Abstract
Aims
Asthma is one of the most common chronic inflammatory diseases of the airways. Calcitriol exerts its action through Vitamin D receptor (VDR), which is a high affinity nuclear receptor. VDR is a transcription factor that alters the transcription of target genes which are involved in a wide spectrum of biological responses. Lower serum vitamin D levels are associated with airway hyperresponsiveness and increased asthma severity. Prohibitin is a ubiquitously expressed protein localized to the cell and mitochondrial membranes and the nucleus.
Methods and results
HBSMCs were cultured and treated with calcitriol and/or TNF-α. The mRNA and protein expression of prohibitin and VDR were analyzed using qPCR and immunoblotting, respectively. In the in vivo studies, female BALB/c mice were fed with special vitamin D-deficient or 2,000 IU/kg of vitamin D-supplemented diet for 13 weeks. Mouse model of allergic airway inflammation was developed by OVA-sensitization and challenge. The expression pattern of TNF-α, prohibitin and VDR in the lung of OVA-sensitized mice was analyzed using immunofluorescence. Calcitriol significantly increased and TNF-α decreased the protein and mRNA expression of prohibitin and VDR in HBSMCs. There was significantly increased expression of TNF-α and decreased expression of VDR and prohibitin in the lung of vitamin D-deficient mouse model of allergic airway inflammation.
Conclusion
These results suggest that under inflammatory conditions there is decreased expression of VDR resulting in decreased expression of prohibitin, which is a vitamin D target gene. Vitamin D deficiency causes increase in the expression of TNF-α, thereby increasing inflammation and decreases the expression of VDR and prohibitin. Supplementation with vitamin D reduces the levels of TNF-α, thereby increasing the expression of VDR and prohibitin that could be responsible for reducing allergic airway inflammation.
Keywords: Allergic airway inflammation, Asthma, Human Bronchial Smooth Cells, Inflammation, Prohibitin, TNF-α, Vitamin D receptor, Vitamin D
Introduction
Asthma is one of the most common chronic inflammatory diseases of the airways (Barnes, 2008). There is increase in the prevalence, mortality and morbidity of asthma within past 40 years in United States and world-wide (McGee et al, 2010). Nearly 300 million people in the world are suffering from asthma, and every decade there is increase in nearly 50% of the cases (Braman, 2006; Gupta and Agrawal, 2010).
Calcitriol, 1,25-Dihydroxyvitamin D3, is an active metabolite of Vitamin D3, and is synthesized in a highly regulated multi-step process (Deeb et al., 2007). Calcitriol exerts its action through vitamin D receptor (VDR), which is a high affinity nuclear receptor (Carlberg and Seuter, 2007). VDR is a member of super-family of steroid nuclear receptors (Damera et al., 2009). VDR is a transcription factor that interacts with its co-regulators and alters the transcription of target gene which is involved in a wide spectrum of biological responses (Bosse et al., 2009).
There is increasing evidence that vitamin D has a role in allergic airway diseases. Vitamin D is required for the lung growth in utero and its deficiency may lead to the development of asthma in the offspring of pregnant women (Edelson et al., 1994; Nguyen et al., 2004; Nguyen et al., 1996). Low levels of serum 25(OH)D are associated with: (i) severity of asthma and allergy in children, (ii) elevated total IgE and eosinophils count, (iii) increase asthma-related hospitalization, (iv) more use of anti-inflammatory medications, and (v) high AHR and increased levels of TNF-α in the BALF (Brehm et al., 2009; Sutherland et al.,2010). Vitamin D deficiency leads to decreased lung volume, decreased lung function and altered lung structure (Zosky et al., 2011). Children with therapy-resistant severe asthma having lower vitamin D levels have increased airway smooth muscle (ASM) mass and worse asthma control and lung function (Gupta et al., 2011). Vitamin D supplementation with high doses in pregnant women reduces asthma risk by 40% in children aged 3-5 years (Litonjua and Weiss, 2007). These studies emphasize a critical role of vitamin D in modulating the immune response in allergic airway diseases.
Prohibitin (PHB) is a ubiquitously expressed protein localized to the cell and mitochondrial membranes as well as the nucleus. PHB has been implicated in numerous functions including regulation of proliferation, apoptosis, transcription and mitochondrial protein folding (Theiss et al., 2009b). PHB interacts with tumor suppressor proteins and transcription factors, inhibits oxidative stress and mitochondrial dysfunction, acts as a cell-surface receptor for the vasculature of adipose tissue, and modulates cell proliferation and apoptosis (Fusaro et al., 2003; Lee et al., 2010; Theiss et al., 2007; Wang et al., 1999). The expression of PHB is decreased in patients with inflammatory bowel disease (Theiss et al., 2007). PHB prevents inflammation-associated oxidative stress and may play an important role in decreasing the severity of colitis by acting as a regulator of antioxidant response (Theiss et al., 2009b). In breast cancer cells, PHB is up-regulated by 1α(OH)D5 treatment recognizing prohibitin as a vitamin D target gene (Peng et al., 2006).
Tumor necrosis alpha (TNF-α) is a pro-inflammatory cytokine (Gupta et al., 2011) and has a major role in airway inflammation and airway remodeling in asthma (Berry et al., 2006). In mouse models of asthma, deficiencies in TNF-α or its receptor or treatment with anti-TNF-α antibody results in the attenuation of antigen-induced airway hyperresponsiveness and eosinophilic airway inflammation (Berry et al., 2007).
Immune modulating potential of Vitamin D has been implicated in various inflammatory diseases. Given that TNF-α plays a major proinflammatory role in airway inflammation, in the present study, we assessed the regulation of PHB and VDR expression by TNF-α. We also examined the association between vitamin D deficiency and allergic asthma in a mouse model. Since PHB is identified as a vitamin D responsive gene and it has a role in reducing inflammation, we determined if vitamin D deficiency and allergic asthma has an effect on the expression of VDR, PHB and TNF-α in the lung.
Materials and Methods
Cell Culture and Cell Stimulation
Primary human bronchial smooth muscle cells (HBMSC) were obtained from ScienCell Research laboratories. Cells were cultured in 25 cm2 cell culture flasks in smooth muscle cell medium (SMCM) containing 10% FBS and were maintained at 5% CO2 at 37°C. Cells in passage 3-7 maintained their SMC phenotype and were used in all experiments. Cells were characterized for smooth muscle cell markers including smooth muscle α-actin and smooth muscle heavy chain by immunofluorescence. All experiments were done in three biologically independent samples. Cultured HBSMCs (70-80% confluent cells) were growth arrested by serum starvation for 24 h by replacing the FBS containing SMCM with FBS free DMEM (Dulbecco’s modified eagle’s medium). After 24 hr cells were stimulated with calcitriol (D1530 Sigma-Aldrich, St. Louis, MO) at various concentrations (0.1 - 100 nM) or ethanol (<0.05%) as vehicle in fresh DMEM for 24 hr. Recombinant TNF-α (PeproTech, Inc., NJ) was used at a dose of 10ng/ml. After stimulation, cells were harvested for RNA and protein. Each experiment was run in triplicate.
Animals and diets
Female BALB/c mice were purchased from Harlan Laboratories (Indianapolis, IN). Mice were maintained in a pathogen-free environment at Creighton University. Food and water were provided ad libitum. Female mice were divided in two groups. The first group were fed with vitamin D-deficient diet (n=7) (Harlan Laboratories, Madison, WI) and the second group with vitamin D-sufficient diet (n=7) (Harlan Laboratories, Madison, WI). In the first group, vitamin D deficient diet was given to breeding male and female mice. These were fed on a special diet containing 1.2% calcium to prevent them from hypocalcemic tetany. The diet was also fortified with vitamin A, E and K. Female offsprings were weaned on the same diet. The second group of mice was fed on the purified diet containing 2,000 IU/kg of vitamin D3 starting at day 21. The research protocol of this study was approved by the Institutional Animal Care and Use Committee of Creighton University.
Induction of allergic airway inflammation
The sensitization protocol was started at six weeks of age, as established in our laboratory (Makinde and Agrawal 2011; Shao et al., 2009). Briefly, mice were sensitized with 20 μg i.p. injections of OVA (Sigma-Aldrich, St. Louis, MO) emulsified in 2.25 mg of Inject alum (Pierce Biotechnology, Rockford, IL) on Days 0 and 14 (Figure 1). Animals were challenged with 1% OVA for three consecutive days from day 28 to day 30 and with 5% OVA on day 32. On day 33, airway hyperresponsiveness (AHR) and enhanced pause in response to aerosolized acetyl β-methylcholine (Sigma-Aldrich) were measured in the all mice using whole-body plethysmography (Buxco Electronics, Troy, NY). The mice with established airway hyperresponsiveness (AHR) were then challenged with 5% OVA by aerosol on day 44. On day 45, airway hyperresponsiveness (AHR) and enhanced pause in response to aerosolized acetyl β-methylcholine (Sigma-Aldrich) was measured in all mice (Figure 1).
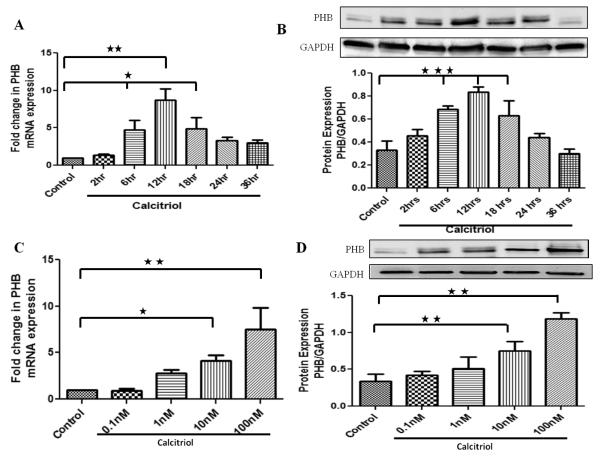
Figure 1. Effect of calcitriol on mRNA and protein expression of prohibitin in a time (A,B) and dose (C,D) -dependent manner in HBSMCs.
Cultured HBSMCs were serum starved for 24 hr followed by treatment with calcitriol (100 nM) at different time periods (0-36hrs) (A,B) and with different doses of calcitriol (0.1nM-100nM) for 12 hr (C,D). The mRNA and protein were isolated from whole cell lysate and subjected to qPCR and immunoblotting, respectively. Data is shown as mean ± SEM from three individual samples in each experiment; *p<0.05, **p<0.01, ***p<0.001.
RNA Isolation, Reverse Transcription and RT-PCR
Total cellular RNA was extracted from adherent cells and mice lung tissue for examining VDR and prohibitin mRNA transcripts. The total RNA was isolated using the Trizol reagent (Sigma, St. Louis, MO) method and RNA was quantified using Nano Drop (Thermo scientific, Rockford, IL). Total RNA (1 μg) was used for the synthesis of first strand of cDNA with oligo-dT (1μg), 5× reaction buffer, MgCl2, dNTPmix and Improm II reverse transcriptase as per Improm II reverse transcription kit (Promega, Madison, WI). With the synthesis of first strand of cDNA quantitative PCR was done using 8 μl cDNA, 10 μl SYBR green PCR master mix (Bio-Rad Lab, Hercules, CA) and forward and reverse primers (10 pmol/μl) (Integrated DNA Technologies, San Diego, CA) using a 7500 Real Time PCR system (CFX96, Bio-Rad Labs, Hercules, CA). The following primer sequences were used in PCR: GAPDH: Forward-5′GGGAAGGTGAAGGTCGGAGT3′, Reverse-5′TTGAGGTCAATGAAGGGGTCA3′; Prohibitin (PHB): Forward- 5′GGGCACAGAGCTGTCATCTT3′, Reverse-5′TGACTGGCACATTACGTGGT3′; VDR: Forward-5′CTTCAGGCGAAGCATGAAGC3′, Reverse-5′CCTTCATCATGCCGATGTCC3′.
The PCR cycling conditions were 5 min at 95°C , 40 cycles of 30 sec at 95°C, 30 sec at 52-58°C (depending upon the primer annealing temperatures) and 30 sec at 72°C. Each real-time PCR was carried out using 10 individual samples in duplicates and the threshold cycle values were averaged. Calculation of relative normalized gene expression was done using the BioRad CFX manager software based on the ΔΔCt method. The results were normalized against housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Western Blot Analysis
The cell monolayer was washed with serum free medium and the cells were detached by trypsinization and centrifuged at 300 g for 5 min. Cell lysis was done by the addition of 50 μl of ice-cold RIPA (radioimmunoprecipitation) buffer [0.05 min Tris buffer (pH 7.2), 0.15 min NaCl, 1% Triton X-100, 1% deoxycholate, 0.1% sodium dodecyl sulfate (SDS)] with 1% protease inhibitor (P8340, SIGMA) and the cell extract was kept on ice and vortexed periodically for a total duration of 30 min. The lysate was sonicated and transferred to eppendorf microcentrifuge tubes and centrifuged at 14,000 g for 10 min at 4°C. The pellet was discarded, and the protein lysates were stored at −70°C for analysis of protein expression.
The protein was quantified and 50 μg of each protein sample was resolved by electrophoresis using 10-20% polyacryamide gels (BioRad, Hercules, CA), after mixing with Laemmli loading buffer with 10% mercaptoethanol. The lysate was separated by gel electrophoresis and transferred onto nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA). Non-specific proteins were blocked by incubating the membranes in blocking buffer (5% w/v nonfat dry milk, 0.05% Tween 20 in PBS). The membranes were then blocked and probed with a specific primary antibody and incubated overnight at 4°C with gentle rocking. The following antibodies (1:500 dilutions) were used: TNF-α (ab6671) (rabbit; Abcam, MA), Prohibitin (ab28172) (rabbit; Abcam, MA), VDR (sc-1008) (rabbit; SantaCruz Biotechnology, CA). Protein expression in whole cell lysate was normalized against GAPDH. After 5-6 washes (10 minutes each) with washing buffer (0.05% Tween20 in PBS), HRP-conjugated secondary antibodies (1:2000) (Novus Biologicals, Littleton, CO) was incubated with the membrane for 1 hr at room temperature with gentle rocking. The membrane was washed three times with washing buffer and the immunoreactive bands were visualized using ECL chemiluminescence detection reagents (Pierce, Rockford, IL). The emission was detected in EpiChemi darkroom and the image was captured with BioChemi CCD camera.
Immunofluorescence
Lung lobes were fixed in 4% formalin and paraffin embedded. Thin sections of 5μm thickness were cut from paraffin blocks and mounted on microscope slides. The sections were also stained using specific antibodies to prohibitin, TNF-α and VDR for immunofluorescence. The sections were treated with DAKO target retrieval solution in a steamer for 20 min, then cooled for 20 min and washed with PBS. The non-specific binding was blocked for 2 hr at room temperature by using a blocking solution of normal goat or normal donkey serum (1:200 dilution), 0.25% Triton and PBS. The sections were incubated with the primary antibody (1:200 dilution) in blocking solution overnight at 4°C. After washing with PBS, the tissue sections were incubated with Cy3-conjugated secondary antibody for 2 hr at room temp. They were washed with PBS and fixed using Vectashield mounting media with DAPI. The tissues were observed using an Olympus inverted fluorescence microscope (Olympus BX51). Negative controls of sections were run without incubation with the primary antibody.
Statistical Analysis
Values of all measurements are reported as mean ± SEM. The Graph Pad Prism 4.0 biochemical statistical package (Graph Pad Software, Inc, San Diego, CA) software was used to analyze data and plot graphs. Statistical analysis was performed using one-way ANOVA to analyze statistically significant differences between groups. The P value of <0.05 was considered significant (*P < 0.05, **P < 0.01, ***P < 0.001).
Results
Serum vitamin D (25-OH D) levels
Serum vitamin D levels in deficient mice were 5.00 ± 0.25 ng/ml (n=7) and serum vitamin D levels in mice supplemented with 2,000 IU of vitamin D3 were 31.00 ± 0.75 ng/ml (n=7) at 13 weeks of age.
Calcitriol increases the expression of prohibitin in HBSMCs in a time- and dose-dependent manner
HBSMCs were treated with calcitriol (100nM) at different time periods (0 -36 hrs). The mRNA and protein expression of prohibitin in HBSMCs in response to calcitriol increased in a time-dependent manner with the maximum expression at 12 hr (Figure 1A, 1B).
HBSMCs were treated with different concentration of calcitriol (1-100 nM) for 12hr. Calcitriol (10-100nM) significantly increased mRNA and protein expression of prohibitin (Figure 1 C, 1D). Therefore, in the following experiments HBSMCs were treated with a dose of 100nM of calcitriol for 12hr.
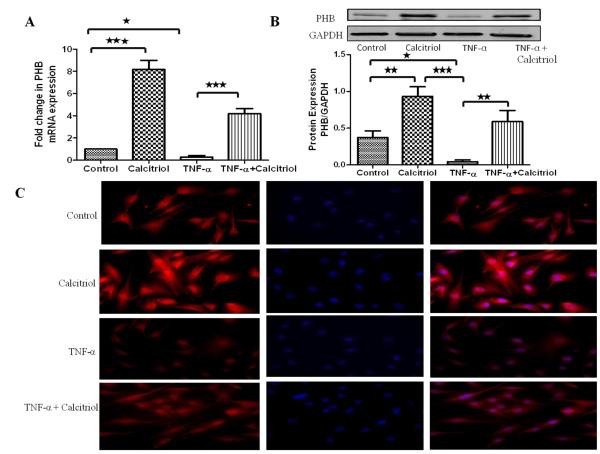
TNF-α decreases the calcitriol-induced mRNA transcript and protein expression of prohibitin in HBSMCs
The effect of the pro-inflammatory cytokine, TNF-α, on the expression of prohibitin was examined. HBSMCs were treated with calcitriol (100 nM) and/or TNF-α (10 ng/ml) for 12hr, followed by RNA and protein isolation for qPCR and immunoblotting, respectively. Following calcitriol treatment there was ~8-fold increase in mRNA expression (Figure 2A) and significant increase in protein expression of prohibitin (Figure 2B). On the other hand, treatment of the cells with TNF-α alone decreased the expression of prohibitin. However, stimulation with calcitriol abolished the TNF-α-induced downregulation of mRNA and protein expression of prohibitin. The findings from the PCR and Western blot were consistent with the immunocytochemical studies (Figure 2C).
Figure 2. Effect of TNF-α in the absence and presence of calcitriol on mRNA and protein expression of prohibitin in HBSMCs: A-B.
Cultured HBSMCs were serum starved for 24 hours followed by treatment with TNF-α (10ng/ml) ± calcitriol (100 nM) for 12 hours. The mRNA and protein were isolated from whole cell lysates and subjected to qPCR and immunoblotting, respectively. Data is shown as mean ± SEM from three individual samples in each experiment; *p <0.05, **p<0.01, ***p<0.001. C: HBSMCs cultured in chamber slides were quiesced before addition of TNF-α (10 ng/ml) ± calcitriol (100 nM) for 12 hr. HBSMCs were fixed, permeabilized and stained with rabbit anti-prohibitin and goat anti-rabbit cy3 as secondary antibody. Red color indicates prohibitin expression and nuclei are stained blue with DAPI.
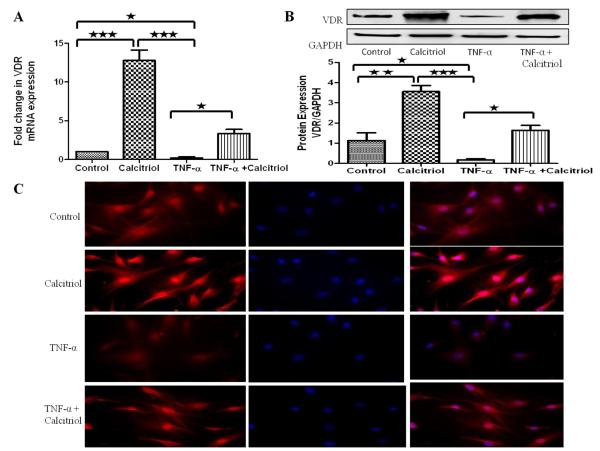
TNF-α- decreases the calcitriol-induced mRNA transcript and protein expression of VDR in HBSMCs
HBSMCs were treated with calcitriol (100 nM) and/or TNF-α (10ng/ml) for 24hr, followed by RNA and protein isolation for qPCR and immunoblotting, respectively. Calcitriol treatment resulted in ~12-fold increase in mRNA expression (Figure 3A) and significant increase in protein expression (Figure 3B) of VDR. However, TNF-α alone decreased the expression of VDR. Calcitriol stimulation abolished the TNF-α-induced decreased mRNA and protein expression of VDR. The findings from the PCR and Western blot results were consistent with the immunocytochemical studies (Figure 3C).
Figure 3. Effect of TNF-α + Calcitriol on mRNA and protein expression of VDR in HBSMCs: A-B.
Cultured HBSMCs were serum starved for 24 hr followed by treatment with TNF-α (10ng/ml) ± calcitriol (100 nM) for 24 hr. The mRNA and protein were isolated from whole cell lysate and subjected to qPCR and immunoblotting, respectively. Data is shown as mean ± SEM from three individual samples in each experiment; *p <0.05, **p<0.01, ***p<0.001. C: HBSMCs cultured in chamber slides were quiesced before addition of TNF-α (10ng/ml) ± calcitriol (100 nM) for 24 hours. HBSMCs were fixed, permeabilized and stained with rabbit anti-VDR and goat anti-rabbit cy3 as secondary antibody. Red color indicates VDR expression and nuclei are stained blue with DAPI.
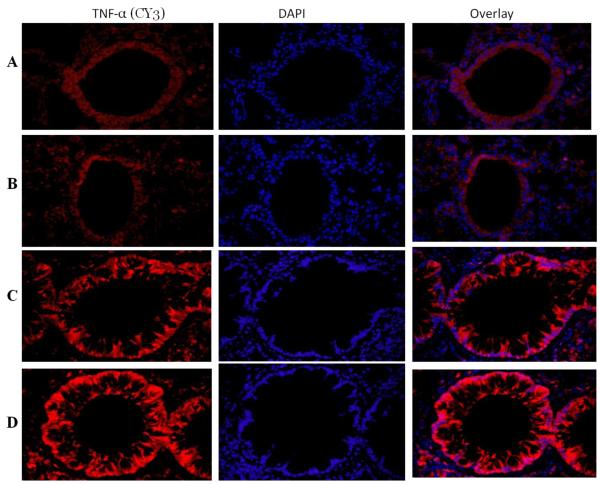
Expression of TNF-α in the lung of vitamin D-deficient and vitamin D-sufficient OVA –sensitized and challenged mice
Mild expression of TNF-α was observed in the lung of both vitamin D-sufficient and vitamin D-deficient PBS control mice (Figure 4A, B). There was a significant increase in the expression of TNF-α in vitamin D-sufficient OVA-sensitized and -challenged mice compared to the PBS control mice (Figure 4C). However, the expression of TNF-α in OVA sensitized and -challenged mice with vitamin D deficiency was significantly higher than in the OVA sensitized and - challenged mice with vitamin D sufficiency (Figure 4D).
Figure 4. TNF-α expression in lung tissue.
Immunofluorescence of TNF-α expression in the lung of vitamin D-sufficient (C) and vitamin D-deficient (D) OVA-sensitized and challenged mice compared to PBS control vitamin D-sufficient (A) and vitamin D-deficient (B) OVA-sensitized and challenged mice, respectively (600× magnification). Sections were stained using rabbit anti-TNF-α antibody and goat anti-rabbit cy3 as secondary antibody. DAPI was used to stain the nuclei.
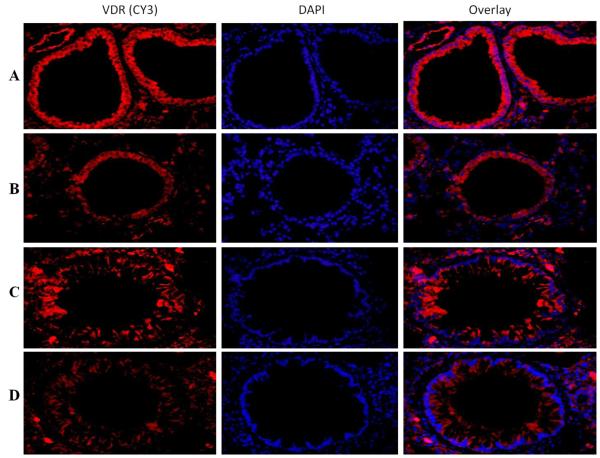
Expression of VDR in the lung of vitamin D-deficient and vitamin D-sufficient OVA–sensitized and challenged mice
VDR was significantly expressed in vitamin D sufficient PBS control mice compared to moderate expression of VDR in vitamin D-deficient PBS control mice. (Figure 5A, B). There was a significant decrease in the expression of VDR in vitamin D-sufficient OVA-sensitized and challenged mice compared to the PBS control mice (Figure 5C). Interestingly, the expression of VDR in the vitamin D-deficient mice was further downregulated compared to vitamin D-sufficient OVA-sensitized and challenged mice (Figure 5D).
Figure 5. VDR expression in lung tissue.
Immunofluorescence of VDR expression in the lung of vitamin D-sufficient OVA (C) and vitamin D-deficient (D) OVA-sensitized and challenged mice compared to PBS control vitamin D-sufficient (A) and vitamin D-deficient (B) OVA-sensitized and challenged mice, respectively (600× magnification). Sections were stained using rabbit anti-VDR antibody and goat anti-rabbit cy3 as secondary antibody. DAPI was used to stain the nuclei.
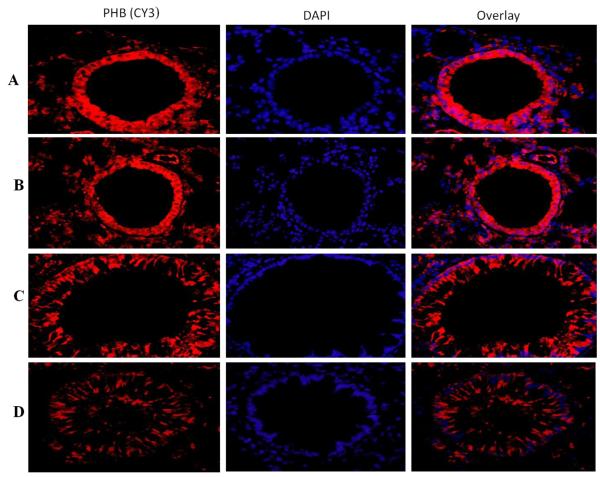
Expression of prohibitin in the lung of vitamin D deficient and vitamin D sufficient OVA–sensitized and challenged mice
A significant expression of prohibitin was observed in the lung tissue of both vitamin D sufficient and vitamin D deficient PBS control mice (Figure 6A, B). There was a significant decrease in the expression of prohibitin vitamin D sufficient OVA sensitized mice compared to the PBS control mice (Figure 6C). However, the expression of prohibitin in OVA-sensitized and challenged mice with vitamin D deficiency was significantly lower than in vitamin D sufficient OVA-sensitized and challenged mice (Figure 6D).
Figure 6. PHB expression in lung tissue.
Immunofluorescence of prohibitin (PHB) expression in the lung of vitamin D-sufficient OVA (C) and vitamin D-deficient (D) OVA-sensitized and challenged mice compared to PBS control vitamin D-sufficient (A) and vitamin D-deficient (B) OVA-sensitized and challenged mice, respectively (600× magnification). Sections were stained using rabbit anti-VDR antibody and goat anti-rabbit cy3 as secondary antibody. DAPI was used to stain the nuclei.
Discussion
TNF-α is a deleterious pro-inflammatory cytokine that activates many potential genes responsible for inflammation, production of inflammatory cytokines and increases the severity of asthma (Berry et al., 2007). Asthma is associated with increased expression of TNF-α (Berry et al., 2006). In our study, vitamin D deficiency increased the expression of TNF-α in the lungs of mice with allergic airway inflammation. Our results support the findings of Sutherland and colleagues that low vitamin D levels are associated with increased levels of TNF-α in asthmatic human subjects (Sutherland et al., 2010). Furthermore, our data suggest that vitamin D deficiency enhances airway inflammation in bronchial asthma.
Immunomodulatory potential of vitamin D has been implicated in various inflammatory diseases (Mathieu and Adorini, 2002). Vitamin D deficiency predisposes to many chronic diseases, including type 1 diabetes, rheumatoid arthritis, Crohn’s disease, infectious diseases, and cancers (Wu and Sun, 2011). Calcitriol, the biologically active form of vitamin D, exerts its effects through VDR (Carlberg and Seuter, 2007). Studies have established the role of VDR in cell proliferation, differentiation, and immunomodulation (Nagpal et al., 2005). VDR is present in most cell types of the immune system, particularly in antigen presenting cells (APCs), including macrophages and dendritic cells, as well as in both CD4+ and CD8+ T cells (Mathieu and Adorini, 2002; Veldman et al., 2000). The expression of VDR is considerably decreased in the colonic mucosa of patients with inflammatory bowel disease compared to normal patients (Wada et al., 2009). Vitamin D3 deficiency together with the dysfunction of VDR can cause poor bone development and health, and may increase the risk of many chronic inflammatory diseases and cancers (Peterlik and Cross, 2005). In our study, we demonstrate that under inflammatory conditions there is decreased expression of VDR in the lungs of OVA-sensitized and -challenged mice, supporting a recent finding (Wada et al., 2009). These studies suggest the immunomodulatory and anti-inflammatory function of vitamin D in various tissues. However, the molecular mechanism underlying the role of vitamin D in the pathophysiology of asthma has not been elucidated.
PHB is a protein involved in diverse cellular processes, including transcriptional regulation of various genes (Theiss and Sitaraman 2011). Increased expression of PHB harbors great potential for the development of therapeutic agents for obesity, diabetes, cancer and inflammatory diseases (Theiss and Sitaraman 2011). There is a decreased expression of PHB in colonic mucosa in patients with inflammatory bowel disease (Hsieh et al., 2006). Similarly, a study in Caco2-BBE human intestinal epithelial cell revealed decreased expression of PHB on stimulation with TNF-α (Theiss et al., 2009a). Administration of intraperitoneal injection of TNF-α in mice decreased the protein expression of PHB in colon mucosa compared to mice injected with PBS as a control (Theiss et al., 2009a).
NF-κB is a key transcriptional factor that co-ordinates the expression of various immune and inflammatory genes (Wertz and Dixit, 2010). Over-expression of PHB in human intestinal epithelial cell and transgenic mice over-expressing PHB results in marked decrease in TNF-α-induced nuclear translocation of the NF-κB resulting in decreased colonic inflammation (Theiss et al., 2009a). Restoration of PHB levels represents a potential therapeutic approach in IBD (Theiss et al., 2009b) suggesting anti-inflammatory properties of PHB. In our study, we found that TNF-α significantly decreased the expression of PHB in HBSMCs. In addition, treatment of HBSMCs with TNF-α and calcitriol, the decrease in PHB expression was abolished confirming the previous studies that PHB is a vitamin D target gene (Peng et al., 2006). In our in vivo studies, vitamin D deficiency resulted in further increase in TNF-α expression and decreased expression of PHB compared to vitamin D-sufficient mice lungs. There are potential VDR/RXR binding sites in the promoter region of PHB gene which makes it a vitamin D responsive gene (Peng et al., 2006). In our study, treatment with calcitriol increased the expression of PHB in HBSMCs. Our data is in accordance with Peng and colleagues where vitamin D analogue 1α-Hydroxy-24-ethyl-cholecalciferol [1α(OH)D5]1α(OH)D5 treatment significantly increased PHB expression (Peng et al., 2006).
Conclusion
Based on our findings, we conclude that an inflammatory environment decreases the expression of VDR and PHB. Vitamin D deficiency causes further increase in the expression of TNF-α, thereby increasing airway inflammation and further decreasing the expression of VDR and PHB in the lung. Supplementation with vitamin D in bronchial asthma might reduce the levels of TNF-α, thereby increasing the expression of VDR and PHB and thus could be therapeutically effective in preventing and/or reversing allergic airway inflammation.
Acknowledgments
This work was supported by NIH grants R01HL085680 and R01AI75315 to DKA
List of abbreviations
- AHR
Airway Hyperresponsiveness
- HBSMC
Human Bronchial Smooth Muscle Cell
- PHB
Prohibitin
- TNF-α
Tumor Necrosis Factor-α
- VDR
Vitamin D Receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Invest. 2008;118:3546–56. doi: 10.1172/JCI36130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M, Brightling C, Pavord I, Wardlaw A. TNF-alpha in asthma. Curr Opin Pharmacol. 2007;7:279–82. doi: 10.1016/j.coph.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Berry MA, Hargadon B, Shelley M, Parker D, Shaw DE, Green RH, Bradding P, Brightling CE, Wardlaw AJ, Pavord ID. Evidence of a role of tumor necrosis factor alpha in refractory asthma. N Engl J Med. 2006;354:697–708. doi: 10.1056/NEJMoa050580. [DOI] [PubMed] [Google Scholar]
- Bosse Y, Lemire M, Poon AH, Daley D, He JQ, Sandford A, White JH, James AL, Musk AW, Palmer LJ, Raby BA, Weiss ST, Kozyrskyj AL, Becker A, Hudson TJ, Laprise C. Asthma and genes encoding components of the vitamin D pathway. Respir Res. 2009;10:98. doi: 10.1186/1465-9921-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braman SS. The global burden of asthma. Chest. 2006;130:4S–12S. doi: 10.1378/chest.130.1_suppl.4S. [DOI] [PubMed] [Google Scholar]
- Brehm JM, Celedon JC, Soto-Quiros ME, Avila L, Hunninghake GM, Forno E, Laskey D, Sylvia JS, Hollis BW, Weiss ST, Litonjua AA. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med. 2009;179:765–71. doi: 10.1164/rccm.200808-1361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg C, Seuter S. The vitamin D receptor. Dermatol Clin. 2007;25:515–23. viii. doi: 10.1016/j.det.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Damera G, Fogle HW, Lim P, Goncharova EA, Zhao H, Banerjee A, Tliba O, Krymskaya VP, Panettieri RA., Jr. Vitamin D inhibits growth of human airway smooth muscle cells through growth factor-induced phosphorylation of retinoblastoma protein and checkpoint kinase 1. Br J Pharmacol. 2009;158:1429–41. doi: 10.1111/j.1476-5381.2009.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- Edelson JD, Chan S, Jassal D, Post M, Tanswell AK. Vitamin D stimulates DNA synthesis in alveolar type-II cells. Biochim Biophys Acta. 1994;1221:159–66. doi: 10.1016/0167-4889(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Fusaro G, Dasgupta P, Rastogi S, Joshi B, Chellappan S. Prohibitin induces the transcriptional activity of p53 and is exported from the nucleus upon apoptotic signaling. J Biol Chem. 2003;278:47853–61. doi: 10.1074/jbc.M305171200. [DOI] [PubMed] [Google Scholar]
- Gupta A, Sjoukes A, Richards D, Banya W, Hawrylowicz C, Bush A, Saglani S. Relationship between serum vitamin D, disease severity, and airway remodeling in children with asthma. Am J Respir Crit Care Med. 2011;184:1342–9. doi: 10.1164/rccm.201107-1239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta GK, Agrawal DK. CpG oligodeoxynucleotides as TLR9 agonists: therapeutic application in allergy and asthma. BioDrugs. 2010;24:225–35. doi: 10.2165/11536140-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Gupta GK, Dhar K, Del Core MG, Hunter WJ, 3rd, Hatzoudis GI, Agrawal DK. Suppressor of cytokine signaling-3 and intimal hyperplasia in porcine coronary arteries following coronary intervention. Exp Mol Pathol. 2011;91:346–52. doi: 10.1016/j.yexmp.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh SY, Shih TC, Yeh CY, Lin CJ, Chou YY, Lee YS. Comparative proteomic studies on the pathogenesis of human ulcerative colitis. Proteomics. 2006;6:5322–31. doi: 10.1002/pmic.200500541. [DOI] [PubMed] [Google Scholar]
- Lee JH, Nguyen KH, Mishra S, Nyomba BL. Prohibitin is expressed in pancreatic beta-cells and protects against oxidative and proapoptotic effects of ethanol. Febs J. 2010;277:488–500. doi: 10.1111/j.1742-4658.2009.07505.x. [DOI] [PubMed] [Google Scholar]
- Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol. 2007;120:1031–5. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- Makinde TO, Agrawal DK. Increased expression of angiopoietins and Tie2 in the lungs of chronic asthmatic mice. Am J Respir Cell Mol Biol. 2011;44:384–93. doi: 10.1165/rcmb.2009-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu C, Adorini L. The coming of age of 1,25-dihydroxyvitamin D(3) analogs as immunomodulatory agents. Trends Mol Med. 2002;8:174–9. doi: 10.1016/s1471-4914(02)02294-3. [DOI] [PubMed] [Google Scholar]
- McGee HS, Stallworth AL, Agrawal T, Shao Z, Lorence L, Agrawal DK. Fms-like tyrosine kinase 3 ligand decreases T helper type 17 cells and suppressors of cytokine signaling proteins in the lung of house dust mite-sensitized and - challenged mice. Am J Respir Cell Mol Biol. 2010;43:520–9. doi: 10.1165/rcmb.2009-0241OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26:662–87. doi: 10.1210/er.2004-0002. [DOI] [PubMed] [Google Scholar]
- Nguyen M, Trubert CL, Rizk-Rabin M, Rehan VK, Besancon F, Cayre YE, Garabedian M. 1,25-Dihydroxyvitamin D3 and fetal lung maturation: immunogold detection of VDR expression in pneumocytes type II cells and effect on fructose 1,6 bisphosphatase. J Steroid Biochem Mol Biol. 2004;89-90:93–7. doi: 10.1016/j.jsbmb.2004.03.054. [DOI] [PubMed] [Google Scholar]
- Nguyen TM, Guillozo H, Marin L, Tordet C, Koite S, Garabedian M. Evidence for a vitamin D paracrine system regulating maturation of developing rat lung epithelium. Am J Physiol. 1996;271:L392–9. doi: 10.1152/ajplung.1996.271.3.L392. [DOI] [PubMed] [Google Scholar]
- Peng X, Mehta R, Wang S, Chellappan S, Mehta RG. Prohibitin is a novel target gene of vitamin D involved in its antiproliferative action in breast cancer cells. Cancer Res. 2006;66:7361–9. doi: 10.1158/0008-5472.CAN-06-1004. [DOI] [PubMed] [Google Scholar]
- Peterlik M, Cross HS. Vitamin D and calcium deficits predispose for multiple chronic diseases. Eur J Clin Invest. 2005;35:290–304. doi: 10.1111/j.1365-2362.2005.01487.x. [DOI] [PubMed] [Google Scholar]
- Shao Z, Makinde TO, McGee HS, Wang X, Agrawal DK. Fms-like tyrosine kinase 3 ligand regulates migratory pattern and antigen uptake of lung dendritic cell subsets in a murine model of allergic airway inflammation. J Immunol. 2009;183:7531–38. doi: 10.4049/jimmunol.0901341. [DOI] [PubMed] [Google Scholar]
- Sutherland ER, Goleva E, Jackson LP, Stevens AD, Leung DY. Vitamin D levels, lung function, and steroid response in adult asthma. Am J Respir Crit Care Med. 2010;181:699–704. doi: 10.1164/rccm.200911-1710OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiss AL, Idell RD, Srinivasan S, Klapproth JM, Jones DP, Merlin D, Sitaraman SV. Prohibitin protects against oxidative stress in intestinal epithelial cells. FASEB J. 2007;21:197–206. doi: 10.1096/fj.06-6801com. [DOI] [PubMed] [Google Scholar]
- Theiss AL, Jenkins AK, Okoro NI, Klapproth JM, Merlin D, Sitaraman SV. Prohibitin inhibits tumor necrosis factor alpha-induced nuclear factor-kappa B nuclear translocation via the novel mechanism of decreasing importin alpha3 expression. Mol Biol Cell. 2009a;20:4412–23. doi: 10.1091/mbc.E09-05-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiss AL, Sitaraman SV. The role and therapeutic potential of prohibitin in disease. Biochim Biophys Acta. 2011;1813:1137–43. doi: 10.1016/j.bbamcr.2011.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiss AL, Vijay-Kumar M, Obertone TS, Jones DP, Hansen JM, Gewirtz AT, Merlin D, Sitaraman SV. Prohibitin is a novel regulator of antioxidant response that attenuates colonic inflammation in mice. Gastroenterology. 2009b;137:199–208. 208, e1–6. doi: 10.1053/j.gastro.2009.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldman CM, Cantorna MT, DeLuca HF. Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch Biochem Biophys. 2000;374:334–8. doi: 10.1006/abbi.1999.1605. [DOI] [PubMed] [Google Scholar]
- Wada K, Tanaka H, Maeda K, Inoue T, Noda E, Amano R, Kubo N, Muguruma K, Yamada N, Yashiro M, Sawada T, Nakata B, Ohira M, Hirakawa K. Vitamin D receptor expression is associated with colon cancer in ulcerative colitis. Oncol Rep. 2009;22:1021–5. doi: 10.3892/or_00000530. [DOI] [PubMed] [Google Scholar]
- Wang S, Nath N, Adlam M, Chellappan S. Prohibitin, a potential tumor suppressor, interacts with RB and regulates E2F function. Oncogene. 1999;18:3501–10. doi: 10.1038/sj.onc.1202684. [DOI] [PubMed] [Google Scholar]
- Wertz IE, Dixit VM. Signaling to NF-kappaB: regulation by ubiquitination. Cold Spring Harb Perspect Biol. 2010;2:a003350. doi: 10.1101/cshperspect.a003350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Sun J. Vitamin D, vitamin D receptor, and macroautophagy in inflammation and infection. Discov Med. 2011;11:325–35. [PMC free article] [PubMed] [Google Scholar]
- Zosky GR, Berry LJ, Elliot JG, James AL, Gorman S, Hart PH. Vitamin D deficiency causes deficits in lung function and alters lung structure. Am J Respir Crit Care Med. 2011;183:1336–43. doi: 10.1164/rccm.201010-1596OC. [DOI] [PubMed] [Google Scholar]