Abstract
This study aimed to construct a eukaryotic expression plasmid containing the G250/MN/CA IX (G250) and human granulocyte-macrophage colony stimulating factor (hGM-CSF) genes, and to detect the expression of these proteins in vitro by recombinant plasmids in eukaryotic cells. pORF-hGM-CSF and pcDNA3.0-G250 were used as the template to amplify G250 and hGM-CSF by routine polymerase chain reaction (PCR). The two PCR products were cloned into the eukaryotic vector pVAX1, in order to construct a recombinant plasmid pVAX1-G250-hGM, and the plasmid was transfected into human embryonic kidney 293 cells. The protein expression was then determined by immunocytochemistry, atomic force microscopy, ELISA and Western blotting. DNA sequencing showed that the cloned G250 and hGM-CSF sequences were consistent with the reported Gene Bank ones. Moreover, a high expression was noted following recombinant plasmid transfection of the G250 and hGM-CSF proteins. Thus, the eukaryotic expression vector pVAX1-G250-hGM containing G250 and hGM-CSF was constructed, allowing for the investigation of the anti-G250 antigen vaccine and immune response mechanisms of biological immunotherapy in renal cell carcinoma.
Keywords: renal cell carcinoma, G250/MN/CA IX gene, human granulocyte-macrophage colony stimulating factor
Introduction
Renal cell carcinoma (RCC) is a common malignant tumor of the urinary system. One out of nearly a million individuals succumb to the disease each year worldwide and the incidence is on the increase (1). RCC patients frequently present with subclinical disease and 20–30% of patients are admitted (2). However, traditional radical nephrectomy in RCC in the early stage has a positive effect, which is not the case in advanced stage and metastatic RCC. Simultaneously, RCC is not sensitive to radiotherapy and chemotherapy (3). Since RCC is a highly immunogenic tumor, advances in molecular and immune biology allow for the potential use of tumor vaccines as immune therapy (4). G250/MN/CA IX (MN antigen receptor/carbonic anhydrase-9) is one of the tumor markers that possess favorable tumor specificity (5). The specific expression of G250/MN/CA IX in RCC renders it a key target for cancer diagnosis and treatment. In this study, a eukaryotic expression vector, containing the G250/MN/CA IX and human granulocyte-macrophage colony stimulating factor (hGM-CSF) genes, was constructed and transfected into human embryonic kidney 293 (HEK 293) cells. The fusion protein expression and immunoreactivity were then detected to establish anti-renal cell carcinoma vaccines for studies based on the G250 gene.
Materials and methods
Materials
E. coli Top 10, vector pVAX1, recombinant plasmid pORF-hGM-CSF and pcDNA3.0-G250 were obtained from the Department of Microbiology and Immunology, Medical College, Jinan University, Guangzhou, China. Ex-Taq DNA polymerase, T4 DNA ligase, restriction enzymes XbaI, HindIII and KpnI, 1 kb DNA marker, 100 bp DNA marker, plasmid mini and gel extraction kits were from Takara Bio Inc., Japan. The target gene sequencing analysis was from Shanghai Sangon Biological Engineering Technology and Services Company, China. Lipofectamine-2000 and Opti-MEM were from Invitrogen Corporation (Carlsbad, CA, USA), and the immunohistochemical kit was from Zhongshan Company, China. The ELISA kit was purchased from R&D Inc., USA, and the G250 antibody was from Abcam Inc., USA.
Construction and identification of recombinant plasmids
Based on the CDS sequence of the G250 gene in Gene Bank (NCBI: BC014950), primer 5.0 was used to design the primers 5′-GCAAGCTTTTCCAATGCACGTACAG-3′ (forward) and 5′-TCGGGTACCGGCTCCAGTCTC-3′ (reverse) with the appropriate restriction endonuclease sites and omission of the termination codon which was used to amplify G250 (Fig. 1). The PCR fragments and plasmid pVAX1 were digested by KpnI and HindIII. The cleaved products were ligated using T4 DNA ligase at 16°C overnight. The ligated products were transformed into the competent E. coli Top 10, and antibiotic selection and the restriction endonuclease assay (Fig. 2) were used to screen and identify positive clones. DNA sequencing analysis was performed using Sanger dideoxy chain termination.
Figure 1.

Electrophoresis of the PCR product. M1: 1 kb DNA ladder marker; lanes 1 and 2: PCR product of the G250 gene; M2: 100 bp DNA ladder marker; lanes 3 and 4: PCR product of the hGM-CSF gene.
Figure 2.
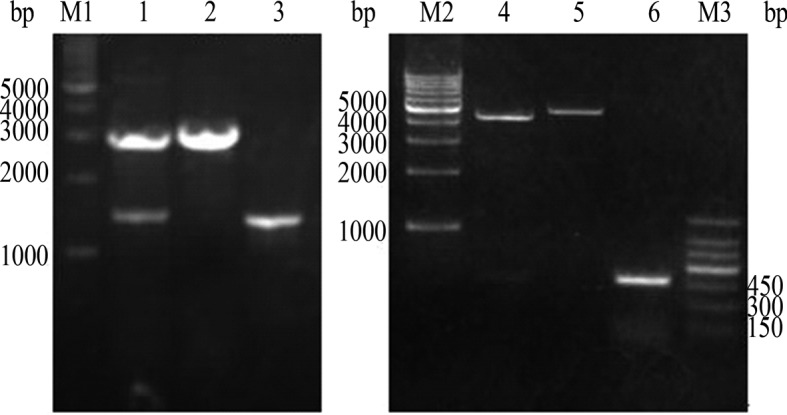
Restriction map of recombinant plasmids. M1: 1 kb DNA ladder marker; lane 1: pVAX1-G250/HindIII + KpnI; lane 2: pVAX1/HindIII + KpnI; lane 3: PCR product of G250. M2: 1 kb DNA ladder mark; lane 4: pVAX1-G250-GM/KpnI + XbaI; lane 5: pVAX1-CAIX/KpnI + XbaI; lane 6: PCR product of hGM-CSF; M3: 150 bp DNA ladder marker.
The coding sequences of the hGM-CSF fragments were synthesized by PCR from pORF-hGM-CSF using specific primers: 5′-TATGGTACCGGATCAGGAGGTTCTATGTGG CTGCAGAGCCT-3′ (forward) and 5′-GGGTCTAGATATCA TGTCGAGCTAGCGAATTCACT-3′ (reverse), which were cloned into the KpnI and XbaI sites of pVAX1-G250 using standard cloning techniques (Fig. 1). The recombinant plasmids were purified and double digested with KpnI and XbaI (Fig. 2). The procedure involving the sequence analysis of the recombinant plasmids was terminated by the Shanghai Bio-Engineering Company and recombinant plasmid pVAX1-G250-GM was successfully constructed.
Cell transfection
HEK 293 cells were digested with 0.25% trypsin and diluted to 1–4×105 cells/ml. The cells were plated in 6-well plates with 2 ml medium per well. When the cells achieved 60–70% confluence, 4 μg purified plasmid was transfected to the prepared cells using 8 μl lipofectamine-2000 reagent. After 48 h, the living cells were examined directly and photographed under an inverted fluorescence microscope.
Immunocytochemistry staining
Non-transfected cells were regarded as the blank comparison and pVAX1-transfected cells as the negative comparison. Immunocytochemistry staining was performed according to the manufacturer's instructions and mouse anti-G250 antibody was used as the primary antibody (Fig. 3).
Figure 3.
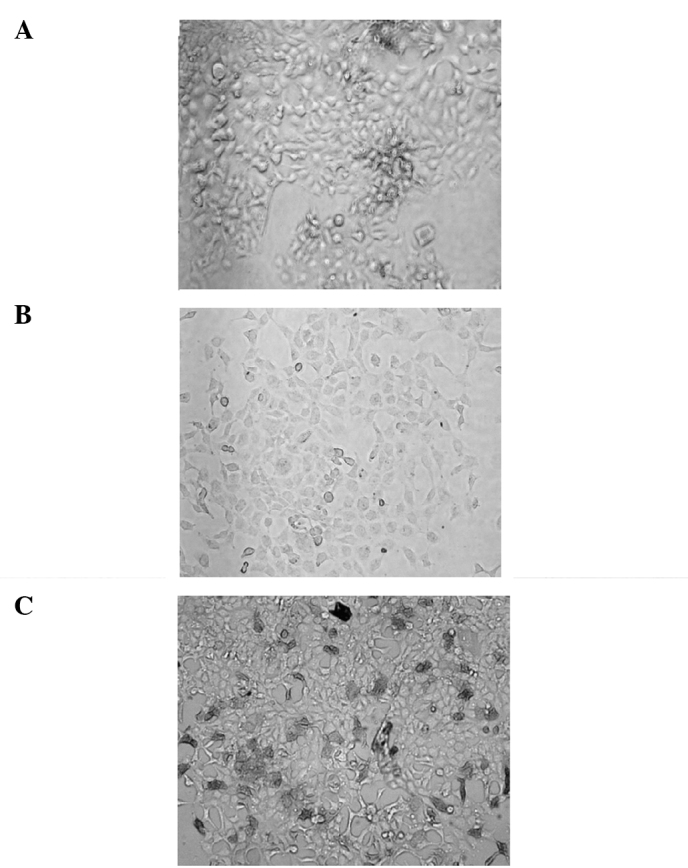
Identification of the protein expression in HEK 293 cells by immunocytochemistry staining (magnification, ×100). (A) Negative group (non-transfected). (B) Negative group (transfected pVAX1). (C) Positive group (transfected pVAX1-G250-GM).
Atomic force microscopy
After being dried, samples were immunoblotted, visualized using DAB chromogen and scanned by atomic force microscopy (AFM) (AutoProbe CP Research, ThermoMicroscopes Inc., USA). The samples were placed in XY scanning stage, and then the monitor was used to locate the scanning area of the sample and scanning image. The test was conducted using 100 μm scanners, UL20B silicon probe, and a force constant of 2.8 N/m. Equipment configuration software (ThermoMicroscopes pro-scan image processing software version 2.1) was used for image data acquisition and processing. Images were smoothed to eliminate the scan direction and ensure a low level of background noise (Fig. 4).
Figure 4.
Atomic force microscopy. (A) Normal HEK 293 cells. (B) HEK 293 cells transfected with pVAX1. (C) HEK 293 cells transfected with pVAX1-G250-GM.
ELISA
Double-antibody sandwich ELISA was used to detect the hGM-CSF protein level. The results were shown as the χ̄ ± s and the significant level of difference between the values was analyzed using SPSS 13.0 software. P<0.05 was considered to be statistically significant (Table I, Fig. 5).
Table I.
Expression value of hGM-CSF protein by ELISA.
| Group | 24-h hGM-CSF (pg/ml) | 48-h hGM-CSF (pg/ml) |
|---|---|---|
| pVAX1 | 53.97±0.53 | 54.10±0.79 |
| pVAX1CAIX-hGM | 482.47±5.86a | 513.36±4.45a |
Figure 5.

Expression value of hGM-CSF protein by ELISA. (A) Transfected pVAX1 (time, 24 h). (B) Transfected pVAX1-G250-GM (time, 24 h). (C) Transfected pVAX1 (time, 48 h). (D) Transfected pVAX1-G250-GM (time, 48 h).
Western blotting
A Western blot analysis of the fusion proteins was performed according to the standard method. The purified protein was separated on 120 g/l SDS-PAGE and transferred to a nitrocellulose membrane. Anti-G250 antibody at 1:1,000 dilution was used as the primary antibody to detect the G250 protein. The blots were developed using the ECL method with HRP-labeled anti-goat IgG at a dilution of 1:6,000 (Fig. 6).
Figure 6.
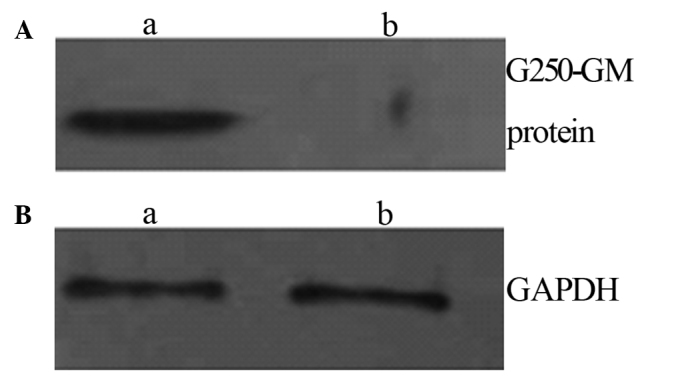
Western blot analysis of protein expressed in HEK 293 cells. Lysates of HEK 293 cells transfected with: (A-a) recombinant plasmid pVAX1-G250-GM; (A-b) blank plasmid pVAX1; (B-a) recombinant plasmid pVAX1-G250-GM and (B-b) blank plasmid pVAX1.
Results
PCR products of G250 and hGM-CSF genes
Results of an agarose gel electrophoresis assay showed that the size of the specific PCR amplification products were ~1.48 and 0.48 kb for the G250 and hGM-CSF genes, respectively. The results were in agreement with the anticipated fragment.
Recombinant plasmid detection by restriction enzyme digestion
pVAX1-G250 was double digested by KpnI and HindIII. Results of an agarose gel electrophoresis assay showed the two fragments to be ~3.0 and 1.5 kb for the G250 and hGM-CSF genes, respectively. pVAX1-G250-GM was double digested by KpnI and XbaI, and the two fragments were found to be ~4.5 and 0.48 kb for the G250 and hGM-CSF genes, respectively, were noted.
Sequencing identification
The recombinant plasmid pVAX1-G250-GM was examined by sequencing. The results showed that it was identical to the reported G250 gene sequence (NCBI accession: BC014950) and the hGM-CSF gene sequence (NCBI accession: M10663).
Identification of fusion protein by immunocytochemistry staining
Immunocytochemistry staining results showed that the expression of the G250 protein was negative in the transfected pVAX1 group. However, in the pVAX1-G250-GM-transfected group the expression of G250 protein was positive (Fig. 3).
Atomic force microscopy
Normal HEK 293 cells had a smooth surface, with a rich-pseudopod extension and a cell height that reached 1,250 μm. When transfected with pVAX1, the cell surfaces became rough and the cell height was 1,161 μm. pVAX1-G250-GM was transfected into cells and the cell height became significant (~7,450 nm).
ELISA analysis
The expression values of the hGM-CSF protein of the pVAX1 control group were 53.97±0.53 and 54.10±0.79 pg/ml (χ̄ ± s, n=3), whereas the expression values of the hGM-CSF protein of the pVAX1-G250-GM-transfected group were 482.47±5.86 and 513.36±4.45 pg/ml. The results were considered to be statistically significant (P<0.05).
Western blotting
The proteins which bound to the G250 antibody were detected following recombinant plasmid pVAX1-G250-hGM transfection into the HEK 293 cells.
Discussion
RCC is a highly immunogenic tumor that induces the host immune response. RCC primary tumors and their metastasis were found to be relatively stable over time. The tumors occasionally spontaneously regressed, indicating that immune mechanisms play a key role in RCC. G250/MN/CA IX, a good tissue-specific RCC-associated antigen, has been identified and cloned from a variety of RCC cell lines. This antigen has 459 amino acids lying in the plasma and nuclear membranes, with a molecular weight between 58 and 54 ku (6). Clear cell carcinoma of the kidneys, and the majority of other types of RCC, express G250 antigen. Approximately 88% of tumor metastases also express the G250 antigen (7), which regulates cell proliferation in hypoxic conditions. G250 possesses HLA-A2.1-restricted epitopes (8), and the G250 transduction in peripheral blood mononuclear cells produced cytotoxic T cells, which inhibited the tumor cells expressed in the growth of G250. For these reasons, G250 is a key target for RCC immunotherapy (9).
In recent years, increasing importance has been attached to gene therapy as adjuvant treatment in DNA vaccination. As a preponderant adjuvant, hGM-CSF has the ability to activate endotheliocytes and macrophagocytes through a variety of mechanisms. It also regulates the amount of and function of antigen-presenting cells and enhances the cytoactive level of cytotoxic T lymphocyte and natural killer cells to strengthen the immune level. hGM-CSF is considered to be an adjuvant therapy that assists in the preparation of DNA vaccination and more favorable experimental results (10,11). Tani et al (12) prepared hGM-CSF gene-modified autologous tumor cells from type IV tumors and used gene-modified tumor cells to treat RCC patients. The results showed that the vaccine induced a tumoral immune response, indicating that it was able to enhance the anti-tumor effect. Simultaneously, hGM-CSF combined with autologous tumor cells increased the number of lymphocytes, resulting in slow disease progression and extended patient survival (13).
Concerning safety requirements, we used vector pVAX1, which is approved by the FDA and can be applied to the human body. Linkers were added when the upstream primers were designed in order to amplify hGM-CSF. To produce G250 protein, the linkers were added between the fusion proteins and the hGM-CSF fold in natural three-dimensional structures while maintaining intrinsic immunogenicity. To assess the character of the fusion proteins, we transfected the recombinant plasmids into HEK 293 cells using the liposome transfection method and detected the proteins with immunocytochemistry staining and Western blotting. The results showed that cells transfected with recombinant plasmids in immunocytochemistry expressed protein, whereas the control group had no significant change in color. This expression shows that G250 protein is immunoreactive. Immunocytochemistry usually uses conventional light or electron microscopy. These classical methods lack dynamic, three-dimensional and single-molecule measurement ability. This study utilized AFM, a novel surface imaging technique that detected nanometer positioning resolution, in order to scale antigen-antibody complex positioning, qualitative, quantitative and three-dimensional display on the membrane surface. AFM detects, not only single-molecule single atoms, but also specific biological molecules or non-specific binding between the three-dimensional morphology of the dynamic process of change. Cells transfected with recombinant plasmid ultrastructures exhibit surface roughness and the height changes significantly, due to the recombinant plasmid which, since it contains G250, binds with the antibody, thereby reducing the interaction between the cells. Notably, the use of ELISA has shown an accurate expression of hGM-CSF fusion protein.
In conclusion, we constructed a eukaryotic vector containing G250 and hGM-CSF and detected the expression of the two proteins. However, further investigations regarding immunogenicity and safety of the vaccine are required, as well as examination of the vaccination of the anti-G250 antigen and its immune response mechanism of biological immunotherapy in RCC.
Acknowledgements
This study was supported by the Key specialist of Guangdong Provincial 11th Five-Year Plan.
References
- 1.Kirkali Z, Tuzel E, Mungan MU. Recent advances in kidney cancer and metastatic disease. BJU Int. 2001;88:818–824. doi: 10.1046/j.1464-4096.2001.02442.x. [DOI] [PubMed] [Google Scholar]
- 2.Ljungberg B, Hanbury DC, Kuczyk MA, Merseburger AS, Mulders PF, Patard JJ, Sinescu IC. European Association of Urology Guideline Group for renal cell carcinoma. Eur Urol. 2007;51:1502–1510. doi: 10.1016/j.eururo.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 3.Bleumer I, Oosterwijk E, De Mulder PF. Immunotherapy for renal cell carcinoma. Eur Urol. 2003;44:65–75. doi: 10.1016/s0302-2838(03)00191-x. [DOI] [PubMed] [Google Scholar]
- 4.Van Poppel H, Joniau S, van Gool SW. Vaccine therapy in patients with renal cell carcinoma. European Urology. 2009;55:1333–1344. doi: 10.1016/j.eururo.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 5.Potter CP, Harris AL. Diagnostic, prognostic and therapeutic implications on carbonic anhydrases in cancer. Br J Cancer. 2003;89:2–7. doi: 10.1038/sj.bjc.6600936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Opavský R, Pastoreková S, Zelník V, Gibadulinová A, Stanbridge EJ, Závada J, Kettmann R, Pastorek J. Human MN/CA9 gene, a novel member of the carbonic anhydrase family: structure and exon to protein domain relationships. Genomics. 1996;33:480–487. doi: 10.1006/geno.1996.0223. [DOI] [PubMed] [Google Scholar]
- 7.Oosterwijk E, Ruiter DJ, Hoedemaeker PJ, Pauwels EK, Jonas U, Zwartendijk J, Warnaar SO. Monoclonal antibody G250 recognizes a determinant present in renal cell carcinoma and absent from normal kidney. Int J Cancer. 1986;38:489–494. doi: 10.1002/ijc.2910380406. [DOI] [PubMed] [Google Scholar]
- 8.Vissers JL, De Vries IJ, Schreurs MW, Engelen LP, Oosterwijk E, Figdor CG, Adema GJ. The renal cell carcinoma-associated antigen G250 encodes a human leukocyte antigen (HLA)-A2.1-restricted epitope recognized by cytotoxic T lymphocytes. Cancer Res. 1999;59:5554–5559. [PubMed] [Google Scholar]
- 9.Dorai T, Sawczuk IS, Pastorek J, Wiernik PH, Dutcher JP. The role of carbonic anhydrase IX overexpression in kidney cancer. Eur J Cancer. 2005;41:2935–2947. doi: 10.1016/j.ejca.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Cruciani M, Mengoli C, Serpelloni G, Mazzi R, Bosco O, Malena M. Granulocyte macrophage colony-stimulating factor as an adjuvant for hepatitis B vaccination: a meta-analysis. Vaccine. 2007;25:709–718. doi: 10.1016/j.vaccine.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Miyashita T, Shah FA, Marti G, Wang J, Armstrong T, Bonde P, Gibson MK, Yoshimura K, Montgomery EA, Duncan MD, Jaffee EM, Harmon JW. Vaccine impedes the development of reflux-induced esophageal cancer in a surgical rat model: efficacy of the vaccine in a pre-Barrett's esophagus setting. J Gastrointest Surg. 2008;12:2–9. doi: 10.1007/s11605-007-0337-2. [DOI] [PubMed] [Google Scholar]
- 12.Tani K, Azuma M, Nakazaki Y, et al. Phase I study of autologous tumor vaccines transduced with the GM-CSF gene in four patients with stage IV renal cell cancer in Japan: clinical and immunological findings. Mol Ther. 2004;10:799–816. doi: 10.1016/j.ymthe.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Schwaab T, Tretter CP, Gibson JJ, Cole BF, Schned AR, Harris R, Wallen EM, Fisher JL, Waugh MG, Truman D, Stempkowski LM, Crosby NA, Heaney JA, Ernstoff MS. Immunological effects of granulocyte-macrophage colony-stimulating factor and autologous tumor vaccine in patients with renal cell carcinoma. J Urol. 2004;171:1036–1042. doi: 10.1097/01.ju.0000113275.91953.5d. [DOI] [PubMed] [Google Scholar]



