Abstract
Breast cancer occurs at a high frequency in women and, given this fact, a primary focus of breast cancer research has been the study of estrogen receptor α (ER) signaling. However, androgens are known to play a role in normal breast physiology and therefore androgen receptor (AR) signaling is becoming increasingly recognized as an important contributor towards breast carcinogenesis. Moreover, the high frequency of AR expression in breast cancer makes it an attractive therapeutic target, but the ability to exploit AR for therapy has been difficult. Here we review the historical use of androgen/anti-androgen therapies in breast cancer, the challenges of accurately modeling nuclear hormone receptor signaling in vitro, and the presence and prognostic significance of AR in breast cancer.
Keywords: Androgen receptor, MAP kinase, breast cancer, p21, androgens
Introduction
The development of targeted therapies has always been a goal of cancer research. The ideal targeted therapy by definition would be specific for the tumor and not affect other tissues in the body. While there are many examples of effective targeted therapies in a variety of cancers none fully achieve this definition; that is, side effects of medications always occur. Though there may never be a drug that achieves a perfect level of specificity, certainly there is opportunity for new targeted therapies to be as effective as or better than current standards of care. Therefore, the challenge for developing better therapies has recently relied upon the discovery and validation of targets that are mostly or exclusively tumor-specific, including products of gene mutations and/or translocations. An alternative strategy that has been successful for cancer therapy is to develop drugs against targets that are frequently expressed at increased levels in cancerous tissues. Examples include HER2, a member of the epidermal growth factor receptor (EGFR) family, and estrogen receptor α (ER). Both have been successfully exploited as targeted therapies for breast cancer patients [1,2]. However, many patients are not eligible for these therapies because their tumors lack expression of these receptors, and/or their tumors become resistant to the therapeutic agent. Thus, there is a continuing need to expand the repertoire of targeted anti-cancer therapies. This review focuses on the androgen receptor (AR) and its future potential as a new generation of endocrine therapies for the treatment of breast cancer.
History of targeting AR for therapy in breast cancer
It has been appreciated now for over a century that hormones play a critical role in breast carcinogenesis [3,4] and therefore this provides a rationale for their use as anti-cancer therapies. Given the drastically higher prevalence of breast cancer in women compared to men, research has focused on the dominant sex hormone in females, that is, estrogens. However, because male sex hormones also play a role in normal female breast physiology, research has also been conducted in the use of androgens for breast cancer therapy. In principle one might hypothesize that breast cancer may be a result of a hormonal “imbalance” of estrogens to androgens and that this ratio is vastly different between women and men. Thus, empirically and simplistically, exogenous administration of androgens could lead to an effective therapy for the treatment of breast cancer. For the first half of the 20th century, therapies utilizing androgens were mainly reserved for postmenopausal women; treatment for premenopausal patients initially consisted of oophorectomy either surgically by removing the ovaries or chemically by administration of androgens. However, androgen administration was discontinued when physicians became aware that androgens can be converted to estrogens [5]. As demonstrated by the results of experiments with surgical oophorectomy, it was clear that estrogen increased the formation of breast cancers and stimulated most of these malignancies to grow [3,4]. Despite setbacks in the premenopausal setting, multiple studies in the postmenopausal setting validated the efficacy of androgen therapy [6-8]. Studies showed a disease regression rate of ~20-25% in women treated with androgen therapy which was slightly less effective than the ~25-35% rate demonstrated for women treated with estrogen-based therapy [9,10].
While androgen therapy had moderate efficacy, the administration of excess androgen had significant negative side effects including increased aggressive behavior and hirsutism [7]. Efforts were made to minimize masculinizing side effects by developing androgen analogs, but similar efforts into developing improved estrogen based therapies were also underway. Eventually, targeting AR as a therapy for breast cancer fell out of favor in the 1970s with the advent of what would later be termed Selective Estrogen Receptor Modulators or SERMs [11]. This new class of drugs, with tamoxifen being the archetype, became the primary treatment modality not simply due to greater efficacy over conventional hormonal therapies but importantly because of a much favorable toxicity profile [10].
Because of the success of tamoxifen and other ER targeted therapies for the prevention and treatment of breast cancer (e.g. raloxifene, aromatase inhibitors, faslodex) very few clinical trials in the 1970s, 80s, and 90s focused on targeted androgen based therapy. Yet the idea of AR directed therapy for breast cancer was not completely abandoned as documented by a relatively few number of clinical trials during this period [12-14]. Although there are only a scant number of published reports examining the clinical feasibility of targeting AR, much more work has been done in the laboratory setting. Collectively, these studies helped elucidate the role of AR in breast cancer and the mechanism of action of drugs targeting AR. With this laboratory work as a basis, therapies targeted to AR in breast cancer are re-emerging in the clinic. New clinical trials have been initiated within the last few years using agents directed against AR or against androgen production (see clinicaltrials.gov: NCT00468715, NCT00516542,NCT00755885, NCT00972023). The effectiveness of these androgen based therapies remains to be seen but will become more apparent in subsequent years. Additionally, with more data regarding the presence of AR and the mechanism of action of androgens in breast cancer, researchers have the opportunity to develop more efficacious targeted androgen therapies.
Action of androgens in the breast
Elucidating the effect of nuclear hormones in breast cancer has been a continual challenge. An immense body of work has been undertaken to understand nuclear hormone receptor signaling but these studies have not always provided clarity into how these pathways affect breast carcinogenesis. In this section we review these studies, acknowledge the hurdles that have been encountered, and attempt to provide insight into the results that have been generated over the past few decades.
In part, the lack of understanding of AR signaling as related to breast carcinogenesis is a direct result of the lack of ideal model systems that accurately recapitulate AR biology in human breast epithelial cells. In vitro models using cell lines established from primary human breast tumors have been the standard method for analyzing nuclear hormone receptor signaling in breast tissues. However, there are relatively few breast cancer cell lines that express AR as the sole sex hormone receptor, and those that do exist harbor genetic mutations/alterations that could potentially affect AR signaling. For example, the MDA-MB-453 breast cancer cell line is AR positive and ER negative, but also contains homozygous deletion of TP53, a homozygous PTEN missense mutation, HER2 amplification and an oncogenic mutation in PIK3CA (Sanger COSMIC database) [15,16].
The use of cancer cell line models has prevailed for years but has not always generated definitive results. A report by Birrell et al. examined the response to androgens in four commonly used breast cancer cell lines [17]. Androgen treatment of one or more of these cell lines has been investigated in numerous reports from a variety of other groups including our own [17-34]. Table 1 summarizes results from these published reports. The response to androgen treatment in a variety of other breast cancer cell lines also has been detailed by many other groups, but for brevity only the four cell lines investigated by Birrell et al. are included. Often the reports are in agreement with one another in respect to the response seen in a given cell line, but on occasion there are opposing results. This is of concern because irreproducibility obstructs the advancement of these AR ligands into in vivo studies or early phase clinical trials. Therefore the cause or causes of these disparate findings is worthy of further consideration.
Table 1.
Growth response of breast cancer cell lines to androgen receptor ligand
| Cell line | AR ligand | Concentration | Growth response | Assay | Grown in | Reference |
|---|---|---|---|---|---|---|
| MDA-MB-453 | DHT | 1nM | (+)(+)(+) | methylene blue whole cell binding | 96-well plate | Birrell et al. 1995 |
| mibolerone | 1nM | (+)(+)(+) | methylene blue whole cell binding | 96-well plate | Birrell et al. 1995 | |
| R1881 | 10nM | (+)(+)(+) | cell count using Vi-cell XR | T25 flask | Garay et al. | |
| MCF-7 | DHT | 1nM | (+)(+)(+) | methylene blue whole cell binding | 96-well plate | Birrell et al. 1995 |
| mibolerone | 1nM | (+)(+)(+) | methylene blue whole cell binding | 96-well plate | Birrell et al. 1995 | |
| DHT | 0.1nM - 10nM | (-) | cell count using hemacytometer w/trypan blue | 24-well plate | Cops et al. 2008 | |
| mibolerone | 0.1nM - 10nM | (-) | cell count using hemacytometer w/trypan blue | 24-well plate | Cops et al. 2008 | |
| R1881 | 10nM | (-)(-)(-) | cell count using Vi-cell XR | T25 flask | Garay et al. | |
| mibolerone | 10nM | (-)(-)(-) | cell count using Vi-cell XR | T25 flask | Garay et al. | |
| DHT | 0.1nM - 100nM | (-)(-)(-) | cell count using hemacytometer | 6-well plate | Greeve et al. 2004 | |
| DHT | 1nM - 1μM | (+)(+)(+) | cell count using hemacytometer | 4x-cluster dish | Hackenberg et al. 1988 | |
| DHT | 1nM - 100nM | (+)(+)(+) | tritiated thymidine incorporation | 24-well plate | Lin et al. 2009 | |
| DHT | 100nM | (+)(+)(+) | net protein synthesis | 60mm dish | Lippman et al. 1976 | |
| AD | 1nM - 10nM | (-) | MTT colorimetric assay | 96-well plate | Macedo et al. 2006 | |
| DHT | 0.01nM - 1nM | (-)(-)(-) | MTT colorimetric assay | 96-well plate | Macedo et al. 2006 | |
| T | 100nM | (+)(+)(+) | methylene blue whole cell binding | 60mm dish | Maggiolini et al. 1999 | |
| DHT | 1nM - 100nM | (-)(-)(-) | MTT colorimetric assay | 96-well plate | Ortmann et al. 2002 | |
| T | 1nM - 100nM | (-)(-)(-) | MTT colorimetric assay | 96-well plate | Ortmann et al. 2002 | |
| DHT | 10nM | NR | N/A | N/A | Zava and McGuire 1978 | |
| DHT | 1nM | (+)(+)(+) | N/A | N/A | Zava and McGuire 1978 | |
| T47D | DHT | 1nM | (-)(-)(-) | methylene blue whole cell binding | 96-well plate | Birrell et al. 1995 |
| mibolerone | 1nM | (-)(-)(-) | methylene blue whole cell binding | 96-well plate | Birrell et al. 1995 | |
| DHT | N/A | NR | cell count and tritiated thymidine incorpoation | N/A | Chalbos et al. 1982 | |
| DHT | 0.1nM - 10nM | (-) | cell count using hemacytometer w/trypan blue | 24-well plate | Cops et al. 2008 | |
| mibolerone | 0.1nM - 10nM | (-)(-)(-) | cell count using hemacytometer w/trypan blue | 24-well plate | Cops et al. 2008 | |
| R1881 | 10nM | NR | cell count using Vi-cell XR | T25 flask | Garay et al. | |
| DHT | 10nM | NR | quantification of total DNA and total protein | T75 flask | Horwitz et al. 1985 | |
| T | 10nM | NR | quantification of total DNA and total protein | T75 flask | Horwitz et al. 1985 | |
| DHT | 1nM - 100nM | (-)(-)(-) | MTT colorimetric assay | 96-well plate | Ortmann et al. 2002 | |
| T | 1nM - 100nM | (-)(-)(-) | MTT colorimetric assay | 96-well plate | Ortmann et al. 2002 | |
| DHT | 0.1nM - 1μM | (-)(-)(-) | cell count using Coulter Counter | 6-well plate | Reese et al. 1988 | |
| DHT | 10nM - 100nM | (-) | cell count using hemacytometer | T25 flask | Sutherland et al. 1988 | |
| T | 10nM - 100nM | (-) | cell count using hemacytometer | T25 flask | Sutherland et al. 1988 | |
| ZR-75-1 | DHT | 1nM | (-)(-)(-) | methylene blue whole cell binding | 96-well plate | Birrell et al. 1995 |
| mibolerone | 1nM | (-)(-)(-) | methylene blue whole cell binding | 96-well plate | Birrell et al. 1995 | |
| DHT | ~0.67nM | (-)(-)(-) | tumor volume | athymic mice | Dauvois et al. 1991 | |
| MPA^ | 300μg/day | (-)(-)(-) | tumor volume | athymic mice | Dauvois et al. 1991 | |
| DHT | 1nM | (-)(-)(-) | cell count using Coulter Counter ZM | 6-well plate | de Launoit et al. 1991 | |
| R1881 | 10nM | NR | cell count using Vi-cell XR | T25 flask | Garay et al. | |
| DHT | 10nM - 1μM | (-)(-)(-) | FACS quantification of subdiploid population | N/A | Kandouz et al. 1999 | |
| DHT | 0.01nM - 10nM | (-)(-)(-) | cell count using Coulter Counter ZM | 24-well plate | Poulin et al. 1988 | |
| T | 0.01nM - 10nM | (-)(-)(-) | cell count using Coulter Counter ZM | 24-well plate | Poulin et al. 1988 |
Abbreviations: DHT = dihydrotestosterone, AD = androstenedione, T= testosterone, MPA = medroxyprogesterone acetate. Growth effect is scored weak () or strong ()()() based on report by cited authors; NR = no response.
MPA is a synthetic progestin but has been shown to bind androgen receptor and have androgenic effects
Numerous experimental variables can be the source of incongruent results. As Table 1 shows, different methods for assessing cell growth could be an explanation for experimental discrepancy between studies, but this is not likely. A more probable confounding issue is the growth conditions used in a particular assay. Density of cells at time of drug administration can skew observed proliferative or antiproliferative results (unpublished observations and [23]). Our own unpublished observations and those of others have also shown that a confounding variable in the study of nuclear hormone receptors is serum conditions. Untreated serum can have exogenous and therefore unknown levels of hormones such as estrogen or testosterone. Reduction, though not complete removal, of exogenous growth factors and hormones is possible by treating serum with charcoal-dextran stripping or other methods; this can minimize the influence of exogenous hormones but not completely eliminate it from an experiment. Additionally, in some reports, growth inhibition by AR ligand is only examined in the presence of growth stimulation by another factor (often estrogen) [23,24,26,27,33]. Using estrogen to supplement cell growth while examining sensitivity to AR ligand can alter results because ER and AR interact within a cell and can modulate the ability of the other to transactivate target genes [35].
Another confounding factor is the use of different ligands. Most studies have used dihydrotesterone (DHT, the more potent and non-aromatizable form of testosterone) or R1881 (a non-aromatizable synthetic analogue of testosterone) to minimize the conversion of androgen to estrogen by cells in culture. An important issue to consider is the specificity of these ligands. While all ligands in Table 1 have been shown to bind to AR, their ability to bind other receptors and cause conflicting responses has also been documented. Medroxyprogesterone acetate (MPA) provides an excellent example of this phenomenon. MPA is a synthetic progestin but it has been shown to bind to AR and results in androgenic signaling [36-38]. Thus, Dauvois et al. include it alongside DHT as an AR ligand in their analyses. The study from Zava and McGuire represents a similar situation. In their study, low doses (10nM) of DHT bind to AR and it is translocated to the nucleus but has no effect on cell proliferation. However, a higher concentration (1 μM) stimulates proliferation [19]. The conundrum is that while nuclear translocation of AR was seen at this higher concentration so was nuclear translocation of ER. Since ER translocation was not seen at the lower concentration, the observed growth effects may have been mediated by ER rather than AR. Yet another illustrative example is the selective estrogen receptor downregulator, Faslodex (fulvestrant or ICI 182,780). This anti-estrogen clearly binds to ER and downregulates its expression but it has also been shown to have the same effect on AR in cultured cells [39]. Despite the limitations of cell based models, a better understanding of sex hormone signaling in breast cancer can be achieved through implementation of experimentally defined systems. The limitations of cell line models certainly should be considered with care and concern when considering the development of new targeted molecules for the treatment of cancer.
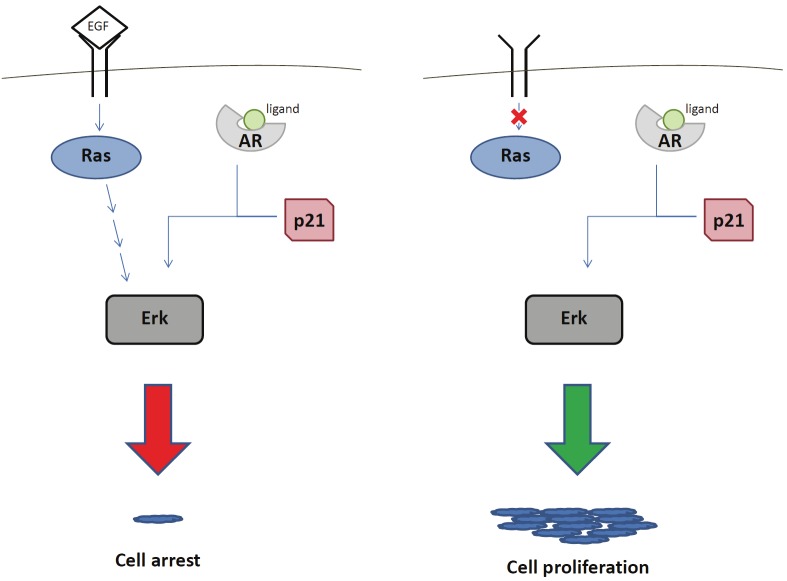
To this end, much effort has been invested into understanding the mechanism of action of AR in human epithelial cells. This includes research on intramolecular interactions within a single AR molecule, intermolecular interactions of AR upon dimerization, cellular localization of AR with and without ligand, protein turnover, and associations with other cellular proteins. It would be impossible to give each of these topics a thorough discussion in the length of this abbreviated review; therefore, we will focus briefly on the interaction of AR with other cellular proteins. In an effort to elucidate AR nuclear hormone signaling in breast epithelial cells, our lab has created a non-tumorigenic breast cell line in which AR is the only expressed steroid nuclear hormone receptor [40]. Previously we have uncovered some of the mechanisms of ER signaling by creating a similar in vitro system [41,42]. Utilizing this in vitro AR model, we have identified p21 as an important molecule in AR signaling. Similar results have also been reported in prostate and colon cancer cell lines [43]. The role of p21 in breast cancer has been appreciated previously as it is an important regulator of the cell cycle. Numerous other reports have shown that p21 is affected by ER signaling [44-47] and our lab has shown that the presence of functional p21 is necessary for sensitivity to tamoxifen treatment [42]. It is also known that p21 contains a putative androgen response element in its promoter [48]. Experiments using our model demonstrate that p21 is necessary for AR to signal through the MAP kinase pathway, as measured by ERK phosphorylation [40]. It is unclear, however, whether this is due to a direct or indirect interaction between p21 and AR. What is intriguing and perhaps therapeutically relevant is that the response to AR signaling is dictated by additional MAP kinase activity within the cell. For example, under conditions in which MAP kinase is already being stimulated by epidermal growth factor receptor signaling, additional AR ligand binding with R1881 causes cell cycle arrest. However, in the absence of a MAP kinase pathway activation, the signaling from AR ligand binding with R1881 results in cell proliferation (Figure 1). Somewhat ironically, the therapeutic implication is to overload a tumor with growth promoting signals leading to cell cycle arrest. Indeed, recent evidence suggests supraphysiologic doses of androgens may be useful for the treatment of castration resistant prostate cancer and ongoing clinical trials are testing this concept [49]. While more details of the exact mechanism and other molecules involved in AR and MAP Kinase crosstalk remain to be deciphered, this has provided a starting point to understanding the intricate molecular interactions that result from nuclear hormone receptor signaling.
Figure 1.
Schematic of signaling crosstalk between androgen receptor and epidermal growth factor receptor pathways. Concurrent signaling via androgen receptor with the epidermal growth factor receptor (left) leads to cell cycle arrest. Signaling via one or the other pathway (right) leads to growth proliferation. p21 is required for AR signaling in either situation.
AR expression in breast cancer and its prognostic value
As mentioned above, the presence of AR expression in breast cancer has always been appreciated but the ability to utilize this information effectively for therapy remains elusive. Although androgen therapy lost its status as a modality for managing breast cancer in the 1970s, laboratory investigation into the presence of AR in breast cancer continued and through the years began to gather momentum [50-52]. Aided by new, more sensitive methods to detect AR, studies demonstrated that AR, somewhat paradoxically, is the most commonly expressed nuclear hormone receptor in breast cancer [52-56]. Despite this fact, AR levels are not routinely assessed because they have not been shown to predict responses to currently used therapies. In contrast, assays for ER and progesterone receptor (PR) are performed routinely and have been shown to predict for response to currently approved endocrine therapies.
The success of ER/PR and HER2 targeted therapies has shifted interest in AR to those breast cancers that lack ER/PR and/or HER2 expression, so called triple negative disease. In addition, AR targeted therapies may also be important for breast cancers that have developed resistance to current hormone and HER2 directed therapies. Interestingly, AR is expressed in the majority of ER negative breast cancers with apocrine differentiation [57]. These tumors often have amplification of HER2 making them amenable to HER2 targeted therapies such as trastuzumab [1,58]. Recent work detailing the gene expression of triple negative breast cancers supports these findings [59]. However, it has also been shown both in vitro and in vivo that combinatorial therapy targeting both the MAP kinase pathway and AR is an effective means of reducing tumor cell viability and tumor burden [60]. Whether used as a singular modality or in combination with other systemic agents, AR directed therapies could be a valuable treatment for a large proportion of breast cancers.
Perhaps the most cogent argument for targeting AR is that approximately 10-35% of tumors without ER/PR expression or HER2 amplification (triple negative tumors) express AR [61,62]. A study by Moinfar et al. examined sex hormone receptor status as well as HER2 amplification in various grades of carcinoma in situ and invasive cancer [63]. They found an inverse correlation between histopathological grade and the expression of all sex hormone receptors in breast tumors. As tumor grade progressed from 1 to 3, AR expression decreased from 95% to 76% in DCIS and 88% to 47% in invasive carcinoma. In the same analysis, ER expression decreased from 100% to 8% in DCIS and to 9.5% in invasive carcinoma, with increasing tumor grade. These findings have been validated by another independent report [54]. In addition, it has been shown that, albeit at a low percentage (25%), AR is the sole sex hormone receptor expressed in distant breast cancer metastases [52]. As stated, although AR expression decreases with higher tumor grade, the levels of expression remain significantly higher than ER, making AR a potentially valuable target for new therapies.
Interestingly, in the study by Moinfar et al., HER2 expression increases as tumor grade increases – 0% in grade 1 to 84% and 42% in grade 3 DCIS and invasive carcinoma respectively [63]. It is well known that this heightened HER2 expression is often a result of gene amplification [64], but gene amplification of other important mediators of breast carcinogenesis, such as nuclear hormone receptors, is less well-documented. Recently our lab examined the possibility of AR amplification as a possible etiologic factor of breast cancer as has been implied with ER amplification [65]. Examination of a tissue microarray consisting of multiple cores from 18 separate samples showed no amplification of AR [40,66]. Replicates of these samples were similarly analyzed for AR expression; 62% of samples (21 of 34) had high levels of AR expression. Therefore, AR is overexpressed in the majority of breast tumors but its overexpression is not a result of gene amplification as is commonly seen with HER2. Consistent with this finding, recent reports did not find a high rate of ER amplification in breast cancers in contrast to the original report from Holst et al. [67-71]. Regardless, the frequency of AR expression in primary breast cancer samples justifies its consideration as a potential therapeutic target.
Although the ability to target AR for breast cancer therapy has yet to be effectively deployed, many studies have looked at the utility of AR in breast cancer as a prognostic or predictive marker in breast cancer. AR expression has been linked favorably to response to the synthetic progesterone analogue MPA, with high levels of AR expression predicting for remission of tumors treated with MPA [72,73]. Whether this is a result of the drug acting directly through AR is unclear, however. In another report by Rakha et al., AR was shown to be the most valuable prognostic factor when compared to a number of other markers including ER, PR, HER2, EGFR, and p53. Expression of AR in lymph-node positive tumors was prognostic for a better disease-free interval and overall survival, and loss of AR expression correlated closely with higher tumor grade, higher recurrence, and metastases [74]. Agoff et al. also found that expression of AR correlated with longer time to relapse of disease [75]. These studies focused on triple negative breast cancers, but the same correlation was found when ER positive tumors were examined. Castellano et al. determined in an ER positive cohort of patients that AR positivity was a prognostic marker for longer time to relapse and disease specific survival [76]. Collectively, these recent studies continue to support data from more historic reports demonstrating that AR expression in tumors correlates with good prognosis [51].
Because of the potential prognostic importance of AR, there also has been a desire to identify more assessable surrogate biomarkers for AR expression in breast cancer. For example, prostate specific antigen (PSA) is a well-known androgen- responsive gene and detection in the blood is used as a biomarker for prostate cancer. Secretion of PSA by breast cells, either benign or malignant, has been evaluated [77]. Also, correlation of PSA levels with breast cancer risk has been determined using feasible bioassays on samples collected via minimally invasive procedures such as collection of nipple aspirate fluid [78] or patient serum [79]. In the latter study, there is an association between higher detectable levels of secreted PSA and histological grade of the breast tumor. However, other reports present conflicting data about this association [80,81]. Further studies are warranted before the routine use of PSA as reliable marker for breast cancer detection can be implemented.
While useful as a prognostic factor, AR lacks a causative association between its expression and carcinogenesis. This has led researchers to explore the correlation of other factors in breast carcinogenesis with AR. One report did find a non-significant increase in breast cancer incidence in women taking a testosterone patch for increased libido over control patients, but the study lacked conclusive evidence to link increased androgens with breast cancer risk [82]. A series of studies tried to determine a causative role of AR in breast tumorigenesis involving the BRCA1 and BRCA2 tumor suppressor genes. BRCA genes require inactivation of both alleles for a phenotypic effect. In individuals with a germline mutation of one allele, other genes can modify the susceptibility of the second wild type BRCA allele to inactivation, rendering it more permissive to alterations that lead to its loss or silencing. In the case of AR, the length of a trinucleotide repeat (CAG) in the N-terminus of the gene has been linked to increased risk of developing breast cancer in BRCA mutation carriers; AR therefore may have a role in breast carcinogenesis in these kindreds [83]. This initial report was bolstered by a subsequent discovery that BRCA1 enhances AR activity by binding to the activation function domain in the N-terminal portion of the protein [84]. However, the validity of this hypothesis has been challenged [85-87]. As another example, Gonzalez-Angulo et al. explored the relationship in breast cancer between AR levels and mutations in the PI3-kinase alpha catalytic subunit, PIK3CA. They found AR tends to be more highly expressed in cancers with a particular PIK3CA mutation in the kinase domain of the protein [88], a finding further supported by recent studies by Pietenpol and colleagues [59]. Importantly, this correlation also holds in the subset of cancers that are negative for expression of ER and PR. This information leads to the intriguing idea of coupling androgen-based therapy with therapies targeting other important pathways.
Conclusion
Molecular analysis of tumors is becoming more detailed allowing therapies to become tailored to individual tumor types from patient to patient. Thus, it is becoming clear that no single treatment will be the panacea for cancer; so, if multi-faceted treatment is to be successful, it is necessary to utilize as many targets for therapy as logistically possible. AR is an excellent target for therapy because it has been shown historically to be an effective target for prostate cancer treatment and is commonly expressed in the majority of breast cancers. A clear and comprehensive understanding of the mechanism of AR signaling in the breast has yet to be revealed, but with continued laboratory efforts there is hope that soon we will have viable breast cancer therapies that exploit AR signaling.
Acknowledgments
Grant Support: The Avon Foundation (B.H.P.), NIH CA109274 (J.P.Garay is a recipient of a Research Supplement to Promote Diversity in Health-Related Research, B.H.P.).
References
- 1.Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman G, Slamon DJ. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J. Clin. Oncol. 1999;17:2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 2.Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists' Collaborative Group. Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- 3.Beatson GT. On the treatment of inoperable cases of carcinoma of the mamma: suggestions for a new method of treatment, with illustrative cases. Lancet. 1896;148:104–107. [PMC free article] [PubMed] [Google Scholar]
- 4.Boyd S. Remarks on Oophorectomy in the Treatment of Cancer of the Breast. Br Med J. 1899;1:257–262. doi: 10.1136/bmj.1.1988.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.West CD, Damast BL, Sarro SD, Pearson OH. Conversion of testosterone to estrogens in castrated, adrenalectomized human females. J Biol Chem. 1956;218:409–418. [PubMed] [Google Scholar]
- 6.Adair FE, Herrmann JB. The use of testosterone propionate in the treatment of advanced carcinoma of the breast. Annals of Surg. 1946;123:1023–1035. [PubMed] [Google Scholar]
- 7.Kennedy BJ. Fluoxymesterone therapy in advanced breast cancer. N Engl J Med. 1958;259:673–675. doi: 10.1056/NEJM195810022591404. [DOI] [PubMed] [Google Scholar]
- 8.Testosterone propionate therapy in breast cancer. JAMA. 1964;188:1069–1072. doi: 10.1001/jama.1964.03060380037009. [DOI] [PubMed] [Google Scholar]
- 9.Council on Drugs; Subcommittee on Breast and Genital Cancer; Committee on Research; A.M.A. Androgens and estrogens in the treatment of disseminated mammary carcinoma - Retrospective study of 944 patients. JAMA. 1960;172:1271–1283. [Google Scholar]
- 10.Cole MP, Jones CT, Todd ID. A new antioestrogenic agent in late breast cancer. An early clinical appraisal of ICI46474. Br J Cancer. 1971;25:270–275. doi: 10.1038/bjc.1971.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jordan VC, Robinson SP. Species-specific pharmacology of antiestrogens: role of metabolism. Fed Proc. 1987;46:1870–1874. [PubMed] [Google Scholar]
- 12.Goldenberg IS, Waters N, Ravdin RS, Ansfield FJ, Segaloff A. Androgenic therapy for advanced breast cancer in women. A report of the cooperative breast cancer group. Jama. 1973;223:1267–1268. [PubMed] [Google Scholar]
- 13.Manni A, Arafah BM, Pearson OH. Androgen-induced remissions after antiestrogen and hypophysectomy in stage IV breast cancer. Cancer. 1981;48:2507–2509. doi: 10.1002/1097-0142(19811201)48:11<2507::aid-cncr2820481127>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 14.Ingle JN, Twito DI, Schaid DJ, Cullinan SA, Krook JE, Mailliard JA, Tschetter LK, Long HJ, Gerstner JG, Windschitl HE, Levitt R, Pfeifle DM. Combination hormonal therapy with tamoxifen plus fluoxymesterone versus tamoxifen alone in postmenopausal women with metastatic breast cancer. An updated analysis. Cancer. 1991;67:886–891. doi: 10.1002/1097-0142(19910215)67:4<886::aid-cncr2820670405>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 15.Wasielewski M, Elstrodt F, Klijn JG, Berns EM, Schutte M. Thirteen new p53 gene mutants identified among 41 human breast cancer cell lines. Breast Cancer Res Treat. 2006;99:97–101. doi: 10.1007/s10549-006-9186-z. [DOI] [PubMed] [Google Scholar]
- 16.She QB, Chandarlapaty S, Ye Q, Lobo J, Haskell KM, Leander KR, DeFeo-Jones D, Huber HE, Rosen N. Breast tumor cells with PI3K mutation or HER2 amplification are selectively addicted to Akt signaling. PLoS ONE. 2008;3:e3065. doi: 10.1371/journal.pone.0003065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birrell SN, Bentel JM, Hickey TE, Ricciardelli C, Weger MA, Horsfall DJ, Tilley WD. Androgens induce divergent proliferative responses in human breast cancer cell lines. J Steroid Biochem Mol Biol. 1995;52:459–467. doi: 10.1016/0960-0760(95)00005-k. [DOI] [PubMed] [Google Scholar]
- 18.Lippman M, Bolan G, Huff K. The effects of androgens and antiandrogens on hormone-responsive human breast cancer in long-term tissue culture. Cancer Res. 1976;36:4610–4618. [PubMed] [Google Scholar]
- 19.Zava DT, McGuire WL. Androgen action through estrogen receptor in a human breast cancer cell line. Endocrinology. 1978;103:624–631. doi: 10.1210/endo-103-2-624. [DOI] [PubMed] [Google Scholar]
- 20.Chalbos D, Vignon F, Keydar I, Rochefort H. Estrogens stimulate cell proliferation and induce secretory proteins in a human breast cancer cell line (T47D) J Clin Endocrinol Metab. 1982;55:276–283. doi: 10.1210/jcem-55-2-276. [DOI] [PubMed] [Google Scholar]
- 21.Horwitz KB, Freidenberg GR. Growth inhibition and increase of insulin receptors in antiestrogen-resistant T47DCO human breast cancer cells by progestins: implications for endocrine therapies. Cancer Res. 1985;45:167–173. [PubMed] [Google Scholar]
- 22.Hackenberg R, Hofmann J, Holzel F, Schulz KD. Stimulatory effects of androgen and antiandrogen on the in vitro proliferation of human mammary carcinoma cells. J Cancer Res Clin Oncol. 1988;114:593–601. doi: 10.1007/BF00398183. [DOI] [PubMed] [Google Scholar]
- 23.Poulin R, Baker D, Labrie F. Androgens inhibit basal and estrogen-induced cell proliferation in the ZR-75-1 human breast cancer cell line. Breast Cancer Res Treat. 1988;12:213–225. doi: 10.1007/BF01805942. [DOI] [PubMed] [Google Scholar]
- 24.Reese CC, Warshaw ML, Murai JT, Siiteri PK. Alternative models for estrogen and androgen regulation of human breast cancer cell (T47D) growth. Ann N Y Acad Sci. 1988;538:112–121. doi: 10.1111/j.1749-6632.1988.tb48856.x. [DOI] [PubMed] [Google Scholar]
- 25.Sutherland RL, Hall RE, Pang GY, Musgrove EA, Clarke CL. Effect of medroxyprogesterone acetate on proliferation and cell cycle kinetics of human mammary carcinoma cells. Cancer Res. 1988;48:5084–5091. [PubMed] [Google Scholar]
- 26.Dauvois S, Geng CS, Levesque C, Merand Y, Labrie F. Additive inhibitory effects of an androgen and the antiestrogen EM-170 on estradiol-stimulated growth of human ZR-75-1 breast tumors in athymic mice. Cancer Res. 1991;51:3131–3135. [PubMed] [Google Scholar]
- 27.de Launoit Y, Dauvois S, Dufour M, Simard J, Labrie F. Inhibition of cell cycle kinetics and proliferation by the androgen 5 alpha-dihydrotestosterone and antiestrogen N,n-butyl-N-methyl-11-[16' alpha-chloro-3',17 beta-dihydroxy-estra-1',3',5'-(10')triene-7' alpha-yl] undecanamide in human breast cancer ZR-75-1 cells. Cancer Res. 1991;51:2797–2802. [PubMed] [Google Scholar]
- 28.Kandouz M, Lombet A, Perrot JY, Jacob D, Carvajal S, Kazem A, Rostene W, Therwath A, Gompel A. Proapoptotic effects of antiestrogens, progestins and androgen in breast cancer cells. J Steroid Biochem Mol Biol. 1999;69:463–471. doi: 10.1016/s0960-0760(99)00069-2. [DOI] [PubMed] [Google Scholar]
- 29.Maggiolini M, Donze O, Jeannin E, Ando S, Picard D. Adrenal androgens stimulate the proliferation of breast cancer cells as direct activators of estrogen receptor alpha. Cancer Res. 1999;59:4864–4869. [PubMed] [Google Scholar]
- 30.Ortmann J, Prifti S, Bohlmann MK, Rehberger-Schneider S, Strowitzki T, Rabe T. Testosterone and 5 alpha-dihydrotestosterone inhibit in vitro growth of human breast cancer cell lines. Gynecol Endocrinol. 2002;16:113–120. [PubMed] [Google Scholar]
- 31.Greeve MA, Allan RK, Harvey JM, Bentel JM. Inhibition of MCF-7 breast cancer cell proliferation by 5alpha-dihydrotestosterone; a role for p21(Cip1/Waf1) J Mol Endocrinol. 2004;32:793–810. doi: 10.1677/jme.0.0320793. [DOI] [PubMed] [Google Scholar]
- 32.Macedo LF, Guo Z, Tilghman SL, Sabnis GJ, Qiu Y, Brodie A. Role of androgens on MCF-7 breast cancer cell growth and on the inhibitory effect of letrozole. Cancer Res. 2006;66:7775–7782. doi: 10.1158/0008-5472.CAN-05-3984. [DOI] [PubMed] [Google Scholar]
- 33.Cops EJ, Bianco-Miotto T, Moore NL, Clarke CL, Birrell SN, Butler LM, Tilley WD. Antiproliferative actions of the synthetic androgen, mibolerone, in breast cancer cells are mediated by both androgen and progesterone receptors. J Steroid Biochem Mol Biol. 2008;110:236–243. doi: 10.1016/j.jsbmb.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 34.Lin HY, Sun M, Lin C, Tang HY, London D, Shih A, Davis FB, Davis PJ. Androgen-induced human breast cancer cell proliferation is mediated by discrete mechanisms in estrogen receptor-alpha-positive and -negative breast cancer cells. J Steroid Biochem Mol Biol. 2009;113:182–188. doi: 10.1016/j.jsbmb.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 35.Panet-Raymond V, Gottlieb B, Beitel LK, Pinsky L, Trifiro MA. Interactions between androgen and estrogen receptors and the effects on their transactivational properties. Mol Cell Endocrinol. 2000;167:139–150. doi: 10.1016/s0303-7207(00)00279-3. [DOI] [PubMed] [Google Scholar]
- 36.Poulin R, Baker D, Poirier D, Labrie F. Androgen and glucocorticoid receptor-mediated inhibition of cell proliferation by medroxyprogesterone acetate in ZR-75-1 human breast cancer cells. Breast Cancer Res Treat. 1989;13:161–172. doi: 10.1007/BF01806528. [DOI] [PubMed] [Google Scholar]
- 37.Bentel JM, Birrell SN, Pickering MA, Holds DJ, Horsfall DJ, Tilley WD. Androgen receptor agonist activity of the synthetic progestin, medroxyprogesterone acetate, in human breast cancer cells. Mol Cell Endocrinol. 1999;154:11–20. doi: 10.1016/s0303-7207(99)00109-4. [DOI] [PubMed] [Google Scholar]
- 38.Ghatge RP, Jacobsen BM, Schittone SA, Horwitz KB. The progestational and androgenic properties of medroxyprogesterone acetate: gene regulatory overlap with dihydrotestosterone in breast cancer cells. Breast Cancer Res. 2005;7:R1036–1050. doi: 10.1186/bcr1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhattacharyya RS, Krishnan AV, Swami S, Feldman D. Fulvestrant (ICI 182,780) downregulates androgen receptor expression and diminishes androgenic responses in LNCaP human prostate cancer cells. Mol Cancer Ther. 2006;5:1539–1549. doi: 10.1158/1535-7163.MCT-06-0065. [DOI] [PubMed] [Google Scholar]
- 40.Garay JP, Karakas B, Abukhdeir AM, Cosgrove DP, Gustin JP, Higgins MJ, Konishi H, Konishi Y, Lauring J, Mohseni M, Wang GM, Sherman-Baust CA, Morin PJ, Toubaji A, Meeker A, De Marzo AM, Lewis G, Subhawong A, Argani P, Park BH. The growth response to androgen receptor signaling in human breast cells is dependent on p21 and mediated by MAP kinase activation. Breast Cancer Res. 2012;14:R27. doi: 10.1186/bcr3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abukhdeir AM, Blair BG, Brenner K, Karakas B, Konishi H, Lim J, Sahasranaman V, Huang Y, Keen J, Davidson N, Vitolo MI, Bachman KE, Park BH. Physiologic estrogen receptor alpha signaling in non-tumorigenic human mammary epithelial cells. Breast Cancer Res Treat. 2006;99:23–33. doi: 10.1007/s10549-006-9177-0. [DOI] [PubMed] [Google Scholar]
- 42.Abukhdeir AM, Vitolo MI, Argani P, De Marzo AM, Karakas B, Konishi H, Gustin JP, Lauring J, Garay JP, Pendleton C, Konishi Y, Blair BG, Brenner K, Garrett-Mayer E, Carraway H, Bachman KE, Park BH. Tamoxifen-stimulated growth of breast cancer due to p21 loss. Proc Natl Acad Sci USA. 2008;105:288–293. doi: 10.1073/pnas.0710887105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang F, Kokontis J, Lin Y, Liao S, Lin A, Xiang J. Androgen via p21 inhibits tumor necrosis factor alpha-induced JNK activation and apoptosis. J Biol Chem. 2009;284:32353–32358. doi: 10.1074/jbc.M109.042994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Planas-Silva MD, Weinberg RA. Estrogen-dependent cyclin E-cdk2 activation through p21 redistribution. Mol Cell Biol. 1997;17:4059–4069. doi: 10.1128/mcb.17.7.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cariou S, Donovan JC, Flanagan WM, Milic A, Bhattacharya N, Slingerland JM. Downregulation of p21WAF1/CIP1 or p27Kip1 abrogates antiestrogen-mediated cell cycle arrest in human breast cancer cells. Proc Natl Acad Sci USA. 2000;97:9042–9046. doi: 10.1073/pnas.160016897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carroll JS, Prall OW, Musgrove EA, Sutherland RL. A pure estrogen antagonist inhibits cyclin E-Cdk2 activity in MCF-7 breast cancer cells and induces accumulation of p130-E2F4 complexes characteristic of quiescence. J Biol Chem. 2000;275:38221–38229. doi: 10.1074/jbc.M004424200. [DOI] [PubMed] [Google Scholar]
- 47.Skildum AJ, Mukherjee S, Conrad SE. The cyclin-dependent kinase inhibitor p21WAF1/Cip1 is an antiestrogen-regulated inhibitor of Cdk4 in human breast cancer cells. J Biol Chem. 2002;277:5145–5152. doi: 10.1074/jbc.M109179200. [DOI] [PubMed] [Google Scholar]
- 48.Lu S, Liu M, Epner DE, Tsai SY, Tsai MJ. Androgen regulation of the cyclin-dependent kinase inhibitor p21 gene through an androgen response element in the proximal promoter. Mol Endocrinol. 1999;13:376–384. doi: 10.1210/mend.13.3.0254. [DOI] [PubMed] [Google Scholar]
- 49.Isaacs JT, D'Antonio JM, Chen S, Antony L, Dalrymple SP, Ndikuyeze GH, Luo J, Denmeade SR. Adaptive auto-regulation of androgen receptor provides a paradigm shifting rationale for bipolar androgen therapy (BAT) for castrate resistant human prostate cancer. Prostate. 2012 doi: 10.1002/pros.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Allegra JC, Lippman ME, Thompson EB, Simon R, Barlock A, Green L, Huff KK, Do HM, Aitken SC. Distribution, frequency, and quantitative analysis of estrogen, progesterone, androgen, and glucocorticoid receptors in human breast cancer. Cancer Res. 1979;39:1447–1454. [PubMed] [Google Scholar]
- 51.Bryan RM, Mercer RJ, Bennett RC, Rennie GC, Tat HL, Morgan FJ. Androgen receptors in breast cancer. Cancer. 1984;54:2436–2440. doi: 10.1002/1097-0142(19841201)54:11<2436::aid-cncr2820541121>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 52.Lea OA, Kvinnsland S, Thorsen T. Improved measurement of androgen receptors in human breast cancer. Cancer Res. 1989;49:7162–7167. [PubMed] [Google Scholar]
- 53.Park S, Koo J, Park HS, Kim JH, Choi SY, Lee JH, Park BW, Lee KS. Expression of androgen receptors in primary breast cancer. Ann Oncol. 2010;21:488–492. doi: 10.1093/annonc/mdp510. [DOI] [PubMed] [Google Scholar]
- 54.Hanley K, Wang J, Bourne P, Yang Q, Gao AC, Lyman G, Tang P. Lack of expression of androgen receptor may play a critical role in transformation from in situ to invasive basal subtype of high-grade ductal carcinoma of the breast. Hum Pathol. 2008;39:386–392. doi: 10.1016/j.humpath.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 55.Isola JJ. Immunohistochemical demonstration of androgen receptor in breast cancer and its relationship to other prognostic factors. J Pathol. 1993;170:31–35. doi: 10.1002/path.1711700106. [DOI] [PubMed] [Google Scholar]
- 56.Schippinger W, Regitnig P, Dandachi N, Wernecke KD, Bauernhofer T, Samonigg H, Moinfar F. Evaluation of the prognostic significance of androgen receptor expression in metastatic breast cancer. Virchows Arch. 2006;449:24–30. doi: 10.1007/s00428-006-0213-6. [DOI] [PubMed] [Google Scholar]
- 57.Niemeier LA, Dabbs DJ, Beriwal S, Striebel JM, Bhargava R. Androgen receptor in breast cancer: expression in estrogen receptor-positive tumors and in estrogen receptor-negative tumors with apocrine differentiation. Mod Pathol. 2010;23:205–212. doi: 10.1038/modpathol.2009.159. [DOI] [PubMed] [Google Scholar]
- 58.Carter P, Presta L, Gorman CM, Ridgway JB, Henner D, Wong WL, Rowland AM, Kotts C, Carver ME, Shepard HM. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci USA. 1992;89:4285–4289. doi: 10.1073/pnas.89.10.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Naderi A, Liu J. Inhibition of androgen receptor and Cdc25A phosphatase as a combination targeted therapy in molecular apocrine breast cancer. Cancer Lett. 2010;298:74–87. doi: 10.1016/j.canlet.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 61.Ogawa Y, Hai E, Matsumoto K, Ikeda K, Tokunaga S, Nagahara H, Sakurai K, Inoue T, Nishiguchi Y. Androgen receptor expression in breast cancer: relationship with clinicopathological factors and biomarkers. Int J Clin Oncol. 2008;13:431–435. doi: 10.1007/s10147-008-0770-6. [DOI] [PubMed] [Google Scholar]
- 62.Fawell SE, White R, Hoare S, Sydenham M, Page M, Parker MG. Inhibition of estrogen receptor-DNA binding by the "pure" antiestrogen ICI 164,384 appears to be mediated by impaired receptor dimerization. Proc Natl Acad Sci USA. 1990;87:6883–6887. doi: 10.1073/pnas.87.17.6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moinfar F, Okcu M, Tsybrovskyy O, Regitnig P, Lax SF, Weybora W, Ratschek M, Tavassoli FA, Denk H. Androgen receptors frequently are expressed in breast carcinomas: potential relevance to new therapeutic strategies. Cancer. 2003;98:703–711. doi: 10.1002/cncr.11532. [DOI] [PubMed] [Google Scholar]
- 64.Yokota J, Yamamoto T, Toyoshima K, Terada M, Sugimura T, Battifora H, Cline MJ. Amplification of c-erbB-2 oncogene in human adenocarcinomas in vivo. Lancet. 1986;1:765–767. doi: 10.1016/s0140-6736(86)91782-4. [DOI] [PubMed] [Google Scholar]
- 65.Holst F, Stahl PR, Ruiz C, Hellwinkel O, Jehan Z, Wendland M, Lebeau A, Terracciano L, Al-Kuraya K, Janicke F, Sauter G, Simon R. Estrogen receptor alpha (ESR1) gene amplification is frequent in breast cancer. Nat Genet. 2007;39:655–660. doi: 10.1038/ng2006. [DOI] [PubMed] [Google Scholar]
- 66.Garay JP, Karakas B, Abukhdeir AM, Cosgrove DP, Gustin JP, Higgins MJ, Konishi H, Konishi Y, Lauring J, Mohseni M, Wang GM, Jelovac D, Weeraratna A, Sherman Baust CA, Morin PJ, Toubaji A, Meeker A, De Marzo AM, Lewis G, Subhawong A, Argani P, Park BH. The growth response to androgen receptor signaling in ERalpha-negative human breast cells is dependent on p21 and mediated by MAPK activation. Breast Cancer Res. 2012;14:R27. doi: 10.1186/bcr3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brown LA, Hoog J, Chin SF, Tao Y, Zayed AA, Chin K, Teschendorff AE, Quackenbush JF, Marioni JC, Leung S, Perou CM, Neilsen TO, Ellis M, Gray JW, Bernard PS, Huntsman DG, Caldas C. ESR1 gene amplification in breast cancer: a common phenomenon? Nat Genet. 2008;40:806–807. doi: 10.1038/ng0708-806. author reply 810-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Horlings HM, Bergamaschi A, Nordgard SH, Kim YH, Han W, Noh DY, Salari K, Joosse SA, Reyal F, Lingjaerde OC, Kristensen VN, Borresen-Dale AL, Pollack J, van de Vijver MJ. ESR1 gene amplification in breast cancer: a common phenomenon? Nat Genet. 2008;40:807–808. doi: 10.1038/ng0708-807. author reply 810-802. [DOI] [PubMed] [Google Scholar]
- 69.Vincent-Salomon A, Raynal V, Lucchesi C, Gruel N, Delattre O. ESR1 gene amplification in breast cancer: a common phenomenon? Nat Genet. 2008;40:809. doi: 10.1038/ng0708-809a. author reply 810-802. [DOI] [PubMed] [Google Scholar]
- 70.Reis-Filho JS, Drury S, Lambros MB, Marchio C, Johnson N, Natrajan R, Salter J, Levey P, Fletcher O, Peto J, Ashworth A, Dowsett M. ESR1 gene amplification in breast cancer: a common phenomenon? Nat Genet. 2008;40:809–810. doi: 10.1038/ng0708-809b. author reply 810-802. [DOI] [PubMed] [Google Scholar]
- 71.Albertson DG. Conflicting evidence on the frequency of ESR1 amplification in breast cancer. Nat Genet. 2008;40:821–822. doi: 10.1038/ng0708-821. [DOI] [PubMed] [Google Scholar]
- 72.Birrell SN, Roder DM, Horsfall DJ, Bentel JM, Tilley WD. Medroxyprogesterone acetate therapy in advanced breast cancer: the predictive value of androgen receptor expression. J. Clin. Oncol. 1995;13:1572–1577. doi: 10.1200/JCO.1995.13.7.1572. [DOI] [PubMed] [Google Scholar]
- 73.Buchanan G, Birrell SN, Peters AA, Bianco-Miotto T, Ramsay K, Cops EJ, Yang M, Harris JM, Simila HA, Moore NL, Bentel JM, Ricciardelli C, Horsfall DJ, Butler LM, Tilley WD. Decreased androgen receptor levels and receptor function in breast cancer contribute to the failure of response to medroxyprogesterone acetate. Cancer Res. 2005;65:8487–8496. doi: 10.1158/0008-5472.CAN-04-3077. [DOI] [PubMed] [Google Scholar]
- 74.Rakha EA, El-Sayed ME, Green AR, Lee AH, Robertson JF, Ellis IO. Prognostic markers in triple-negative breast cancer. Cancer. 2007;109:25–32. doi: 10.1002/cncr.22381. [DOI] [PubMed] [Google Scholar]
- 75.Agoff SN, Swanson PE, Linden H, Hawes SE, Lawton TJ. Androgen receptor expression in estrogen receptor-negative breast cancer. Immunohistochemical, clinical, and prognostic associations. Am J Clin Pathol. 2003;120:725–731. doi: 10.1309/42F0-0D0D-JD0J-5EDT. [DOI] [PubMed] [Google Scholar]
- 76.Castellano I, Allia E, Accortanzo V, Vandone AM, Chiusa L, Arisio R, Durando A, Donadio M, Bussolati G, Coates AS, Viale G, Sapino A. Androgen receptor expression is a significant prognostic factor in estrogen receptor positive breast cancers. Breast Cancer Res Treat. 2010;124:607–617. doi: 10.1007/s10549-010-0761-y. [DOI] [PubMed] [Google Scholar]
- 77.Yu H, Diamandis EP, Levesque M, Giai M, Roagna R, Ponzone R, Sismondi P, Monne M, Croce CM. Prostate specific antigen in breast cancer, benign breast disease and normal breast tissue. Breast Cancer Res Treat. 1996;40:171–178. doi: 10.1007/BF01806212. [DOI] [PubMed] [Google Scholar]
- 78.Sauter ER, Lininger J, Magklara A, Hewett JE, Diamandis EP. Association of kallikrein expression in nipple aspirate fluid with breast cancer risk. Int J Cancer. 2004;108:588–591. doi: 10.1002/ijc.11607. [DOI] [PubMed] [Google Scholar]
- 79.Black MH, Giai M, Ponzone R, Sismondi P, Yu H, Diamandis EP. Serum total and free prostate-specific antigen for breast cancer diagnosis in women. Clin Cancer Res. 2000;6:467–473. [PubMed] [Google Scholar]
- 80.Miller MK, Unger PD, Bleiweiss IJ. Immunohistochemical analysis of prostate specific antigen in breast cancer. Breast Cancer Res Treat. 2001;68:111–116. doi: 10.1023/a:1011959127928. [DOI] [PubMed] [Google Scholar]
- 81.Kraus TS, Cohen C, Siddiqui MT. Prostatespecific antigen and hormone receptor expression in male and female breast carcinoma. Diagn Pathol. 2010;5:63. doi: 10.1186/1746-1596-5-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Davis SR, Moreau M, Kroll R, Bouchard C, Panay N, Gass M, Braunstein GD, Hirschberg AL, Rodenberg C, Pack S, Koch H, Moufarege A, Studd J. Testosterone for low libido in postmenopausal women not taking estrogen. N Engl J Med. 2008;359:2005–2017. doi: 10.1056/NEJMoa0707302. [DOI] [PubMed] [Google Scholar]
- 83.Rebbeck TR, Kantoff PW, Krithivas K, Neuhausen S, Blackwood MA, Godwin AK, Daly MB, Narod SA, Garber JE, Lynch HT, Weber BL, Brown M. Modification of BRCA1-associated breast cancer risk by the polymorphic androgen-receptor CAG repeat. Am J Hum Genet. 1999;64:1371–1377. doi: 10.1086/302366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park JJ, Irvine RA, Buchanan G, Koh SS, Park JM, Tilley WD, Stallcup MR, Press MF, Coetzee GA. Breast cancer susceptibility gene 1 (BRCAI) is a coactivator of the androgen receptor. Cancer Res. 2000;60:5946–5949. [PubMed] [Google Scholar]
- 85.Kadouri L, Easton DF, Edwards S, Hubert A, Kote-Jarai Z, Glaser B, Durocher F, Abeliovich D, Peretz T, Eeles RA. CAG and GGC repeat polymorphisms in the androgen receptor gene and breast cancer susceptibility in BRCA1/2 carriers and non-carriers. Br J Cancer. 2001;85:36–40. doi: 10.1054/bjoc.2001.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Spurdle AB, Antoniou AC, Duffy DL, Pandeya N, Kelemen L, Chen X, Peock S, Cook MR, Smith PL, Purdie DM, Newman B, Dite GS, Apicella C, Southey MC, Giles GG, Hopper JL, Chenevix-Trench G, Easton DF. The androgen receptor CAG repeat polymorphism and modification of breast cancer risk in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. 2005;7:R176–183. doi: 10.1186/bcr971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cox DG, Blanche H, Pearce CL, Calle EE, Colditz GA, Pike MC, Albanes D, Allen NE, Amiano P, Berglund G, Boeing H, Buring J, Burtt N, Canzian F, Chanock S, Clavel-Chapelon F, Feigelson HS, Freedman M, Haiman CA, Hankinson SE, Henderson BE, Hoover R, Hunter DJ, Kaaks R, Kolonel L, Kraft P, LeMarchand L, Lund E, Palli D, Peeters PH, Riboli E, Stram DO, Thun M, Tjonneland A, Trichopoulos D, Yeager M. A comprehensive analysis of the androgen receptor gene and risk of breast cancer: results from the National Cancer Institute Breast and Prostate Cancer Cohort Consortium (BPC3) Breast Cancer Res. 2006;8:R54. doi: 10.1186/bcr1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gonzalez-Angulo AM, Stemke-Hale K, Palla SL, Carey M, Agarwal R, Meric-Berstam F, Traina TA, Hudis C, Hortobagyi GN, Gerald WL, Mills GB, Hennessy BT. Androgen receptor levels and association with PIK3CA mutations and prognosis in breast cancer. Clin Cancer Res. 2009;15:2472–2478. doi: 10.1158/1078-0432.CCR-08-1763. [DOI] [PubMed] [Google Scholar]