Abstract
Neural and mesenchymal stem cells have extensive tropism for malignant glioma. The tumor tropism of induced pluripotent stem (iPS) cells was tested using the Matrigel invasion assay. Mouse iPS cells showed a significant tropism to the conditioned media prepared from six rodent and human glioma cell lines and this tropism to the glioma conditioned media was partially blocked by the neutralizing antibodies for four major tumor-associated growth factors [stem cell factor (SCF), platelet-derived growth factor BB (PDGF-BB), stromal-derived factor-1α (SDF-1α) and vascular endothelial growth factor (VEGF)], which are secreted from the malignant gliomas. The tropism of the iPS cells was enhanced by the growth factors in a concentration-dependent manner from 0.1 to 100 ng/ml. The receptors for those growth factors (c-Kit, ICAM-1, CXCR4 and VEGFR2), measured by reverse transcriptase-polymerase chain reaction, were highly up-regulated in the mouse iPS cells compared to the mouse fibroblasts. The results showed that the specific growth factors secreted from the gliomas strongly attracted the iPS cells. Therefore, gene therapies using iPS cells as vectors to deliver anti-tumor agents are novel strategies for the treatment of malignant gliomas that deeply infiltrate the brain.
Keywords: induced pluripotent stem cells, glioma, migration, growth factors
Introduction
Malignant glioma is the most common subtype of primary brain tumors. Glioblastoma multiforme, the most malignant glioma subtype, is associated with a median survival duration in the range of 6–18 months (1,2). Due to their specific properties, including infiltrative growth and resistance to tumoricidal agents, numerous advances in conventional therapeutic approaches, such as extensive surgical excision and adjuvant radio- and chemotherapy have been unsuccessful (3,4). This poor outcome relates, at least in part, to the inability to deliver therapeutic agents to the tumor (5). Accumulating evidences of tropism of stem cells for malignant glioma (6) show that gene therapies, using stem cells as the vehicles for therapeutic agents, have emerged as a promising treatment modality for malignant glioma. Furthermore, a number of pre-clinical trials of stem cell-based gene therapies have shown that neural stem cells (NSCs) are effective tumor-specific delivery vehicles for transgenes to malignant glioma (7–12). Similarly, evidence indicated that bone marrow-derived mesenchymal stem cells (MSCs) are effective vehicles for the delivery of gene therapies to malignant glioma (5,13). In previous studies, the extensive tropism of NSC and MSC for malignant glioma was observed, as well as a sufficient effect of the gene therapy using a suicide gene, the herpes simplex virus-thymidine kinase (HSVtk) gene, and a prodrug, ganciclovir (GCV) (14–18).
The mechanism underlying the tropism of NSC and MSC for tumors in general, and malignant gliomas in particular, has recently been identified as including soluble factors, cell adhesion molecules and extracellular matrix components (19–22). Malignant gliomas produce growth factors, cytokines and chemokines, which then mediate the tropism of NSC and MSC for malignant glioma. Previous studies using in vitro migration assays suggested that the exposure of NSC and MSC to specific growth factors, particularly stem cell factor (SCF) (21), platelet-derived growth factor BB (PDGF-BB) (22), stromal-derived factor-1α (SDF-1α) (19) and vascular endothelial growth factor (VEGF) (20), enhanced the migration of NSC and MSC (19–22).
Induced pluripotent stem (iPS) cells have been established both in rodents and humans, and various pre-clinical studies have been performed in the field of regeneration therapy (23). As previously noted, NSCs and MSCs are excellent vehicles for gene delivery to gliomas. Thus, the use of iPS cells from patients is likely to be more ideal in terms of the quality control of the cells and the invasiveness of cell collection. In the present study, the tumor-tropic activity of iPS cells was examined to evaluate whether the cells could be utilized as vehicles for glioma gene therapies.
Materials and methods
Cell culture
The mouse iPS cells, iPS-MEF-Ng-20D-17 established by Yamanaka et al (23), were obtained from Riken Biosource Center (Tsukuba, Japan) and were cultured on mitotically inactivated mouse embryonic fibroblasts in the medium composed of Dulbecco’s modified Eagle’s medium (DMEM) high glucose 1X (Invitrogen, Tokyo, Japan) supplemented with 15% fetal bovine serum (FBS; Sigma-Aldrich Japan, Tokyo, Japan), 0.1 mM MEM non-essential amino acids (Invitrogen), 0.1 mM 2-mercaptoethanol (Sigma-Aldrich Japan) and 1,000 U/ml leukemia inhibitory factor (ESGRO; Millipore, Temecula, CA, USA) on a gelatin-coated dish at 37°C in a 5% CO2 humidified atmosphere according to the protocol previously reported (24). Experiments were performed using the mouse iPS cells during passages 2–4. The mouse glioma cell line GL261 and the rat glioma cell line C6 were purchased from Health Science Research Resources Bank (Osaka, Japan), and the human glioma cell lines A172, T98G, YKG1 and U87 from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were grown in DMEM (Sigma-Aldrich, Japan) supplemented with 10% FBS, penicillin (100 IU/ml) and streptomycin (100 μg/ml) at 37°C in a humidified atmosphere of 5% CO2. The mouse iPS cells were dissociated at 37°C for 2 min using 0.25% trypsin with 1 mM EDTA, and the glioma cell lines were dissociated using 0.25% trypsin with 1 mM EDTA for 3 min.
Migration of induced pluripotent stem cells towards the glioma-conditioned media and specific growth factors
The in vitro migratory capacity of iPS cells was assessed using the 24-well Matrigel Invasion Chamber (BD Biosciences Discovery Labware, Bedford, MA, USA), which contained an 8-μm pore size PET membrane treated with Matrigel Basement Membrane Matrix in the insert (25). First, 0.5 ml DMEM was added to the interior of the inserts and the bottom of the wells and allowed to rehydrate for 2 h at 37°C in a 5% CO2 humidified atmosphere. The DMEM was then carefully removed without disturbing the layer of Matrigel Matrix on the membrane. The mouse iPS cells were washed twice in phosphate-buffered saline (PBS) and resuspended to 1×105 cells/ml. Cell suspension (0.5 ml) (5×104 cells) was added to the upper insert. The lower chamber was filled with 0.75 ml of conditioned medium (CM) of the glioma cell lines as well as unconditioned medium (DMEM) as a control. CM was obtained by collecting, centrifuging and filtering medium from GL261, C6, A172, T98G, YKG1 and U87 clones (1×106), which were cultured in 10 ml of DMEM without FBS for 48 h. For the migration stimulation assays, the specific growth factors SCF, PDGF-BB, SDF-1α and VEGF were added to the lower chamber at concentrations from 0.1 to 100 ng/ml. For the specific growth factors blocking experiments, CM from the GL261 mouse glioma cell line was incubated with anti-SCF, anti-PDGF-BB, anti-SDF-1α and anti-VEGF neutralizing antibodies (Abcam PLC, Tokyo, Japan) for 3 h prior to transfer into the lower chamber at concentrations of 1 and 10 μg/ml.
Following incubation of the Matrigel Invasion Chambers for 24 h at 37°C in a 5% CO2 humidified atmosphere, the non-invading cells and/or Matrigel Matrix were removed from the upper surface of the membrane in the inserts with a cotton swab. The cells migrating to the lower surface of the membrane were stained with the Diff-Quick kit (International Reagents, Hyogo, Japan), which was achieved by sequentially transferring the inserts to air dry. The nuclei of the migrated cells were counted in 4 high-power fields (HPF) per membrane using a magnification of ×200. All experiments were conducted in triplicate and results were expressed as the mean number of cells migrating per field ± SD.
Expression of the receptors for growth factors
The status of the growth factor receptors of mouse iPS cells was analyzed by reverse transcriptase-polymerase chain reaction (RT-PCR). The receptors used for the growth factors SCF, PDGF-BB, SDF-1α and VEGF were c-Kit, intercellular adhesion molecule-1 (ICAM-1), CXC chemokine receptor 4 (CXCR4) and vascular endotherial growth factor receptor 2 (VEGFR2), respectively. Total RNA was extracted from the mouse iPS cells and from the control mouse fibroblasts using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. cDNA was generated from 1 μg of total RNA from each sample. The primers used were: c-Kit, forward, 5′-CAGAGGCTTAGCGGAGTGAAGTG-3′ and reverse, 5′-TCCCTGGATTGGCAGCATTAC-3′; ICAM-1, forward, 5′-AACTGTGGCACCGTGCAGTC-3′ and reverse, 5′-AGGG TGAGGTCCTTGCCTACTTG-3′; CXCR4, forward, 5′-CCG GTACCTCGCTATTGTCCAC-3′ and reverse, 5′-GGATCCAG ACGCCCACACATAGA-3′; VEGFR2, forward, 5′-TCTCC GTTATTGCTTCTGTTAG-3′ and reverse, 5′-GTGATACC TTGCACAGAGTGACAC-3′; β-actin, forward, 5′-TCAGGT CATCACTATCGGCAAT-3′ and reverse, 5′-AAAGAAAGGGT GTAAAACGCA-3′. The PCR conditions consisted of an initial denaturation at 94°C for 2 min followed by 30 cycles of denaturation at 94°C for 30 sec, annealing at 50°C for 30 sec and extension at 72°C for 30 sec in a thermal cycler (LightCycler, Roche Diagnostics K.K., Tokyo, Japan). The integrated density values were determined by extrapolation using the cRNA standard curve, and normalized with that of β-actin. The fold increase was calculated from the results of three independent experiments.
Statistical analysis
The data (mean ± SD) were analyzed using the two-tailed unpaired Student’s t-test with 95% confidence interval for a two-group comparison. Differences were considered significant at p<0.01.
Results
Migration of induced pluripotent stem cells towards the glioma-conditioned media and specific growth factors
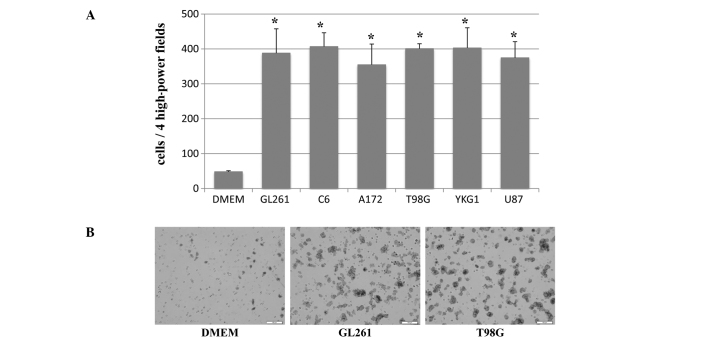
Directional migration of mouse iPS cells towards the CM prepared from six different glioma cell lines (GL261, C6, A172, T98G, YKG1 and U87) was analyzed using the 24-well Matrigel Invasion Chamber. A high number of mouse iPS cells were observed migrating towards the CM of GL261, C6, A172, T98G, YKG1 and U87 (388±70, 407±40, 355±59, 401±14, 403±58 and 375±46 per 4HPF, respectively), whereas few cells migrated towards the unconditioned medium with or without FBS (89±6 and 48±3 per 4HPF, respectively) (Fig. 1). Every CM from glioma cell lines significantly stimulated the directional migration of mouse iPS cells compared to unconditioned medium with or without FBS (p<0.001).
Figure 1.
(A) Migration of the mouse iPS cells induced by conditioned media (CM) prepared from six rodent (GL261 and C6) and human (A172, T98G, YKG1 and U87) glioma cell lines and unconditioned medium Dulbecco’s modified Eagle’s medium (DMEM) in the 24-well Matrigel Invasion Chamber. The migration was significantly increased by the glioma CM (triplicate, means ± SD, *p<0.001). (B) Representative photomicrographs of the micropore membranes from DMEM, GL261-CM and T98G-CM (bars, 100 μm).
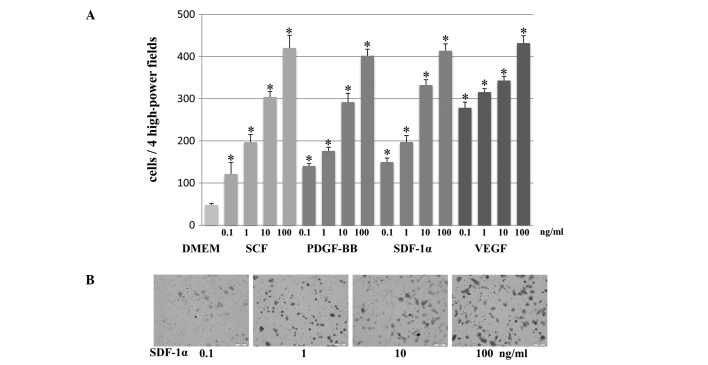
The migration of mouse iPS cells towards increasing concentrations of specific growth factors, such as SCF, SDF-1α VEGF and PDGF-BB (Fig. 2) was then assessed. Specifically, 0.1–100 ng/ml of specific growth factors were placed in the lower chambers. Migration was assayed after 24 h by counting the number of mouse iPS cells in 4HPF on the membrane. The migration of mouse iPS cells increased significantly with the stimulation of each specific growth factor (SCF, SDF-1α VEGF and PDGF-BB) at concentrations of 0.1, 1, 10 and 100 ng/ml compared to the unconditioned medium without FBS (p<0.001). Furthermore, the number of migrating mouse iPS cells increased dose-dependently with concentrations of each specific growth factor from 0.1 to 100 ng/ml.
Figure 2.
(A) Chemotactic migration of the mouse iPS cells by increasing concentrations of specific growth factors [stem cell factor, platelet-derived growth factor BB, stromal-derived factor-1α (SDF-1α) and vascular endothelial growth factor] in the 24-well Matrigel Invasion Chamber. The migration significantly increased with the stimulation of each growth factor in a concentration-dependent manner at concentrations of 0.1, 1, 10 and 100 ng/ml compared to Dulbecco’s modified Eagle’s medium (triplicate, means ± SD, *p<0.001). (B) Representative photomicrographs of the micropore membranes of SDF-1α at concentrations from 0.1 to 100 ng/ml (bars, 100 μm).
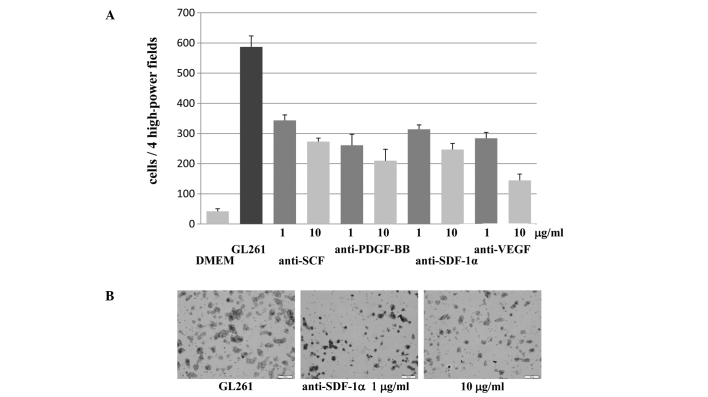
To investigate whether the increase in the migration of mouse iPS cells towards CM prepared from glioma cell lines was specifically attributable to the presence of specific growth factors, migration assays were performed using CM from the GL261 mouse glioma cell line treated with increasing concentrations of the inhibitory monoclonal anti-SCF, SDF-1α, VEGF and PDGF-BB antibodies that neutralized the activity of SCF, SDF-1α, VEGF and PDGF-BB, respectively. The high level of the migration of mouse iPS cells that resulted following exposure to native CM from the GL261 mouse glioma cell line was significantly attenuated by treatment with the inhibitory monoclonal anti-SCF, anti-SDF-1α, anti-VEGF and anti-PDGF-BB antibodies (Fig. 3). The application of the antibodies without any CM did not show any effect on mouse iPS cell migration (data not shown). The inhibition was dose-dependent, with 10 μg/ml of antibodies resulting in more effective inhibition of mouse iPS cell migration compared to 1 μg/ml of antibodies. Anti-SCF, anti-SDF-1α, anti-VEGF and anti-PDGF-BB antibodies (10 μg/ml) blocked the effects of CM by 46.5, 42.1, 24.6 and 35.7%, respectively. On the other hand, 1 μg/ml of anti-SCF, anti-SDF-1α, anti-VEGF and anti-PDGF-BB antibodies blocked the effects of CM by 58.5, 53.5, 48.4 and 44.5%, respectively. Furthermore, the combination of all the antibodies showed a stronger attenuating effect of mouse iPS cell migration, with 10 μg/ml blocked by 18.2% and 1 μg/ml by 32.2%. Taken together, these results indicate that tumor-derived specific growth factors (SCF, SDF-1α VEGF and PDGF-BB) promote the migration of mouse iPS cells towards gliomas in vitro.
Figure 3.
(A) Migration assays were performed using GL261-CM treated with the inhibitory monoclonal neutralizing antibodies of the growth factors at concentrations of 1 and 10 μg/ml. Enhanced migrations of the mouse-induced pluripotent stem cells by GL261-CM mouse were significantly attenuated by the treatment with anti-stem cell factor, anti-platelet-derived growth factor BB, anti-stromal-derived factor-1α and anti-vascular endothelial growth factor antibodies (triplicate, means ± SD, *p<0.001). (B) Representative photomicrographs of the micropore membranes of GL261-CM treated with 0, 1 and 10 μg/ml of the anti-SDF-1α antibody (bars, 100 μm).
Expression of the receptors for growth factors
The expression of four different growth factor receptors, c-Kit, CXCR4, VEGFR2 and ICAM-1, was analyzed in mouse iPS cells using RT-PCR (Fig. 4). As a positive control, mouse fibroblasts were analyzed in parallel. All of the growth factor receptors were found to be expressed by the mouse iPS cells. When the values were normalized to β-actin expresssion, c-Kit, CXCR4, VEGFR2 and ICAM-1 mRNA expression in mouse iPS cells was significantly up-regulated compared to the mouse fibroblasts (p<0.001).
Figure 4.
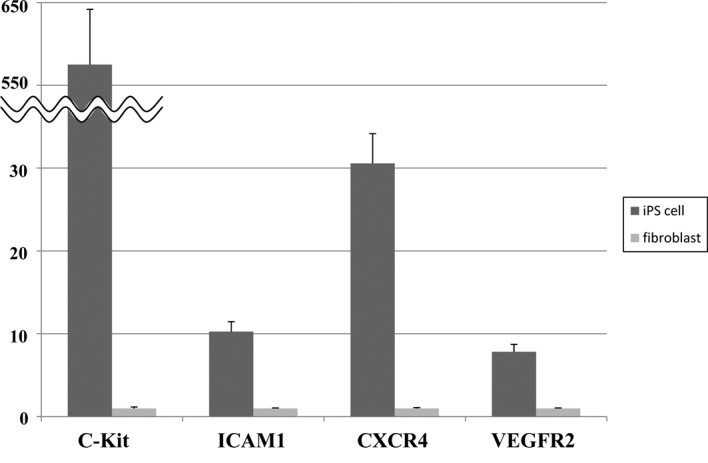
Expression of the receptors for four growth factors (c-Kit, ICAM-1, CXCR4 and VEGFR2) in the mouse-induced pluripotent stem cells and mouse fibroblasts was analyzed by reverse transcriptase-polymerase chain reaction (RT-PCR). The values were normalized to β-actin expression. The mRNA expression of the growth factor receptors was significantly up-regulated in the mouse iPS cells compared to the mouse fibroblast (means ± SD, *p<0.001).
Discussion
Following the establishment of mouse iPS cells generated from mouse skin fibroblasts by the retroviral introduction of four transcription factors (Oct3/4, Sox2, c-Myc and Klf4) (23), various cell transplantation studies were performed due to the high reproductive and pluripotent ability of iPS cells (26–29). For iPS cells to function appropriately, the migratory activity of iPS cells to the appropriate regions is crucial. Although numerous studies have shown the in vitro and in vivo tumor-tropic migratory ability of NSCs and MSCs to malignant gliomas (6,7,9,11,12,15,30,31), studies of migratory activity of iPS cells have yet to be performed.
This study aimed to examine the migratory capacity of mouse iPS cells towards gliomas in order to evaluate the usefulness of iPS cells as vehicles for glioma gene therapies. The migration of mouse iPS cells was significantly stimulated by CM from six rodent and human glioma cell lines as well as four specific growth factors that are secreted by gliomas (SCF, PDGF-BB, SDF-1α and VEGF) (19–22). Furthermore, the expression of the corresponding receptors (c-Kit, ICAM-1, CXCR4 and VEGFR2) was also significantly up-regulated in the mouse iPS cells. These findings indicated that soluble factors secreted by malignant glioma cells, including the four growth factors examined, were potent inducers of mouse iPS cell migration.
However, factors other than these four growth factors that stimulate the migration of mouse iPS cells may be produced by the glioma cells to various extents, depending on the cell line. The heterogeneity of growth factor production presumably expresses the in vivo differences in the biological behavior of malignant gliomas, including the proliferative and invasive natures associated with the expression profiles of cytokines, interleukins and growth factors, such as transforming growth factor-β1 (TGF-β1) (32–34) and matrix metalloproteinases (35). Other specific growth factors known to be expressed in malignant gliomas are fibroblast growth factor-1 (FGF-1), PDGF-AA, insulin-like growth factor-1 (IGF-1), scatter factor/hepatocyte growth factor (SF/HGF) and TGF-α, -β1 and -β2 (36,37). A number of these factors already play a role in the stimulation of NSC migration (31).
The results of the present study showed that the four specific growth factors (SCF, PDGF-BB, SDF-1α and VEGF) serve as attractants for mouse iPS cells. Subsequently, blocking experiments were performed by adding the inhibitory monoclonal antibodies for those factors in the CM from GL261 mouse glioma cells known to secrete numerous specific growth factors. Stimulated mouse iPS cell migration by GL261 CM was significantly inhibited by all the neutralizing antibodies examined, indicating that the presence of these specific growth factors was significantly responsible for the chemoattractant capacity of the CM.
Various types of stem cells are known to express the receptors for SCF, PDGF-BB, SDF-1α and VEGF (c-Kit, ICAM-1,CXCR4 and VEGFR2, respectively) (38,39). These receptors were highly expressed in the mouse iPS cells. The presence of ligand/receptor combinations of the chemoattractive factors, such as SCF/c-Kit, PDGF-BB/ICAM-1, SDF-1α/CXCR4 and VEGF/VEGFR2, allow malignant glioma and iPS cells to communicate with each other and, consequently, facilitate the migration of iPS cells to gliomas.
The present study showed, for the first time, that mouse iPS cells exerted marked tropism to glioma CM and at least four specific growth factors (SCF, PDGF-BB, SDF-1α and VEGF). The tropism was blocked by the neutralizing antibodies for these growth factors. The majority of the malignant glioma cells are supposed to secrete numerous soluble factors that attract mouse iPS cells, although the amount is variable among the tumors. Additionally, mouse iPS cells recognize a broad spectrum of signals from malignant glioma cells as migration triggers. These observations suggest that iPS cells are likely to aid as therapeutic vehicles for the treatment of malignant gliomas if they are genetically modified to express therapeutic transgenes that encode oncolytic agents, apoptosis-inducing factors, interleukins, factors that inhibit angiogenesis and the suicide genes.
We have investigated the use of NSCs and MSCs as therapeutic vehicles for a suicide gene therapy, HSVtk/GCV, and obtained encouraging results in pre-clinical models (14–18). However, the use of this strategy for patients is hampered by significant limitations, such as the isolation of clinically viable and legally utilizable sources and ethical problems (20). Similarly, iPS cells remain in pre-clinical phases and also experience ethical problems, including cell tumorigenesis (40,41). If tumor formation of the iPS cells is adequately regulated and all of the variables affecting safety issues are rigorously evaluated, the clinical use of iPS cell-based therapies may become a useful tool in the field of regeneration therapy. The results of the present study strongly suggest that the use of iPS cells as therapeutic vehicles for the delivery of suicide genes is a novel strategy for the treatment of malignant gliomas. Additional studies are required to compare the migration characteristics of iPS cells to those of NSCs and MSCs, particularly under in vivo conditions.
Acknowledgements
This study was supported in part by the Japan Society for the Promotion of Science Young Investigators Grants (S.K.).
References
- 1.Michotte A, Neyns B, Chaskis C, Sadones J, In’t Veld P. Neuropathological and molecular aspects of low-grade and high-grade gliomas. Acta Neurol Belg. 2004;104:148–153. [PubMed] [Google Scholar]
- 2.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Surawicz TS, Davis F, Freels S, Laws ER, Jr, Menck HR. Brain tumor survival: results from the National Cancer Data Base. J Neurooncol. 1998;40:151–160. doi: 10.1023/a:1006091608586. [DOI] [PubMed] [Google Scholar]
- 4.DeAngelis LM. Brain tumors. N Engl J Med. 2001;344:114–123. doi: 10.1056/NEJM200101113440207. [DOI] [PubMed] [Google Scholar]
- 5.Nakamizo A, Marini F, Amano T, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 6.Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benedetti S, Pirola B, Pollo B, et al. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med. 2000;6:447–450. doi: 10.1038/74710. [DOI] [PubMed] [Google Scholar]
- 8.Ehtesham M, Kabos P, Gutierrez MA, et al. Induction of glioblastoma apoptosis using neural stem cell-mediated delivery of tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res. 2002;62:7170–7174. [PubMed] [Google Scholar]
- 9.Ehtesham M, Kabos P, Kabosova A, Neuman T, Black KL, Yu JS. The use of interleukin 12-secreting neural stem cells for the treatment of intracranial glioma. Cancer Res. 2002;62:5657–5663. [PubMed] [Google Scholar]
- 10.Kim SK, Cargioli TG, Machluf M, et al. PEX-producing human neural stem cells inhibit tumor growth in a mouse glioma model. Clin Cancer Res. 2005;11:5965–5970. doi: 10.1158/1078-0432.CCR-05-0371. [DOI] [PubMed] [Google Scholar]
- 11.Kim SK, Kim SU, Park IH, et al. Human neural stem cells target experimental intracranial medulloblastoma and deliver a therapeutic gene leading to tumor regression. Clin Cancer Res. 2006;12:5550–5556. doi: 10.1158/1078-0432.CCR-05-2508. [DOI] [PubMed] [Google Scholar]
- 12.Lee DH, Ahn Y, Kim SU, et al. Targeting rat brainstem glioma using human neural stem cells and human mesenchymal stem cells. Clin Cancer Res. 2009;15:4925–4934. doi: 10.1158/1078-0432.CCR-08-3076. [DOI] [PubMed] [Google Scholar]
- 13.Sasportas LS, Kasmieh R, Wakimoto H, et al. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc Natl Acad Sci USA. 2009;106:4822–4827. doi: 10.1073/pnas.0806647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amano S, Li S, Gu C, et al. Use of genetically engineered bone marrow-derived mesenchymal stem cells for glioma gene therapy. Int J Oncol. 2009;35:1265–1270. doi: 10.3892/ijo_00000443. [DOI] [PubMed] [Google Scholar]
- 15.Li S, Gao Y, Tokuyama T, et al. Genetically engineered neural stem cells migrate and suppress glioma cell growth at distant intracranial sites. Cancer Lett. 2007;251:220–227. doi: 10.1016/j.canlet.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 16.Li S, Tokuyama T, Yamamoto J, Koide M, Yokota N, Namba H. Potent bystander effect in suicide gene therapy using neural stem cells transduced with herpes simplex virus thymidine kinase gene. Oncology. 2005;69:503–508. doi: 10.1159/000091032. [DOI] [PubMed] [Google Scholar]
- 17.Li S, Tokuyama T, Yamamoto J, Koide M, Yokota N, Namba H. Bystander effect-mediated gene therapy of gliomas using genetically engineered neural stem cells. Cancer Gene Ther. 2005;12:600–607. doi: 10.1038/sj.cgt.7700826. [DOI] [PubMed] [Google Scholar]
- 18.Namba H, Tagawa M, Iwadate Y, Kimura M, Sueyoshi K, Sakiyama S. Bystander effect-mediated therapy of experimental brain tumor by genetically engineered tumor cells. Hum Gene Ther. 1998;9:5–11. doi: 10.1089/hum.1998.9.1-5. [DOI] [PubMed] [Google Scholar]
- 19.Schichor C, Birnbaum T, Etminan N, et al. Vascular endothelial growth factor A contributes to glioma-induced migration of human marrow stromal cells (hMSC) Exp Neurol. 2006;199:301–310. doi: 10.1016/j.expneurol.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 20.Ehtesham M, Yuan X, Kabos P, et al. Glioma tropic neural stem cells consist of astrocytic precursors and their migratory capacity is mediated by CXCR4. Neoplasia. 2004;6:287–293. doi: 10.1593/neo.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serfozo P, Schlarman MS, Pierret C, Maria BL, Kirk MD. Selective migration of neuralized embryonic stem cells to stem cell factor and media conditioned by glioma cell lines. Cancer Cell Int. 2006;6:1. doi: 10.1186/1475-2867-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hata N, Shinojima N, Gumin J, et al. Platelet-derived growth factor BB mediates the tropism of human mesenchymal stem cells for malignant gliomas. Neurosurgery. 2010;66:144–157. doi: 10.1227/01.NEU.0000363149.58885.2E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi K, Okita K, Nakagawa M, Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2007;2:3081–3089. doi: 10.1038/nprot.2007.418. [DOI] [PubMed] [Google Scholar]
- 25.Mohanam S, Sawaya R, McCutcheon I, Ali-Osman F, Boyd D, Rao JS. Modulation of in vitro invasion of human glioblastoma cells by urokinase-type plasminogen activator receptor antibody. Cancer Res. 1993;53:4143–4147. [PubMed] [Google Scholar]
- 26.Amabile G, Meissner A. Induced pluripotent stem cells: current progress and potential for regenerative medicine. Trends Mol Med. 2009;15:59–68. doi: 10.1016/j.molmed.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Koch P, Kokaia Z, Lindvall O, Brustle O. Emerging concepts in neural stem cell research: autologous repair and cell-based disease modelling. Lancet Neurol. 2009;8:819–829. doi: 10.1016/S1474-4422(09)70202-9. [DOI] [PubMed] [Google Scholar]
- 28.Ronaghi M, Erceg S, Moreno-Manzano V, Stojkovic M. Challenges of stem cell therapy for spinal cord injury: human embryonic stem cells, endogenous neural stem cells, or induced pluripotent stem cells? Stem Cells. 2010;28:93–99. doi: 10.1002/stem.253. [DOI] [PubMed] [Google Scholar]
- 29.Yu J, Thomson JA. Pluripotent stem cell lines. Genes Dev. 2008;22:1987–1997. doi: 10.1101/gad.1689808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng P, Gao ZQ, Liu YH, Xue YX. Platelet-derived growth factor BB promotes the migration of bone marrow-derived mesenchymal stem cells towards C6 glioma and up-regulates the expression of intracellular adhesion molecule-1. Neurosci Lett. 2009;451:52–56. doi: 10.1016/j.neulet.2008.12.044. [DOI] [PubMed] [Google Scholar]
- 31.Heese O, Disko A, Zirkel D, Westphal M, Lamszus K. Neural stem cell migration toward gliomas in vitro. Neuro Oncol. 2005;7:476–484. doi: 10.1215/S1152851704000754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brat DJ, Bellail AC, van Meir EG. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol. 2005;7:122–133. doi: 10.1215/S1152851704001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mentlein R, Held-Feindt J. Pleiotrophin, an angiogenic and mitogenic growth factor, is expressed in human gliomas. J Neurochem. 2002;83:747–753. doi: 10.1046/j.1471-4159.2002.01179.x. [DOI] [PubMed] [Google Scholar]
- 34.Teicher BA. Malignant cells, directors of the malignant process: role of transforming growth factor-beta. Cancer Metastasis Rev. 2001;20:133–143. doi: 10.1023/a:1013177011767. [DOI] [PubMed] [Google Scholar]
- 35.Nagashima G, Suzuki R, Asai J, Fujimoto T. Immunohistochemical analysis of reactive astrocytes around glioblastoma: an immunohistochemical study of postmortem glioblastoma cases. Clin Neurol Neurosurg. 2002;104:125–131. doi: 10.1016/s0303-8467(01)00197-4. [DOI] [PubMed] [Google Scholar]
- 36.Dunn IF, Heese O, Black PM. Growth factors in glioma angiogenesis: FGFs, PDGF, EGF, and TGFs. J Neurooncol. 2000;50:121–137. doi: 10.1023/a:1006436624862. [DOI] [PubMed] [Google Scholar]
- 37.Hamel W, Westphal M. Growth factors in gliomas revisited. Acta Neurochir. 2000;142:113–138. doi: 10.1007/s007010050015. [DOI] [PubMed] [Google Scholar]
- 38.Das AV, James J, Zhao X, Rahnenfuhrer J, Ahmad I. Identification of c-Kit receptor as a regulator of adult neural stem cells in the mammalian eye: interactions with Notch signaling. Dev Biol. 2004;273:87–105. doi: 10.1016/j.ydbio.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 39.Tran PB, Ren D, Veldhouse TJ, Miller RJ. Chemokine receptors are expressed widely by embryonic and adult neural progenitor cells. J Neurosci Res. 2004;76:20–34. doi: 10.1002/jnr.20001. [DOI] [PubMed] [Google Scholar]
- 40.Baudino TA, McKay C, Pendeville-Samain H, et al. c-Myc is essential for vasculogenesis and angiogenesis during development and tumor progression. Genes Dev. 2002;16:2530–2543. doi: 10.1101/gad.1024602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miura K, Okada Y, Aoi T, et al. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27:743–745. doi: 10.1038/nbt.1554. [DOI] [PubMed] [Google Scholar]