Abstract
Purpose
We conducted a phase I study of hepatic arterial infusion (HAI) cisplatin and systemic chemotherapy in patients with advanced cancer and dominant liver involvement.
Methods
Patients were treated with HAI cisplatin 100–125 mg/m2 (and 3,000 IU heparin) intraarterially and liposomal doxorubicin (doxil) 20–35 mg/m2 IV (day 1) every 28 days. A “3 + 3” study design was used.
Results
Thirty patients were treated (median age, 56 years). Diagnoses were breast cancer (n = 11), colorectal cancer (n = 8), ocular melanoma (n = 4), and other (n = 7). The median number of prior therapies was 5. The maximum tolerated dose (MTD) was at the 100/35 mg/m2 level. Dose-limiting toxicities were Grade 4 neutropenia (2 of 4 patients), and Grade 4 thrombocytopenia (n = 1) at the cisplatin 125 mg/m2 and systemic doxil 35 mg/m2 dose level. The most common toxicities were nausea/vomiting and fatigue. Of 24 patients evaluable for response, 4 (17%) had a partial response (PR) and 7 (29%) had stable disease (SD) for ≥4 months. Of the 11 patients with breast cancer, 3 (27%) had a PR and 5 (45%) had SD for ≥4 months. Of 4 patients with ocular melanoma, 1 had a PR and 1 SD for 4 months. One patient with hepatocellular carcinoma had SD for 4 months. Of 12 evaluable patients treated at the MTD, 2 (17%) had a PR and 5 (42%) had SD.
Conclusion
The MTD was HAI cisplatin 100 mg/m2 and systemic doxil 35 mg/m2. This regimen demonstrated anti-tumor activity, especially in breast cancer.
Keywords: Hepatic arterial infusion, Cisplatin, Doxil, Phase I trial
Introduction
Liver metastases from solid tumors are associated with a poor prognosis.
Overall, 15–25% of patients with colorectal cancer present with liver metastases, and another 25–50% develop hepatic metastasis following resection of the primary tumor [1–3].
Approximately 85% of patients with metastatic ocular melanoma have liver involvement, and in half of these patients the metastatic tumor is limited to the liver [4]. Despite the frequent confinement of metastatic ocular melanoma to the liver, resection or radiofrequency ablation of liver metastasis is rarely possible because of multifocal involvement of the liver in these patients [5].
Although resection of liver metastases from colorectal cancer can produce long-term survival in selected patients with solitary liver metastases, the efficacy of liver resection as a solitary treatment is limited by the number of patients with resectable disease, and even after resection most patients develop recurrent disease in the liver [6].
Hepatic arterial infusion (HAI) has been used to treat hepatic metastases from any type of cancer (most commonly colorectal), or primary cancers including hepatocellular carcinoma, and biliary tract cancer. The rationale for using HAI is based on the concept that malignant lesions derive most of their blood supply from the hepatic artery, in contrast to normal hepatocytes that are supplied through the portal venous circulation [7]. Cytotoxics administered via the hepatic artery are thought to be extracted during their initial pass through the hepatic parenchyma, therefore maximizing drug concentration in the liver metastases [7].
In 1989, a controlled clinical trial of HAI consisting of 5-fluoro-2′-deoxyuridine (FUDR) for hepatic metastases from colorectal carcinoma via continuous intraarterial versus continuous intraarterial/intravenous (IV) therapy demonstrated that the combination of intraarterial and IV therapy prevented extrahepatic spread during therapy in most patients, and survival was significantly prolonged in patients with metastatic regression [8]. Results of subsequent clinical trials of intrahepatic therapy were encouraging [9], and regional adjuvant therapy with FUDR was shown to improve survival in colorectal patients with liver involvement [10, 11].
The rationale for combining HAI cisplatin and IV liposomal doxorubicin is based on the previously reported encouraging local antitumor activity with intraarterial hepatic infusion/embolization with cisplatin with or without other cytotoxic agents [12] and a 27% partial response rate seen with IV cisplatin and IV liposomal doxorubicin in a phase I study in advanced solid tumors [13].
Patients and methods
Eligibility criteria included a histologically confirmed diagnosis of malignancy and liver involvement as the dominant site of metastasis, defined as hepatic metastases constituting ≥ 50% of all tumor burden; Karnofsky performance status ≥60%; and adequate renal (serum creatinine ≤1.5 mg/dL or a calculated creatinine clearance >50 mL/min), hepatic (total bilirubin ≤1.5 mg/dL, AST ≤5 times upper normal reference value, or ALT ≤5 times upper normal reference value) and bone marrow function (ANC ≥ 1.5 × 109/L; PLT ≥ 100 × 109/L). Female patients with childbearing potential were eligible if they had a negative urine or serum HCG test. Pediatric patients were eligible at the discretion of the primary investigator. Patients were eligible to start therapy if >21 days from day 1 of prior therapy had elapsed, and they had complete recovery from all associated toxicities.
Exclusion criteria included clinical or radiographic evidence of ascites, pregnancy, hypersensitivity to platinum compounds or anthracyclins, inability to complete an informed consent process and adhere to the protocol treatment plan and follow-up requirements, prothrombin time >20 s or INR >2.0, portal vein thrombosis, Grade 2 peripheral neuropathy, or medical history or clinical evidence of congestive heart failure.
All patients signed informed consent forms fully disclosing the investigational nature of the trial prior to enrollment. The study protocol was approved by the M. D. Anderson Cancer Center Institutional Review Board.
Treatment
Patients were admitted for treatment at M. D. Anderson Cancer Center. A hepatic intraarterial catheter was placed by an interventional radiologist using the femoral approach. A 5-French angiographic catheter was utilized to select the celiac and/or superior mesenteric artery, and a co-axial 3 French microcatheter was advanced into the desired hepatic artery. Hepatic artery flow evaluation was then performed following the injection of 5 mCi Technetium-99 m macro-aggregated albumin particles through the HAI catheter used to simulate the distribution of chemotherapeutic agent. The nuclear medicine flow study was also used to identify any evidence of extra-hepatic flow to reduce the risk of gastrointestinal complications. The catheter was removed at the end of the cisplatin infusion.
Patients were treated with HAI cisplatin 100–125 mg/m2 intraarterially in normal saline 250 mL with 3,000 IU heparin intraarterially over 2 h on day 1, followed by liposomal doxorubicin 20–35 mg/m2 IV mixed in 100 cc 5% dextrose water over 1 h on day 1. Cycles were repeated every 4 weeks. Dose escalation of cisplatin and liposomal doxorubicin is shown in Table 1. Patients also received dexamethasone 20 mg IV on day 1 prior to chemotherapy followed by 4 mg IV every 12 h for 5 days.
Table 1.
Dose escalation scheme of cisplatin and liposomal doxorubicin
| Dose level | Cisplatin (mg/m2) | Liposomal doxorubicin (mg/m2) |
|---|---|---|
| 1 | 100 | 20 |
| 2 | 100 | 25 |
| 3 | 100 | 30 |
| 4 | 100 | 35 |
| 5 | 125 | 35 |
Patient monitoring
Patients were monitored every 4 weeks by physical examination, complete blood counts and differential, chemistry laboratory studies, vital signs, periodic serial electrocardiograms, liver function tests and renal function tests every 2 weeks, chest X-ray and assessment of adverse events. All patients had initial tumor staging and assessment after completion of every two cycles of therapy.
Endpoints and statistical considerations
The study was designed using a conventional “3 + 3” study design, followed by an expansion phase composed of 10 patients. Dose-limiting toxicities were assessed during the first cycle and were defined as follows: any Grade 3–4 adverse event as defined in the NCI CTC v3.0 (except those that are expected and related to cisplatin, including myelosuppression, alopecia, nausea and vomiting); any Grade 4 hematologic toxicity (as defined by the NCI CTC) >5 consecutive days or requiring transfusion or growth factor support; and Grade 4 nausea/vomiting > 5 days, and any other Grade 3 non-hematologic toxicity, including symptoms/signs of vascular leak or cytokine release syndrome, or any severe life-threatening complication. The use of growth factors was acceptable during the clinical study.
Best response was assessed by a radiologist from M. D. Anderson Cancer Center starting after 2 cycles of therapy, and after every 2 cycles (1 cycle = 4 weeks) using the RECIST guidelines that were used during the study period [14]. These criteria defined a partial response (PR) as a ≥30% decrease in the sum of the longest diameter of target lesions, excluding complete disappearance of disease (CR). Progressive disease was defined as a ≥20% increase in the sum of the longest diameter of target lesions. Stable disease (SD) was defined as small changes not meeting the criteria for a PR or progressive disease (PD). Waterfall plot analysis illustrated antitumor activity, if any, as previously described [15]. Responses shown in the waterfall plot were grouped according to standard RECIST criteria.
Survival was measured from start of the treatment on protocol until death from any cause or last follow-up. Progression-free survival was measured from start of treatment on protocol until progression or death, whichever occurred first. Toxicities were assessed using NCI CTC, v. 3.0 [16]. A p value of <0.05 was considered statistically significant. Statistical analyses were carried out using SAS 9.1 (SAS Institute, Cary, NC, USA) and S-Plus, version 7.0 (Insightful Corp., Seattle, WA, USA).
Results
Demographics
Thirty-two patients were registered on protocol. Two patients were screen failures because of rapid decline of performance status prior to initiation of therapy (n = 1) and baseline ejection fraction of 25–30% (n = 1). Thirty patients were treated. Their median age was 56 (range 15–76). Twenty-five women and 5 men were treated. The most common diagnoses were breast cancer (n = 11), colorectal cancer (n = 8) and ocular melanoma (n = 4). One patient had each of one of the following diagnoses: melanoma, gastric cancer, hepatocellular carcinoma, pancreatic cancer, neuroendocrine cancer, adenocystic head and neck cancer and leiomyosarcoma. The median number of prior therapies was 5 (range 1–13). Prior therapies are listed in Table 2.
Table 2.
Prior therapies
| Prior therapies | No. of pts (n = 30) | % |
|---|---|---|
| Cytotoxics | ||
| 5-fluorouracil | 23 | 77 |
| Taxanes | 17 | 57 |
| Irinotecan | 16 | 53 |
| Capecitabine | 12 | 40 |
| Anthracyclines | 11 | 37 |
| Oxaliplatin/Cisplatin/Carboplatin | 10/3/6 | 33/10/20 |
| Cyclophosphamide | 7 | 23 |
| Gemcitabine | 6 | 20 |
| Navelbine | 5 | 17 |
| Other | 3 | 10 |
| Targeted agents | ||
| Bevacizumab | 14 | 47 |
| Cetuximab and Panitumamab (Vecitibix) | 11 | 37 |
| Cetuximab | 8 | 27 |
| Herceptin | 4 | 13 |
| Gefitinib/Lapatinib | 2/2 | 7/7 |
| Other | 10 | 33 |
| Hormonal therapy | ||
| Tamoxifen | 4 | 13 |
| Arimidex | 5 | 17 |
| Other | 4 | 13 |
Dose escalation and dose-limiting toxicity
Dose escalation and dose-limiting toxicities are listed in Table 3. The maximum tolerated dose (MTD) of HAI cisplatin was 100 mg/m2 and liposomal doxorubicin was 35 mg/m2. Dose-limiting toxicities were noted at HAI of oxaliplatin 125 mg/m2 and liposomal doxorubicin 35 mg/m2 and included Grade 4 neutropenia (2 of 4 patients), and Grade 4 thrombocytopenia (1 of 4 patients).
Table 3.
Distribution of patients, treatment cycles, and dose-limiting toxicities across tested dose levels
| Cisplatin/Doxil dose level (mg/m2) | No. of patients | No. of pts. completed C1 | No. of pts. with DLTs | Description of DLTs |
|---|---|---|---|---|
| 100/20 | 3 | 3 | 0 | |
| 100/25 | 4 | 4 | 0 | |
| 100/30 | 3 | 3 | 0 | |
| 100/35 | 16a | 16a | 0 | |
| 125/35 | 4 | 4 | 2 | G4 neutropenia (1 patient); G4 thrombocytopenia and neutropenia (1 patient) |
DLT dose-limiting toxicity
Including 10 patients in the dose expansion phase
Toxicity
A total of 79 cycles of HAI cisplatin and systemic IV liposomal doxorubicin were administered. The median number of cycles administered per patient was 4 (range 1–6). Toxicities are summarized in Table 4. Among 30 patients who completed cycle 1, 7 (23%) patients had no toxicity > Grade 1. The most common toxicities were nausea/vomiting (n = 23), fatigue (n = 15) and constipation (n = 13) (Table 4).
Table 4.
Toxicity
| Adverse event | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Nausea/vomiting | 11/10 | 9/8 | 3/1 | |
| Fatigue | 11 | 2 | 2 | |
| Constipation | 11 | 2 | ||
| Anorexia | 9 | 3 | ||
| Myalgia/bone pain | 8 | |||
| Neuropathy | 6 | |||
| Abdominal pain | 2 | 2 | ||
| Renal insufficiency | 1 | 3 | ||
| Diarrhea | 1 | 1 | ||
| Neutropenia | 1 | 2 | 1 | 2 |
| Thrombocytopenia | 2 | 1 | 1 | |
| Anemia | 5 | 6 | 4 | |
| Infection | 3 | |||
| Mucositis | 2 | |||
| Fever | 2 | 2 | ||
| Hearing loss/tinnitus | 2 | 2 | ||
| Dizziness | 1 | |||
| Headache | 3 | |||
| Hypertension | 1 | |||
| Edema | 3 | |||
| Rash | 2 | |||
| Hypomagnesemia | 4 | |||
| Hyperkalemia | 1 | 1 | ||
| Hypokalemia | 1 | 2 | ||
| Hyponatremia | 1 | |||
| Hypocalcemia | 1 |
Response
Of 30 treated patients, 24 patients reached their first restaging evaluation at 2 months. Six patients were not evaluable for response for the following reasons: 4 withdrew consent after the first (n = 3) or the second (n = 1) cycle of treatment because of toxicities: anorexia and fatigue (n = 1); Grade 2 dehydration and Grade 3 anemia (n = 1); Grade 4 neutropenia and Grade 3 nausea (n = 1); Grade 2 nausea/vomiting, hypertension, and weakness (n = 1), and two patients were lost to follow-up after cycle 1.
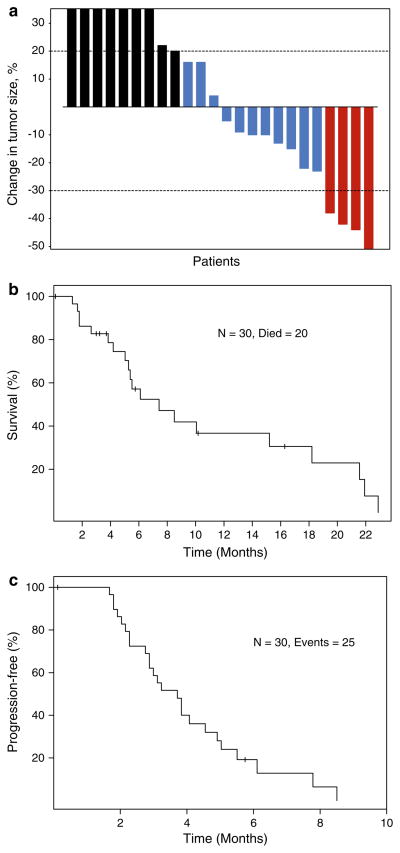
Response is shown in Table 5 and Fig. 1. HAI of cisplatin in combination with systemic liposomal doxorubicin induced a PR in 4 (17%) patients. Tumor size decreased by 38%, 42%, 44% and 51%, for 4, 4, 6 and 4 months, respectively. In addition, seven (29%) patients had SD for at least 4 months. Of the 11 patients with breast cancer, 3 (27%) had a PR and 5 (45%) had SD for ≥4 months. Of 4 patients with ocular melanoma, 1 had a PR (duration, 4 months) and 1 SD (duration, 4 months). One patient with hepatocellular carcinoma had SD for 4 months. Of 12 evaluable patients treated at the MTD, 2 (17%) had a PR and 5 (42%) had SD.
Table 5.
Characteristics of patients treated with hepatic arterial infusion of cisplatin and IV liposomal doxorubicin with stable disease or better and their best response
| Patient No. | Age/Sex | PS | Cisplatin/Doxil (mg/m2) | No. of prior Rx | No. of cycles | Type of cancer | Response | % | Reason off-protocol Comments |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 55/F | 0 | 100/20 | 9 | 5 | Breasta | SD | −10 | Withdrew consent b/c of fatigue, neuropathy |
| 5 | 61/M | 1 | 100/25 | 1 | 4 | Ocular melanoma | PR | −38 | PD |
| 10 | 27/M | 0 | 100/30 | 0 | 4 | Ocular melanoma | SD | −15 | Lost to follow-up |
| 17 | 63/F | 1 | 100/35 | 9 | 4 | Breast | SD | −22 | Withdrew consent b/c of N/V, weight loss |
| 22 | 50/F | 0 | 125/35 | 11 | 4 | Breast | PR | −42 | PD |
| 23 | 54/F | 1 | 100/35 | 8 | 6 | Breast | SD | −13 | PD |
| 24 | 51/F | 1 | 100/35 | 8 | 4 | Breast | PR | −51 | Withdrew consent b/c of need for hospitalization |
| 25 | 57/F | 1 | 100/35 | 4 | 6 | Breast | PR | −44 | PD |
| 28 | 15/F | 1 | 100/35 | 3 | 4 | Hepatocellular | SD | −5 | Further assessment for resection/liver transplant |
| 31 | 61/F | 1 | 100/35 | 6 | 4+ | Breast | SD | −10 | N/A |
| 32 | 40/F | 1 | 100/35 | 5 | 4+ | Breast | SD | −9 | N/A |
N/A non-applicable, PD progressive disease, PR partial response, PS performance status, SD stable disease
Following discontinuation of HAI of cisplatin and IV liposomal doxorubicin because of poor tolerance, the patient was treated with low-dose vinorelbine and trastuzumab every other week for 5 months. Her neuropathy worsened, and subsequently resection of liver metastases was attempted, but the tumor involved major vascular structures, and therefore resection of metastases was not possible
Fig. 1.
a Best response by RECIST to hepatic arterial infusion of cisplatin and intravenous doxil in 24 patients evaluable for response. Each red box indicates a patient with maximum response a partial response (≥30% reduction in tumor size) (n = 4), each blue box indicates a patient with stable disease (maximum response between 29% reduction in tumor size to increase by 19% in tumor size) (n = 11) and each black box indicates a patient with progressive disease (≥20% increase in tumor size) (n = 9). b Kaplan–Meier plot for overall survival. c Kaplan–Meier plot for progression-free survival
Survival and failure-free survival
With a median follow-up of 16.3 months, 20 of 30 patients have died. The median overall survival was 7.4 months (95%CI: 5.3–21.6 months; Fig. 1a). The median overall survival in patients with breast cancer was 8.5 months (95%CI: 5.5–22+ months) compared with 5.3 months (95%CI: 3.8–22 months) in patients with types of cancer other than breast cancer (p = 0.13).
Twenty-five patients had progressive disease. The median progression-free survival was 3.7 months (95%CI: 2.9–5.0 months; Fig. 1c). The median progression-free survival in patients with breast cancer was 5.0 months (95%CI: 3.8–10+ months) compared to 2.9 months (95%CI: 2.2–4.9 months) in patients with types of cancer other than breast cancer (p = 0.009).
Discussion
Our study demonstrated that the MTD of HAI cisplatin in combination with systemic liposomal doxorubicin was 100 mg/m2 and 35 mg/m2, respectively. The regimen was well tolerated, and the most common toxicities were nausea/vomiting and fatigue. PRs were noted in 4 (17%) patients (breast cancer, n = 3; ocular melanoma, n = 1) and 29% of patients had SD. At the MTD, the rates of PR and SD were 17 and 42%, respectively.
Hepatotoxicity, previously reported with HAI of chemotherapy, including biliary sclerosis, was reported in earlier trials in 6–25% of patients treated with FUDR [17] but was not observed in our study, probably because of premedication with corticosteroids to prevent toxicity.
Other investigators have demonstrated that HAI of cisplatin and anthracycline-containing regimens has been associated with favorable clinical outcomes in patients with hepatocellular carcinoma [18–23], unresectable biliary tract cancer [24–26], and metastatic cholangiocarcinoma [27] and advanced gastric cancer [28]. In patients with hepatocellular carcinoma, the reported response rate ranged from 21 to 53%, and the median survival was >1 year [18, 19, 21–23]. In biliary tract cancer, the response rates were 32–40% [24–27], and the median survival ranged from 13 to 18 months [24–27]. Results of this treatment modality in colorectal cancer were disappointing [29].
Keeping in mind that in our clinical trial, the median number of prior therapies per patient was 5 therapies, the response rates are encouraging. An intriguing finding in our study was that among patients with breast cancer, 3 (27%) patients had a PR and 5 (45%) patients had SD for ≥4 months. Although the number of patients with breast cancer was small (n = 11), our results suggest that this treatment modality should be further investigated in Phase II clinical trials for patients with advanced breast cancer and dominant liver metastases. Interestingly, antitumor activity was also noted in 2 of 4 patients with ocular melanoma (PR, 1; and SD, 1) and in 1 patient with hepatocellular carcinoma.
In conclusion, the antitumor activity of HAI of cisplatin and systemic liposomal doxorubicin in patients with advanced breast cancer, ocular melanoma, and hepatocellular carcinoma with dominant liver metastases suggests that this treatment modality should be further explored, particularly in breast cancer.
Acknowledgments
Supported in part by Grant Number RR024148 from the National Center for Research Resources, a component of the NIH Roadmap for Medical Research (http://nihroadmap.nih.gov/clinicalresearch/overview).
Contributor Information
Apostolia M. Tsimberidou, Email: atsimber@mdanderson.org, Phase I Program, Department of Investigational Cancer, Therapeutics, Unit 455, The University of Texas, M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030, USA
Stacy Moulder, Phase I Program, Department of Investigational Cancer, Therapeutics, Unit 455, The University of Texas, M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030, USA.
Siqing Fu, Phase I Program, Department of Investigational Cancer, Therapeutics, Unit 455, The University of Texas, M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030, USA.
Sijin Wen, Department of Biostatistics, M. D. Anderson Cancer Center, Houston, TX, USA.
Aung Naing, Phase I Program, Department of Investigational Cancer, Therapeutics, Unit 455, The University of Texas, M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030, USA.
Agop Y. Bedikian, Department of Melanoma, M. D. Anderson Cancer Center, Houston, TX, USA
Shawn Daring, Phase I Program, Department of Investigational Cancer, Therapeutics, Unit 455, The University of Texas, M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030, USA.
Cynthia Uehara, Phase I Program, Department of Investigational Cancer, Therapeutics, Unit 455, The University of Texas, M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030, USA.
Chaan Ng, Department of Diagnostic Radiology, M. D. Anderson Cancer Center, Houston, TX, USA.
Michael Wallace, Department of Interventional Radiology, M. D. Anderson Cancer Center, Houston, TX, USA.
Luis Camacho, Oncology Consultants, P. A., Houston, TX, USA.
Razelle Kurzrock, Phase I Program, Department of Investigational Cancer, Therapeutics, Unit 455, The University of Texas, M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030, USA.
References
- 1.Power DG, Healey-Bird BR, Kemeny NE. Regional chemotherapy for liver-limited metastatic colorectal cancer. Clin Colorectal Cancer. 2008;7:247–259. doi: 10.3816/CCC.2008.n.032. [DOI] [PubMed] [Google Scholar]
- 2.Khatri VP, Chee KG, Petrelli NJ. Modern multimodality approach to hepatic colorectal metastases: solutions and controversies. Surg Oncol. 2007;16:71–83. doi: 10.1016/j.suronc.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Pawlik TM, Choti MA. Surgical therapy for colorectal metastases to the liver. J Gastrointest Surg. 2007;11:1057–1077. doi: 10.1007/s11605-006-0061-3. [DOI] [PubMed] [Google Scholar]
- 4.Bedikian AY, Kantarjian H, Young SE, et al. Prognosis in metastatic choroidal melanoma. South Med J. 1981;74:574–577. doi: 10.1097/00007611-198105000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Bedikian AY. Metastatic uveal melanoma therapy: current options. Int Ophthalmol Clin. 2006;46:151–166. doi: 10.1097/01.iio.0000195852.08453.de. [DOI] [PubMed] [Google Scholar]
- 6.Curley SA, Izzo F, Abdalla E, et al. Surgical treatment of colorectal cancer metastasis. Cancer Metastasis Rev. 2004;23:165–182. doi: 10.1023/a:1025875332255. [DOI] [PubMed] [Google Scholar]
- 7.Ragnhammar P, Hafstrom L, Nygren P, et al. A systematic overview of chemotherapy effects in colorectal cancer. Acta Oncol. 2001;40:282–308. doi: 10.1080/02841860151116367. [DOI] [PubMed] [Google Scholar]
- 8.Safi F, Bittner R, Roscher R, et al. Regional chemotherapy for hepatic metastases of colorectal carcinoma (continuous intraarterial versus continuous intraarterial/intravenous therapy). Results of a controlled clinical trial. Cancer. 1989;64:379–387. doi: 10.1002/1097-0142(19890715)64:2<379::aid-cncr2820640207>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Kelly H, Goldberg RM. Systemic therapy for metastatic colorectal cancer: current options, current evidence. J Clin Oncol. 2005;23:4553–4560. doi: 10.1200/JCO.2005.17.749. [DOI] [PubMed] [Google Scholar]
- 10.Barber FD, Mavligit G, Kurzrock R. Hepatic arterial infusion chemotherapy for metastatic colorectal cancer: a concise overview. Cancer Treat Rev. 2004;30:425–436. doi: 10.1016/j.ctrv.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Kemeny N, Fata F. Hepatic-arterial chemotherapy. Lancet Oncol. 2001;2:418–428. doi: 10.1016/S1470-2045(00)00419-8. [DOI] [PubMed] [Google Scholar]
- 12.Feun LG, Reddy KR, Yrizarry JM, et al. A phase I study of chemoembolization with cisplatin and lipiodol for primary and metastatic liver cancer. Am J Clin Oncol. 1994;17:405–410. doi: 10.1097/00000421-199410000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Lyass O, Hubert A, Gabizon AA. Phase I study of doxil-cisplatin combination chemotherapy in patients with advanced malignancies. Clin Cancer Res. 2001;7:3040–3046. [PubMed] [Google Scholar]
- 14.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 15.Ratain MJ, Eisen T, Stadler WM, et al. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:2505–2512. doi: 10.1200/JCO.2005.03.6723. [DOI] [PubMed] [Google Scholar]
- 16.http://ctep.cancer.gov/
- 17.Kemeny NE, Ron IG. Hepatic arterial chemotherapy in metastatic colorectal patients. Semin Oncol. 1999;26:524–535. [PubMed] [Google Scholar]
- 18.Jang JW, Bae SH, Choi JY, et al. A combination therapy with transarterial chemo-lipiodolization and systemic chemo-infusion for large extensive hepatocellular carcinoma invading portal vein in comparison with conservative management. Cancer Chemother Pharmacol. 2007;59:9–15. doi: 10.1007/s00280-006-0239-0. [DOI] [PubMed] [Google Scholar]
- 19.Jang JW, Park YM, Bae SH, et al. Therapeutic effcacy of multimodal combination therapy using transcatheter arterial infusion of epirubicin and cisplatin, systemic infusion of 5-fluorouracil, and additional percutaneous ethanol injection for unresectable hepatocellular carcinoma. Cancer Chemother Pharmacol. 2004;54:415–420. doi: 10.1007/s00280-004-0829-7. [DOI] [PubMed] [Google Scholar]
- 20.Sumie S, Yamashita F, Ando E, et al. Interventional radiology for advanced hepatocellular carcinoma: comparison of hepatic artery infusion chemotherapy and transcatheter arterial lipiodol chemoembolization. AJR Am J Roentgenol. 2003;181:1327–1334. doi: 10.2214/ajr.181.5.1811327. [DOI] [PubMed] [Google Scholar]
- 21.Patt YZ, Charnsangavej C, Yoffe B, et al. Hepatic arterial infusion of floxuridine, leucovorin, doxorubicin, and cisplatin for hepatocellular carcinoma: effects of hepatitis B and C viral infection on drug toxicity and patient survival. J Clin Oncol. 1994;12:1204–1211. doi: 10.1200/JCO.1994.12.6.1204. [DOI] [PubMed] [Google Scholar]
- 22.Yodono H, Takekawa SD, Tarusawa K, et al. Combination therapy consisting of arterial infusion chemotherapy (EPF, EAP) and transcatheter arterial embolization (TAE) Cancer Chemother Pharmacol. 1994;33 (Suppl):S79–S83. doi: 10.1007/BF00686673. [DOI] [PubMed] [Google Scholar]
- 23.Yodono H, Sasaki T, Tarusawa K, et al. Arterial infusion chemotherapy for advanced hepatocellular carcinoma using EPF and EAP therapies. Cancer Chemother Pharmacol. 1992;31 (Suppl):S89–S92. doi: 10.1007/BF00687114. [DOI] [PubMed] [Google Scholar]
- 24.Cantore M, Fiorentini G, Mambrini A, et al. Regional combined with systemic chemotherapy in unresectable biliary tract cancers: a phase II study. J Exp Clin Cancer Res. 2003;22:59–64. [PubMed] [Google Scholar]
- 25.Cantore M, Mambrini A, Fiorentini G, et al. Phase II study of hepatic intraarterial epirubicin and cisplatin, with systemic 5-fluorouracil in patients with unresectable biliary tract tumors. Cancer. 2005;103:1402–1407. doi: 10.1002/cncr.20964. [DOI] [PubMed] [Google Scholar]
- 26.Mambrini A, Guglielmi A, Pacetti P, et al. Capecitabine plus hepatic intra-arterial epirubicin and cisplatin in unresectable biliary cancer: a phase II study. Anticancer Res. 2007;27:3009–3013. [PubMed] [Google Scholar]
- 27.Cantore M, Rabbi C, Guadagni S, et al. Intra-arterial hepatic chemotherapy combined with continuous infusion of 5-fluorouracil in patients with metastatic cholangiocarcinoma. Ann Oncol. 2002;13:1687–1688. doi: 10.1093/annonc/mdf262. [DOI] [PubMed] [Google Scholar]
- 28.Iida T, Hirata N, Hirakawa M, et al. Preoperative intraarterial infusion chemotherapy for advanced gastric cancer—a retrospective review of four cases. Radiat Med. 2003;21:172–177. [PubMed] [Google Scholar]
- 29.Leichman CG, Jacobson JR, Modiano M, et al. Hepatic chemoembolization combined with systemic infusion of 5-fluorouracil and bolus leucovorin for patients with metastatic colorectal carcinoma: a Southwest Oncology Group pilot trial. Cancer. 1999;86:775–781. [PubMed] [Google Scholar]