with surrounding fluid. The ascites resolved 3 weeks later and the mass was resected on day of life 2 due to persistent mediastinal shift.
Figure 6.
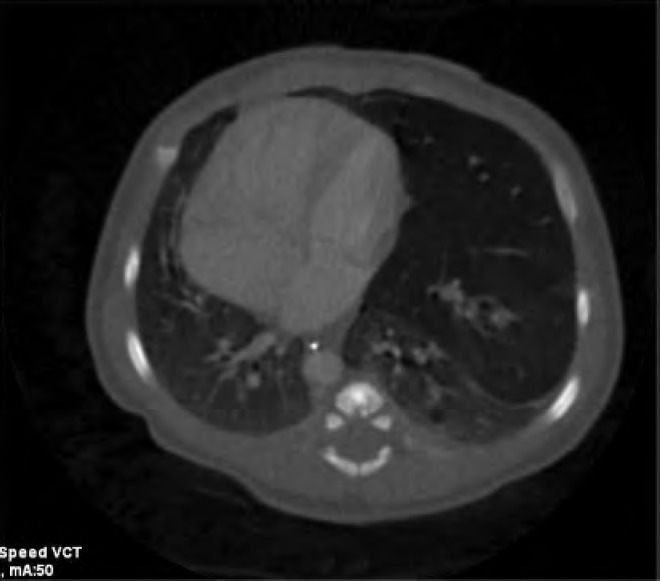
Computed tomography scan of the neonate in Case 2 on day of life 1. Findings are compatible with cystic adenomatoid malformation of the left upper lobe with associated marked hyperinflation causing rightward mediastinal shift and deviation of the descending thoracic aorta. Note less marked involvement of the basilar segments of the left lower lobe. The patient underwent left upper lobe lobectomy at 4 months of life (see Figure 7).
Figure 11.
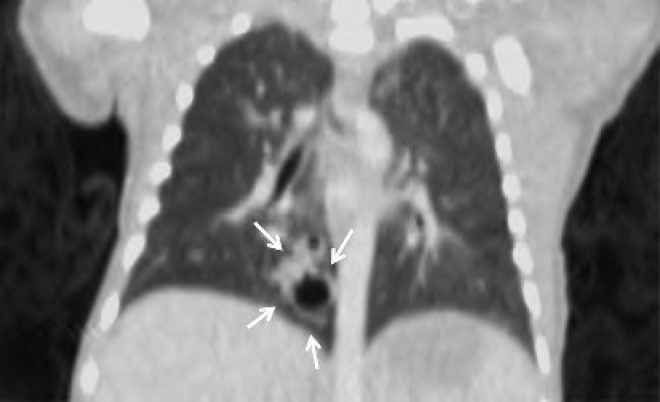
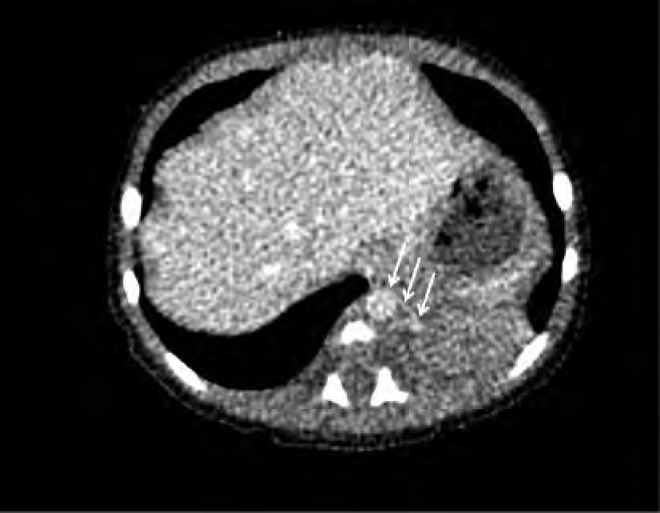
Computed tomography scan of the neonate in Case 4 on day of life 2. There is a solid soft tissue density within the left lower lobe measuring approximately 3 cm 3 2 cm. A small vessel arising from the descending aorta is seen supplying this solid mass (arrows); findings are consistent with sequestration. The baby underwent resection of the mass at 3 months of age.
References
- 1.Laberge JM, Flageole H, Pugash D, et al. Outcome of the prenatally diagnosed congenital cystic adenomatoid lung malformation: a Canadian experience. Fetal Diagn Ther. 2001;16:178–186. doi: 10.1159/000053905. [DOI] [PubMed] [Google Scholar]
- 2.Gornall AS, Budd JL, Draper ES, et al. Congenital cystic adenomatoid malformation: accuracy of prenatal diagnosis, prevalence and outcome in a general population. Prenat Diagn. 2003;23:997–1002. doi: 10.1002/pd.739. [DOI] [PubMed] [Google Scholar]
- 3.Bianchi DW, Crombleholme TM, D’Alton ME, Malone FE, editors. Fetology. Diagnosis and Management of the Fetal Patient. 2nd ed. New York, NY: McGraw Hill; 2010. [Google Scholar]
- 4.Cass DL, Crombleholme TM, Howell LJ, et al. Cystic lung lesions with systemic arterial blood supply: a hybrid of congenital cystic adenomatoid malformation and bronchopulmonary sequestration. J Pediatr Surg. 1997;32:986–990. doi: 10.1016/s0022-3468(97)90383-3. [DOI] [PubMed] [Google Scholar]
- 5.Davenport M, Warne SA, Cacciaguerra S, et al. Current outcome of antenally diagnosed cystic lung disease. J Pediatr Surg. 2004;39:549–556. doi: 10.1016/j.jpedsurg.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 6.Shanti CM, Klein MD. Cystic lung disease. Semin Pediatr Surg. 2008;17:2–8. doi: 10.1053/j.sempedsurg.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Langston C. New concepts in the pathology of congenital lung malformations. Semin Pediatr Surg. 2003;12:17–37. doi: 10.1053/spsu.2003.00001. [DOI] [PubMed] [Google Scholar]
- 8.Stocker JT, Madewell JE, Drake RM. Congenital cystic adenomatoid malformation of the lung. Classification and morphologic spectrum. Hum Pathol. 1977;8:155–171. doi: 10.1016/s0046-8177(77)80078-6. [DOI] [PubMed] [Google Scholar]
- 9.Stocker JT. Cystic lung disease in infants and children. Fetal Pediatr Pathol. 2009;28:155–184. doi: 10.1080/15513810902984095. [DOI] [PubMed] [Google Scholar]
- 10.Priest JR, Williams GM, Hill DA, et al. Pulmonary cysts in early childhood and the risk of malignancy. Pediatr Pulmonol. 2009;44:14–30. doi: 10.1002/ppul.20917. [DOI] [PubMed] [Google Scholar]
- 11.MacSweeney F, Papagiannopoulos K, Goldstraw P, et al. An assessment of the expanded classification of congenital cystic adenomatoid malformations and their relationship to malignant transformation. Am J Surg Pathol. 2003;27:1139–1146. doi: 10.1097/00000478-200308000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Adzick NS, Harrison MR, Glick PL, et al. Fetal cystic adenomatoid malformation: prenatal diagnosis and natural history. J Pediatr Surg. 1985;20:483–488. doi: 10.1016/s0022-3468(85)80470-x. [DOI] [PubMed] [Google Scholar]
- 13.Berrocal T, Madrid C, Novo S, et al. Congenital anomalies of the tracheobronchial tree, lung, and mediastinum: embryology, radiology, and pathology. Radiographics. 2004;24:e17. doi: 10.1148/rg.e17. [DOI] [PubMed] [Google Scholar]
- 14.Laje P, Martinez-Ferro M, Grisoni E, Dudgeon D. Intraabdominal pulmonary sequestration. A case series and review of the literature. J Pediatr Surg. 2006;41:1309–1312. doi: 10.1016/j.jpedsurg.2006.03.049. [DOI] [PubMed] [Google Scholar]
- 15.Ryan CA, Finer NN, Etches PC, et al. Congenital diaphragmatic hernia: associated malformations—cystic adenomatoid malformation, extralobular sequestration, and laryngotracheoesophageal cleft: two case reports. J Pediatr Surg. 1995;30:883–885. doi: 10.1016/0022-3468(95)90772-6. [DOI] [PubMed] [Google Scholar]
- 16.Hubbard AM, Adzick NS, Crombleholme TM, et al. Congenital chest lesions: diagnosis and characterization with prenatal MR imaging. Radiology. 1999;212:43–48. doi: 10.1148/radiology.212.1.r99jl3143. [DOI] [PubMed] [Google Scholar]
- 17.Matsuoka S, Takeuchi K, Yamanaka Y, et al. Comparison of magnetic resonance imaging and ultrasonography in the prenatal diagnosis of congenital thoracic abnormalities. Fetal Diagn Ther. 2003;18:447–453. doi: 10.1159/000073141. [DOI] [PubMed] [Google Scholar]
- 18.Laje P, Liechty KW. Postnatal management and outcome of prenatally diagnosed lung lesions. Prenat Diagn. 2008;28:612–618. doi: 10.1002/pd.1966. [DOI] [PubMed] [Google Scholar]
- 19.Azizkhan RG, Crombleholme TM. Congenital cystic lung disease: contemporary antenatal and postnatal management. Pediatr Surg Int. 2008;24:643–657. doi: 10.1007/s00383-008-2139-3. [DOI] [PubMed] [Google Scholar]
- 20.Conran RM, Stocker JT. Extralobar sequestration with frequently associated congenital cystic adenomatoid malformation, type 2: report of 50 cases. Pediatr Dev Pathol. 1999;2:454–463. doi: 10.1007/s100249900149. [DOI] [PubMed] [Google Scholar]
- 21.Van Raemdonck D, De Boeck K, Devlieger H, et al. Pulmonary sequestration: a comparison between pediatric and adult patients. Eur J Cardiothorac Surg. 2001;19:388–395. doi: 10.1016/s1010-7940(01)00603-0. [DOI] [PubMed] [Google Scholar]
- 22.Wilson RD, Hedrick HL, Liechty KW, et al. Cystic adenomatoid malformation of the lung: review of genetics, prenatal diagnosis, and in utero treatment. Am J Med Genet A. 2006;140:151–155. doi: 10.1002/ajmg.a.31031. [DOI] [PubMed] [Google Scholar]
- 23.Witlox RS, Lopriore E, Oepkes D, Walther FJ. Neonatal outcome after prenatal interventions for congenital lung lesions. Early Hum Dev. 2011;87:611–618. doi: 10.1016/j.earlhumdev.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Crombleholme TM, Coleman B, Hedrick H, et al. Cystic adenomatoid malformation volume ratio predicts outcome in prenatally diagnosed cystic adenomatoid malformation of the lung. J Pediatr Surg. 2002;37:331–338. doi: 10.1053/jpsu.2002.30832. [DOI] [PubMed] [Google Scholar]
- 25.Adzick NS, Harrison MR, Crombleholme TM, et al. Fetal lung lesions: management and outcome. Am J Obstet Gynecol. 1998;179:884–889. doi: 10.1016/s0002-9378(98)70183-8. [DOI] [PubMed] [Google Scholar]
- 26.Knox EM, Kilby MD, Martin WL, Khan KS. In-utero pulmonary drainage in the management of primary hydrothorax and congenital cystic lung lesion: a systematic review. Ultrasound Obstet Gynecol. 2006;28:726–734. doi: 10.1002/uog.3812. [DOI] [PubMed] [Google Scholar]
- 27.Tsao K, Hawgood S, Vu L, et al. Resolution of hydrops fetalis in congenital cystic adenomatoid malformation after prenatal steroid therapy. J Pediatr Surg. 2003;38:508–510. doi: 10.1053/jpsu.2003.50089. [DOI] [PubMed] [Google Scholar]
- 28.Blau H, Barak A, Karmazyn B, et al. Postnatal management of resolving fetal lung lesions. Pediatrics. 2002;109:105–108. doi: 10.1542/peds.109.1.105. [DOI] [PubMed] [Google Scholar]
- 29.Stanton M, Njere I, Ade-Ajayi N, et al. Systematic review and meta-analysis of the postnatal management of congenital cystic lung lesions. J Pediatr Surg. 2009;44:1027–1033. doi: 10.1016/j.jpedsurg.2008.10.118. [DOI] [PubMed] [Google Scholar]
- 30.Tsai AY, Liechty KW, Hedrick HL, et al. Outcomes after postnatal resection of prenatally diagnosed asymptomatic cystic lung lesions. J Pediatr Surg. 2008;43:513–517. doi: 10.1016/j.jpedsurg.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 31.Cass DL, Olutoye OO, Cassady CI, et al. Prenatal diagnosis and outcome of fetal lung masses. J Pediatr Surg. 2011;46:292–298. doi: 10.1016/j.jpedsurg.2010.11.004. [DOI] [PubMed] [Google Scholar]