Abstract
The non-receptor tyrosine kinases of the SRC family (SFK) play important roles in signal transduction induced by a large variety of extracellular stimuli, including growth factors and Integrins. When deregulated, SFKs show oncogenic activity, as originally reported for v-Src, the transforming product of the avian retrovirus RSV, and then, in many human cancers, particularly colorectal cancer (CRC). In CRC, SFK deregulation largely occurs in the absence of mutations of the corresponding genes, but the underlying molecular mechanisms involved are still unclear. In addition to a role in early tumor progression, SFK deregulation may also be important in advanced CRC, as suggested by the association between increased SFK activity and poor clinical outcome. However, SFK contribution to CRC metastasis formation is still poorly documented. Here, we will review recent findings that broaden our understanding of the mechanisms underlying SFK deregulation and signaling in advanced CRC. We will also discuss the implication of these observations for SFK-based therapy in metastatic CRC.
Keywords: Non-receptor tyrosine kinases, signal transduction, colorectal cancer (CRC), early tumor progression, advanced CRC
Introduction
The Src family kinases (SFK) are non-receptor (cytoplasmic) tyrosine kinases (TK) and comprise 8 members, of which three, SRC, FYN and YES, are widely expressed. SFK members share a common modular structure that includes a myristilation site at the N-terminus for membrane targeting, a unique sequence of unclear function, followed by a SH3 and SH2 domain involved in protein-protein interaction and a kinase domain, called SH1. This catalytic sequence is also bordered by two short regulatory sequences, named the SH2-CD-linker and the C-terminal tail [1]. SFKs are activated by a large number of extracellular stimuli, including growth factors and components of the extracellular matrix and they transmit intracellular signals that promote cell proliferation, survival, migration and angiogenesis [1]. When deregulated, SFKs show oncogenic activity as was originally reported for v-Src, the transforming product of the avian retrovirus RSV [2] and then confirmed by analyzing other viral oncoproteins, such as v-Yes, the transforming product of the Yamaguchi avian sarcoma virus [3]. Accordingly, deregulated SFKs induce malignant transformation of rodent cells, probably due to their capacity to induce mitogenic and survival signaling cascades even in the absence of extracellular stimuli [3]. In contrast to viral oncoproteins, activating mutations of cellular SFKs are rarely observed in human cancers [2]. Nevertheless, a significant level of SFK deregulation has been reported in many human cancers, including leukemia and solid tumors of epithelial origin [4]. Remarkably, SFK deregulation is found in 80% of colorectal cancers (CRC) and the extent of the increase in SFK activity (5-10 fold) is consistent with oncogenic activity [5]. In addition, the level SRC and YES activity has been associated with poor clinical prognosis [6,7], suggesting that SKFs have a crucial role in late tumorigenesis. Indeed, SRC is thought to regulate growth, survival and invasion of CRC cells [5]. YES function in CRC is less clear, although a specific role in metastasis formation has been recently proposed for this protein [8,9]. Based on these observations, SFKs are now considered to be attractive therapeutic targets for the treatment of advanced CRC and several small inhibitors are currently evaluated in clinical trials [10,11].
SFK deregulation
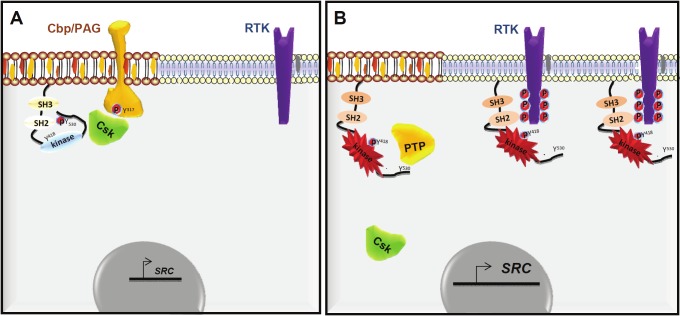
SRC activating mutation(s)
How SFKs are deregulated in colon cancer is an important issue that has been only partially addressed. While RAS is mutated in 30% of human cancers, initial studies could not detect any SRC oncogenic mutation and thus SRC was not considered to play a major role in human cancer [2]. Yet, Irby et al reported that the C-terminally deleted SRC530 mutant, which shows significant oncogenic activity when expressed in rodent fibroblasts, was expressed in 12% of the tested late-stage CRC [12]. This work was the first to show that SRC is an oncogene in human cancers. While other laboratories did not confirm this finding at that time [13-15], sequencing of the whole kinome in a large set of tumor biopsies confirmed the expression of this oncogenic SRC mutant in about 1% of the CRC analyzed [16] (http://www.sanger.ac.uk/genetics/CGP/cosmic). Rare somatic mutations have been detected in the transcripts of the SFK members FYN, LCK and LYN in CRC [17] and some of them might affect their catalytic activity; however the contribution of these somatic mutations to CRC is currently unknown. The fact that these genetic alterations are very rare indicates that the frequently observed SFK deregulation in CRC is induced by other mechanisms.
SRC over-expression
SFK deregulation in CRC may primarily involve protein over-expression [5]. This idea is largely supported by a significant correlation between kinase activation and increased SFK protein expression in tumor biopsies [2]. How SFK are over-expressed in CRC is still unclear. SRC transcriptional activation might be an important determinant of c-Src over-expression in CRC cells, but the signaling pathway(s) involved in this process is currently unknown [18]. Interestingly, elevated SRC and YES protein levels have been observed in human polyps [19-21] and Src levels are increased in cells from intestinal adenomas in Apc-mutated transgenic mice [22], suggesting that the WNT/beta-Catenin pathway might contribute to SFK induction during early tumorigenesis. Intriguingly, a recent report showed an increase in SRC gene copy number (3-5) in several CRC cells lines and CRC explants [23], suggesting that gene amplification may also play a role in this process. Epigenetic mechanisms have also been involved in the induction of several oncogenes. However, in the case of SRC, experimental evidence is missing, although Dham et al [24] proposed the involvement of an HDAC in the increase of SRC transcription in some cancers. Conversely, YES, which is over-expressed in CRC [25], is a direct target of the microRNA-145 in CRC cells [26]. As this microRNA has a tumor suppressor function in CRC [27], miRNA-145 down-regulation during CRC tumor progression may also contribute to YES up-regulation.
CSK inactivation
SFK over-expression alone in a normal cellular context is not sufficient to induce cell transformation, because SFKs are strictly regulated [3]; therefore SFK oncogenic activity relies on additional post-translational modifications. SRC and probably all the other family members are regulated by intramolecular interactions that keep them in a closed conformation. Precisely, the interaction of the SH2 domain with phosphorylated Tyr530 (pTyr530) in the tail and of the SH3 domain with the SH2-CD linker constraint the catalytic domain in its inactive form [28]. Phosphorylation of Tyr530 is therefore crucial and is mediated by members of the CSK family of cytoplasmic TKs [29]. The importance of this mechanism is illustrated by the finding that Csk inactivation in mice leads to aberrant SFK activity and embryonic lethality [30,31]. Similarly, loss of dCsk in Drosophila induces over-proliferation and disorganization of the tissue architecture [32,33]. Opening the conformation by disrupting these interactions de-represses SRC catalytic activity; furthermore, any mutation that affects these intramolecular interactions will stabilize the kinase in an open conformation and will cause aberrant catalytic activity consistent with its oncogenic properties [28]. Indeed, all retroviral and cellular oncoproteins have an altered C-terminal sequence with reduced affinity for the SH2 domain [3]. However, as SFK mutations are very rare in CRC, oncogenic activation might instead involve the alteration of key SFK regulators [2]. Several candidates have been suggested to disrupt the SH2-pTyr530 intramolecular interaction. These include CSK, tyrosine phosphatases that dephosphorylate pTyr530 [34,35] and growth factor receptors via their association with the SFK SH2 domain [36].
As CSK is the major negative SFK regulator in vivo, it could act as a tumor suppressor in human cancers. However, the role of CSK in CRC is still debated. While originally reported downregulated in some CRC cell lines [37], its level was increased along with SFK activity both in primary tumors and in other CRC cell lines [38,39]. These findings are in contradiction with a potential tumor suppressor function, as suggested also by the detection of anti-CSK autoantibodies in some patients with CRC, indicating that this tyrosine kinase is a novel tumor antigen in CRC [38]. Moreover, purified CSK from CRC cells still retains the full capacity to phosphorylate SFK in vitro [40] and no inactivating mutation has been detected in the primary tumors tested so far [16] (http://www.sanger.ac.uk/genetics/CGP/cosmic). Collectively, these data indicate that CSK catalytic inactivation on its own does not play a major role in SFK induction in CRC. Moreover, while SFKs are very good CSK substrates in vitro, they do not interact efficiently with CSK in vivo [2] due to the fact that inactive CSK is strictly cytosolic, while SFKs accumulate at the membrane. Therefore, in vivo SFK inhibition by CSK requires CSK recruitment to the sub-cellular compartments where SFKs reside via interactions with membrane-associated CSK binding proteins [41,42]. Interestingly, this additional step is largely impaired in CRC cells and CSK mislocalization plays an important role in SFK deregulation in CRC [40]. Therefore, CSK cytoplasmic retention together with SFK over-expression may be sufficient to fully induce SFK oncogenic activity in CRC. This mechanism may be particularly important in advanced CRC in which tumor cells are characterized by elevated CSK membrane delocalization and strong SFK activity. Remarkably, although moderately expressed, wild type SRC is very active in advanced CRC cells [43] (unpublished data).
Cbp/PAG down-regulation
Several CSK binding proteins that regulate SFK activity have been identified. These include the cytoskeletal-associated protein Paxillin [44], the signaling protein DOK-1 [45], the caveolar structural protein Caveolin-1 [46], the junctional proteins VE-cadherin [47] and ZO-1 [48] and finally some members of the Transmembrane adaptor protein family (TRAP) [49]. They all define a SFK negative regulatory loop in which SFK phosphorylation triggers their binding to CSK via a SH2-pTyr dependent mechanism. Among these CSK binding proteins, inhibition of Cbp/PAG, a member of the TRAP family [49], appears to largely contribute to CSK inactivation in CRC [40]. Cbp/PAG was originally identified as a CSK binding protein (Cbp) following phosphorylation of Tyr317 [41] and as a novel Phosphoprotein Associated with Glycopshingolipids (PAG), which is controlled by two palmitoylation sites in the juxtamembrane sequence [42]. By recruiting CSK to lipid rafts, Cbp/PAG regulates the activity of SFKs that are localized in these microdomains, including SRC [40] and YES (unpublished data). Interestingly, Cbp/PAG is the only CSK binding protein of the TRAP family expressed in colon and its expression is frequently down-regulated in CRC biopsies, consistent with a tumor suppressor function in this cancer. In agreement with this hypothesis, introduction of Cbp/PAG in metastatic CRC cells that have lost PAG, increased CSK membrane localization and reduced SFK invasive properties, while depletion of residual Cbp/PAG in cells derived from early CRC induced a remarkable increase in SFK activity, promoting cell invasion [40]. This finding makes of Cbp/PAG a central player in CSK regulation and its inhibition contributes to SFK deregulation in CRC.
Cbp/PAG down-regulation in transformed cells has been reported to be mediated by histone modifications through the PI3K/MAPK pathway [50]. Whether this epigenetic mechanism operates in CRC is not known, but as this signaling pathway is frequently deregulated in this cancer, Cbp/PAG-CSK-SFK uncoupling might culminate in CRC harboring oncogenic KRAS or PI3KIA mutations. While a CSK-dependent tumor suppressor function of Cbp/PAG has been clearly established in CRC cells [40], Cbp/PAG also interacts with other signaling proteins, including the E3 ligase SOCS1 [51], the negative Ras regulator RasGAP [52] and also SFKs [53-55]. Therefore Cbp/PAG function in CRC could also affect CSK-independent signaling cascades. For example, Cbp/PAG over-expression inhibits Src transforming activity in mouse fibroblasts in a Csk-independent manner [55]. By sequestering v-Src in lipid rafts through direct association, Cbp/PAG prevents SFK oncogenic signaling outside cell microdomains, such as focal adhesion, that is also required for full cell transformation [55]. This effect has not been observed in CRC cells [40], probably due the low endogenous Cbp/PAG level that may not be sufficient to segregate SRC in rafts. Besides, Cbp/PAG N-terminus can also stimulate the activity of Neu-3 sialidase [56] that controls lipid raft properties [57] and subsequently SFK signaling. Clearly, additional roles for Cbp/PAG in CRC can be expected; however, it may not be the only Cbp involved in SFK deregulation in this cancer. Indeed, we found that Cbp/PAG can control at most half of the whole SFK activity detected in CRC cells; moreover, it specifically regulates SFK invasive activity without affecting SFK-regulated cell growth in vitro [40]. This raises the hypothesis that the SRC-dependent growth promoting response is attained through an additional, yet unknown mechanism. Spatial regulation of SFKs is also important for cellular signaling both in normal and transformed cells [58-61]. For instance, cell survival involves SRC signaling initiated at focal adhesions outside lipid rafts [55]. We thus hypothesize that there are pools of deregulated SFKs which are localized outside lipid rafts and which contribute to the transforming properties of CRC cells. This additional pool of SFKs may not be targeted by Cbp/PAG, but by another CSK binding protein to be identified.
Activation of tyrosine phosphatases
Dephosphorylation of pTyr530 SRC may also contribute to SFK deregulation in CRC. While only the CSK family can phosphorylate SRC at Tyr530, a large number of tyrosine phosphatases have been implicated in its dephosphorylation. These include the receptor tyrosine phosphatases CD45, RPTP alpha and RPTP epsilon as well as the non-receptor tyrosine phosphatases PTP1B, SHP1 and SHP2 [62]. Although their role in SFK deregulation in CRC is poorly documented, they might target specific pools of SFKs, similarly to what described for CSK binding proteins. PTP1B was originally reported to be activated in CRC cells and is considered the main tyrosine phosphatase involved in SRC activation [34]. Conversely, SHP2 activates SRC through an indirect mechanism that involves the disruption of the CSK-Cbp/PAG interaction at the membrane via dephosphorylation of pTyr317 Cbp/PAG [63]. Experimental evidence suggests that this mechanism may operate in early-stage CRC cells that still express Cbp/PAG, thus promoting SFK invasive activity (unpublished data). Therefore, SHP2 may represent another way to uncouple SRC and CSK during the early steps of CRC development. RPTP alpha also has been implicated in SFK deregulation, promoting CRC cell survival [35]. RPTP alpha activation involves the expression of RPTP alpha splice mutants. Although catalytically inactive, these mutants bind to full length RPTP alpha and increase its capacity to dephosphorylate SRC [64]. Finally, inhibition of tyrosine phosphatases may also favor aberrant SRC induction in CRC. Indeed, SRC activation is associated with autophosphorylation of the conserved Tyr418 in the activation loop and its dephosphorylation by PTPL1 inactivates it [65]. Accordingly, PTPL1 inactivation in CRC cells also participates in SRC deregulated activity, leading to anchorage-independent cell growth. Mechanistically, epigenetic silencing of LIM, an adaptor protein that bridges PTPL1 to SRC, prevents PTPL1-dependent SRC inactivation [66]. Overall, SRC can be deregulated by several tyrosine phosphatases, but their exact role in CRC tumor progression is largely unknown.
Association with receptor tyrosine kinases
A complex interplay between deregulated SRC and Receptor Tyrosine Kinases (RTKs) may also participate in the aberrant kinase activity observed in CRC. SFKs can interact directly with activated growth factor receptors in normal cells [67]. This association is regulated by a SH2-pTyr-dependent mechanism that disrupts the SH2-pTyr530 intermolecular interaction, promoting SRC de-repression. For example, active PDGFR stimulates SRC activation and mitogenic activity by direct interaction of the SFK-SH2 domain with pTyr579 PDGFR. SRC activation by RTKs may also be regulated by indirect mechanisms, as suggested by the fact that EGFR does not contain any binding site for the SH2 domain of SRC. Interestingly, several RTKs, such as MET, EGFR and EPHA2 [68-70], are frequently deregulated and/or over-expressed in CRC and in some cases (for instance MET) SRC-like activity might participates in their oncogenic effect [71]. Additionally, SFKs can phosphorylate RTKs, including EGFR, PDGFR and IGFR, at specific Tyr residues, thus modifying their activity and signaling [67]. This suggests that aberrant SRC activation in CRC cells may in turn fully activate these RTKs even in the absence of their ligands. This hypothesis has been recently validated by a quantitative phosphoproteomic analysis in advanced CRC cells [43]. In these cells, deregulated SRC initiates a RTK signaling network that regulates SRC oncogenic activity. Specifically, SRC interacts with and phosphorylates a cluster of RTKs, including MET and EPHA2, promoting CRC cell invasiveness. Activated RTKs appear to be required for maintaining maximal SRC activity [43]. These observations suggest that SRC deregulation may initiate a vicious circle, in which SRC initiates a reverse signaling by interacting with these RTKs, which, in turn, further increase SRC activity, leading to maximal kinase deregulation. How this loop is activated is unclear. We speculate that the modification of lipid raft constituents observed in CRC cells might change RTK membrane partitioning and favor the formation of SFK-RTK complexes. In addition, an increase in growth factor secretion through an autocrine/paracrine loop could participate in the maintenance of maximal RTK activity.
Overall, these observations indicate that SFK oncogenic activation in CRC is promoted by several mechanisms of non-genetic origin (Figure 1). It primarily involves protein over-expression, but also requires catalytic deregulation. Kinase activation might be induced through several mechanisms. Each of them may target a specific pool of SFKs, thus contributing to various aspects of tumor progression. Moreover, Cbp/PAG down-regulation, which is frequently observed in CRC, may play a major role in SFK deregulation during late CRC tumorigenesis. This indicates that SRC deregulation in CRC largely relies on down-regulation of its negative regulators. Nevertheless, quantitative phospho-proteomic studies uncovered an additional important mechanism that operates in advanced stage of CRC and is based on the interplay between SRC and deregulated RTKs. All these mechanisms may explain at least in part why SRC oncogenic mutations are a rare event in CRC.
Figure 1.
Mechanism of SRC deregulation in CRC cells. Regulation of SRC in normal (A) and CRC cells (B).
SFK signaling in CRC
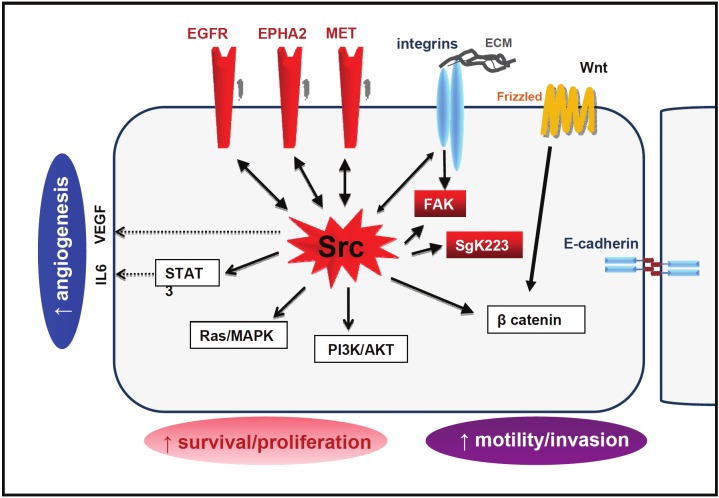
SFKs regulate intracellular signaling induced by growth factors and Integrins in untransformed cells [1]. SFK role in human cancer cells may be more complex as they are not always needed for cell transformation; however, due to their frequent and strong deregulation consistent with oncogenic properties, SFKs might support the tumor properties of most CRC cells. For example, SFKs have a significant impact on RTK/Ras/PI3K and beta-Catenin oncogenic signaling in these cells, although the underlying mechanism is only partially elucidated (Figure 2). Indeed, SFKs control mitogenic and survival signaling cascades that are induced by RTKs and culminate in STAT3 and AKT activation [67]. By phosphorylating adaptors of the Gab/IRS family, SFKs can promote PI3K membrane recruitment [72,73], a process required for AKT activation. Additional mechanisms may, however, be at play because SFKs control AKT activity also in CRC cells harboring oncogenic PI3KCA (unpublished data). SFKs regulate STAT3 signaling by directly inducing its phosphorylation on Tyr705 [74] and by initiating a positive feedback loop driven by the inflammatory cytokine Interleukin-6 that maintains high STAT3 activity [75]. Under hypoxic conditions, SFKs induce VEGF and probably other cytokines that promote angiogenesis [5]. These data indicate that SFKs are important mediators of the angiogenic and inflammatory processes required for tumorigenesis.
Figure 2.
SRC oncogenic signaling cascade in CRC cells. The Tyrosine Kinase network initiated by deregulated SRC is highlighted in red.
Besides the interaction with RTKs, SFK oncogenic functions rely also on their capacity to modify adhesive receptor signaling cascades that control cell migration and invasion (Figure 2). By targeting p120 Catenin [76], TIAM1 [77] or Integrin signaling [78], SRC affects E-cadherin localization at adherent junctions, triggering cell dissociation and scattering. SRC also interacts with Integrins and other components of focal adhesions, such as FAK, thus promoting cell motility [76]. While all these properties are known to play important roles in carcinogenesis, the correlation between SRC and YES deregulated activity in CRC and poor clinical prognosis [6,7] suggests additional roles for SFKs during the metastatic process. This hypothesis is supported by the remarkable capacity of SFKs to promote liver metastasis in mouse xenograft models (unpublished data). The exact roles of SFKs during metastasis formation are still poorly documented. Here, we will focus on some of our recent knowledge on SFK involvement in tumor progression, leading to metastasis formation and that may also apply to CRC.
Epithelial to mesenchyme transition
To disseminate, cells must modify their adhesive properties, motility and invasive capacities. A change in the adhesive properties of cells is often governed by Epithelial to Mesenchyme Transition (EMT) during which E-cadherin is silenced and mesenchymal markers, such as Vimentin and Fibronectin, are induced. This genetic program is regulated by transcription factors of the Snail, Slug, ZEB and Twist families [79]. SKFs have an important role in EMT induction in several carcinoma cells [80,81] and this function largely relies on a RAS-independent signaling cascade [82]. In some CRC cell lines, SKF inhibition restores their adhesive properties and prevents liver metastasis formation when they are injected in nude mice, in accordance with a possible role for SKFs in this process [9,83]. However, no direct causal link between aberrant SFK activity and EMT inducers has been reported in CRC so far. CRC tumorigenesis is primarily induced by the WNT/beta-Catenin pathway [84] that also governs EMT [79]. Aberrant WNT/beta-Catenin signaling is the result of the alteration of various components of this pathway, including oncogenic mutations of beta-Catenin and inactivating mutations of the tumor suppressor and negative regulator APC. All these alterations lead to beta-Catenin stabilization in the cytoplasm and to its translocation to the nucleus, thus promoting its transcriptional activity. SFKs appear to modulate this oncogenic process as illustrated in vivo by the capacity of a SFK inhibitor to reduce intestinal tumor development in the Apc(Min+/-) mouse model [85]. The exact role of SFKs has not been further characterized and the generation of appropriate mouse transgenic models will be very informative, as recently reported in the case of pancreatic cancer [86]. Nevertheless, in cellulo studies have revealed several molecular mechanisms through which SFKs might influence beta-Catenin signaling. Beta-Catenin concentrates at adherent junctions; however, by phosphorylating components of the junctional complex, SFKs diminish cell-cell contacts and promotes beta-Catenin cytoplasmic accumulation [76]. SFKs may also regulate beta-Catenin protein stabilization induced by scattering factors, such as PDGF, EGF and TGF-beta. Upon receptor activation, SFKs might contribute to ABL activation that triggers the formation of p68 RNA helicase-beta-Catenin complexes. This complex excludes APC from the beta-Catenin complex and prevents its proteasomal destruction [87]. Finally, a recent report shows that direct phosphorylation of beta-Catenin by SRC may also increase its transcriptional activity. In EGF-stimulated glioblastoma cells, SRC phosphorylation of beta-Catenin on Tyr333 creates a binding site for the non-metabolic isoform of the pyruvate kinase MPK2. The interaction of MPK2 with beta-Catenin unmasks its nuclear transcriptional activity, leading to cell proliferation [88]. While not addressed, this signaling cascade could also operate in some CRC in which high level of EGFR expression is associated with aberrant SFK activity.
Deregulated SRC promotes cell migration by phosphorylation of important focal adhesion (FA) components, such as CAS, Paxillin or FAK [76]. Recently, two additional SRC substrates (Tensin-3 [89] and SgK269/PEAK1 [90]) that link the Actin cytoskeleton with Integrins have been shown to play important roles in SRC-regulated cell migration. Tensin-3 is an SH2-containing protein localized at FA. Surprisingly, phosphorylation by SRC of specific Tyr in the Tensin-3 SH2 domain favors FA complex formation in an SH2-dependent manner, thus controlling tumor growth and metastasis formation in nude mice [89]. This represents a novel mechanism by which SRC regulates pTyr-SH2 complex formation. PEAK1 is a recently identified atypical cytoplasmic TK that localizes to Actin filaments and FA. PEAK1 belong to the NFK3 family of pseudo-kinases that should be catalytically inactive due to the absence of conserved residues important for enzymatic reaction. However, PEAK1 seems to have TK activity in vitro [90]. In addition, PEAK1 undergoes SRC-induced tyrosine phosphorylation, regulates the p130Cas-CRK-Paxillin and ERK signaling pathways and operates downstream of Integrin to control CRC cell migration [90]. However, it has not been established whether PEAK1 catalytic activity contributes to these oncogenic properties. In addition, we reported a similar function for SgK223, the other member of the NFK3 family, in SRC-induced cell invasion, suggesting that these atypical kinases define a novel class of important SRC oncogenic substrates [43]. Indeed, quantitative phosphoproteomic analysis of mouse tumor xenografts identified Tensin-3 and PEAK1 as two of the main SRC substrates in vivo, further supporting an important role for these molecules in SRC oncogenic signaling in CRC (unpublished data).
Local invasion and dissemination
Cancer cell dissemination requires degradation of the basement membrane. A similar mechanism may operate during extravasation which depends on proteolysis of the endothelial basement membrane [91]. This invasive activity is controlled by specialized, Actin-based membrane protrusions in the ventral part of cancer cells (invadosomes) that degrade the extracellular matrix (ECM) via proteases. The underlying mechanism is currently the focus of much investigation and several components of this signaling cascade have been characterized. By phosphorylating important cytoskeletal components of invadosomes, such as Cortactin or members of the TKS family, SRC plays a pivotal role in this process [91]. SRC also promotes protease secretion by invadosomes for basal membrane degradation. Most of our knowledge on invadosomes has been obtained by using breast cancer cells or SRC-transformed 3T3 fibroblasts. Nevertheless, a role for invadosomes has been recently reported in CRC cell invasion as well [92,93]. In addition to structural components of invadosomes, the RTK PDGFR alpha has been identified as an important SRC mediator in this process [94], further supporting the notion of RTK as mediators of SRC oncogenic signaling. In breast cancer cells, the EMT-inducer Twist 1 has also the capacity to induce invadopodia formation through induction of PDGFR alpha expression and activation and consequently SRC activation [94]. Whether a similar mechanism operates in CRC has not been addressed, but the fact that Twist 1 is over-expressed in some CRC suggests the existence of a similar mechanism [95]. Alternatively, MET and EPHA2 are important SRC substrates in CRC cell invasion as well [43] and they could play similar functions as PDGFR alpha in the regulation of SRC activity.
In addition to invadosomes, SRC also induces Tubulin-based protusions (microtentacles) [96] that might participate in the capillarity retention of cancer cells in the circulation. Surprisingly, microtentacle formation was strongly enhanced by expression of a catalytically inactive form of SRC [96]. This observation suggests the counterintuitive hypothesis that SRC inhibitors could enhance tumor cell reattachment during the early step of metastasis formation. Whether this cellular process also operates in CRC cells deserves further investigation. Finally, aberrant SFK activity also promotes survival of CRC cells during anoikis, a process that occurs during tumor cell dissemination in blood and lymphatic vessels. SFKs initiate an AKT-dependent survival signaling cascade [97] and impair FAS-induced apoptosis by direct phosphorylation and inhibition of Caspase 8 activity [98]. This important SFK activity might promote CRC cell dissemination in the blood and favor colonization to secondary organs.
Metastasis
While suggested by clinical data, experimental evidence for a role of SFKs in later steps of CRC metastasis formation is incomplete. The first indication has been provided by the inhibitory effect of the SFK inhibitor PP2 on the number of liver metastasis in nude mice following inoculation of human CRC cells in the spleen [83]. As this assay allows the direct contact of CRC cells with the portal veins, these findings suggest an additional SFK role in metastasis formation that is distinct from their function in local cancer cell dissemination. Similar results were then obtained also by silencing YES (or SRC, but to a less extent) in CRC cells [9]. Further evidence has been provided by the strong capacity of ectopic SRC expression to increase the number and the size of metastatic nodules generated by CRC cells in which SFK activity was weakly deregulated (unpublished data). While all these data strongly support an important function for SRC and YES in CRC liver metastasis formation, they give no clue about their exact role. Our understanding of the biological mechanisms promoting the later steps of the metastatic process remains elusive. Disseminated cells must acquire the ability to survive and colonize a specific tissue/organ, called the niche. Two molecular inducers of this biological process have been recently uncovered. Production of Tenascin C [99] and Periostin [100] by tumor stromal cells plays crucial roles in breast cancer metastatic colonization of bone and lung, respectively. By intersecting a NOTCH or WNT pathway, they promote cancer cell survival and proliferation in the niche. Interestingly, Periostin has a similar function in CRC liver metastasis [101]. These peptidic components of the stromal environment are not the only players in this process. Indeed, the RTK DDR1, a receptor for Collagen, which is one of the most abundant components of extracellular matrix, has been also involved in bone homing of metastatic lung cancer cells, suggesting that Collagen could control the interaction of cancer cells with the niche [102]. The exact role of this receptor during niche colonization has not been characterized yet.
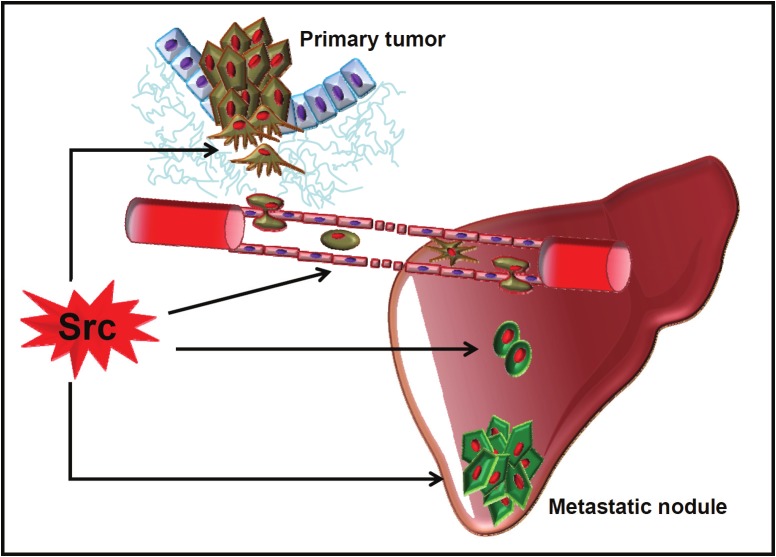
While there is no clear role for SFKs in the homing process, they have a pivotal role in the survival of dormant, disseminated cancer cells in the niche. Specifically, analysis of the gene expression profile of breast cancers highlighted the strong association of a SRC responsive signature with late-onset bone metastasis. SRC controls the survival and outgrowth of these cancer cells in the bone marrow by regulating AKT activation in response to the chemokine CXCL12 and to TRAIL in the microenvironment [103]. Whether SFKs have a similar function in CRC has not been addressed. SFKs may also play a role in macro-metastasis formation. Indeed, Collagen I induces a beta-Integrin1-SRC signaling cascade that triggers proliferation of disseminated, dormant breast tumor cells [104]. Interestingly, experimental data obtained from nude mice suggests that a similar mechanism operates during liver colonization by human CRC, but the molecular mechanisms involved in this process are poorly defined [105,106]. Finally, SFKs might play important roles also in metastatic growth as suggested by the effects of SFK inhibitors on metastatic CRC cell proliferation both in vitro and in liver metastatic models [40,107]. Accordingly, recent data also suggest a role for SFKs in the ability of some cells to initiate a tumor. These cells are called cancer stem cells and are thought to be important determinant in tumor recurrence and metastasis. For example, the hyaluronic receptor CD44 induces sphere formation in culture and CRC cell-induced metastasis in nude mice through a SRC-beta-Integrin1 axis that increases resistance to anoikis [108]. Likewise, SFK pharmacological inhibition abrogates sphere formation and tumorigenicity of metastatic CRC cells [109]. In conclusion, while still poorly documented, recent findings on the role of SFKs in other cancers suggest that these TKs might modulate several important steps in CRC metastasis formation as well (Figure 3).
Figure 3.
The role of SRC in metastatic CRC. Proposed functions of SRC in the metastatic process.
SFK-based therapies for metastatic CRC
The described important roles for SFKs in CRC tumorigenesis indicate that they may be valuable therapeutic targets for the treatment of this cancer. Several ATP-competitive inhibitors that target SFKs have been developed and three of them are currently evaluated in clinical trials [11]. These are the SFK inhibitor saracatinib, the SRC/ABL inhibitor bosutinib and the multi-kinase inhibitor dasatinib that also target SFKs [10]. In accordance with a role for SFKs in cell proliferation, these inhibitors reduce the growth rate of CRC cell lines; however, their biological activity seems to be highly dependent on the type of cell line or primary tumor grafted in nude mice. For example, dasatinib and saracatinib inhibited cell proliferation in less than 20% of the CRC cell lines under study [23,110]. In accordance with a specific biological effect of these molecules, growth inhibition correlated with the level of SKF deregulation [23]. This preclinical data, thus, suggests that these drugs will have a therapeutic activity only on a small fraction of CRC, probably those with highly deregulated SFK activity. Conversely, SFK inhibition by other means has a wider effect on cell proliferation [40,111]. For example, YES silencing by using a specific shRNA strategy reduced proliferation and induced apoptosis of CRC cells, an effect that has not been reported with the available clinical SFK inhibitors [9]. This difference could simply be explained by the conditions used to test cell proliferation (standard versus anchorage-independent growth). Moreover, the incomplete inhibition of the different SFK family members by these inhibitors may leave a residual activity that is sufficient to maintain a transformed phenotype. Therefore, a better pharmacological response is required, possibly by using inhibitors that target efficiently all SFKs within the cell, including those present in lipid rafts, by exploiting the affinity of some compounds for the lipophilic micro-environment. Nevertheless, this strategy may not inhibit SKF kinase-independent signaling which has also been reported in CRC cells [9,112]. In this case, a combined therapeutic approach in which both SFKs and their downstream substrates are targeted might inhibit SFK oncogenic signaling more efficiently. This strategy has been recently validated by using dasatinib in nilotinib-resistant chronic myeloid leukemia [113,114].
In contrast to their weak effect on primary tumor growth, SFK inhibitors have a strong impact on CRC cell migration and invasion [40,83,110,115]. We thus anticipate that these agents might be particularly useful for the treatment of metastatic CRC, specifically to reduce cell dissemination during tumor progression and/or surgery. Moreover, these inhibitors could also hinder CRC metastatic growth and angiogenesis, as observed in the case of liver metastases in nude mice treated with dasatinib [107]. Therefore, these molecules could improve the standard therapy for patients with metastatic CRC. Indeed, dasatinib in combination with oxaliplatin had a remarkable synergistic effect in a murine model of liver metastases from colorectal cancer [107]. The shrinkage of the metastatic nodules was due to reduction of angiogenesis and induction of apoptosis. This remarkable effect was probably mediated by the oxidative stress induced by oxaliplatin, leading to a very robust SFK activation. Such SFK activation could be due to inhibition of regulatory tyrosine phosphatases and/or, possibly, to direct SFK activation by ROS. This idea is supported by the finding that ROS can trigger oxidation of cysteine residues within the SRC sequence, leading to stabilization of the protein in its open and active conformation [116]. Clearly, the combination of SFK inhibitors with oxaliplatin deserves further investigation.
Finally, SFK inhibitors could also improve existing targeted therapies. For example, anti-EGFR antibodies, such as cetuximab, can significantly improve survival in patients with CRC. However, clinical investigations have demonstrated a strong correlation between the therapeutic response to anti-EGFR antibodies and the status of KRAS as no response was observed in tumors with mutated KRAS. Therefore, cetuximab is currently proposed only to patients with tumors harboring wild type KRAS [11]. The underlying mechanism of tumor resistance is still unclear, but it has been proposed that oncogenic KRAS induces constitutive MAPK and probably PI3K signaling pathways that are no longer dependent on EGFR activity. Therefore, in these cancers, EGFR inhibition will not inhibit MAPK effect on cancer cell growth. Interestingly, dasatinib can sensitize CRC cells with mutant KRAS to cetuximab in preclinical models, leading to anti-proliferative effects on tumor growth [117]. Whether this combination reduces metastatic growth as well has not been addressed. This synergistic effect has been attributed to SFK inhibitory effects on the AKT/mTOR, beta-Catenin and STAT oncogenic pathways. These data indicate that mutated KRAS alone is not sufficient to ensure tumor growth and that RTKs and SFKs play additional important roles in this process. A similar mechanism may also be at play in CRC with oncogenic PI3K [118].
Although not demonstrated, the synergistic activity of these drugs may be explained by a more efficient inhibition of the oncogenic signaling pathways that operate in CRC cells. This hypothesis implies that oncogenic signaling must be inhibited to a sufficient level in order to trigger a cell response. Due to the pleiotropic activity of SFKs on the major oncogenic signaling pathways that drive CRC tumor progression, a combination of SFK inhibitors should improve RTK-based targeted therapy. Similarly, the observed interplay between SFKs and RTKs in regulating the invasiveness of metastatic CRC cells suggests that SFK-based therapies might also be improved by the use of a combination of inhibitors that target these downstream TKs in advanced CRC. Therefore, the characterization of the TK signaling network that operates during metastasis formation will have important implications in the design of novel SFK-based therapeutic strategies for metastatic CRC. It can thus be easily anticipated that a combination of appropriate therapeutic agents that target TK oncogenic signaling pathways, including SFKs, may be of therapeutic value in this deadly disease.
Acknowledgements
We thank Clément Chevalier and colleagues for reviewing the manuscript. AS is supported by the “Fondation de France”, SR and CB are INSERM investigators. This work was supported by the Ligue Nationale contre le Cancer (équipe labélisée), INCa and the Fondation de France.
Abbreviations
- SRC
Tyrosine kinases, oncogenic signaling, colorectal cancer, metastasis
References
- 1.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 2.Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4:470–480. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- 3.Roche S, Courtneidge SA. Oncogenic cytoplasmic tyrosine kinases in "oncogenes and tumor suppressors". Oxford New York Tokyo: Oxford University Press; 1997. pp. 97–130. [Google Scholar]
- 4.Kim LC, Song L, Haura EB. Src kinases as therapeutic targets for cancer. Nat Rev Clin Oncol. 2009;6:587–95. doi: 10.1038/nrclinonc.2009.129. [DOI] [PubMed] [Google Scholar]
- 5.Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22:337–358. doi: 10.1023/a:1023772912750. [DOI] [PubMed] [Google Scholar]
- 6.Han NM, Curley SA, Gallick GE. Differential activation of pp60(c-src) and pp62(c-yes) in human colorectal carcinoma liver metastases. Clin Cancer Res. 1996;2:1397–1404. [PubMed] [Google Scholar]
- 7.Aligayer H, Boyd DD, Heiss MM, Abdalla EK, Curley SA, Gallick GE. Activation of Src kinase in primary colorectal carcinoma: an indicator of poor clinical prognosis. Cancer. 2002;94:344–351. doi: 10.1002/cncr.10221. [DOI] [PubMed] [Google Scholar]
- 8.Weis S, Cui J, Barnes L, Cheresh D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J Cell Biol. 2004;167:223–229. doi: 10.1083/jcb.200408130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sancier F, Dumont A, Sirvent A, Paquay de Plater L, Edmonds T, David G, Jan M, de Montrion C, Coge F, Leonce S, Burbridge M, Bruno A, Boutin JA, Lockhart B, Roche S, Cruzalegui F. Specific oncogenic activity of the Src-family tyrosine kinase c-Yes in colon carcinoma cells. PLoS One. 2011;6:e17237. doi: 10.1371/journal.pone.0017237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kopetz S, Shah AN, Gallick GE. Src continues aging: current and future clinical directions. Clin Cancer Res. 2007;13:7232–7236. doi: 10.1158/1078-0432.CCR-07-1902. [DOI] [PubMed] [Google Scholar]
- 11.Hollande F, Pannequin J, Joubert D. The long road to colorectal cancer therapy: searching for the right signals. Drug Resist Updat. 2010;13:44–56. doi: 10.1016/j.drup.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Irby RB, Mao W, Coppola D, Kang J, Loubeau JM, Trudeau W, Karl R, Fujita DJ, Jove R, Yeatman TJ. Activating SRC mutation in a subset of advanced human colon cancers. Nat Genet. 1999;21:187–90. doi: 10.1038/5971. [DOI] [PubMed] [Google Scholar]
- 13.Daigo Y, Furukawa Y, Kawasoe T, Ishiguro H, Fujita M, Sugai S, Nakamori S, Liefers GJ, Tollenaar RA, van de Velde CJ, Nakamura Y. Absence of genetic alteration at codon 531 of the human c-src gene in 479 advanced colorectal cancers from Japanese and Caucasian patients. Cancer Res. 1999;59:4222–4224. [PubMed] [Google Scholar]
- 14.Wang NM, Yeh KT, Tsai CH, Chen SJ, Chang JG. No evidence of correlation between mutation at codon 531 of src and the risk of colon cancer in Chinese. Cancer Lett. 2000;150:201–204. doi: 10.1016/s0304-3835(99)00398-5. [DOI] [PubMed] [Google Scholar]
- 15.Bénistant C, Chapuis H, Motter N, Noletti J, Crapez E, Bali J, Roche S. Deregulation of the cytoplasmic tyrosine kinase cSrc in the absence of a truncating mutation at codon 531 in human Bladder carcinoma. Biochem Biophys Res Comm. 2000;273:425–430. doi: 10.1006/bbrc.2000.2948. [DOI] [PubMed] [Google Scholar]
- 16.Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G, Davies H, Teague J, Butler A, Stevens C, Edkins S, O'Meara S, Vastrik I, Schmidt EE, Avis T, Barthorpe S, Bhamra G, Buck G, Choudhury B, Clements J, Cole J, Dicks E, Forbes S, Gray K, Halliday K, Harrison R, Hills K, Hinton J, Jenkinson A, Jones D, Menzies A, Mironenko T, Perry J, Raine K, Richardson D, Shepherd R, Small A, Tofts C, Varian J, Webb T, West S, Widaa S, Yates A, Cahill DP, Louis DN, Goldstraw P, Nicholson AG, Brasseur F, Looijenga L, Weber BL, Chiew YE, DeFazio A, Greaves MF, Green AR, Campbell P, Birney E, Easton DF, Chenevix-Trench G, Tan MH, Khoo SK, Teh BT, Yuen ST, Leung SY, Wooster R, Futreal PA, Stratton MR. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446:153–158. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruhe JE, Streit S, Hart S, Wong CH, Specht K, Knyazev P, Knyazeva T, Tay LS, Loo HL, Foo P, Wong W, Pok S, Lim SJ, Ong H, Luo M, Ho HK, Peng K, Lee TC, Bezler M, Mann C, Gaertner S, Hoefler H, Iacobelli S, Peter S, Tay A, Brenner S, Venkatesh B, Ullrich A. Genetic alterations in the tyrosine kinase transcriptome of human cancer cell lines. Cancer Res. 2007;67:11368–11376. doi: 10.1158/0008-5472.CAN-07-2703. [DOI] [PubMed] [Google Scholar]
- 18.Dehm S, Senger MA, Bonham K. SRC transcriptional activation in a subset of human colon cancer cell lines. FEBS Lett. 2001;487:367–371. doi: 10.1016/s0014-5793(00)02354-1. [DOI] [PubMed] [Google Scholar]
- 19.Pena SV, Melhem MF, Meisler AI, Cartwright CA. Elevated c-yes tyrosine kinase activity in premalignant lesions of the colon. Gastroenterology. 1995;108:117–124. doi: 10.1016/0016-5085(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 20.Cartwright CA, Meisler AI, Eckhart W. Activation of the pp60c-src protein kinase is an early event in colonic carcinogenesis. Proc Natl Acad Sci USA. 1990;87:558–562. doi: 10.1073/pnas.87.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iravani S, Mao W, Fu L, Karl R, Yeatman T, Jove R, Coppola D. Elevated c-Src protein expression is an early event in colonic neoplasia. Lab Invest. 1998;78:365–371. [PubMed] [Google Scholar]
- 22.Carothers AM, Javid SH, Moran AE, Hunt DH, Redston M, Bertagnolli MM. Deficient E-cadherin adhesion in C57BL/6J-Min/+ mice is associated with increased tyrosine kinase activity and RhoA-dependent actomyosin contractility. Exp Cell Res. 2006;312:387–400. doi: 10.1016/j.yexcr.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 23.Arcaroli JJ, Touban BM, Tan AC, Varella-Garcia M, Powell RW, Eckhardt SG, Elvin P, Gao D, Messersmith WA. Gene array and fluorescence in situ hybridization biomarkers of activity of saracatinib (AZD0530), a Src inhibitor, in a preclinical model of colorectal cancer. Clin Cancer Res. 2010;16:4165–77. doi: 10.1158/1078-0432.CCR-10-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dehm SM, Bonham K. SRC gene expression in human cancer: the role of transcriptional activation. Biochem Cell Biol. 2004;82:263–274. doi: 10.1139/o03-077. [DOI] [PubMed] [Google Scholar]
- 25.Park J, Meisler AI, Cartwright CA. c-Yes tyrosine kinase activity in human colon carcinoma. Oncogene. 1993;8:2627–2635. [PubMed] [Google Scholar]
- 26.Gregersen LH, Jacobsen AB, Frankel LB, Wen J, Krogh A, Lund AH. MicroRNA-145 targets YES and STAT1 in colon cancer cells. PLoS One. 2010;5:e8836. doi: 10.1371/journal.pone.0008836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akao Y, Nakagawa Y, Naoe T. MicroRNA-143 and -145 in colon cancer. DNA Cell Biol. 2007;26:311–320. doi: 10.1089/dna.2006.0550. [DOI] [PubMed] [Google Scholar]
- 28.Boggon TJ, Eck MJ. Structure and regulation of Src family kinases. Oncogene. 2004;23:7918–7927. doi: 10.1038/sj.onc.1208081. [DOI] [PubMed] [Google Scholar]
- 29.Nada S, Okada M, MacAuley A, Cooper JA, Nakagawa H. Cloning of a complementary DNA for a protein-tyrosine kinase that specifically phosphorylates a negative regulatory site of p60csrc. Nature. 1991;351:69–72. doi: 10.1038/351069a0. [DOI] [PubMed] [Google Scholar]
- 30.Imamoto A, Soriano P. Disruption of the csk gene, encoding a negative regulator of Src family tyrosine kinases, leads to neural tube defects and embryonic lethality in mice. Cell. 1993;73:1117–1124. doi: 10.1016/0092-8674(93)90641-3. [DOI] [PubMed] [Google Scholar]
- 31.Nada S, Yagi T, Takeda H, Tokunaga T, Nakagawa H, Ikawa Y, Okada M, Aizawa S. Constitutive activation of Src family kinases in mouse embryos that lack Csk. Cell. 1993;73:1125–1135. doi: 10.1016/0092-8674(93)90642-4. [DOI] [PubMed] [Google Scholar]
- 32.Stewart RA, Li DM, Huang H, Xu T. A genetic screen for modifiers of the lats tumor suppressor gene identifies C-terminal Src kinase as a regulator of cell proliferation in Drosophila. Oncogene. 2003;22:6436–6444. doi: 10.1038/sj.onc.1206820. [DOI] [PubMed] [Google Scholar]
- 33.Read RD, Bach EA, Cagan RL. Drosophila C-terminal Src kinase negatively regulates organ growth and cell proliferation through inhibition of the Src, Jun N-terminal kinase, and STAT pathways. Mol Cell Biol. 2004;24:6676–6689. doi: 10.1128/MCB.24.15.6676-6689.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu S, Bjorge JD, Fujita DJ. PTP1B contributes to the oncogenic properties of colon cancer cells through Src activation. Cancer Res. 2007;67:10129–10137. doi: 10.1158/0008-5472.CAN-06-4338. [DOI] [PubMed] [Google Scholar]
- 35.Zheng X, Resnick RJ, Shalloway D. Apoptosis of estrogen-receptor negative breast cancer and colon cancer cell lines by PTP alpha and src RNAi. Int J Cancer. 2008;122:1999–2007. doi: 10.1002/ijc.23321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mao W, Irby R, Coppola D, Fu L, Wloch M, Turner J, Yu H, Garcia R, Jove R, Yeatman TJ. Activation of c-Src by receptor tyrosine kinases in human colon cancer cells with high metastatic potential. Oncogene. 1997;15:3083–3090. doi: 10.1038/sj.onc.1201496. [DOI] [PubMed] [Google Scholar]
- 37.Cam WR, Masaki T, Shiratori Y, Kato N, Ikenoue T, Okamoto M, Igarashi K, Sano T, Omata M. Reduced C-terminal Src kinase activity is correlated inversely with pp60(c-src) activity in colorectal carcinoma. Cancer. 2001;92:61–70. doi: 10.1002/1097-0142(20010701)92:1<61::aid-cncr1292>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 38.Bénistant C, Bourgaux J, Chapuis H, Mottet N, Roche S, Bali J. The C-terminal Src kinase is a tumour antigen in human carcinoma. Cancer Research. 2001;61:1415–1420. [PubMed] [Google Scholar]
- 39.Watanabe N, Matsuda S, Kuramochi S, Tsuzuku J, Yamamoto T, Endo K. Expression of C-terminal src kinase in human colorectal cancer cell lines. Jpn J Clin Oncol. 1995;25:5–9. [PubMed] [Google Scholar]
- 40.Sirvent A, Benistant C, Pannequin J, Veracini L, Simon V, Bourgaux JF, Hollande F, Cruzalegui F, Roche S. Src family tyrosine kinases-driven colon cancer cell invasion is induced by Csk membrane delocalization. Oncogene. 2010;29:1303–1315. doi: 10.1038/onc.2009.450. [DOI] [PubMed] [Google Scholar]
- 41.Kawabuchi M, Satomi Y, Takao T, Shimonishi Y, Nada S, Nagai K, Tarakhovsky A, Okada M. Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases [In Process Citation] . Nature. 2000;404:999–1003. doi: 10.1038/35010121. [DOI] [PubMed] [Google Scholar]
- 42.Brdicka T, Pavlistova D, Leo A, Bruyns E, Korinek V, Angelisova P, Scherer J, Shevchenko A, Hilgert I, Cerny J, Drbal K, Kuramitsu Y, Kornacker B, Horejsi V, Schraven B. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. J Exp Med. 2000;191:1591–1604. doi: 10.1084/jem.191.9.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leroy C, Fialin C, Sirvent A, Simon V, Urbach S, Poncet J, Robert B, Jouin P, Roche S. Quantitative phosphoproteomics reveals a cluster of tyrosine kinases that mediates SRC invasive activity in advanced colon carcinoma cells. Cancer Res. 2009;69:2279–2286. doi: 10.1158/0008-5472.CAN-08-2354. [DOI] [PubMed] [Google Scholar]
- 44.Schaller MD, Parsons JT. pp125FAK-dependent tyrosine phosphorylation of paxillin creates a high-affinity binding site for Crk. Mol Cell Biol. 1995;15:2635–2645. doi: 10.1128/mcb.15.5.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao M, Janas JA, Niki M, Pandolfi PP, Van Aelst L. Dok-1 independently attenuates Ras/mitogen-activated protein kinase and Src/cmyc pathways to inhibit platelet-derived growth factor-induced mitogenesis. Mol Cell Biol. 2006;26:2479–2489. doi: 10.1128/MCB.26.7.2479-2489.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee H, Volonte D, Galbiati F, Iyengar P, Lublin DM, Bregman DB, Wilson MT, Campos-Gonzalez R, Bouzahzah B, Pestell RG, Scherer PE, Lisanti MP. Constitutive and growth factor-regulated phosphorylation of caveolin-1 occurs at the same site (Tyr-14) in vivo: identification of a c-Src/Cav-1/Grb7 signaling cassette. Mol Endocrinol. 2000;14:1750–1775. doi: 10.1210/mend.14.11.0553. [DOI] [PubMed] [Google Scholar]
- 47.Baumeister U, Funke R, Ebnet K, Vorschmitt H, Koch S, Vestweber D. Association of Csk to VE-cadherin and inhibition of cell proliferation. Embo J. 2005;24:1686–1695. doi: 10.1038/sj.emboj.7600647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saito K, Enya K, Oneyama C, Hikita T, Okada M. Proteomic identification of ZO-1/2 as a novel scaffold for Src/Csk regulatory circuit. Biochem Biophys Res Commun. 2008;366:969–975. doi: 10.1016/j.bbrc.2007.12.055. [DOI] [PubMed] [Google Scholar]
- 49.Horejsi V, Zhang W, Schraven B. Transmembrane adaptor proteins: organizers of immunoreceptor signalling. Nat Rev Immunol. 2004;4:603–616. doi: 10.1038/nri1414. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki K, Oneyama C, Kimura H, Tajima S, Okada M. Down-regulation of the tumor suppressor C-terminal Src kinase (Csk)-binding protein (Cbp)/PAG1 is mediated by epigenetic histone modifications via the mitogen-activated protein kinase (MAPK)/phosphatidylinositol 3-kinase (PI3K) pathway. J Biol Chem; 286:15698–15706. doi: 10.1074/jbc.M110.195362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ingley E, Schneider JR, Payne CJ, McCarthy DJ, Harder KW, Hibbs ML, Klinken SP. Csk-binding protein mediates sequential enzymatic downregulation and degradation of Lyn in erythropoietin-stimulated cells. J Biol Chem. 2006;281:31920–31929. doi: 10.1074/jbc.M602637200. [DOI] [PubMed] [Google Scholar]
- 52.Smida M, Posevitz-Fejfar A, Horejsi V, Schraven B, Lindquist JA. A novel negative regulatory function of the phosphoprotein associated with glycosphingolipid-enriched microdomains: blocking Ras activation. Blood. 2007;110:596–615. doi: 10.1182/blood-2006-07-038752. [DOI] [PubMed] [Google Scholar]
- 53.Tauzin S, Ding H, Khatib K, Ahmad I, Burdevet D, van Echten-Deckert G, Lindquist JA, Schraven B, Din NU, Borisch B, Hoessli DC. Oncogenic association of the Cbp/PAG adaptor protein with the Lyn tyrosine kinase in human B -NHL rafts. Blood. 2008;111:2310–2320. doi: 10.1182/blood-2007-05-090985. [DOI] [PubMed] [Google Scholar]
- 54.Solheim SA, Torgersen KM, Tasken K, Berge T. Regulation of FynT function by dual domain docking on PAG/Cbp. J Biol Chem. 2008;283:2773–2783. doi: 10.1074/jbc.M705215200. [DOI] [PubMed] [Google Scholar]
- 55.Oneyama C, Hikita T, Enya K, Dobenecker MW, Saito K, Nada S, Tarakhovsky A, Okada M. The lipid raft-anchored adaptor protein cbp controls the oncogenic potential of c-Src. Mol Cell. 2008;30:426–436. doi: 10.1016/j.molcel.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 56.Veracini L, Simon V, Richard V, Schraven B, Horejsi V, Roche S, Benistant C. The Csk-binding protein PAG regulates PDGF-induced Src mitogenic signaling via GM1. J Cell Biol. 2008;182:603–614. doi: 10.1083/jcb.200705102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miyagi T, Wada T, Yamaguchi K. Roles of plasma membrane-associated sialidase NEU3 in human cancers. Biochim Biophys Acta. 2008;1780:532–537. doi: 10.1016/j.bbagen.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 58.Veracini L, Franco M, Boureux A, Simon V, Roche S, Benistant C. Two distinct pools of Src family tyrosine kinases regulate PDGF-induced DNA synthesis and actin dorsal ruffles. J Cell Sci. 2006;20:2921–2934. doi: 10.1242/jcs.03015. [DOI] [PubMed] [Google Scholar]
- 59.Collin G, Franco M, Simon V, Benistant C, Roche S. The Tom1L1-clathrin heavy chain complex regulates membrane partitioning of the tyrosine kinase Src required for mitogenic and transforming activities. Mol Cell Biol. 2007;27:7631–7640. doi: 10.1128/MCB.00543-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oneyama C, Iino T, Saito K, Suzuki K, Ogawa A, Okada M. Transforming potential of Src family kinases is limited by the cholesterol-enriched membrane microdomain. Mol Cell Biol. 2009;29:6462–6472. doi: 10.1128/MCB.00941-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chan KT, Cortesio CL, Huttenlocher A. FAK alters invadopodia and focal adhesion composition and dynamics to regulate breast cancer invasion. J Cell Biol. 2009;185:357–370. doi: 10.1083/jcb.200809110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bjorge JD, Jakymiw A, Fujita DJ. Selected glimpses into the activation and function of Src kinase. Oncogene. 2000;19:5620–5635. doi: 10.1038/sj.onc.1203923. [DOI] [PubMed] [Google Scholar]
- 63.Zhang SQ, Yang W, Kontaridis MI, Bivona TG, Wen G, Araki T, Luo J, Thompson JA, Schraven BL, Philips MR, Neel BG. Shp2 regulates SRC family kinase activity and Ras/Erk activation by controlling Csk recruitment. Mol Cell. 2004;13:341–355. doi: 10.1016/s1097-2765(04)00050-4. [DOI] [PubMed] [Google Scholar]
- 64.Huang J, Yao L, Xu R, Wu H, Wang M, White BS, Shalloway D, Zheng X. Activation of Src and transformation by an RPTPalpha splice mutant found in human tumours. EMBO J. 2011;30:3200–3211. doi: 10.1038/emboj.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Palmer A, Zimmer M, Erdmann KS, Eulenburg V, Porthin A, Heumann R, Deutsch U, Klein R. EphrinB phosphorylation and reverse signaling: regulation by Src kinases and PTP-BL phosphatase. Mol Cell. 2002;9:725–737. doi: 10.1016/s1097-2765(02)00488-4. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Y, Tu Y, Zhao J, Chen K, Wu C. Reversion-induced LIM interaction with Src reveals a novel Src inactivation cycle. J Cell Biol. 2009;184:785–792. doi: 10.1083/jcb.200810155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bromann PA, Korkaya H, Courtneidge SA. The interplay between Src family kinases and receptor tyrosine kinases. Oncogene. 2004;23:7957–7968. doi: 10.1038/sj.onc.1208079. [DOI] [PubMed] [Google Scholar]
- 68.Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat Rev Cancer. 2012;12:89–103. doi: 10.1038/nrc3205. [DOI] [PubMed] [Google Scholar]
- 69.Yarom N, Jonker DJ. The role of the epidermal growth factor receptor in the mechanism and treatment of colorectal cancer. Discov Med. 2011;11:95–105. [PubMed] [Google Scholar]
- 70.Herath NI, Boyd AW. The role of Eph receptors and ephrin ligands in colorectal cancer. Int J Cancer. 2010;126:2003–2011. doi: 10.1002/ijc.25147. [DOI] [PubMed] [Google Scholar]
- 71.Nakaigawa N, Weirich G, Schmidt L, Zbar B. Tumorigenesis mediated by MET mutant M1268T is inhibited by dominant-negative Src. Oncogene. 2000;19:2996–3002. doi: 10.1038/sj.onc.1203628. [DOI] [PubMed] [Google Scholar]
- 72.Kong M, Mounier C, Dumas V, Posner BI. Epidermal growth factor-induced DNA synthesis. Key role for Src phosphorylation of the docking protein Gab2. J Biol Chem. 2003;278:5837–5844. doi: 10.1074/jbc.M208286200. [DOI] [PubMed] [Google Scholar]
- 73.Bennett HL, Brummer T, Jeanes A, Yap AS, Daly RJ. Gab2 and Src co-operate in human mammary epithelial cells to promote growth factor independence and disruption of acinar morphogenesis. Oncogene. 2008;27:2693–2704. doi: 10.1038/sj.onc.1210928. [DOI] [PubMed] [Google Scholar]
- 74.Bowman T, Broome MA, Sinibaldi D, Wharton W, Pledger WJ, Sedivy JM, Irby R, Yeatman T, Courtneidge SA, Jove R. Stat3-mediated Myc expression is required for Src transformation and PDGF-induced mitogenesis. Proc Natl Acad Sci USA. 2001;98:7319–7324. doi: 10.1073/pnas.131568898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K. STAT3 activation of miR-21 and miR- 181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell. 2010;39:493–506. doi: 10.1016/j.molcel.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Frame MC. Newest findings on the oldest oncogene; how activated src does it. J Cell Sci. 2004;117:989–998. doi: 10.1242/jcs.01111. [DOI] [PubMed] [Google Scholar]
- 77.Woodcock SA, Rooney C, Liontos M, Connolly Y, Zoumpourlis V, Whetton AD, Gorgoulis VG, Malliri A. SRC-induced disassembly of adherens junctions requires localized phosphorylation and degradation of the rac activator tiam1. Mol Cell. 2009;33:639–653. doi: 10.1016/j.molcel.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 78.Avizienyte E, Wyke AW, Jones RJ, McLean GW, Westhoff MA, Brunton VG, Frame MC. Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signalling. Nat Cell Biol. 2002;4:632–638. doi: 10.1038/ncb829. [DOI] [PubMed] [Google Scholar]
- 79.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 80.Rodier JM, Valles AM, Denoyelle M, Thiery JP, Boyer B. pp60c-src is a positive regulator of growth factor-induced cell scattering in a rat bladder carcinoma cell line. J Cell Biol. 1995;131:761–773. doi: 10.1083/jcb.131.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Irby RB, Yeatman TJ. Increased Src activity disrupts cadherin/catenin-mediated homotypic adhesion in human colon cancer and transformed rodent cells. Cancer Res. 2002;62:2669–2674. [PubMed] [Google Scholar]
- 82.Boyer B, Roche S, Denoyelle M, Thiery JP. Src and Ras are involved in separate pathways in epithelial cell scattering. Embo J. 1997;16:5904–5913. doi: 10.1093/emboj/16.19.5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nam JS, Ino Y, Sakamoto M, Hirohashi S. Src family kinase inhibitor PP2 restores the E-cadherin/catenin cell adhesion system in human cancer cells and reduces cancer metastasis. Clin Cancer Res. 2002;8:2430–2436. [PubMed] [Google Scholar]
- 84.de Lau W, Barker N, Clevers H. WNT signaling in the normal intestine and colorectal cancer. Front Biosci. 2007;12:471–491. doi: 10.2741/2076. [DOI] [PubMed] [Google Scholar]
- 85.Nautiyal J, Banerjee S, Kanwar SS, Yu Y, Patel BB, Sarkar FH, Majumdar AP. Curcumin enhances dasatinib-induced inhibition of growth and transformation of colon cancer cells. Int J Cancer. 2011;128:951–961. doi: 10.1002/ijc.25410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shields DJ, Murphy EA, Desgrosellier JS, Mielgo A, Lau SK, Barnes LA, Lesperance J, Huang M, Schmedt C, Tarin D, Lowy AM, Cheresh DA. Oncogenic Ras/Src cooperativity in pancreatic neoplasia. Oncogene. 2011;30:2123–2134. doi: 10.1038/onc.2010.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang L, Lin C, Liu ZR. P68 RNA helicase mediates PDGF-induced epithelial mesenchymal transition by displacing Axin from beta-catenin. Cell. 2006;127:139–155. doi: 10.1016/j.cell.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 88.Yang W, Xia Y, Ji H, Zheng Y, Liang J, Huang W, Gao X, Aldape K, Lu Z. Nuclear PKM2 regulates beta-catenin transactivation upon EGFR activation. Nature. 2011;480:118–122. doi: 10.1038/nature10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qian X, Li G, Vass WC, Papageorge A, Walker RC, Asnaghi L, Steinbach PJ, Tosato G, Hunter K, Lowy DR. The Tensin-3 protein, including its SH2 domain, is phosphorylated by Src and contributes to tumorigenesis and metastasis. Cancer Cell. 2009;16:246–258. doi: 10.1016/j.ccr.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang Y, Kelber JA, Tran Cao HS, Cantin GT, Lin R, Wang W, Kaushal S, Bristow JM, Edgington TS, Hoffman RM, Bouvet M, Yates JR 3rd, Klemke RL. Pseudopodium-enriched atypical kinase 1 regulates the cytoskeleton and cancer progression. Proc Natl Acad Sci USA. 2010;107:10920–10925. doi: 10.1073/pnas.0914776107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Murphy DA, Courtneidge SA. The 'ins' and 'outs' of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol. 2011;12:413–426. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vitale S, Avizienyte E, Brunton VG, Frame MC. Focal adhesion kinase is not required for Src-induced formation of invadopodia in KM12C colon cancer cells and can interfere with their assembly. Eur J Cell Biol. 2008;87:569–579. doi: 10.1016/j.ejcb.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 93.Schoumacher M, Goldman RD, Louvard D, Vignjevic DM. Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. J Cell Biol. 2010;189:541–556. doi: 10.1083/jcb.200909113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Eckert MA, Lwin TM, Chang AT, Kim J, Danis E, Ohno-Machado L, Yang J. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell. 2011;19:372–386. doi: 10.1016/j.ccr.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Valdes-Mora F, Gomez del Pulgar T, Bandres E, Cejas P, Ramirez de Molina A, Perez-Palacios R, Gallego-Ortega D, Garcia-Cabezas MA, Casado E, Larrauri J, Nistal M, Gonzalez-Baron M, F Garcia-oncillas J, Lacal JC. TWIST1 overexpression is associated with nodal invasion and male sex in primary colorectal cancer. Ann Surg Oncol. 2009;16:78–87. doi: 10.1245/s10434-008-0166-x. [DOI] [PubMed] [Google Scholar]
- 96.Balzer EM, Whipple RA, Thompson K, Boggs AE, Slovic J, Cho EH, Matrone MA, Yoneda T, Mueller SC, Martin SS. c-Src differentially regulates the functions of microtentacles and invadopodia. Oncogene. 2010;29:6402–6408. doi: 10.1038/onc.2010.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Windham TC, Parikh NU, Siwak DR, Summy JM, McConkey DJ, Kraker AJ, Gallick GE. Src activation regulates anoikis in human colon tumor cell lines. Oncogene. 2002;21:7797–7807. doi: 10.1038/sj.onc.1205989. [DOI] [PubMed] [Google Scholar]
- 98.Cursi S, Rufini A, Stagni V, Condo I, Matafora V, Bachi A, Bonifazi AP, Coppola L, Superti-Furga G, Testi R, Barila D. Src kinase phosphorylates Caspase-8 on Tyr380: a novel mechanism of apoptosis suppression. EMBO J. 2006;25:1895–1905. doi: 10.1038/sj.emboj.7601085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Oskarsson T, Acharyya S, Zhang XH, Vanharanta S, Tavazoie SF, Morris PG, Downey RJ, Manova-Todorova K, Brogi E, Massague J. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat Med. 2011;17:867–874. doi: 10.1038/nm.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Malanchi I, Santamaria-Martinez A, Susanto E, Peng H, Lehr HA, Delaloye JF, Huelsken J. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2011;481:85–89. doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- 101.Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu M, Shao R, Anderson RM, Rich JN, Wang XF. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell. 2004;5:329–339. doi: 10.1016/s1535-6108(04)00081-9. [DOI] [PubMed] [Google Scholar]
- 102.Valencia K, Ormazabal C, Zandueta C, Luis-Ravelo D, Anton I, Pajares MJ, Agorreta J, Montuenga LM, Martinez-Canarias S, Leitinger B, Lecanda F. Inhibition of collagen receptor discoidin domain receptor-1 (DDR1) reduces cell survival, homing, and colonization in lung cancer bone metastasis. Clin Cancer Res. 2012;18:969–980. doi: 10.1158/1078-0432.CCR-11-1686. [DOI] [PubMed] [Google Scholar]
- 103.Zhang XH, Wang Q, Gerald W, Hudis CA, Norton L, Smid M, Foekens JA, Massague J. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell. 2009;16:67–78. doi: 10.1016/j.ccr.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Barkan D, El Touny LH, Michalowski AM, Smith JA, Chu I, Davis AS, Webster JD, Hoover S, Simpson RM, Gauldie J, Green JE. Metastatic growth from dormant cells induced by a col-I-enriched fibrotic environment. Cancer Res. 2010;70:5706–5716. doi: 10.1158/0008-5472.CAN-09-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Panis Y, Ribeiro J, Chretien Y, Nordlinger B. Dormant liver metastases: an experimental study. Br J Surg. 1992;79:221–223. doi: 10.1002/bjs.1800790309. [DOI] [PubMed] [Google Scholar]
- 106.Iguchi K, Oh G, Ookawa K, Yanagi K, Sakai M, Yamamoto T, Ishikawa S, Onizuka M. In vivo observation of pulmonary micrometastasis of colon cancer in normal rats. Microvasc Res. 2007;73:206–213. doi: 10.1016/j.mvr.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 107.Kopetz S, Lesslie DP, Dallas NA, Park SI, Johnson M, Parikh NU, Kim MP, Abbruzzese JL, Ellis LM, Chandra J, Gallick GE. Synergistic activity of the SRC family kinase inhibitor dasatinib and oxaliplatin in colon carcinoma cells is mediated by oxidative stress. Cancer Res. 2009;69:3842–3849. doi: 10.1158/0008-5472.CAN-08-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Su YJ, Lai HM, Chang YW, Chen GY, Lee JL. Direct reprogramming of stem cell properties in colon cancer cells by CD44. EMBO J. 2011;30:3186–3199. doi: 10.1038/emboj.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Golas JM, Lucas J, Etienne C, Golas J, Discafani C, Sridharan L, Boghaert E, Arndt K, Ye F, Boschelli DH, Li F, Titsch C, Huselton C, Chaudhary I, Boschelli F. SKI-606, a Src/Abl inhibitor with in vivo activity in colon tumor xenograft models. Cancer Res. 2005;65:5358–5364. doi: 10.1158/0008-5472.CAN-04-2484. [DOI] [PubMed] [Google Scholar]
- 110.Serrels A, Macpherson IR, Evans TR, Lee FY, Clark EA, Sansom OJ, Ashton GH, Frame MC, Brunton VG. Identification of potential biomarkers for measuring inhibition of Src kinase activity in colon cancer cells following treatment with dasatinib. Mol Cancer Ther. 2006;5:3014–3022. doi: 10.1158/1535-7163.MCT-06-0382. [DOI] [PubMed] [Google Scholar]
- 111.Emaduddin M, Bicknell DC, Bodmer WF, Feller SM. Cell growth, global phosphotyrosine elevation, and c-Met phosphorylation through Src family kinases in colorectal cancer cells. Proc Natl Acad Sci USA. 2008;105:2358–2362. doi: 10.1073/pnas.0712176105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brunton VG, Avizienyte E, Fincham VJ, Serrels B, Metcalf CA 3rd, Sawyer TK, Frame MC. Identification of Src-specific phosphorylation site on focal adhesion kinase: dissection of the role of Src SH2 and catalytic functions and their consequences for tumor cell behavior. Cancer Res. 2005;65:1335–1342. doi: 10.1158/0008-5472.CAN-04-1949. [DOI] [PubMed] [Google Scholar]
- 113.Mahon FX, Hayette S, Lagarde V, Belloc F, Turcq B, Nicolini F, Belanger C, Manley PW, Leroy C, Etienne G, Roche S, Pasquet JM. Evidence that resistance to nilotinib may be due to BCR-ABL, Pgp, or Src kinase overexpression. Cancer Res. 2008;68:9809–9816. doi: 10.1158/0008-5472.CAN-08-1008. [DOI] [PubMed] [Google Scholar]
- 114.Gioia R, Leroy C, Drullion C, Lagarde V, Etienne G, Dulucq S, Lippert E, Roche S, Mahon FX, Pasquet JM. Quantitative phosphoproteomics revealed interplay between Syk and Lyn in the resistance to nilotinib in chronic myeloid leukemia cells. Blood. 2011;118:2211–2221. doi: 10.1182/blood-2010-10-313692. [DOI] [PubMed] [Google Scholar]
- 115.Boyd DD, Wang H, Avila H, Parikh NU, Kessler H, Magdolen V, Gallick GE. Combination of an SRC kinase inhibitor with a novel pharmacological antagonist of the urokinase receptor diminishes in vitro colon cancer invasiveness. Clin Cancer Res. 2004;10:1545–1555. doi: 10.1158/1078-0432.ccr-1565-02. [DOI] [PubMed] [Google Scholar]
- 116.Giannoni E, Buricchi F, Raugei G, Ramponi G, Chiarugi P. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol Cell Biol. 2005;25:6391–6403. doi: 10.1128/MCB.25.15.6391-6403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dunn EF, Iida M, Myers RA, Campbell DA, Hintz KA, Armstrong EA, Li C, Wheeler DL. Dasatinib sensitizes KRAS mutant colorectal tumors to cetuximab. Oncogene. 2011;30:561–574. doi: 10.1038/onc.2010.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ebi H, Corcoran RB, Singh A, Chen Z, Song Y, Lifshits E, Ryan DP, Meyerhardt JA, Benes C, Settleman J, Wong KK, Cantley LC, Engelman JA. Receptor tyrosine kinases exert dominant control over PI3K signaling in human KRAS mutant colorectal cancers. J Clin Invest. 2011;121:4311–4321. doi: 10.1172/JCI57909. [DOI] [PMC free article] [PubMed] [Google Scholar]