Abstract
The balance of putative pro- and anti-inflammatory lipoxygenase (LOX)-derived S-hydroxyeicosatetraenoic acids (S-HETEs) in colon mucosa is a potential target for modulating colon cancer risk and progression. The biological effects of S-HETEs and R-HETEs (produced by distinct pathways) may differ, but levels of these compounds in colon are unknown. The objective of this study was to develop chiral methods to characterize HETE enantiomers in colonic mucosa and evaluate the effects of fish oil on HETE formation. C57BL/6 mice (COX-1 null, COX-2 null, wild-type) were fed a diet supplemented with either olive oil or menhaden oil for 11 weeks, and R/S-HETEs in colonic mucosa were quantified by chiral LC-MS/MS. The R-enantiomer comprised 60-72% of 5-HETE, 18-58% of 15-HETE and 1-16% of 12-HETE in colonic mucosa, suggesting that non-LOX sources contribute to HETE profiles. Fish oil reduced levels of both R- and S-HETEs, and increased the preponderance of the R-enantiomers (particularly 12- and 15-HETEs). There was apparent shunting of arachidonic acid to12/15-LOX in the COX-1 null animals. This is the first report of the enantiomeric composition of HETEs in the colon in vivo.
Keywords: experimental, lipoxygenase, hydroxyeicosatetraenoic acid, enantiomer, colon
INTRODUCTION
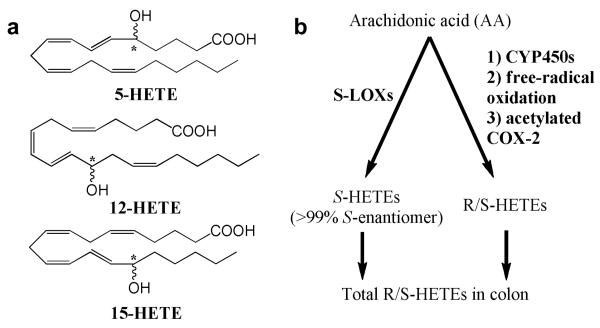
Formation of pro-inflammatory eicosanoids is a potential target for prevention of colorectal cancer. The role of cyclooxygenases (COX) and their arachidonic acid metabolites, particularly prostaglandin E2(PGE2), in colorectal cancer has been studied much more extensively than the role of lipoxygenase (LOX) products. Upregulation of COX-2 and increased PGE2 synthesis have been considered hallmarks of colorectal cancer progression (1-3). The target for cancer prevention, however, is normal colon. For studying early changes, it therefore may be more pertinent to evaluate the role of eicosanoids that are characteristically higher in normal than tumor tissue, such as the lipoxygenase (LOX) product 15-S-hydroxyeicosatetraenoic acid (HETE) (4-7). LOXs convert arachidonic acid to 5-, 12-, and 15-S-HETEs (Fig. 1a). LOXs are stereospecific: S-LOXs, which predominate in the colon, form greater than 99% S-HETEs and less than 1% R-HETEs (8-11). Although R-LOXs exist, they have not been detected in the colon (12).
Figure 1.
A: Structures of measured HETEs in colonic mucosa. Chiral carbons are indicated by *. B: Pathways of arachidonic acid metabolism leading to the formation of R- and S-HETEs. The enantiomeric composition of non S-LOX mediated pathways are unknown.
LOX and HETE levels in colonic mucosa are correlated with inflammation and hyperproliferation. Compared to normal mucosa, transformed mucosa has reduced 15-LOX and increased 5- and 12-LOX expression (6, 7, 13-15). 15-HETE appears to have protective and anti-inflammatory activities, while 5- and 12-HETEs are thought to be pro-inflammatory (16-18). The literature thus supports the hypothesis that LOX expression levels and the levels of their HETE products in colon mucosa are useful biomarkers for assessing colonic inflammation and colorectal cancer risk (4, 19).
The utility of HETEs as biomarkers of LOX status is confounded by the fact that non-LOX sources also contribute to HETEs pools (20) (Fig. 1b). Non-enzymatic oxidation of arachidonic acid generates racemic mixtures (1:1) of R- and S-HETEs (20, 21). CYP450s also generate HETEs through LOX-like bis-allylic oxidation of arachidonic acid (22, 23). Human CYP450s synthesize predominantly R-HETEs in vitro (24), but the stereospecificity of CYP450-mediated synthesis appears to be tissue-specific and the resulting R/S ratios in the colon are unknown (23, 25). Finally, acetylated COX-2 (formed by aspirin) produces 11-R and 15-R- HETEs instead of PGH2 (the precursor of PGE2) in intestinal cells and tumors (26, 27). The relative contributions of LOXs vs. these other sources to total HETE levels and the resulting stereochemical ratios (R/S) present in colon mucosa are unknown since non-chiral HPLC measures total (R + S) HETEs, and common ELISA methods are fairly specific for S-HETEs. Chiral analysis is therefore necessary to define the stereochemistry of HETEs in colonic mucosa.
The objective of this study was to develop chiral HPLC methods for analysis of the enantiomeric composition of 5-, 12-, and 15-HETEs in colonic mucosa and to characterize the ratios of enantiomers for each HETE in vivo. We present an example of how both R- and S-HETEs levels in colonic mucosa can be modulated by dietary manipulation of fatty acids in mice with and without intact COX enzyme. The results show the range of levels of each enantiomer in the colon and large effects of fish oil, which demonstrates both the feasibility and necessity of characterizing HETEs stereochemistry in vivo. These data can be used to guide further characterization of HETEs stereochemistry in the colon and other tissues, as well as mechanistic work on the biological effects of these eicosanoids in colon cells. In addition, the effects of genetic deletion of COX-1 or COX-2 (which are more common targets for colon cancer prevention) are shown, and we hypothesized that this could make more substrate fatty acids available for LOX action.
MATERIALS AND METHODS
Animals and Diets
All animal protocols for this experiment were approved by the University Committee on Use and Care of Animals at the University of Michigan. Six-week old female C57BL/6 mice (wild-type, COX-1 null, or COX-2 null, n=20 per group) were obtained from Taconic (Hudson, NY). Mice were fed one of two modified AIN-93 diets (n=10 per genotype per group) ad libitum for 11 weeks. The modified AIN-93 had a basal fat composition (6% by wt.) comprised of a broad mixture of fatty acids present in European and American diets, composed of coconut oil (45 wt. % supplying saturated fats), olive oil (30 wt. % supplying oleate), corn oil (15 wt. % supplying linoleic acid) and soybean oil (10 wt. % supplying α-linolenic acid). The basal diet was modified with an additional 6% by wt. olive oil [oleate diet, as olive oil contains large amounts of oleic acid (18:1ω9)] or 6% by wt. menhaden oil [fish oil diet, rich in EPA (20:5ω3) and DHA (22:6ω3)] (Table 1). Mice were maintained on a 12 h light/dark cycle. At the end of the study, animals were sacrificed by isoflurane inhalation and decapitation.
Table 1.
Fatty acid composition of the diets. Mice were fed a modified AIN–93 diet containing 6% western fat blend and either 6% olive oil (oleate diet, representing a diet high in MUFA) or 6% fish oil (high PUFA)†
| fatty acid | diet |
||
|---|---|---|---|
| oleate | fish oil | ||
|
Composition (g/kg diet) |
stearic acid (18:0) | 2.7 | 3.3 |
| oleic acid (18:1ω9) | 59.3 | 30.2 | |
| linoleic acid (18:2ω6) | 15.9 | 11.3 | |
| linolenic acid (18:3ω3) | 1.4 | 1.5 | |
| arachidonic acid (20:4ω6) | 0 | 0.7 | |
| EPA (20:5ω3) | 0 | 7.9 | |
| DHA (22:6ω3) | 0 | 5.2 | |
|
| |||
| SFA‡ | 36.2 | 46.2 | |
| MUFA | 62.3 | 34.6 | |
| PUFA | 17.1 | 31.3 | |
| ω3 | 1.1 | 17.5 | |
| ω6 | 16.0 | 12.1 | |
| ω9 | 61.9 | 34.4 | |
|
| |||
| Ratios | ω3/ω6 | 0.1 | 1.4 |
| ω9/ω6 | 3.9 | 2.9 | |
| arachidonic acid/EPA | – | 11.3 | |
| EPA/DHA | – | 1.5 | |
MUFA: mono-unsaturated fatty acids, PUFA: poly-unsaturated fatty acids
SFA: saturated fatty acids,
The colon was immediately removed and rinsed with cold PBS containing indomethacin (5.6 μg/mL). The colon was sliced vertically, and the mucosa was then scraped off, snap frozen in liquid nitrogen, and stored at −80°C prior to processing (28, 29). Blood was collected following sacrifice from the neck. Serum was prepared by centrifugation and stored at −80°C prior to processing. Blood from animals sacrificed on the same day in each diet*genotype group was pooled to increase volume available, resulting in three pooled blood samples per group since animals were sacrificed in batches on three different days.
Frozen mucosal samples (~140 mg) were pulverized using a Multisample Bio-Pulverizer (Research Products International Corp., Mt. Prospect, IL) that was cooled with dry ice and liquid nitrogen. Homogenates were then prepared in 500 μL cold PBS containing 5.6 μg/mL indomethacin. The suspension was further homogenized by ultrasonication in ice water for 3 min (20 s sonication, 20 s cooling cycle), further diluted with 500 μL cold PBS/indomethacin, snap frozen, and stored at −80°C for no more than one week prior to analysis of eicosanoids. A small portion (10 μL) of the homogenate was analyzed for protein content using Advanced Protein Assay (Cytoskeleton, Denver, CO).
Chiral Analysis of HETEs
For extraction of HETEs for chiral analysis, 400 μL of the homogenate was added to 12 × 75 mm glass tubes on ice, along with 1N citric acid (20 μL), 30 mM disodium EDTA (50 μL), and deuterated internal standards (10 μL of a solution containing 200 ng/mL 12-S-HETE-d8, 100 ng/mL 5-S-HETE-d8, 100 ng/mL 15-S-HETE-d8 in methanol). The resulting solution was then extracted twice with 2 mL hexane: ethyl acetate (1:1 v/v, containing 0.1% BHT w/v) by vortexing (3 min), centrifugation (2000 × g, 10 min, 4°C), and collection of the organic layer. The pooled extracts were evaporated under vacuum and reconstituted with 100 μL cold methanol: ammonium acetate buffer (10 mM, pH 8.5) (80:20 v/v), sonicated, centrifuged, and placed in Total Recovery HPLC vials (Waters, Milford, MA) for analysis by chiral LC-MS/MS (40-60 μL injections).
Chiral HPLC separation was performed on a Waters 2695 separations module, employing a Chiral-Pak AD-RH analytical column (2.1 × 150 mm, 5 μm particle size) (Chiral Technologies, West Chester, PA) based upon the method of Carter et al. (30). The column was maintained at 40°C. Gradient elution was performed with a binary solvent system: phase A: 95% H2O, 5% ACN, 0.025% formic acid; phase B: 5% H2O, 95% ACN, 0.025% formic acid (30). The system flow rate was 0.2 mL/min. The linear gradient program was as follows: 50% B (0-10 min), 60% B (25 min), 100% B (27-30 min), 50% B (31-40 min). Samples were maintained at 10°C prior to injection. The effluent was introduced into a Finnigan TSQ Quantum Ultra triple quadrupole mass spectrometer, electrospray ionization (ESI) and detection of negative ions. The ESI voltage was −3 kV, and the capillary temperature was 350°C, with nitrogen as the nebulizing and sheath gases (31). Following ionization, deprotonated molecular ions ([M–H]–) were fragmented by collision-induced dissociation using argon gas, and detection was performed by selected reaction monitoring (SRM) of fragment ions (see Table 2). The SRM mass span was ± 0.15 Da, with scan dwell time of 0.2 s per transition. Quantification was performed by the stable isotope dilution IS method. All eicosanoid standards were obtained from Cayman Chemical (Ann Arbor, MI). Both R- and S-enantiomers of 5-, 12-, and 15-HETEs were calculated relative to the corresponding S-HETE-d8 IS. HETE levels in colon mucosa were normalized to protein levels.
Table 2.
Selected reaction monitoring (SRM) transitions for chiral LC-MS/MS analysis of R- and S-HETE enantiomers
| Compound | [M–H]– (m/z) |
SRM Transition | Collision Energy (eV) |
Tube Lens (V) |
|---|---|---|---|---|
| 5-R/S-HETEs† | 319 | 319.5 → 115.27 | 27 | −42 |
| 5- S-HETE-d8 | 327 | 327.50 → 116.30 | 28 | −102 |
| 12- R/S-HETEs† | 319 | 319.24 → 179.09 | 26 | −55 |
| 12-S-HETE-d8 | 327 | 327.24 → 184.09 | 22 | −55 |
| 15- R/S-HETEs† | 319 | 319.31 → 219.30 | 20 | −52 |
| 15- S-HETE-d8 | 327 | 327.20 → 226.05 | 20 | −52 |
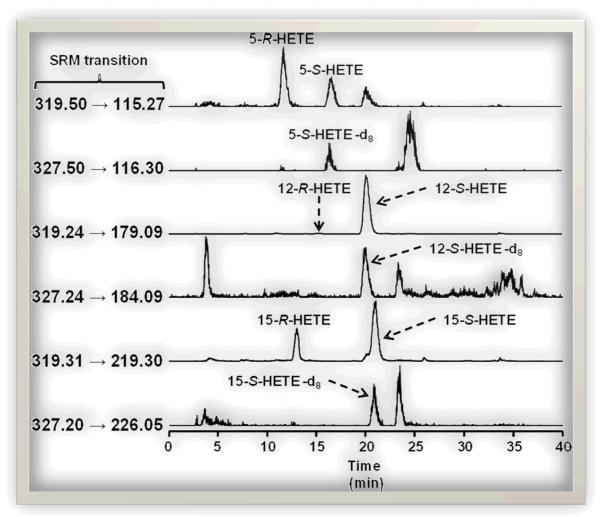
In chiral LC, the R- and S-enantiomers of each HETE are resolved and elute as 2 distinct peaks. Both peaks for each HETE were quantified relative to the respective S-HETE-d8 internal standard.
Fatty Acid Analysis
For extraction of fatty acids, 10 μL of internal standard (17:0, 1 mg/mL in hexane) was added to 100 μL of serum. Fatty acids were extracted with Folch reagent and fatty acid methyl esters (FAMEs) were prepared using METH-PREP II derivatization reagent (0.2N methanolic (m-trifluoromethylphenyl)trimethylammonium hydroxide, Alltech, Deerfield, IL). For quantification, standard curves were prepared using mixtures of fatty acids in varying concentrations derivatized in the same manner as serum fatty acids. GC analysis was performed on an Hewlett Packard 5890 GC with a 5971 MSD (Santa Clara CA) and a SP-2330 capillary column 30 m × 0.32 mm, 0.2 μm film thickness (Supelco, Bellefonte, PA) as previously described (32). Selected ion monitoring was used that was optimized for each fatty acid.
Data Analysis
Statistical analyses were performed by 2-way ANOVA using Least-Squares Difference (LSD) for multiple comparisons (PASW Statistics software v.18, SPSS, Chicago, IL). Significance was defined as P < 0.05.
RESULTS
Fatty Acids
Fish oil feeding effectively modified the fatty acid composition of serum (Table 3), with no differences between genotypes within each diet. Mice fed the oleate diet had serum fatty acid composition ranging from 41-50% arachidonic acid, which was significantly reduced by the fish oil diet for all genotypes to 14-16% arachidonic acid. EPA was significantly increased in all genotypes from roughly 0.2% in mice fed the oleate diet to 12-13% in mice fed the fish oil diet. This resulted in a significant decrease in the ratio of highly unsaturated 20 carbon fatty acids (AA/EPA) from 198:1-297:1 in mice fed the oleate diet to roughly 1:1 in mice fed the fish oil diet. This indicates a shift in fatty acid availability from predominantly ω6 arachidonic acid to a balance of arachidonic acid and ω3 EPA. Similar to EPA, DHA was significantly increased in all genotypes from roughly 6-7% in mice fed the oleate diet to 16-17% in mice fed the fish oil diet. The shift in the relative availability of ω6/ω3 fatty acids for LOX and COX metabolism is reflected by the ω3 HUFA (highly unsaturated fatty acid) score [the % of highly unsaturated fatty acids (≥20 carbons and ≥3 double bonds) that are ω3] (33). The ω3 HUFA scores were 10-14% in mice fed the oleate diet, and increased significantly to 61-65% in mice fed the fish oil diet.
Table 3.
Levels of fatty acids in serum as a % of total fatty acids
| % of total fatty acids†‡ |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Genotype | Diet | SFA§ | MUFA∥ | linoleic (18:2ω6) |
linolenic (18:3ω3) |
arachidonic (20:4ω6) |
EPA (20:5ω3) |
DHA (22:6ω3) |
% ω3 HUFAζ |
| COX-1 null | oleate | 20.2 ± 1.6 | 16.7 ± 1.9 | 12.8 ± 1.2 | 0.252 ± 0.048 | 41.1 ± 4.9 | 0.206 ± 0.011 | 6.61 ± 0.16 | 14.0 ± 1.5 |
| fish oil | 23.3 ± 0.50 | 15.8 ± 0.56 | 13.5 ± 0.65 | 0.395 ± 0.022b | 16.0 ± 0.78c | 13.2 ± 0.028c | 16.0 ± 0.32c | 62.3 ± 1.3c | |
| COX-2 null | oleate | 19.0 ± 1.6 | 15.1 ± 0.72 | 12.3 ± 0.65 | 0.221 ± 0.020 | 44.9 ± 3.5 | 0.207 ± 0.015 | 6.59 ± 0.83 | 13.0 ± 2.3 |
| fish oil | 24.0 ± 0.30a | 15.3 ± 0.50 | 15.7 ± 0.93a | 0.405 ± 0.019c | 14.1 ± 1.0c | 12.0 ± 0.34c | 16.8 ± 0.50c | 65.0 ± 1.6c | |
| wild-type | oleate | 17.4 ± 0.51 | 14.9 ± 0.59 | 9.90 ± 0.19 | 0.183 ± 0.012 | 50.0 ± 1.2 | 0.177 ± 0.028 | 5.79 ± 0.11 | 10.4 ± 0.33 |
| fish oil | 23.7 ± 0.68b | 15.0 ± 1.2 | 14.8 ± 1.1b | 0.361 ± 0.016c | 16.3 ± 0.72c | 12.5 ± 0.77c | 15.5 ± 1.2c | 61.2 ± 0.77c | |
Values represent mean ± SEM
Means that differ significantly within each column are denoted as follows: significantly different than oleate diet for mice with the same genotype at aP < 0.05, bP < 0.01, cP < 0.001. No differences were detected between genotypes within the same diet
SFA: saturated fatty acids (12:0, 14:0, 16:0)
MUFA: monounsaturated fatty acids (16:1, 18:1)
The % of highly unsaturated fatty acids (≥20 carbons and ≥3 double bonds) that are ω3
HETEs Enantiomers
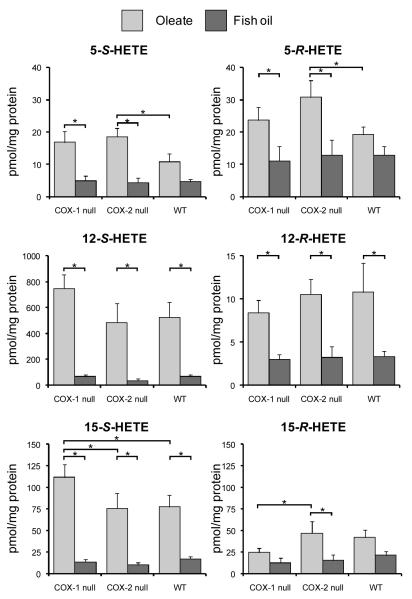
The chiral HPLC method achieved excellent resolution of the enantiomers of each HETE regioisomer, with typical resolution of ≥ 5 min between R- and S-forms (Fig. 2). The absolute levels of R- and S-HETEs in mouse colon mucosa are shown in Fig. 3, and the chiral composition of each HETE regioisomer (% of the total comprised of the R-enantiomer) is shown in Table 4. The results indicate that in animals fed the oleate fat control diet, levels of 12-S-HETE were highest followed by 15-S-HETE and low levels of 5-S-HETE. For R-HETES in all groups, 15-R-HETE was generally present at the highest levels in the colonic mucosa, followed by 5-R-HETE and with very low levels of 12-R-HETE. The R-enantiomer comprised 60-72% of 5-HETE, 18-58% of 15-HETE and 1-16% of 12-HETE, depending on the treatment group. In mice fed fish oil, the % of total HETEs comprised by the R-enantiomer was generally elevated compared to oleate diet (Table 4), particularly for 12- and 15-HETEs. This suggests that the relative contribution of non-LOX pathways such as CYP450 and free-radical oxidation (which produce appreciable % of R-enantiomers) is elevated relative to LOX pathways (which largely generate S-HETEs) when fish oil is administered. This is the first report of the enantiomeric composition of HETEs in the colon in vivo.
Figure 2.
Selected reaction monitoring (SRM) chromatograms demonstrating the resolution of R- and S-HETEs in mouse colonic mucosa by chiral LC-MS/MS.
Figure 3.
Levels of R- and S-HETEs in mouse colon mucosa. Error bars represent SEM. Brackets indicate significant differences between means (*P<0.05). Note the different scales for 12-S-HETE and 12-R-HETE.
Table 4.
Enantiomeric composition of 5-, 12- and 15-HETEs in mouse colon mucosa.
| Treatment |
% R-enantiomer†‡ |
|||
|---|---|---|---|---|
| Genotype | Diet | 5-HETE | 12-HETE | 15-HETE |
| COX-1 null | oleate | 59.5 ± 2.7 | 1.3 ± 0.2 | 18.0 ± 2.3a |
| fish oil | 58.8 ± 5.0a | 7.3 ± 2.5c | 46.9 ± 5.7c | |
| COX-2 null | oleate | 61.9 ± 2.4 | 4.5 ± 1.7 | 39.7 ± 5.6b |
| fish oil | 67.4 ± 8.0 | 15.7 ± 6.9abc | 57.6 ± 6.6c | |
| wild-type | oleate | 65.6 ± 2.7 | 2.0 ± 0.5 | 34.3 ± 3.9 |
| fish oil | 72.2 ± 2.5 | 6.6 ± 1.9 | 54.7 ± 4.2c | |
Values represent mean ± SEM
Within each column, statistically significant differences (P<0.05) between means are indicated by superscripts: asignificantly different from wild-type mice fed the same diet, bsignificantly different from COX-1 null mice fed the same diet, csignificantly different from mice fed the oleate diet in the same genotype
Mice fed fish oil had reduced levels of both R- and S-HETEs compared to mice fed an oleate fat diet, reflecting the shift in the ratio of arachidonic acid/EPA substrate pools produced by fish oil. The decrease in 5-S-HETE with fish oil feeding was observed in all groups but was not statistically significant in wild-type mice with intact COX activity (Fig. 3). COX genotype had less of an effect on HETE levels than diet, as could be expected. Levels of both 5-S and 5-R-HETEs were higher in COX-2 null mice versus wild type. Levels of 12-S-HETE were unaffected by COX status, although a non-significant increase was observed in COX-1 mice. Levels of 15-HETEs tended to mirror that of 12-HETEs, but the higher levels of the S-enantiomer in COX-1 null versus wild type mice was statistically significant for 15-S-HETE. These data suggests possible shunting of arachidonic acid to12/15-LOX in COX-1 null animals.
DISCUSSION
The relative levels of the HETE regioisomers in animals fed the oleate diet are consistent with previous reports of the relative levels of HETEs in both normal and inflamed colonic mucosa of rodents decreasing in the order 12-HETE > 15-HETE > 5-HETE (34). In human colonic mucosa, reported 15-HETE levels were between 2- and 100-fold higher than 12-HETE, with 5-HETE being present at much lower concentrations (7, 19, 34, 35). These species differences are likely due in part to the fact that 12-S and 15-S-HETEs are produced by a 12/15-LOX in rodents (which produces mainly 12-HETE), whereas they are produced by two distinct enzymes in humans (36, 37). Therefore, in humans, the relative R/S ratios for 12- and 15-HETEs also may differ from those seen in mice.
The relatively low levels of 5-S-HETE relative to the 12- and 15-S-HETEs may be due to the low expression of 5-LOX in normal mucosa (13), as well as consumption of the 5-S-H(P)ETE intermediate (the 5-LOX product) by the LT pathway, rather than reduction to 5-S- HETE. In normal colon, COX-1 is present but COX-2 expression is generally very low (38-40). COX-2 deletion did, however, increase levels of 5-S-HETE and 5-R-HETE relative to the wild type (Fig. 3), indicating that COX-2 may have a regulatory role for 5-LOX or that substrate was shunted to the LOX pathway.
Fish oil was highly effective for decreasing levels of both S- and R-HETEs, and this may be due in part to the reduced arachidonic acid levels. In addition to altering arachidonic acid levels, the levels of the ω3 fatty acids EPA and DHA relative to arachidonic acid are critical for determining eicosanoid synthesis. EPA may compete with arachidonic acid as a substrate for LOXs (41-43) and also appears to reduce LOX expression (44). An alternative explanation may be that EPA in fish oil inhibited LOX-mediated oxidation of arachidonic acid (45, 46), similar to the inhibition of COX-1 by EPA (47). These results are consistent with a previous report that feeding fish oil lowers levels of total 12- and 15-HETEs in rat colonic mucosa (48).
In addition to decreased levels of HETEs after fish oil feeding, the enantiomeric composition of HETEs differed for each HETE regioisomer, as well as between diets and genotypes (Table 4). Therefore, the contribution of LOX vs. non-LOX sources to the enantiomeric composition of HETEs in colonic mucosa warrants further investigation. The present data suggest that the balance of R/S-HETEs could be modified by non-toxic dietary approaches and/or pharmacological strategies. The bioactivity of the R- vs. S-forms remains to be clarified, but it should be possible to target optimal profiles of pro- and anti-inflammatory enantiomers in the colon with preventive strategies. Therefore, chiral analysis of HETEs in vivo is necessary to accurately assess the impact of dietary or pharmacological interventions targeting HETEs, as such treatments clearly affect enantiomeric composition as well as absolute levels.
The balance of HETEs in the colon may be important for colon cancer prevention. Data is sparse, but the impact of 5-, 12-, and 15-HETEs on pathways and outcomes central to inflammation and cancer (COX-2 expression, PGE2 synthesis, proliferation, apoptosis, etc.) appear to differ: 5- and 12-HETEs appear to be pro-carcinogenic while 15-HETE appears to be anti-carcinogenic (17, 18). Additionally, the potential exists for increased production of R-HETEs by non-enzymatic oxidation in inflamed tissues. The profiles of total HETEs present in colon have been shown to be altered during colonic inflammation and carcinogenesis, and this could be related to increased formation of R-HETEs (19, 34, 49, 50). It would therefore be of interest to evaluate the enantiomeric composition of HETEs in tissues during progression from normal to disease states.
Limited available data indicate that in addition to the differences in activity between positional isomers, the biological activities of R- vs. S-HETEs in the colon differ as well (51-53). Specifically, 12-R-HETE stimulates proliferation (52). However, the anti-inflammatory and anti-cancer activities of R- vs. S-HETEs on outcomes central to inflammation and cancer progression (proliferation, apoptosis, etc.) in colon cells have not been systematically compared for all the major HETEs, possibly due to the lack of data on the levels of enantiomers in colonic mucosa which is needed to design such studies. Furthermore, no data exist on the impact of R/S-HETEs on early responses to inflammatory stress (i.e. NF-κB signaling). This makes it difficult to interpret the biological significance of these results at the present time.
The present data suggest that R-HETEs are present in substantial quantities in colon mucosa relative to S-HETEs, and the concentrations reported here can guide use of appropriate, HETE levels for in vitro mechanistic studies. The presence of substantial levels of R-HETEs in the colon signifies that more research is needed on non-LOX-mediated synthesis of HETEs, enantiomeric ratios occurring in vivo in normal vs. inflamed/cancerous colonic mucosa, and on the biological roles of both R/S-HETEs in the colon.
ACKNOWLEDGEMENTS
We thank Dr. Robert Langenbach for providing the transgenic animals (originally obtained from Taconic) and Dr. William Lands for advice on formulation of the rodent diets. Patrick Brown assisted with statistical data analysis, and Dr. Kathleen Noon assisted with chiral chromatographic LC-MS-MS methods.
Financial Support: Supported by the Michigan Institute for Clinical & Health Research (grant GM 48864), the University of Michigan Comprehensive Cancer Center (grant P30-CA46592), NCI grant RO1 CA120381, NIH grant R01 GM68848, and the Kutsche Memorial Endowment in Internal Medicine at the University of Michigan Medical School. A. Neilson was supported by the NCI T32 Cancer Biology Training Program at the University of Michigan (grant 5T32CA009676-18)
Abbreviations
- COX
cyclooxygenase
- EPA
eicosapentaenoic acid
- ESI
electrospray ionization
- DHA
docosahexaenoic acid
- HETE
hydroxyeicosatetraenoic acid
- HODE
hydroxyoctadecadienoic acid
- HUFA
highly unsaturated fatty acid
- LOX
lipoxygenase
- MUFA
monounsaturated fatty acid
- PGE2
prostaglandin E2
- SRM
selected reaction monitoring
Footnotes
Conflict of interest: The authors have no conflicts of interest to declare.
REFERENCES
- [1].Chapkin RS, Mcmurray DN, Lupton JR. Colon cancer, fatty acids and anti-inflammatory compounds. Curr Opin Gastroen. 2007;23:48–54. doi: 10.1097/MOG.0b013e32801145d7. [DOI] [PubMed] [Google Scholar]
- [2].Mclean MH, Murray GI, Fyfe N, Hold GL, Mowat N. a. G., et al. COX-2 expression in sporadic colorectal adenomatous polyps is linked to adenoma characteristics. Histopathology. 2008;52:806–15. doi: 10.1111/j.1365-2559.2008.03038.x. [DOI] [PubMed] [Google Scholar]
- [3].Pugh S, Thomas GA. Patients with adenomatous polyps and carcinomas have increased colonic mucosal prostaglandin E2. Gut. 1994;35:675–8. doi: 10.1136/gut.35.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Shureiqi I, Wojno KJ, Poore JA, Reddy RG, Moussalli MJ, et al. Decreased 13-S-hydroxyoctadecadienoic acid levels and 15-lipoxygenase-1 expression in human colon cancers. Carcinogenesis. 1999;20:1985–95. doi: 10.1093/carcin/20.10.1985. [DOI] [PubMed] [Google Scholar]
- [5].Wu Y, Fang B, Yang XQ, Wang L, Chen D, et al. Therapeutic molecular targeting of 15-lipoxygenase-1 in colon cancer. Mol Ther. 2008;16:886–92. doi: 10.1038/mt.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yuri M, Sasahira T, Nakai K, Ishimaru S, Ohmori H, et al. Reversal of expression of 15-lipoxygenase-1 to cyclooxygenase-2 is associated with development of colonic cancer. Histopathology. 2007;51:520–7. doi: 10.1111/j.1365-2559.2007.02799.x. [DOI] [PubMed] [Google Scholar]
- [7].Zijlstra FJ, Vandijk APM, Wilson JHP, Vanriemsdijkoverbeeke IC, Vincent JE, et al. 15-HETE is the main eicosanoid formed by human colonic mucosa. Agents Actions. 1992:C53–C9. [PubMed] [Google Scholar]
- [8].Coffa G, Brash AR. A single active site residue directs oxygenation stereospecificity in lipoxygenases: Stereocontrol is linked to the position of oxygenation. P Natl Acad Sci USA. 2004;101:15579–84. doi: 10.1073/pnas.0406727101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schwarz K, Walther M, Anton M, Gerth C, Feussner I, et al. Structural basis for lipoxygenase specificity - Conversion of the human leukocyte 5-lipoxygenase to a 15-lipoxygenating enzyme species by site-directed mutagenesis. J Biol Chem. 2001;276:773–9. doi: 10.1074/jbc.M005114200. [DOI] [PubMed] [Google Scholar]
- [10].Huang LS, Kang JS, Kim MR, Sok DE. Oxygenation of arachidonoyl lysophospholipids by lipoxygenases from soybean, porcine leukocyte, or rabbit reticulocyte. J Agric Food Chem. 2008;56:1224–32. doi: 10.1021/jf073016i. [DOI] [PubMed] [Google Scholar]
- [11].Walther M, Roffeis J, Jansen C, Anton M, Ivanov I, et al. Structural basis for pH-dependent alterations of reaction specificity of vertebrate lipoxygenase isoforms. Biochim Biophys Acta. 2009;1791:827–35. doi: 10.1016/j.bbalip.2009.05.007. [DOI] [PubMed] [Google Scholar]
- [12].Schneider C, Brash AR. Lipoxygenase-catalyzed formation of R-configuration hydroperoxides. Prostag Oth Lipid M. 2002;68-9:291–301. doi: 10.1016/s0090-6980(02)00041-2. [DOI] [PubMed] [Google Scholar]
- [13].Melstrom LG, Bentrem DJ, Salabat MR, Kennedy TJ, Ding XZ, et al. Overexpression of 5-Lipoxygenase in colon polyps and cancer and the effect of 5-LOX inhibitors in vitro and in a murine model. Clin Cancer Res. 2008;14:6525–30. doi: 10.1158/1078-0432.CCR-07-4631. [DOI] [PubMed] [Google Scholar]
- [14].Kennedy TJ, Talamonti M, Ujiki M, Ding XZ, Ternent CA, et al. Lipoxygenase expression in colon polyps and inhibition of colon cancer growth by lipoxygenase blockade. J Am Coll Surgeons. 2004;199:S78–S. [Google Scholar]
- [15].Merchant N, Chung DH, Townsend CM, Heslin MJ. Tumor-associated down-regulation of 15-lipoxygenase-1 is reversed by celecoxib in colorectal cancer - Discussion. Ann Surg. 2005;241:946–7. doi: 10.1097/01.sla.0000164177.95620.c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ye YN, Liu ESL, Shin VY, Wu WKK, Cho CH. Contributory role of 5-lipoxygenase and its association with angiogenesis in the promotion of inflammation-associated colonic tumorigenesis by cigarette smoking. Toxicology. 2004;203:179–88. doi: 10.1016/j.tox.2004.06.004. [DOI] [PubMed] [Google Scholar]
- [17].Vandijk APM, Mccafferty DM, Wilson JHP, Zijlstra FJ. 15-hydroxy-eicosatetraenoic acid has minor antiinflammatory properties in colitis. Agents Actions. 1993;38:C120–C1. doi: 10.1007/BF01991157. [DOI] [PubMed] [Google Scholar]
- [18].De Carvalho DD, Sadolk A, Bourgarel-Rey V, Gattacceca F, Penel C, et al. Nox1 downstream of 12-lipoxygenase controls cell proliferation but not cell spreading of colon cancer cells. Int J Cancer. 2008;122:1757–64. doi: 10.1002/ijc.23300. [DOI] [PubMed] [Google Scholar]
- [19].Shureiqi I, Chen DN, Day RS, Zuo XS, Hochman FL, et al. Profiling lipoxygenase metabolism in specific steps of colorectal tumorigenesis. Cancer Prev Res. 2010;3:829–38. doi: 10.1158/1940-6207.CAPR-09-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Niknami M, Dong Q, Witting PK. Pitfalls in the use of arachidonic acid oxidation products to assign lipoxygenase activity in cancer cells. Free Radical Res. 2009;43:951. doi: 10.1080/10715760903145013. [DOI] [PubMed] [Google Scholar]
- [21].Zeldin DC, Foley J, Goldsworthy SM, Cook ME, Boyle JE, et al. CYP2J subfamily cytochrome P450s in the gastrointestinal tract: Expression, localization, and potential functional significance. Mol Pharmacol. 1997;51:931–43. doi: 10.1124/mol.51.6.931. [DOI] [PubMed] [Google Scholar]
- [22].Capdevila JH, Falck JR. Biochemical and molecular properties of the cytochrome P450 arachidonic acid monooxygenases. Prostag Oth Lipid M. 2002;68-9:325–44. doi: 10.1016/s0090-6980(02)00038-2. [DOI] [PubMed] [Google Scholar]
- [23].Capdevila J, Yadagiri P, Manna S, Falck JR. Absolute-configuration of the hydroxyeicosatetraenoic acids (HETES) formed during catalytic oxygenation of arachidonicacid by microsomal cytochrome-P-450. Biochem Bioph Res Co. 1986;141:1007–11. doi: 10.1016/s0006-291x(86)80144-9. [DOI] [PubMed] [Google Scholar]
- [24].Bylund J, Ericsson J, Oliw EH. Analysis of cytochrome P450 metabolites of arachidonic and linoleic acids by liquid chromatography-mass spectrometry with ion trap MS2. Anal Biochem. 1998;265:55–68. doi: 10.1006/abio.1998.2897. [DOI] [PubMed] [Google Scholar]
- [25].Conners MS, Stoltz RA, Schwartzman ML. Chiral analysis of 12-hydroxyeicosatetraenoic acid formed by calf corneal epithelial microsomes. J Ocul Pharmacol Th. 1996;12:19–26. doi: 10.1089/jop.1996.12.19. [DOI] [PubMed] [Google Scholar]
- [26].Rowlinson SW, Crews BC, Goodwin DC, Schneider C, Gierse JK, et al. Spatial requirements for 15-(R)-hydroxy-5Z,8Z,11Z,13E-eicosatetraenoic acid synthesis within the cyclooxygenase active site of murine COX-2 - Why acetylated COX-1 does not synthesize 15-(R)-HETE. J Biol Chem. 2000;275:6586–91. doi: 10.1074/jbc.275.9.6586. [DOI] [PubMed] [Google Scholar]
- [27].Lee SH, Rangiah K, Williams MV, Wehr AY, Dubois RN, et al. Cyclooxygenase-2-mediated metabolism of arachidonic acid to 15-oxo-eicosatetraenoic acid by rat intestinal epithelial cells. Chem Res Toxicol. 2007;20:1665–75. doi: 10.1021/tx700130p. [DOI] [PubMed] [Google Scholar]
- [28].Jia Q, Lupton JR, Smith R, Weeks BR, Callaway E, et al. Reduced Colitis-Associated Colon Cancer in Fat-1 (n-3 Fatty Acid Desaturase) Transgenic Mice. Cancer Res. 2008;68:3985–91. doi: 10.1158/0008-5472.CAN-07-6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gravaghi C, La Perle KMD, Ogrodwski P, Kang JX, Quimby F, et al. Cox-2 expression, PGE2 and cytokines production are inhibited by endogenously synthesized n-3 PUFAs in inflamed colon of fat-1 mice. J Nutr Biochem. 2011;22:360–5. doi: 10.1016/j.jnutbio.2010.03.003. [DOI] [PubMed] [Google Scholar]
- [30].Carter MD, Reese Harry S, Wright DW. Identification of hydroxyeicosatetraenoic acid components of schistosomal hemozoin. Biochem Bioph Res Co. 2007;363:867–72. doi: 10.1016/j.bbrc.2007.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Masoodi M, Mir AA, Petasis NA, Serhan CN, Nicolaou A. Simultaneous lipidomic analysis of three families of bioactive lipid mediators leukotrienes, resolvins, protectins and related hydroxy-fatty acids by liquid chromatography/electrospray ionisation tandem mass spectrometry. Rapid Commun Mass Sp. 2008;22:75–83. doi: 10.1002/rcm.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Djuric Z, Ren JW, Blythe J, Vanloon G, Sen A. A Mediterranean dietary intervention in healthy American women changes plasma carotenoids and fatty acids in distinct clusters. Nutrition Research. 2009;29:156–63. doi: 10.1016/j.nutres.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Stark KD. The percentage of n-3 highly unsaturated fatty acids in total HUFA as a biomarker for omega-3 fatty acid status in tissues. Lipids. 2008;43:45–53. doi: 10.1007/s11745-007-3128-3. [DOI] [PubMed] [Google Scholar]
- [34].Zijlstra FJ, Vandijk APM, Garrelds IM, Ouwendijk RJT, Wilson JHP. Species-differences in the pattern of eicosanoids produced by inflamed and noninflamed tissue. Agents Actions. 1992:C73–C5. [PubMed] [Google Scholar]
- [35].Zijlstra FJ, Vandijk APM, Ouwendijk RJT, Vanriemsdijkoverbeeke IC, Wilson JHP. Eicosanoid production by the mucosa in inflammatory bowel-disease after 5-ASA treatment. Agents Actions. 1993;38:C122–C4. doi: 10.1007/BF01991158. [DOI] [PubMed] [Google Scholar]
- [36].Brash AR. Lipoxygenases: Occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem. 1999;274:23679–82. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- [37].Funk CD, Chen X-S, Johnson EN, Zhao L. Lipoxygenase genes and their targeted disruption. Prostag Oth Lipid M. 2002;68-69:303–12. doi: 10.1016/s0090-6980(02)00036-9. [DOI] [PubMed] [Google Scholar]
- [38].Al-Salihi MA, Terrece Pearman A, Doan T, Reichert EC, Rosenberg DW, et al. Transgenic expression of cyclooxygenase-2 in mouse intestine epithelium is insufficient to initiate tumorigenesis but promotes tumor progression. Cancer Lett. 2009;273:225–32. doi: 10.1016/j.canlet.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ishikawa T-O, Herschman HR. Tumor formation in a mouse model of colitis-associated colon cancer does not require COX-1 or COX-2 expression. Carcinogenesis. 2010;31:729–36. doi: 10.1093/carcin/bgq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, et al. Suppression of intestinal polyposis in Apc”716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87:803–9. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- [41].Calder PC. N-3 polyunsaturated fatty acids and inflammation: From molecular biology to the clinic. Lipids. 2003;38:343–52. doi: 10.1007/s11745-003-1068-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lee TH, Hoover RL, Williams JD, Sperling RI, Ravalese J, et al. Effect of dietary enrichment with eicosapentaenoic and docosahexaenoic acids on in vitro neutrophil and monocyte leukotriene generation and neutrophil function. New Engl J Med. 1985;312:1217–24. doi: 10.1056/NEJM198505093121903. [DOI] [PubMed] [Google Scholar]
- [43].Sperling RI, Benincaso AI, Knoell CT, Larkin JK, Austen KF, et al. Dietary omega-3 polyunsaturated fatty-acids inhibit phosphoinositide formation and chemotaxis in neutrophils. J Clin Invest. 1993;91:651–60. doi: 10.1172/JCI116245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Thomas CP, Davison Z, Heard CM. Probing the skin permeation of fish oil/EPA and ketoprofen-3. Effects on epidermal COX-2 and LOX. Prostag Leukotr Ess. 2007;76:357–62. doi: 10.1016/j.plefa.2007.05.002. [DOI] [PubMed] [Google Scholar]
- [45].Prescott SM. The effect of eicosapentaenoic acid on leukotriene B production by human neutrophils. J Biol Chem. 1984;259:7615–21. [PubMed] [Google Scholar]
- [46].Oh-Hashi K, Takahashi T, Watanabe S, Kobayashi T, Okuyama H, et al. Effect of replacing a high linoleate oil with a low linoleate, high alpha-linolenate oil, as compared with supplementing EPA or DHA, on reducing lipid mediator production in rat polymorphonuclear leukocytes. Biol Pharm Bull. 1998;21:558–64. doi: 10.1248/bpb.21.558. [DOI] [PubMed] [Google Scholar]
- [47].Wada M, Delong CJ, Hong YH, Rieke CJ, Song I, et al. Enzymes and receptors of prostaglandin pathways with arachidonic acid-derived versus eicosapentaenoic acid-derived substrates and products. J Biol Chem. 2007;282:22254–66. doi: 10.1074/jbc.M703169200. [DOI] [PubMed] [Google Scholar]
- [48].Vanamala J, Glagolenko A, Yang P, Carroll RJ, Murphy ME, et al. Dietary fish oil and pectin enhance colonocyte apoptosis in part through suppression of PPAR{delta}/PGE2 and elevation of PGE3. Carcinogenesis. 2008;29:790–6. doi: 10.1093/carcin/bgm256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Naito Y, Takagi T, Yoshikawa T. Molecular fingerprints of neutrophil-dependent oxidative stress in inflammatory bowel disease. J Gastroenterol. 2007;42:787–98. doi: 10.1007/s00535-007-2096-y. [DOI] [PubMed] [Google Scholar]
- [50].Rainis T, Maor I, Lanir A, Shnizer S, Lavy A. Enhanced oxidative stress and leucocyte activation in neoplastic tissues of the colon. Digest Dis Sci. 2007;52:526–30. doi: 10.1007/s10620-006-9177-2. [DOI] [PubMed] [Google Scholar]
- [51].Chen GG, Xu H, Lee JFY, Subramaniam M, Leung KL, et al. 15-hydroxy-eicosatetraenoic acid arrests growth of colorectal cancer cells via a peroxisome proliferator-activated receptor gamma-dependent pathway. Int J Cancer. 2003;107:837–43. doi: 10.1002/ijc.11447. [DOI] [PubMed] [Google Scholar]
- [52].Bortuzzo C, Hanif R, Kashfi K, Staianocoico L, Shiff SJ, et al. The effect of leukotrienes B and selected HETEs on the proliferation of colon cancer cells. BBA-Lipid Lipid Met. 1996;1300:240–6. doi: 10.1016/0005-2760(96)00003-3. [DOI] [PubMed] [Google Scholar]
- [53].Di Mari JF, Saada JI, Mifflin RC, Valentich JD, Powell DW. HETEs enhance IL-1-mediated COX-2 expression via augmentation of message stability in human colonic myofibroblasts. Am J Physiol-Gastr L. 2007;293:G719–G28. doi: 10.1152/ajpgi.00117.2007. [DOI] [PubMed] [Google Scholar]