Abstract
Background
MRI has unparalleled soft-tissue imaging capabilities. The presence of devices such as pacemakers and implantable cardioverter/defibrillators (ICDs), however, is historically considered a contraindication to MRI. These devices are now smaller, with less magnetic material and improved electromagnetic interference protection. Our aim was to determine whether these modern systems can be used in an MR environment.
Methods and Results
We tested in vitro and in vivo lead heating, device function, force acting on the device, and image distortion at 1.5 T. Clinical MR protocols and in vivo measurements yielded temperature changes <0.5°C. Older (manufactured before 2000) ICDs were damaged by the MR scans. Newer ICD systems and most pacemakers, however, were not. The maximal force acting on newer devices was <100 g. Modern (manufactured after 2000) ICD systems were implanted in dogs (n=18), and after 4 weeks, 3- to 4-hour MR scans were performed (n=15). No device dysfunction occurred. The images were of high quality with distortion dependent on the scan sequence and plane. Pacing threshold and intracardiac electrogram amplitude were unchanged over the 8 weeks, except in 1 animal that, after MRI, had a transient (<12 hours) capture failure. Pathological data of the scanned animals revealed very limited necrosis or fibrosis at the tip of the lead area, which was not different from controls (n=3) not subjected to MRI.
Conclusions
These data suggest that certain modern pacemaker and ICD systems may indeed be MRI safe. This may have major clinical implications for current imaging practices.
Keywords: defibrillators, implantable; imaging; magnetic resonance imaging; pacemakers; safety
Magnetic resonance imaging (MRI) has many advantages compared with x-ray– based diagnostic techniques, including its nonionizing nature and unparalleled ability to discriminate different soft tissues without contrast media. MRI has now become the image modality of choice for imaging the brain, spine, musculoskeletal system, head and neck, and other tissue structures.1 The number of patients with implantable cardiovascular devices is increasing steadily. The presence of these devices, however, is currently considered a contraindication to MRI.1–4 Nevertheless, there is an increasing need to image patients with implantable devices because of the advantages offered by MRI.
MR scanners use a high-strength static magnetic field as well as powerful radiofrequency and gradient magnetic fields to produce images.1,2 In the past, it has been noted that the MR environment and MRI scanning have the potential to induce several hazardous effects, among which are device inhibition, rapid pacing, mechanical pull and rotation of the device, device failure, device reprogramming, and lead heating.2–9 Current devices, however, are smaller, with less magnetic material and improved electromagnetic interference (EMI) protection.
We sought to determine whether current pacemaker and implantable cardioverter/defibrillator (ICD) systems are suitable for use in the MR environment. We investigated whether the devices are fully MRI compatible (function appropriately and without significant image distortion), are MR safe (function appropriately in the MR environment but distort the image), or may not be usable in an MR scanner.3,4 To evaluate the safety and feasibility of devices and leads subjected to MRI, we conducted in vitro and in vivo experiments and measured lead heating, force and torque induced by the MR scanner, device damage, memory loss, and device function. In addition, we used a series of animals implanted chronically with ICDs to examine for device dysfunction, distortion of the MR image produced by the devices, histological tissue effects, and compromise of pacing caused by MR–device interactions.
Methods
Seventeen different ICD models and 9 different pacemaker models were tested in a 1.5-T MR scanner (General Electric). All animal studies were reviewed and approved by the Animal Care and Use Committee of Johns Hopkins University.
Two major types of MRI sequence scans were used. First, a sequence was used that represented a worst-case (nonclinical, maximal heating) scenario with high specific absorption rate (SAR). The SAR is a measure of the absorption of electromagnetic energy in the body. For maximal heating, a fast spoiled gradient-echo (FSPGR) sequence was used with flip angle of 170° and a peak SAR of 3.54 W/kg, which was applied for 10 minutes. Second, standard clinical sequences were used. These latter sequences included many clinical scan sequences used in daily practice (Data Supplement Table): segmented k-space (FASTCARD) gradient recalled echo (GRE), FSPGR, spin-echo (SE) T1, fast spin-echo (FSE) with T2 blood suppression, 3D FSE, MR angiography using time of flight and phase contrast, fast imaging employing steady state acquisition (FIESTA), and tagging.
Heating
Temperature changes were measured in 10 ICD and 33 pacemaker leads, using an EMI-immune probe (FOT-M; Fiso-Technologies) with a range of −20°C to 85°C and resolution of 0.1°C. The probe was connected to the electrode tip (and in ICD leads was also coupled to the distal and proximal coils) and was coupled by means of an optic fiber to a registration unit outside the MRI room. Each tested pacemaker or defibrillator lead was connected to a device generator and placed in a container (40×30×20 cm) filled with 0.45% NaCl, which provides electric conductance similar to tissue8 and permits conductive fluid to surround the device. The normal anatomic distribution of leads in a patient was simulated. A semicircle configuration of the leads in the coronal plane was chosen to achieve a maximal magnetic induction area. The measurements were repeated with the use of regular clinical MR protocols, which have lower SAR (<1.4 W/kg), and in a gel that simulates both electric and heat conductivities of tissue8,9 (6.5% acrylamide, 0.08% ammonium persulfate [Aldrich Chemical], 0.3% bis-acrylamide [EM Sciences], 0.05% TEMED, and 0.35% NaCl [Sigma]).
An open-chest canine model (n=3) was used to measure heating in vivo, applying the maximal energy as well as clinically relevant MRI protocols. Pacemaker and ICD leads, each connected to several temperature probes, were inserted into the right ventricular cavity through the right atrial appendage and connected to a pacemaker or ICD generator that was implanted in subcutaneous pockets in the chest wall.
Force
The force exerted by each pacemaker or ICD was measured with the use of an aluminum (MR-compatible) force transducer (MB-5, Interface). The steel case surrounding the aluminum transducer elements was removed to eliminate MR-induced forces. Torque was measured by mounting the devices on a rotating platform (100×100 mm), with the use of a similar MR-compatible force transducer. Force transducer signals were amplified (Gould), digitized with the use of a PowerLab/16s (ADInstruments), and stored for offline analysis.
Function
The devices were interrogated after 3 hours of MRI. Each device was connected to a lead in a 0.45% NaCl bath, in normal anatomic distribution in a patient. The pacing function was programmed to VVI 50/min.
In Vivo ICD Implantation
ICD systems (3 models: Marquis-7224, Epic-V235, Prizm2-1860; n=6 for each model) were implanted in 18 adult mongrel dogs (30 to 35 kg). Under fluoroscopic guidance and in sterile conditions, an active fixation lead was inserted through the left jugular vein to the right ventricle apex. The proximal part of the lead was tunneled subcutaneously and connected to an ICD that was inserted in a pocket created in the subcutaneous tissues of the upper left thorax. The ICD was programmed to “therapy off” so that no shocks were delivered, and arrhythmia detection was turned on. The pacing function was programmed to VVI 50/min.
Four weeks after the implantation, which allowed all wounds to heal, the animals underwent a prolonged MRI scan (3 to 4 hours, including most clinical MRI sequences used and the worst-case scenario protocol; net scan time, 1.9±0.4 hours). Anesthesia was induced with sodium thiopental (15 to 20 mg/kg IV) and maintained with 1% to 1.5% isoflurane on an anesthesia ventilator throughout the MR scan. The animals were monitored during the study for arrhythmias.
Device interrogation included pacing threshold testing, R-wave amplitude testing, lead impedance, battery status, memory, arrhythmia detection, and changes in any programmed parameters. Device interrogations were performed once a week after implantation, just before and after the MR scan, after 24 hours, and once a week for the next 4 weeks. After the final interrogation, the animals were euthanized. The heart tissue surrounding the lead tip was fixed in 10% buffered formaldehyde for 48 hours. After fixation, the tissue was opened in a parasagittal fashion parallel to the longitudinal axis of the lead. After photodocumentation, the lead was carefully removed, the tissue blocks were embedded in paraffin, and 5-μm sections were obtained. These blocks were stained with hematoxylineosin, Masson trichome for fibrous tissue, and Movat pentachrome to show elastin and mucopolysaccharides in addition to fibrous tissue.
Image Distortion
Images were performed in the dog model with the clinical scanning protocols, similar to the aforementioned protocols. Image distortion was analyzed by measuring the area of void in the MR image. The distortion was analyzed at the level of the heart and at the level of the device.
Results
Heating
Lead temperatures in vitro increased 1.5°C to 5.7°C for pacemaker leads and 0.2°C to 7.2°C for ICD leads tested (Table 1). Maximal heating was noted at the tip of the lead. The maximal temperature increase for any lead was produced by the highest energy-scanning protocol, with a flip angle of 170°, which is a worst-case scenario protocol designed to induce maximal SAR and is not used clinically. For 18 of 23 regular clinical MRI protocols, the maximal heating was <0.5°C. Clinical MR protocols with the highest SAR (>1.4 W/kg), such as real-time FIESTA, FSPGR with flip angle of 40°, or SE, had a maximal temperature rise of 0.9°C. As opposed to lead tips, the generators themselves did not heat under any of the scanning protocols.
TABLE 1.
Maximal Heating of Pacemaker and ICD Leads
| Length, cm | Name | Maximal Heating, °C |
|---|---|---|
| Pacemaker leads | ||
| 45 | M-4592 (a) | 1.2 |
| M-5594 (a) | 1.3 | |
| M-5554 (a) | 2.0 | |
| M-4068* | 2.2 | |
| M-5076* | 3.1 | |
| 46 | S-1346T | 1.4 |
| S-1488T | 3.5 | |
| S-1488TC* | 5.3 | |
| 52 | S-1388K | 1.1 |
| M-4092 | 1.5 | |
| M-5068* | 1.5 | |
| M-5054 | 1.7 | |
| M-5092 | 2.4 | |
| M-4023 | 2.5 | |
| M-4024 | 2.5 | |
| M-5024 | 2.8 | |
| S-1488T | 2.8 | |
| M-4068* | 3.4 | |
| S-1346T | 3.9 | |
| 53 | M-5554 (a) | 1.6 |
| M-5594 (a) | 2 | |
| 58 | M-5054 | 1.1 |
| M-5076 | 1.4 | |
| M-5092 | 1.5 | |
| M-4068* | 1.6 | |
| M-4023 | 1.9 | |
| M-5068* | 2.1 | |
| M-4092 | 2.3 | |
| S-1388KT | 2.5 | |
| M-2188 (c) | 3.5 | |
| M-4033 | 4.2 | |
| 65 | M-2188 (c) | 5.7 |
| ICD leads | ||
| 58 | M-6945* | 3.0 |
| 65 | M-6945* | 7.2 |
| 75 | M-6945* | 0.8 |
| 65 | M-6944 | 1.8 |
| 65 | M-6942 | 0.3 |
| 65 | M-6947* | 0.2 |
| 65 | M-6932 | 1.2 |
| 65 | S-1580* | 1.6 |
| 65 | S-1571 | 2.5 |
| 64 | G-Reliance, 64 cm | 1.5 |
(a) indicates atrial lead; (c), coronary sinus lead; G, Guidant; M, Medtronic; and S, St Jude.
Active fixation.
Polyacrylamide gel simulates the electric and heat conductivities of tissue.8,9 When the lead tips were embedded deep (3 to 4 cm) in the gel, according to the maximal energy protocol, substantial heating (up to 35°C) was noted. Clinical MRI protocols yielded <3.9°C. However, when the position of the lead in the heart was simulated, ie, with penetration into the gel only 2 to 3 mm, heating was similar to that obtained with the use of 0.45% NaCl.
For in vivo comparisons, with leads placed in the right ventricle of dogs with acute implantation, the maximal heating was 0.2°C, even with the maximal energy protocol.
Force
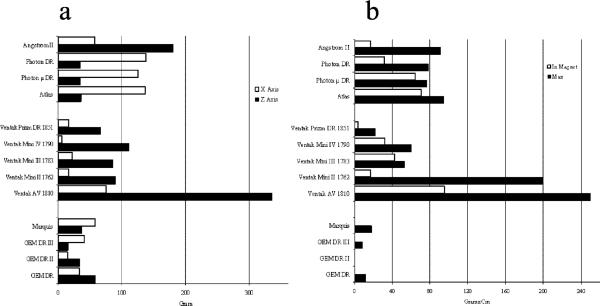
Force on the generators was highest at the entrance to the MR scanner near its edges, and maximal torques were observed 10 cm further inside, toward the MR bore center. The maximal forces acting on the newer ICD models (manufactured after 2000) ranged from 16 to 103 g (Figure 1a). Maximal torque was 23 to 90 g×cm (Figure 1b). Older devices generated values 3 to 5 times higher. The force and torque generated by pacemakers was negligible (<16 g and 10 g×cm, respectively).
Figure 1.
a, Maximal force. b, Torque.
Function
ICDs
Several ICDs manufactured before 2000 were damaged irreversibly, and interrogation of these devices could not be performed (Table 2). Minor decreases in battery voltage were observed with several devices immediately after the scan, which resolved after several days. The majority of devices manufactured after 2000 had no changes in function.
TABLE 2.
ICD Device Function After MR Scanning
| Device | Model | Market Release Year | No. of Devices Tested | Battery Change | Parameter Change | Memory Change | Interrogation Problems | Safe Scan | |
|---|---|---|---|---|---|---|---|---|---|
| St Jude | Angstrom-II | V180HV3 | 1998 | 1 | … | … | Yes‡ | No | ± |
| Photon-μ DR† | V232 | 2001 | 1 | … | … | … | No | + | |
| Photon-DR† | V230HV | 2000 | 2 | … | … | … | No | + | |
| Atlas† | V240 | 2001 | 1 | … | … | … | No | + | |
| Epic† | V235 | 2002 | 5 | … | … | … | No | + | |
| Guidant* | Ventak-AV | 1810 | 1997 | 1 | NA | NA | NA | Yes§ | − |
| MINI-II | 1762 | 1996 | 2 | NA | NA | NA | Yes§ | − | |
| MINI-III | 1783 | 1998 | 1 | NA | NA | NA | Yes§ | − | |
| MINI-IV | 1790 | 1998 | 2 | Yes | Yes | … | Yes§ | − | |
| Prizm-VR | 1850 | 2000 | 1 | Minor∥ | … | … | No | ± | |
| Prizm-DR | 1851 | 2000 | 1 | Minor∥ | … | … | No | ± | |
| Prizm-2VR | 1860 | 2000 | 5 | Minor∥ | … | … | No | ± | |
| Prizm-2DR | 1861 | 2000 | 3 | Minor∥ | … | … | No | ± | |
| Medtronic* | GEM-IDR | 7271 | 1998 | 4 | … | Yes | Yes | Yes¶ | − |
| GEM-IIDR | 7273 | 1999 | 2 | … | … | … | No | + | |
| GEM-IIIDR | 7275 | 2000 | 1 | … | … | … | No | + | |
| Marquis-DR | 7274 | 2002 | 5 | … | … | … | No | + |
NA indicates not available.
During MR in “monitor-only” mode.
During MR in pacing mode (without detection/therapy).
After MR study, several ventricular tachycardia episodes were deleted from memory.
The interrogator displayed: “Out of range, a pulse generator fault has occurred; unable to identify the device.”
Battery depletion occurred after scan; returned to normal after 1 to 2 weeks.
In 3 GEM-I devices, ICD electric reset occurred. In another, interrogation could not be done.
The devices detected MR-induced noise and interpreted the noise as ventricular tachyarrhythmia.
Pacemakers
Most pacemakers tested did not show changes in battery or pacing parameters. These included the following: Kappa-DR703 (n=3), InSync-III-8042 (n=3), Prodigy-DR-7860 (n=1), Vigor-DR-1232 (n=1), Discovery-DR-1274 (n=1), Identity-DR-5370 (n=2), Entity-DR-5326L (n=2), and Affinity-SR-5130R (n=2). The only pacemaker that changed as a result of MR scanning was a Kappa-DR-403, in which “electric reset” occurred. This occurred in all 3 devices tested, with no change in battery status. Nonetheless, unlike ICDs, all pacemakers could be interrogated and reprogrammed.
In Vivo Studies
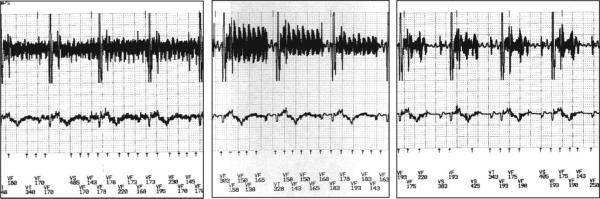
All 15 ICDs implanted and scanned for 3 to 4 hours had intact function. With the use of surface ECG monitoring, no arrhythmias were detected during the scan. Devices from 1 manufacturer had a small decrease in battery voltage noted, which resolved after a few days. In 1 animal, a failure to capture pacing occurred immediately after the scan, which resolved after 12 hours. In all other animals (n=14), no changes in pacing thresholds or intraventricular electrogram amplitudes were noted immediately after the MR scan or at follow-up. As with the ICDs in the in vitro studies, the ICDs detected noise during MR scanning, which was interpreted as ventricular fibrillation (Figure 2).
Figure 2.
EMI noise from different MR scan protocols interpreted by the device as ventricular fibrillation (VF). VT indicates ventricular tachyarrhythmia; VS, ventricular sense.
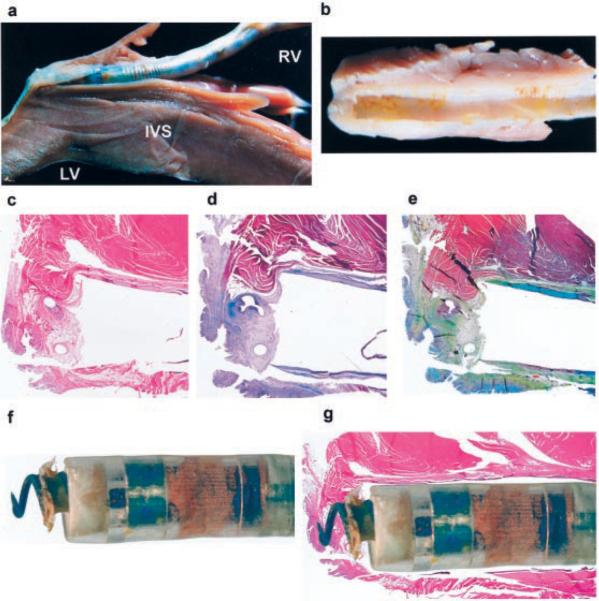
In all 15 animals that were scanned after chronic implantation, histopathological analysis revealed no or very limited necrosis or fibrosis around the tip of the lead (Figure 3), which was not different from findings in control animals (n=3) not undergoing MR scans.
Figure 3.
Gross and microscopic examination of the heart. a, The heart is opened in a parasagittal fashion, parallel to the orientation of the lead. The lead is well anchored at apex of the right ventricle (RV). The RV free wall is seen above the lead. The trabeculation of the RV side of the septum is prominent below the lead. Note the absence of scar tissue in the muscular portion of the RV. LV indicates left ventricle; IVS, interventricular septum. b, The fibrous “cuff” that covers the lead near the apex is shown after removal of the lead. c, Low-magnification view of a histo-logical section showing the lead implantation site with the fibrous tissue surrounding the lead and the anchoring screw. The myocardium is unremarkable; note the absence of inflammatory infiltrates or scarring within the muscle parenchyma. The only fibrous tissue present is at the anchoring site where the anchoring screw has been deployed. Note the 2 circular orifices left during removal of the anchoring screw of the lead (hematoxylin-eosin stain). d, Masson trichrome stain for fibrous tissue (blue staining) confirms the fibrous nature of the cuff. However, there is no evidence of interstitial or replacement fibrosis in the myocardium (red staining). e, Movat pentachrome stain shows mild elastosis within the fibrous cuff but no fibrosis or elastosis in the myocardium. f, Close-up view of the lead showing the anchoring screw delivered and some dried tissue on it. g, Composite of light microscopy of the anchoring site with a superimposed image of the lead. Note matching of the gross image and absence of myocardial fibrosis in areas surrounding the location of electrodes in the lead.
Image Distortion
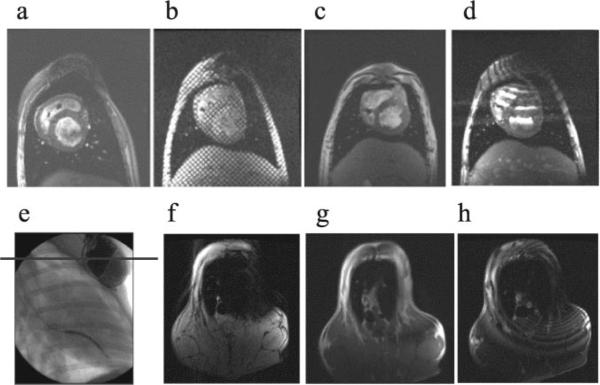
Artifacts due to the devices and leads were observed in some protocols and less in others. Most of the distortion was dependent on scan protocol and image plane: FSE and FIESTA sequences had significant distortion. Fast GRE (FGRE), tagging, and FSPGR sequences, however, yielded good images (Figure 4, a through d). Larger artifacts were observed in image planes roughly parallel to the planes defined by the device itself (Figure 4, e through h). Most distortion was at a distance of 10 to 15 cm around the device generator.
Figure 4.
MRI at heart level with the use of different MR scanning sequences: FSPGR (a), tagging (b), FGRE (c), and FIESTA (d). Imaging at device level (e through h) shows different artifacts depending on scan sequence: angiography showing the level where the axial scan was done (e), FSPGR (f), FGRE (g), and FIESTA (h).
Discussion
The presence of pacemaker and ICD systems is considered a contraindication to MRI. In the present study we tested several of the main concerns that would prohibit MRI scanning (lead heating, forces and torques acting on the device, device function, inhibition of pacing, arrhythmia induction, image quality, and tissue damage). We found that a number of pacemaker systems and selected ICD systems manufactured after 2000 may be safe for MRI. Although these results and the results of others10–15 are encouraging, clinicians must be aware that manufacturers do not claim that their devices currently are MR safe or MR compatible.
Importance of Obtaining MR Scans in Patients With Implanted Devices
MRI continues to be critical for noninvasive imaging in many areas of the body. Because of superior soft tissue discrimination, imaging of the spine and brain is often critical to assess disorders such as tumors, stroke, and inflammatory and infectious diseases.10 Similar considerations make MRI the noninvasive examination of choice to assess internal derangement of most joints and a wide range of other musculoskeletal disorders.6 More recently, MRI has been applied successfully to assess myocardial wall motion, perfusion, and viability.16,17 In many cases, patients with cardiovascular disease who may have the greatest benefit from MRI are also the individuals increasingly likely to have implanted devices. The first cardiac pacemaker was implanted in 1958, and the first ICD was implanted in 1980. Since then, it is estimated that >2 million patients have had these devices implanted. Cardiac pacemakers are the most common electrically active implants found in patients who may be referred for MR procedures.2,12 Thus, it is critical that there is an understanding of the risks of these devices in the MR environment, with the goal of designing devices that are MR compatible.
Theoretical and Real Safety Concerns
The safety of MRI in patients with implanted pacemaker and ICD systems has been debated for years. Recently, it has been suggested that MRI at 0.5 T12–15 and at 1.5 T11 can be safely performed in patients with implanted pacemakers in carefully selected clinical circumstances when appropriate strategies (programming to an asynchronous mode, adequate monitoring techniques, restricted radiofrequency exposure) are used. The presence of implantable devices during routine MRI, however, is considered a contraindication for MRI,3,4 and there are no cardiac devices that currently have achieved Food and Drug Administration (FDA) clearance for MR compatibility. This is in the context in which modern devices are smaller, have less magnetic material, and have improved EMI safety.
Heating at the Lead Tip
Previous studies testing the potential for production of heat in the body by conventional MRI found temperature increases within acceptable levels.7,18 Even MR procedures performed with a very high averaged SAR can be physiologically tolerated by an individual with normal thermoregulatory function.18,19
In the present study temperature increase of the lead tip in a canine model in vivo was minimal. In vitro, in 0.45% NaCl or placed to a depth of 2 to 3 mm in gel, we found, similar to Shellock,19,20 that the temperature change correlated with the level of SAR. MR protocols within current FDA SAR limitations (1.4 W/kg) resulted in nonsignificant changes in lead temperature. However, when leads were imbedded deeply inside gel (simulating nonperfused tissue), the temperature rise was substantial. These data from leads imbedded deeply within gel indicate the important effect of the conductive medium on heating. However, this does not simulate the real-life situation in patients because leads, even with active fixation, are only 2 to 3 mm deep in the myocardium. The major reason that the leads do not heat up in vivo is likely the cooling effect of blood flow through the heart tissue and around the lead tip–tissue interface.18 Our in vivo chronic studies in animals confirmed no signs of thermal injury. These findings suggest that carefully chosen MRI protocols with low SAR produce insignificant heating of the leads.
In 1 animal, a temporary deterioration in pacing capture threshold occurred immediately after the scan. The capture threshold returned to baseline after 12 hours, and 4 weeks later, the tissue surrounding the lead was normal histologically. We presume that some edema occurred at the lead tip–tissue interface, which subsequently resolved.
Force and Torque on Devices
For all pacemakers, the measured force and torque were small (<14 g and 10 g×cm, respectively). For ICD models manufactured after 2000, the force and torque (16 to 103 g and 23 to 90 g×cm, respectively) were much lower than older-generation devices. Similar findings using different methods have been reported by other investigators.21 For reference, a 100-g weight is near the limit of detection of sensation by the body. We speculate that several weeks after ICD implantation, when there is complete healing of the surgical wounds and fibrous tissue has surrounded the lead tip and generator, there will be no danger of lead dislodgment or tissue rupture. From studies of patients with pacemakers undergoing MRI, there have been no reported complaints of pain or a sensation of pulling.11,13,14
ICD Function
The effects of EMI on pacemaker and ICD systems depends on the intensity of the electromagnetic field, the frequency spectrum of the signal, the distance and positioning (angle) of the device relative to the source, the electrode configuration (unipolar or bipolar), nonprogrammable device characteristics, programmed settings, and patient characteristics. In the present study we demonstrated that ICDs from different manufacturers and manufacturing years differ in susceptibility to MRI.
During the study the devices were in “monitor-on/therapy-off” mode. The devices interpreted the EMI produced by the scanner as arrhythmia. No therapy, however, was delivered. In ICDs, when the reed switch is turned “on” by the static magnetic field of the scanner, the devices continue their pacing function but all monitoring (and therapy) is turned off. This latter effect is a desirable occurrence during an MR scan. Our results suggest that when a patient with an ICD is being prepared for MRI, the device should be programmed to “therapy off” to avoid delivering therapy as a result of interpretation of noise as tachyarrhythmia. Therefore, for an ICD, whether the reed switch is activated is of little importance.
According to our findings, patients with ICDs that could not be interrogated after the scan (Table 2) should be absolutely prohibited from undergoing an MRI scan because device replacement would be needed after the procedure. Devices that change to “therapy on” on electric reset (placing the patient at risk for a noise-induced shock) and probably those that showed battery deterioration (even temporarily) should probably not undergo MRI. All other newer devices seem to be safe.
Few studies with MRI in patients with ICDs have been published, but the reported data are in agreement with our findings. Gimbel et al22 reported similar findings regarding GEM-II-7273 (no damage), VENTAK-AV-1810, and MINI-IV-1790 (irreversible damage) in patients who underwent inadvertent MRI scanning when no special precautions were taken. Anfinsen et al23 reported similar findings (ie, battery problems and false arrhythmia detection) with a Prizm-VR-1850 in a patient in whom MRI of the brain was performed without realization that an ICD had been implanted 8 days previously, with false detection of electromagnetic noise during the MRI as ventricular fibrillation.
Pacemakers
Pacemakers appear to be MRI safe. The scanner exerts minimal force or torque on modern pacemakers. There was no modification of the function of the pacemaker temporarily or permanently by the MR fields, and no significant heating was induced in the lead tips. We recommend setting the device to VOO for pacer-dependent patients and to ODO for nondependent patients. The reed switch may be closed by the static magnetic field, because fields as low as 15 G can close the switch. Closing of the reed switch is not hazardous, however, because it results only in placing the pacemaker into an asynchronous mode, which is done routinely during some types of pacemaker interrogation.11
There are practical concerns in imaging patients with pacemakers. First, routine imaging of these patients by MR centers would not be appropriate because of lack of personnel at these centers to assess pacemaker type and pacemaker dependency of the patient. Second, in some circumstances, such as lead failure, leads are left in place, and replacement leads are positioned. We did not assess the impact of these nonfunctional remaining leads. Certain lead configurations with wire loops in the chest wall are observed occasionally; these loops could result in significant heating and burns.
MR physicians should be present during the examination to use pulse sequences with low SAR levels and to reduce examination time to as brief as is necessary to obtain diagnostic information. Physiological MR-compatible monitoring equipment should be used during the examination, and resuscitation equipment should be available. Finally, pacemaker assessment and programming immediately before and after the MR scan should be performed.
Image Quality
Image distortion was dependent on the imaging plane and protocol used. Most image distortion was in the area adjacent to the generator. Therefore, organ visualization beyond this distance, such as knees, lower spine, liver, or brain, will not be affected by the presence of the pacemaker or ICD and may even change the device classification to MRI compatible.
Limitations
Our findings and conclusions can be applied only to those devices and clinical situations tested. Technological advances in pacemaker and ICD development mean that new models appear each year. Until those aspects of modern devices that render them immune to MR scanner fields are defined fully, even newer devices using different technologies will have to be tested individually because newer pacing and ICD technologies may not a priori have improved MRI immunity. Further efforts at understanding the characteristics of these devices that render them MR insensitive are needed before broad revision of practice patterns at MR centers is undertaken. Experimental results obtained in vitro or in healthy laboratory animals exposed to radiofrequency-induced heating during MR procedures cannot be automatically extrapolated to predict thermal or other physiological changes in human subjects, particularly those who are pacemaker dependent or have heart disease.3,4
Recommendations for MRI in Patients With ICDs
Patients with certain ICD devices could be eligible for MR scans under appropriate medically necessary circumstances. For example, MRI would only be considered when other noninvasive imaging tests (eg, CT, ultrasound) were unable to provide diagnostic test results. A potential approach for patients in these circumstances would be informed consent after extensive explanations about potential hazards, including death. The device would be programmed to “therapy off.” MR scanning would consist of the minimum scanning necessary to answer the appropriate clinical question. Noninvasive monitoring of blood pressure, pulse oximetry, and ECG during the MR scan would be available, as well as resuscitation equipment in the MR suite. A physician knowledgeable in pacemaker and/or ICD function should be present during the scan. The MR facility must have an emergency resuscitation plan, with the patient moved rapidly out of the scanner to the control room for immediate resuscitation as needed, including defibrillation. Finally, device interrogation and reprogramming immediately after completion of the MR scan would be necessary.
In conclusion, the data presented in this study suggest that certain pacemaker and ICD systems may be compatible with MR scanning at 1.5 T. Further in vivo studies and carefully monitored patient studies are needed before firm recommendations can be made. If these systems can be shown to be fully MRI compatible (to function appropriately and not distort the image) or at least MRI safe (to function appropriately but distort the image), many patients could benefit from the advantages of MRI and obtain information not readily obtained from other imaging modalities.
Our findings may help to change the clinical imaging indications and contraindications of a large number of patients and to revise current imaging practices worldwide.
Supplementary Material
Acknowledgments
This study was supported by Medtronic; St Jude; grant RO1 HL-64795 from the National Heart, Lung, and Blood Institute; and the Bogle Foundation for the Johns Hopkins ARVD program. Dr Roguin was supported by the Israel Pacing Foundation, Israel Medical Association Ami Cohen grant, Israel Heart Society, and American Physician Fellowship grant. Dr Halperin is a Fellow of the American Heart Association. Devices and leads were provided by Medtronic, St Jude, and Guidant. The corporate sponsors had no role beyond supplying study equipment and did not participate in the analysis of the results or in the writing of the manuscript.
Footnotes
The Data Supplement Table is available online with this article at http://www.circulationaha.org.
Disclosure Dr Halperin is a consultant for Medtronic, and Dr Berger is a consultant for Guidant.
References
- 1.Niehaus M, Tebbenjohanns J. Electromagnetic interference in patients with implanted pacemakers or cardioverter-defibrillators. Heart. 2001;86:246–248. doi: 10.1136/heart.86.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinski SL, Trohman RG. Interference in implanted cardiac devices, part II. Pacing Clin Electrophysiol. 2002;25:1496–1509. doi: 10.1046/j.1460-9592.2002.01496.x. [DOI] [PubMed] [Google Scholar]
- 3.Kanal E, Borgstede JP, Barkovich AJ, et al. American College of Radiology white paper on MR-safety. AJR Am J Roentgenol. 2002;178:1335–1347. doi: 10.2214/ajr.178.6.1781335. [DOI] [PubMed] [Google Scholar]
- 4.Shellock FG, Crues JV., III MR-safety and the American College of Radiology white paper. AJR Am J Roentgenol. 2002;178:1349–352. doi: 10.2214/ajr.178.6.1781349. [DOI] [PubMed] [Google Scholar]
- 5.Achenbach S, Moshage W, Diem B, et al. Effects of MRI on cardiac pacemakers and electrodes. Am Heart J. 1997;134:467–473. doi: 10.1016/s0002-8703(97)70083-8. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed S, Shellock FG. MRI safety: implications for cardiovascular patients. J Cardiovasc Magn Reson. 2001;3:171–182. doi: 10.1081/jcmr-100107466. [DOI] [PubMed] [Google Scholar]
- 7.Shellock FG, Shellock VJ. Cardiovascular catheters and accessories: ex-vivo testing of ferromagnetism, heating, and artifacts associated with MRI. J Cardiovasc Magn Reson. 1998;8:1338–1342. doi: 10.1002/jmri.1880080625. [DOI] [PubMed] [Google Scholar]
- 8.US Food and Drug Administration Magnetic resonance diagnostic device: panel recommendation and report on petitions for MR reclassification. Fed Reg. 1988;53:7575–7579. [Google Scholar]
- 9.US FDA Center for Devices and Radiological Health Magnetic Resonance Working Group [Accessed March 5, 2003];A primer on medical device interactions with magnetic resonance imaging systems. Available at: http://www.fda.gov/cdrh/ode/primerf6.html.
- 10.Gimbel JR, Kanal E. Can patients with implantable pacemakers safely undergo MRI? J Am Coll Cardiol. 2004;43:1325–1327. doi: 10.1016/j.jacc.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Martin ET, Coman JA, Shellock FG, et al. MRI and cardiac pacemaker safety at 1.5-Tesla. J Am Coll Cardiol. 2004;43:1315–1324. doi: 10.1016/j.jacc.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Shellock FG, O'Neil M, Ivans V, et al. Cardiac pacemakers and implantable cardiac defibrillators are unaffected by operation of an extremity MR system. AJR Am J Roentgenol. 1999;172:165–170. doi: 10.2214/ajr.172.1.9888762. [DOI] [PubMed] [Google Scholar]
- 13.Duru F, Luechinger R, Candinas R. MRI in patients with cardiac pacemakers. Radiology. 2001;219:856–858. doi: 10.1148/radiology.219.3.r01jn42856a. [DOI] [PubMed] [Google Scholar]
- 14.Sommer T, Vahlhaus C, Lauck G, et al. MRI and cardiac pacemakers: in-vitro evaluation and in-vivo studies in 51 patients at 0.5 T. Radiology. 2000;215:869–879. doi: 10.1148/radiology.215.3.r00jn08869. [DOI] [PubMed] [Google Scholar]
- 15.Vahlhaus C, Sommer T, Lewalter T, et al. Interference with cardiac pacemakers by MRI: are there irreversible changes at 0.5 Tesla? Pacing Clin Electrophysiol. 2001;24:489–495. doi: 10.1046/j.1460-9592.2001.00489.x. [DOI] [PubMed] [Google Scholar]
- 16.Hundley WG, Morgan TM, Neagle CM, et al. MRI determination of cardiac prognosis. Circulation. 2002;106:2328–2333. doi: 10.1161/01.cir.0000036017.46437.02. [DOI] [PubMed] [Google Scholar]
- 17.Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced MRI to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 18.Finelli DA, Rezai AR, Ruggieri PM, et al. MRI-related heating of deep brain stimulation electrodes: in vitro study. AJNR Am J Neuroradiol. 2002;23:1795–1802. [PMC free article] [PubMed] [Google Scholar]
- 19.Shellock FG. Thermal responses in human subjects exposed to MRI. Ann N Y Acad Sci. 1992;649:260–272. doi: 10.1111/j.1749-6632.1992.tb49615.x. [DOI] [PubMed] [Google Scholar]
- 20.Shellock FG. Metallic neurosurgical implants: evaluation of magnetic field interactions, heating, and artifacts at 1.5 Tesla. J Magn Reson Imaging. 2001;14:295–299. doi: 10.1002/jmri.1185. [DOI] [PubMed] [Google Scholar]
- 21.Luechinger R, Duru F, Scheidegger MB, et al. Force and torque effects of a 1.5-Tesla MRI scanner on cardiac pacemakers and ICDs. Pacing Clin Electrophysiol. 2001;24:199–205. doi: 10.1046/j.1460-9592.2001.00199.x. [DOI] [PubMed] [Google Scholar]
- 22.Gimbel J, Trohman R, Lindsay W, et al. Strategies for safe performance of MRI in ICD patients. PACE. 2002;25(4 Part II):618. Abstract. [Google Scholar]
- 23.Anfinsen OG, Berntsen RF, Aass H, et al. Implantable cardioverter defibrillator dysfunction during and after MRI. Pacing Clin Electrophysiol. 2002;25:1400–1402. doi: 10.1046/j.1460-9592.2002.01400.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.