Abstract
Regulation of immune responses to self and foreign antigens is critically dependent on suppressive CD4+ T cells characterized by expression of Foxp3. The large majority of regulatory T (Treg) cells develop in the thymus as a stable suppressive lineage. However, under the proper physiological conditions, conventional peripheral CD4+ T lymphocytes also develop into Treg cells, particularly in the gut mucosa and inflammatory tissue sites. This review will focus on our current understanding of the immunological and molecular signals controlling the development of thymic derived natural (n)Treg and peripheral converted induced (i)Treg cells. Given the importance of Foxp3 in the development of these cells, particular attention is placed on how such signals are integrated to induce and maintain the expression of this signature transcriptional regulator of Treg cells.
Introduction
CD4+ Foxp3+ Treg cells are a dedicated population of cells that maintain self-tolerance and immune homeostasis. Besides suppressing autoreactive T cells, Treg cells help regulate the magnitude of immune responses to infectious agents and tumors. The transcriptional regulator Foxp3 has been recognized as a lineage-specific marker of Treg cells [1, 2]. The essential role for Foxp3 in Treg development has been shown in animal models and in humans that express mutations in the Foxp3 gene [3–8]. In these cases Treg cells are not produced and lethal autoimmunity ensues. Furthermore, Foxp3 must be induced for Treg development and its expression is actively maintained in mature Treg cells for their suppressive function [7–11].
Natural occurring Treg (nTreg) cells develop within the thymus after expression of Foxp3 at a relatively late stage of thymopoiesis that is primarily confined to “single positive” (SP) CD4+ T cells. nTreg cells represent a minor population of thymocytes, roughly 4% of the SP CD4+ cells [12]. TCR, co-stimulatory, and IL-2 signals are required for thymic development of Treg cells. After exiting the thymus, nTreg cells are shaped by basal environmental cues and inflammatory responses that regulate their suppressive program, migration and homeostasis [13].
Foxp3 can also be expressed by conventional T cells in the periphery to generate suppressive induced Treg (iTreg) cells. These cells have been implicated in maintaining tolerance in tissues sites and to food antigens and commensal bacteria within the gut mucosa. The overall contribution of iTreg cells to the total pool of peripheral Treg cells under basal and inflammatory conditions remains under debate. TCR repertoire analyses of peripheral Treg cells in lymphoid tissues have been estimated to be from 5–20% of all Treg cells [14, 15]. However, the extent these cells might dominate the Treg pool within tissues at the site of immune responses remains unknown.
It should be noted that the in the mouse the detection of Foxp3 is usually synonymous with a cells being a Treg. One exception is that low levels of Foxp3 are not sufficient to direct the Treg suppressive program, but this has only been noted under experimental settings [11, 16]. Thus, in the mouse, Foxp3 is a reliable marker for functionally suppressive Treg cells. In man, however, Foxp3 is also readily seen by a subpopulation of T effectors cells. Thus, detection of Foxp3 in human T cells does not rigorously identify Treg cells. Typically a combination of markers that comprise Foxp3, CD25, CD127, and CD45RO and CD45RA are required for more definitive identification of human Treg cells [17].
In this review, we will discuss recent advances in investigating the factors and mechanisms involved in Treg development and lineage stability. We will focus our attention primarily on mouse Foxp3+ Treg cells as the factors controlling their development have been relatively well described. We will not cover other suppressive T cell populations such as IL-10 producing Tr1 cells or TGF-β-producing Th3 cells.
Thymic development of nTreg cells
The development of Treg cells occurs during a late stage of thymopoiesis as Foxp3 expression is noted primarily in SP CD4+ cells. A few Foxp3+ thymocytes are also detected in “double positive” CD4+ CD8+ cells. However, most of these represent doublets on FACS analysis consisting of a CD4+ CD8+ Foxp3neg and a CD4+ Foxp3+ cell [18]. The most proximal precursor to Foxp3+ Treg cells is a CD4+ CD25+ Foxp3neg thymocyte that under the proper conditions further matures into a Foxp3+ Treg cell that expresses suppressive function [19, 20]. Whether other attributes of Treg cells are acquired in thymocytes that precede these developmental steps remains to be determined, although some suggestive data support this view (discussed below). Consistent with later commitment to the Treg lineage, nTreg cells are preferentially found in the thymic medulla where negative selection usually takes place [12]. Importantly, altered architecture of the thymic medulla is found in mice expressing mutant NF-κB inducing kinase or lacking TNF-associated receptor 6 and Treg development is impaired [21, 22]. Although most Treg development occurs within the medulla, cortical epithelial cells support development of a few Foxp3+ thymocytes [23–25], raising the possibility that Treg development may also be initiated for a few Treg cells in the thymic cortex.
Immunological signaling for nTreg development
TCR requirements
Engagement of the TCR is essential for nTreg development. Treg development requires signaling through the TCR at a level greater than required for positive selection. This was shown when MHC class II-restricted transgenic TCRs were expressed in the Rag2-deficient background where positive selection resulted in development of conventional CD4+ SP thymocytes, but not Treg cells [26]. Self-antigens, nevertheless, drive nTreg development through TCR interactions but at an affinity higher than that required for positive selection. This was strikingly shown in a TCR transgenic system where Treg development was preferred when the TCR and its cognate antigen, which support a high affinity interaction, were co-expressed within the thymus. In contrast, few Treg cells developed when their TCR encounters a mutated cognate antigen with a lower affinity [27–29]. Consistent with increased avidity for self-antigens, enforced expression of a nTreg TCR in conventional T cells enhanced their expansion and autoimmune potential after transfer into lymphopenic recipients [30].
Two general models have been proposed to account for these types of findings. Model 1 suggests that nTreg cells are rescued from negative selection, perhaps by expression of Foxp3 or other molecules. This notion is supported by the observation that nTreg frequency, but not numbers, increased upon encountering increasing levels of cognate antigen within a certain range while conventional CD4+ SP thymocytes underwent massive deletion [31]. Model 2 suggests that precursor cells chose to develop into Treg or T conventional cells based on thymic cues and TCR affinity [27–29]. For Model 1, the TCR repertoire of Treg and T conventional cells may overlap whereas for Model 2 Treg and conventional CD4+ T cells likely express unrelated TCR repertoires.
Current data suggest that Model 1 or 2 does not individually readily explain Treg development. Direct TCR sequencing of TCRs from Treg and conventional CD4+ SP thymocytes do not definitively distinguish between these models. These studies reveal that TCR repertories between these two populations are largely distinct, supporting Model 2, but with some obvious overlap, supporting Model 1[15, 30, 32]. The TCR repertoire expressed by activated CD4+ T cells from Foxp3-deficient mice readily shares specificities with Treg cells in the corresponding wild-type background [33]. This observation is not consistent with Model 1 as the overlapping TCRs in Foxp3-deficient mice are expected to be deleted through negative selection. In addition, mice that express a transgenic TCR isolated from nTreg cells readily support development of conventional CD4+ SP T cells rather than nTreg cells [34, 35]. This finding indicates that nTreg TCRs are readily expressed on conventional T cells and argues against Model 2. However, the same transgenic TCR from nTreg cells favors the generation of nTreg cells when present at a low frequency suggesting that there is a limited niche or resources instructing T cells to develop into Treg cells [34, 35]. Collectively, all these data are consistent with a model, which contain aspects of Models 1 and 2, where developing thymocytes must express a TCR with an affinity for self-peptide higher than that required for positive selection, but usually lower than that leading to negative selection. These T cells must then receive key instructive signals, which are limiting, to adopt a Treg cell fate.
Co-stimulatory requirements
Another important signal involved in Treg development is co-stimulatory signaling through CD28 that at least partially depends on the Lck binding motif in its cytoplasmic tail [36, 37]. CD28- and B7-deficient mice contain a substantial reduction in nTreg cells in the thymus and peripheral immune tissues [36, 38]. One interpretation from these experiments is that impaired CD28 co-stimulation leads to lower IL-2 that is responsible for impaired nTreg development and homeostasis. Expression of active STAT5, a target of IL-2R signaling, in CD28−/− mice improved thymic development of Treg cells, consistent with co-stimulation providing IL-2 for Treg production [19]. However, elegant mixed bone marrow chimera experiments demonstrated that thymocytes originating from CD28−/− bone marrow-derived precursors failed to generate Treg cells even when IL-2 was available from T cells derived from WT precursor cells [36]. This result suggests that CD28 provides an intrinsic signal for Treg development, beyond its possible contribution to optimize IL-2 production. Indeed, CD28−/− mice contain lower number of Treg precursors and those present are unresponsive to IL-2, consistent with a direct role of CD28 in Treg development [37]. Thus, along with promoting IL-2 production, another role for CD28 signaling may be to improve cytokine responsiveness of developing Treg cells.
Requirement for IL-2
Mice deficient in IL-2, IL-2Rα or IL-2Rβ are characterized by extensive lympho-proliferation and die of severe lethal autoimmunity early in life [39–41]. These abnormalities are readily accounted for by failed development and homeostasis of nTreg cells [42–44]. This syndrome is very analogous to autoimmunity associated with Foxp3-deficienct mice, although mice without IL-2R signaling live somewhat longer probably due to attenuated effector responses by IL-2 non-responsive autoreactive T cells. Thus, IL-2R signaling represents another essential signal for nTreg development.
In the absence of IL-2R signaling, mice contain a reduced number of immature thymic nTreg cells that essentially lack expression of CD25 and express reduced levels of Foxp3 [16, 43]. Recent work supports a two-step model of thymic Treg development [19, 20]. TCR engagement and co-stimulatory signals confer Foxp3neg Treg precursors to respond to IL-2 that matures these precursors by upregulating Foxp3 and CD25. Besides these activities, it is commonly believed that IL-2 promotes the growth and survival of developing Treg cells. However, when Bim-deficiency was crossed onto IL-2-deficient mice, Treg numbers were corrected by limiting apoptosis, but autoimmunity still resulted [45]. These data support a model where IL-2R signaling is critical for functional maturation of nTreg cells.
Although IL-2/IL-2R-deficient mice contain Foxp3lo immature Treg cells, γc-deficient mice are devoid of Foxp3+ T cells [44, 46]. Double knockout mice that cannot support IL-2 and IL-7 signaling recapitulate the γc phenotype, i.e. no Foxp3+ T cells [47, 48], indicating a contribution by IL-7 in Treg development. However, the role of IL-7 remains unknown, although it has been proposed to act much earlier than IL -2, i.e. at the “double-negative” stage of thymic development [49]. In vitro, among γc-dependent cytokines, only IL-2, IL-7 and IL-15, transduce signals in thymic Treg cells [48] and only IL-2 and IL-15 support maturation of CD4+CD25+Foxp3neg Treg precursor cells into Treg cells [20]. However, in vivo, mice singly or doubly deficient in responsiveness to IL-7 and IL-15 contain a normal proportion of mature Treg cells [47]. Furthermore, transgenic expression of IL-2Rβ in thymocytes of IL-2Rβ and IL7Rα double knockout mice fully restore nTreg development [47]. Thymic stromal lymphopoeitin (TSLP) is a cytokine that utilizes IL-7Rα as one subunit of the TSLPR [50, 51]. However, TSLP did not support mouse thymic progenitors to developing into Treg cells in vitro [48] and no defects in Treg development were noted in TSLPR−/− mice [52], indicating that TSLP is not an essential non-redundant cytokine during Treg development. Collectively, these findings indicate that IL-7, IL-15 and TSLP are dispensable for mouse Treg development and firmly establish that IL-2 is the dominant γc-dependent cytokine for nTreg development. However, it remains possible that TSLP is active in the human thymus for Treg cells. TSLP is produced by the epithelial cells of the Hassall’s corpuscles and has been implicated to promote the conversion of human CD4+ Foxp3− precursors to Foxp3+ Treg cells in DC dependent manner [53, 54].
Contribution by TGF-β
TGF-β has also been implicated in nTreg development, but its role remains somewhat controversial. Similar to Foxp3- and IL-2-deficient mice, TGF-β1−/− mice also die of severe autoimmunity, consistent with an important role for this cytokine in immune tolerance [55]. Aspects of this disease are attributed in part to defects in Treg cells [56]. Assigning a role for TGF-β1 in nTreg development is complicated as it is an important mediator of Treg suppression [56]. Thymic numbers of Treg cells are normal in 8–10 day old TGF-β1- and TGF-βRII-deficienct mice [56–58]. Additionally, the development of Foxp3+ Treg cells from Foxp3neg precursors was unaffected by addition or blockage of TGF-β [19, 20]. These findings raise the possibility that TGF-β is not involved in thymic Treg development.
In mice lacking TGF-βRI in T lineage cells, thymic Treg cells are significantly decreased in 3 to 5 day old neonatal mice, during the initial generation of nTreg cells. After 1 week, these limited numbers of Treg cells rapidly proliferate primarily by IL-2, yielding a normal compartment of thymic Treg cells [59]. In other experiments, neonatal mice with T cell-specific deletion of TGF-βRII showed increased negative selection and decreased nTreg development. The lower number of Treg cells was attributed to enhanced apoptosis through increased expression of pro-apoptotic Bim, Bak and Bax and lower pro-survival Bcl2 . Correspondingly, deletion of Bim in TGF-βRII−/− mice increased the number of nTreg cells [60]. Collectively, these findings suggest that TGF-β provides survival signals during early Treg development rather than to drive Treg lineage commitment.
Key signaling pathways in nTreg development
Proximal TCR signaling pathway
It is essential to elucidate how the aforementioned immune molecules induce signals that contribute to Foxp3 expression, the signature transcription factor in Treg cells. Figure 1 depicts the main pathways active in nTreg and iTreg with respect to Foxp3 expression and these are each discussed more fully below. Followed by TCR and co-stimulatory signal transduction, several transcription factors are activated, such as NF-κB, NFAT, and AP1, which subsequently bind to the Foxp3 promoter or enhancer regions and directly contribute to Foxp3 expression. Not surprisingly, impaired TCR signaling affects Treg production. For example, mutation of the C-terminal SH2-domain of Zap-70, resulted in autoimmunity through altered thymic selection that increased self-reactive T cells and decreased nTreg production [61, 62]. Furthermore, the absence of PLC-γ1, another mediator of TCR signaling, markedly reduced thymic Treg and T conventional cells [63]. These findings indicate that key intermediates of TCR signaling are involved in signal transduction for both Treg and T conventional cells.
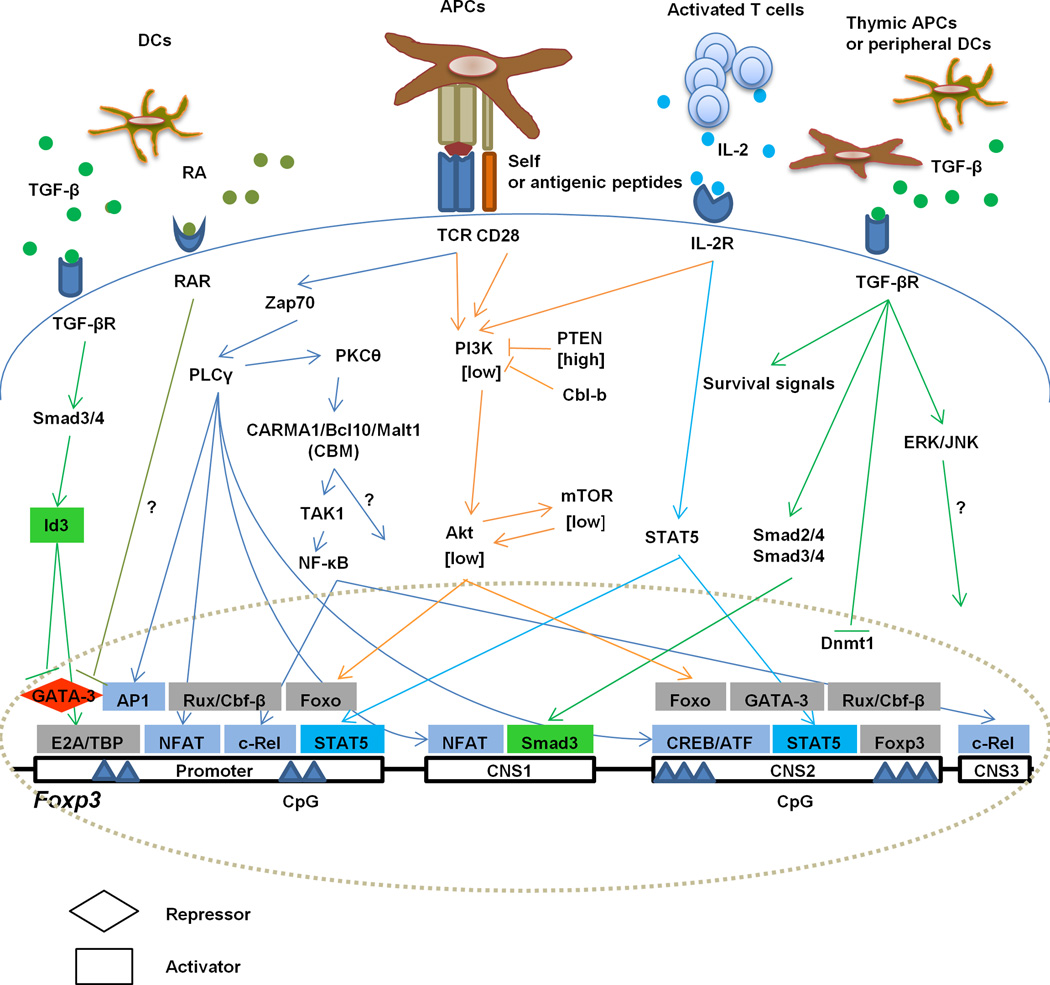
Fig. 1.
Molecular pathways for Treg development and stability. Both nTreg and iTreg cells require TCR activation and cytokines for their development. TCR engagement initiates signaling when nTreg precursor cells recognize self-peptides in the thymus or peripheral CD4+ T are activated by foreign antigens, respectively. IL-2R signaling depends on IL-2 secreted by thymocytes undergoing selection or by activated T cells in the periphery. TGF-β provides survival signals for nTreg cells but in conjunction with RA drives iTreg development. Foxp3 transcription depends on DNA binding factors to the promoter and to 3 CNSs. CNS1 is responsible for iTreg induction. CNS2 regulates the heritable expression of Foxp3 and contributes to the stability of the Treg cells. CNS3 is essential for nTreg development. Shown are TCR and cytokine-dependent signaling pathways and transcription factors that are activated to directly regulate Foxp3 transcription.
PI3-Akt-mTOR pathway
An important point is the extent signaling varies in Treg vs. T conventional cells. In this regard, the PI3K/Akt pathway is differentially activated. The TCR, CD28, and IL-2 all readily activate the PI3K pathway in conventional T cells, but this pathway is attenuated in nTreg cells [64, 65]. For example, expression of active Akt in thymocytes did not affect the development of conventional T cells, but impaired nTreg cells [66].Conversely, blockade of the PI3K pathway with LY294002 or rapamycin promoted Treg cell maturation [67]. Thus, relatively low PI3K/Akt activation is an important determinant for development into Treg cells.
Diminished activation of the PI3K pathway appears to be important to maintain active levels of Foxo1 and Foxo3. These transcription factors bind to the Foxp3 promoter and conserved non-coding sequence (CNS)-2 to facilitate Foxp3 expression [68]. When the PI3K pathway is active, Foxo1 and Foxo3 are phosphorylated, which represent inactive forms that do not support Foxp3 transcription. Importantly, T cell-specific deletion of Foxo1 leads to autoimmune disorders that are associated with enhanced Teff function and diminished Treg development and function [69, 70]. More aggressive autoimmunity occurs in mice lacking both Foxo1 and Foxo3, including a more striking reduction in Treg cells [68, 70]. The lack of Foxo1 and Foxo3 also increases Th1 and Th17 development, suggesting that these factors also constrain effector responses.
NF-κB pathway and C-rel
The lack of other mediators of TCR signaling, such as PKCθ, CARMA1, Bcl10 or TAK1, preferentially impairs Treg production [71–74]. The CARMA1/Bcl10/Malt1 (CBM) complex is required for NF-κB activation. CARMA1-deficient mice lack Foxp3 nTreg cells and their precursor cells [75, 76]. Expression of active STAT5 or Bcl-2 in CARMA1-deficient thymocytes does not rescue nTreg development, implying that the main role of the CBM complex is independent of promoting cytokine responsiveness or providing survival signals [75]. Thus, the CMB complex appears to be required for an early step in Treg development.
Constitutively active NF-κB rescues Treg development in TAK1- or CARMA1-deficienct mice [77], linking activation of CARMA1 to the NF-κB pathway. However, Treg development is not rescued in CARMA1/CYLD-double deficient mice, where the lack of the deubiquitinase CYLD activates NF-κB through TAK1/IKK. In an analogous manner, blockade of canonical pathway of NF-κB activation by transgenic expression of a trans-dominant form of IκBα only partially reduces nTreg numbers [78]. Overall, these findings imply that CARMA1 regulates Treg development only in part through NF-κB. Notably, c-Rel directly binds to the Foxp3 promoter and CNS3, indicating a direct role of NF-κB activation in Treg development [77, 79, 80]. Importantly, deletion of CNS3 inhibits Foxp3 expression during nTreg development, illustrating the importance of transcriptional regulators targeted to this element [80]. Whether Rel A also directly regulates Foxp3 is controversial [77, 79–81].
NF-AT and AP-1 pathway
TCR signaling also activates NFAT and AP1 through binding to the Foxp3 promoter [82], implicating these molecules in Treg development. Consistent with this view, cyclosporine A, which blocks the calcineurin pathway and NF-AT, but not MAPK inhibitors that affects AP-1 , lowers Foxp3 expression in Treg cells [82]. Thus, NFAT may be required while AP-1 may be dispensable for Treg production.
IL-2-STAT5 axis
IL-2R signaling activates a number of pathways in activated T cells, including the MAPK, PI3K and the STAT5 pathways [83]. However, STAT5 activation is the main IL-2R-dependent pathway active in Treg cell as the PI3K pathway is inhibited by high PTEN levels in Treg cells [64, 65]. The importance of STAT5 in Treg cells is directly shown by manipulating this pathway in the context of absent IL-2R signaling. Mice deficient in STAT5 exhibit a substantial decrease of Treg cells and largely recapitulate the phenotype of IL-2- and IL-2R-deficient mice [46, 84]. Furthermore, transgenic expression of active STAT5b in the thymus of IL-2Rβ-deficient mice reconstituted Foxp3+ thymic Treg cells and rescued Treg developmental defects associated with impaired IL-2R signaling [46]. At the molecular level, STAT5 directly regulates Foxp3 expression by binding to the Foxp3 promoter and CNS2 [46, 80]. When thymic CD4+CD25+CD122+ Foxp3neg Treg precursor cells are stimulated with IL-2, STAT5 is activated in most cells but Foxp3 is expressed in only 50% of the cells [20]. Thus, some Treg precursors require signaling beyond IL-2, probably through TCR and CD28, for their maturation. This likely reflects heterogeneity in Treg precursor population.
In vivo the quantitative level of IL-2R signaling that leads to Treg maturation is set at a low threshold. Indeed mice that express mutant IL-2Rβ with attenuated IL-2R-dependent STAT5 activation readily support Treg development, leading to a population of mature Treg cells that exhibit substantial suppressive activity against autoreactive T cells [85]. The ability of developing Treg cells to productively sense low IL-2 is likely important as IL-2 producing cells are rare in the thymus. This exquisite sensitivity of Treg precursors to minimal IL-2R signaling ensures that key molecules of mature Treg cells, such as Foxp3, CD25, CTLA4, and TGF-β, are readily expressed. Nevertheless, a substantial portion of the Treg gene program remains IL-2 dependent under low IL-2R signaling including several functional intermediates, such as IL-10 and granzyme B [85], representing a risk for autoimmunity.
De novo development of iTreg cells in vitro and in vivo
With the emergence of Foxp3 as a reliable marker of Treg cells in the mouse, substantial data demonstrate that conventional peripheral CD4+ T cells develop into suppressive Foxp3+ T cells. Naïve CD4+ Foxp3neg T cells readily develop into suppressive Foxp3+ iTreg cells after culture with anti-CD3, anti-CD28, TGF-β and IL-2 [86]. Administration of nominal antigen in a tolerogenic form to mice that contained CD4+ T cells, which expressed the respective antigen-specific MHC class II-restricted transgenic TCR, also induces suppressive Foxp3+ iTreg cells [87–89]. The use of TCR transgenic T cells on Rag2−/− genetic background, which completely lack nTreg cells, ensures that iTreg cells are not derived by contaminating nTreg cells. In addition, some naïve conventional CD4+T cells convert into iTreg cells after transfer into lymphopenic hosts [90–92].
Compared to nTreg, which are exclusively generated in the thymus, iTreg development seems more versatile. iTreg cells are thought to be an important population in the gut mucosa to maintain tolerance to commensal flora and food antigens [93–95]. In several other physiological setting, iTreg cells are also found, including transplanted tissue allografts, within the tumor microenvironment, and chronic inflammatory sites [96–98]. In all these cases, iTreg cells are thought to be antigen-specific and in many cases mediate beneficial effects to limit tissue rejection, inflammation, and immune responses but sometime are detrimental by interfering with immune responses to chronic infections or tumors.
Factors influencing iTreg development
TCR stimulation
Many of the same stimuli that shape nTreg development also contribute to iTreg development. Since TCRs of nTreg are stringently selected, an important question is whether TCR stimulation differs during iTreg generation. Low antigen dose favors iTreg conversion whereas high level of antigen activates conventional Teff cells [88, 99]. Interestingly, a low affinity peptide agonist poorly generates iTreg cells while a low dose of a high affinity agonist peptide supports iTreg cells production. However, decreasing the cumulative TCR stimulation of the low affinity agonist significantly increases iTreg conversion [100]. Collectively, these findings indicate that the strength of TCR acts as a checkpoint to control peripheral Foxp3 induction that is favored by lower occupancy of the TCR than required for Teff development.
Weak TCR signaling favors iTreg conversion through low activation of PI3K/mTOR [66, 67]. Furthermore, the development of Th1, Th2 and Th17 effector cells is not preferred by low mTOR signaling because responsiveness to inflammatory cytokines required for activation of key STATs is impaired [101]. Reciprocally, in the absence of the E3 ligase Cbl-b, PI3K/Akt activation increases and iTreg production decreases largely through an increased level of phosphorylated Foxo proteins [102]. Thus, low TCR-dependent signaling that favors iTreg cells utilizes a mechanism similar to that discussed above for nTreg development.
Co-stimulatory signaling
For iTreg development CD28 functions solely to promote IL-2 production [103]. Moreover, strong CD28 ligation is detrimental for iTreg development [104, 105]. Correspondingly, iTreg conversion increases when naïve T cells express CD28 containing a mutation in the Lck binding motif on its cytoplasmic tail, lowering CD28-dependent signals [106]. Blockade of CTLA4, which facilitates co-stimulatory signaling, impairs iTreg production [107]. Overall, these data suggest that TCR and co-stimulatory signaling that support iTreg and Teff development are considerably different and Treg cells are favored by lower signal transduction through these molecules.
TGF-β signaling
TGF-β signaling is essential for iTreg production in vitro and in vivo [88, 89, 108, 109]. TGF-β also supports iTreg production under conditions of high TCR signaling that normally favors Teff cells [100]. TGF-β signaling promotes NFAT and Smad3 binding to Foxp3 CNS1 enhancer, leading to histone acetylation and Foxp3 transcription [110]. TGF-β antagonizes the activity of Dnmt1 to facilitate Foxp3 induction in conventional CD4+ T cells [111]. Furthermore, deletion of CNS1 impairs iTreg but not nTreg development [80]. Thus, TGF-β-dependent signaling is directly linked to induction of Foxp3 in iTreg cells. However, blockade of Smad2 and Smad3 only partially inhibits TGF-β-induced Foxp3 induction [112], suggesting that iTreg cells may also depend upon TGF-β-dependent activation of the ERK and JNK/ MAPK pathways.
Under some circumstances peripheral conventional T cells converts into Foxp3+ iTreg cells in cultures under conditions supporting IL-2R, but not TGF-βR signaling. Peripheral CD4+ CD25+ CD62int CD69+ Foxp3neg T cells preferentially develop into iTreg cells by IL-2 [113]. This phenotype is identical to nTreg precursors and such peripheral T cells may be poised to convert into Foxp3+ Treg cells by an instructive IL-2 signal. Thus, a similar mechanism might operate to generate nTreg cells and some iTreg cells. In a related manner, the TCR repertoire iTreg cells obtained after conventional T cells were transferred into lymphopenic recipients overlapped to a greater degree than expected with nTreg cells [14]. Such a result may also represent development of a cell poised to acquire Foxp3. Thus, some iTreg cells may originate from thymic CD4+CD25+Foxp3neg precursors cells that exit the thymus and acquire Foxp3 expression and suppressive function in periphery after encountering self-antigens and IL-2 in an apparent TGF-β independent fashion. The extent the majority of iTreg cells are derived from such IL-2-poised cells remains to be determined.
IL-2-STAT5 axis
IL-2 is essential for peripheral conversion of conventional CD4+ T cells to Foxp3+ iTreg cells in vitro [86, 114]. The culture of conventional T cells with TGF-β without IL-2 does not yield iTreg cells. This function of IL-2 is non-redundant and cannot be substituted by other γc-dependent cytokines [86, 114]. Analogous to nTreg cells, the development of iTreg also depends on STAT5 activation after IL-2R signaling [85]. An important unresolved question is the extent IL-2R signaling is essential for iTreg development in vivo.
Role of retinoic acid (RA) and commensal bacteria
Besides the above immunological signals, iTreg development is influence by other physiological mediators. All-trans RA, a vitamin A metabolite, is highly expressed in the gut mucosa and enhances iTreg conversion in synergy with TGF-β [92, 105, 115, 116]. RA endows iTreg cells with a gut homing phenotype and supports iTreg generation even under strong co-stimulation [105]. CD103+ DCs, which are mainly found in the MLN and lamina propria of small intestine, enhance the conversion of naïve T cells to Foxp3+ iTreg cells through their production of TGF-β and RA [92, 116]. In peripheral lymphoid tissues, CD8+ CD205+ DCs favor iTreg development by producing TGF-β [117]. Lamina propria DCs enhance iTreg production and limit Teff differentiation in part through activation of Wnt/β-catenin pathway, which promotes enzymes required for vitamin A metabolism and inhibits expression of pro-inflammatory cytokines [118]. Thus, micro-environmental cues within the gut mucosa facilitate iTreg generation even under inflammatory conditions.
The molecular mechanism by which RA promotes Foxp3 expression has not yet been well characterized. RA readily promotes iTreg generation by antigen-experienced T cells. Memory cells synthesizing IL-4, IL-21, and IFNγ are more resistant to iTreg conversion. RA acts to relieve this inhibition, which facilitates iTreg production [119]. Much of this effect was shown to be indirectly related to the in vivo environment where iTreg conversion takes place. However, GATA-3 has been reported to be a transcriptional repressor of Foxp3 expression by directly binding its promoter [120]. Therefore, it is tempting to speculate that some the effect by which RA promotes iTreg cells might also be due to reducing GATA-3-dependent repression of Foxp3. However, regulation of Foxp3 by GATA-3 is likely complex as GATA-3 has recently been reported to also be a positive regulator of Foxp3 transcription by binding to CNS2 [121].
The gut is a favored site for iTreg development , suggesting that gut microbiota may contribute to iTreg generation. Indeed, oral tolerance is readily induced by feeding OVA in specific-pathogen free (SPF), but not germ free-mice, which is accompanied by increased numbers of Treg cells in gut-associated lymphoid tissue (GALT) in SPF mice [122]. This result suggests that gut microbiota contributes to immune tolerance in this tissue. However, the influence on both nTreg and iTreg cells by the gut flora is complex and positively or negatively impacts Treg cells depending upon its composition. For example, treatment of mice with the gut-specific antibiotic vancomycin increases the frequency of Treg cells in the small intestine of the lamina propria while Th17 cells decreases [123, 124]. Oral inoculation of neonatal mice with Clostridium species increases Foxp3+ Treg cells in colonic lamina propria [125]. Polysaccharide A produced by Bacteroides fragilis promotes peripheral iTreg conversion in GALT in a TLR2-dependent manner [126]. On the other hand, gut flora DNA signaling through TLR9 on DCs leads to increased inflammatory cytokines and limits iTreg conversion [127]. These latter experiments directly show that iTreg conversion is impacted by gut microbiota. Collectively, commensal bacteria link the innate and adaptive immune response within the gut and are essential to regulate the balance between tolerance and immunity, exhibiting both a positive and negative influence on nTreg and iTreg cells.
The inter-relationship between iTreg and Th17 lineages
There is a clear inter-relationship between the development of Th17 and iTreg cells. Both cell types share TGF-β for their production. TGF-β, acting along with IL-6 or other inflammatory mediators, supports Th17 cells [128–130]. However, TGF-β in the presence of IL-2 or RA favors iTreg cells and opposes Th17 development [92, 115, 116, 131]. The mechanism underlying this phenomenon has been associated with IL-2-dependent STAT5 signaling in limiting Th17 differentiation [131]. The cell fate choice, therefore, between iTreg and Th17 development is driven by environmental cues.
At the molecular level, the cell fate choice between Th17 and iTreg cells is in part determined by the levels of several transcriptional regulators. For example, Foxp3 has been shown to interfere with the activity of RORγt and RORα, key transcriptional regulators for Th17 development [132, 133, 134]. However, RORγt and RORα does not affect the activity Foxp3, rather STAT3 down-regulates Foxp3 [132]. In fact the ratio of STAT5:STAT3 determines whether a cell adopts an iTreg or a Th17 cell fate, the former which is supported by IL-2 and the latter by IL-6 and IL-21 [135].
TGF-β also reciprocally controls the differentiation of iTreg and Th17 through the DNA binding inhibitor, Id3, through a two-step process. First, TGF-β-induces Id3 that inhibits binding of GATA-3, a repressor of Foxp3 transcription, to the Foxp3 promoter. Second, this in turn facilitates binding of E2A to regulatory elements in Foxp3 to promote Foxp3 transcription. In the absence of Id3, therefore, naïve T cells preferentially differentiate into Th17 cells [136].
The stability of Treg cells
Another import issue is whether nTreg and iTreg cell represent stable lineages vs. the capacity to be reprogrammed into Teff cells. This has implications concerning the basis by which the immune system is regulated and is an important consideration in the application of adoptive Treg therapy to suppress unwanted immune responses. There is an emerging picture that some Treg cells lose Foxp3 expression and de-differentiates into other Teff lineages. For example, upon transfer of nTreg cells from the Peyer’s patch into lymphopenic recipients, Foxp3 was lost and these cells acquired T-helper activity to promote IgA production [137]. A small population within the CD25− Treg subset is plastic whereas the CD25+ subset stably maintains Foxp3 expression [138, 139], suggesting IL-2R signaling may function to sustain Foxp3 expression. The culture of nTreg with mAbs to CD3 and CD28, in the presence of TGF-β and IL-6, leads to the loss of Foxp3 and expression of IL-17 [132, 140]. Similarly, in the presence of TGF-β, inflammatory signals mediated by IL-6, IL-1 and IL-23 activate the Th17 transcriptional program in iTreg cells [132].
A fundamental point is the stability of Treg lineage cells in vivo in lympho-replete mice. Several recent reports investigated this issue by developing sophisticated reporter mice that trace the stability of Foxp3+ Treg cells [139, 141]. Both studies showed that the large majority (>80%) of Treg cells are stable. However, the degree of ex-Treg cells varied between the two reports. Ex-Treg cells represented nearly 20% of cells that were at one time in the Treg lineage cell and increased under inflammatory conditions. These ex-Treg cells expressed an activated/memory phenotype with Teff function [139]. In the other report, ex-Treg cells were rare (<5%) and were not more prevalent in mice that were challenged with Listeria to induce an inflammatory response, irradiated to induce lymphopenia, or stimulated to develop autoimmunity [141]. The reasons for these differences in ex-Treg levels might reflect differences in the approaches used to develop the mice for fate mapping and/or because of differences in the timing in which ex-Treg cells were examined.
After thymic development, Runx/Cbf-β binding to CNS2 of Foxp3 is required to maintain stable expression of Foxp3 and functional activity of mature peripheral nTreg cells [142]. The binding of Runx/Cbf-β to the Foxp3 promoter and CNS2 leads to an active chromatin state rather than directly inducing gene expression [142]. Deletion of CNS2 does not abolish thymic development of Treg cells in neonatal mice, but eventually leads to diminished numbers of peripheral Foxp3+ Treg cells [80].
Demethylation of CpG at CNS2 has been linked to the heritable expression of Foxp3 in Treg cells [143, 144]. In vitro derived iTreg cells, which are somewhat unstable, and ex-Treg cells showed increased CpG methylation of CNS2, consistent with Foxp3 transcriptional inactivity [139, 143]. Interestingly, in vivo generated iTreg cells, which stably express Foxp3, exhibit demeythylated CpG [144]. Foxp3 appears to stabilize its own expression by binding to CNS2 through a Runx/Cbf-β complex [80]. Collectively, these finding indicate that transcriptional regulation mediated by factors associated with CNS2 are required to maintain Foxp3 expression after development into Treg cells.
Concluding Remarks
A rather detailed view is emerging concerning how immunological signals are integrated within the cell to promote the expression of Foxp3 that leads to development of nTreg and iTreg cells. However, a number of key questions remain. For nTreg cells, one important issue is that there is only a rudimentary understanding of Foxp3-independent events that promote developing thymocytes into the Treg lineage. Although expression of Foxp3 is essential for Treg development, some thymocytes, destined to become Treg cells, exhibit traits of Treg cells without expression of Foxp3 [145, 146]. Key questions are: what are molecular determinants beyond Foxp3 that contribute to the nTreg lineage; what is the earliest Treg precursor cell; and how do TCR selection events influence this process? Although substantial data support iTreg cells as one option after T cells respond and adapt during peripheral immune responses, questions remain concerning the extent that iTreg cells are represented within the entire pool of CD4+ Foxp3+ T cells and their overall stability. Definition of markers distinctively present or absent in iTreg cells, such as their apparent lack of Helios [147], should aid in resolving these points.
Acknowledgements
Our work is supported by grants R01AI055815, R01CA045957 and P01CA109094 from the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor Foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 2.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 3.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 4.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 5.Chatila TA, Blaeser F, Ho N, Lederman HM, Voulgaropoulos C, Helms C, et al. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J Clin Invest. 2000;106:R75–R81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 7.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 8.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 9.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 10.Williams LM, Rudensky AY. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat Immunol. 2007;8:277–284. doi: 10.1038/ni1437. [DOI] [PubMed] [Google Scholar]
- 11.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 12.Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of Foxp3 expression during ontogeny. J Exp Med. 2005;202:901–906. doi: 10.1084/jem.20050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11:119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lathrop SK, Santacruz NA, Pham D, Luo J, Hsieh CS. Antigen-specific peripheral shaping of the natural regulatory T cell population. J Exp Med. 2008;205:3105–3117. doi: 10.1084/jem.20081359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pacholczyk R, Ignatowicz H, Kraj P, Ignatowicz L. Origin and T cell receptor diversity of Foxp3+CD4+CD25+ T cells. Immunity. 2006;25:249–259. doi: 10.1016/j.immuni.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 16.Bayer AL, Yu A, Malek TR. Function of the IL-2R for thymic and peripheral CD4+CD25+ Foxp3+ T regulatory cells. J Immunol. 2007;178:4062–4071. doi: 10.4049/jimmunol.178.7.4062. [DOI] [PubMed] [Google Scholar]
- 17.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Lee HM, Hsieh CS. Rare development of Foxp3+ thymocytes in the CD4+CD8+ subset. J Immunol. 2009;183:2261–2266. doi: 10.4049/jimmunol.0901304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burchill MA, Yang J, Vang KB, Moon JJ, Chu HH, Lio CW, et al. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity. 2008;28:112–121. doi: 10.1016/j.immuni.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28:100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kajiura F, Sun S, Nomura T, Izumi K, Ueno T, Bando Y, et al. NF-κB-inducing kinase establishes self-tolerance in a thymic stroma-dependent manner. J Immunol. 2004;172:2067–2075. doi: 10.4049/jimmunol.172.4.2067. [DOI] [PubMed] [Google Scholar]
- 22.Akiyama T, Maeda S, Yamane S, Ogino K, Kasai M, Kajiura F, et al. Dependence of self-tolerance on TRAF6-directed development of thymic stroma. Science. 2005;308:248–251. doi: 10.1126/science.1105677. [DOI] [PubMed] [Google Scholar]
- 23.Bensinger SJ, Bandeira A, Jordan MS, Caton AJ, Laufer TM. Major histocompatibility complex class II-positive cortical epithelium mediates the selection of CD4+25+ immunoregulatory T cells. J Exp Med. 2001;194:427–438. doi: 10.1084/jem.194.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribot J, Enault G, Pilipenko S, Huchenq A, Calise M, Hudrisier D, et al. Shaping of the autoreactive regulatory T cell repertoire by thymic cortical positive selection. J Immunol. 2007;179:6741–6748. doi: 10.4049/jimmunol.179.10.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liston A, Nutsch KM, Farr AG, Lund JM, Rasmussen JP, Koni PA, et al. Differentiation of regulatory Foxp3+ T cells in the thymic cortex. Proc Natl Acad Sci U S A. 2008;105:11903–11908. doi: 10.1073/pnas.0801506105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, et al. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- 27.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 28.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3:756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 29.Kawahata K, Misaki Y, Yamauchi M, Tsunekawa S, Setoguchi K, Miyazaki J, et al. Generation of CD4+CD25+ regulatory T cells from autoreactive T cells simultaneously with their negative selection in the thymus and from nonautoreactive T cells by endogenous TCR expression. J Immunol. 2002;168:4399–4405. doi: 10.4049/jimmunol.168.9.4399. [DOI] [PubMed] [Google Scholar]
- 30.Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 31.van Santen HM, Benoist C, Mathis D. Number of T reg cells that differentiate does not increase upon encounter of agonist ligand on thymic epithelial cells. J Exp Med. 2004;200:1221–1230. doi: 10.1084/jem.20041022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong J, Obst R, Correia-Neves M, Losyev G, Mathis D, Benoist C. Adaptation of TCR repertoires to self-peptides in regulatory and nonregulatory CD4+ T cells. J Immunol. 2007;178:7032–7041. doi: 10.4049/jimmunol.178.11.7032. [DOI] [PubMed] [Google Scholar]
- 33.Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol. 2006;7:401–410. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- 34.Bautista JL, Lio CW, Lathrop SK, Forbush K, Liang Y, Luo J, et al. Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nat Immunol. 2009;10:610–617. doi: 10.1038/ni.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leung MW, Shen S, Lafaille JJ. TCR-dependent differentiation of thymic Foxp3+ cells is limited to small clonal sizes. J Exp Med. 2009;206:2121–2130. doi: 10.1084/jem.20091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat Immunol. 2005;6:152–162. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- 37.Lio CW, Dodson LF, Deppong CM, Hsieh CS, Green JM. CD28 facilitates the generation of Foxp3− cytokine responsive regulatory T cell precursors. J Immunol. 2010;184:6007–6013. doi: 10.4049/jimmunol.1000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 39.Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 40.Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW. Interleukin-2 receptor α chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki H, Kundig TM, Furlonger C, Wakeham A, Timms E, Matsuyama T, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor β. Science. 1995;268:1472–1476. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 42.Almeida AR, Legrand N, Papiernik M, Freitas AA. Homeostasis of peripheral CD4+ T cells: IL-2Rα and IL-2 shape a population of regulatory cells that controls CD4+ T cell numbers. J Immunol. 2002;169:4850–4860. doi: 10.4049/jimmunol.169.9.4850. [DOI] [PubMed] [Google Scholar]
- 43.Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rβ-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–178. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 44.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 45.Barron L, Dooms H, Hoyer KK, Kuswanto W, Hofmann J, O'Gorman WE, et al. Cutting edge: mechanisms of IL-2-dependent maintenance of functional regulatory T cells. J Immunol. 2010;185:6426–6430. doi: 10.4049/jimmunol.0903940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor β-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 47.Bayer AL, Lee JY, de la Barrera A, Surh CD, Malek TR. A function for IL-7R for CD4+CD25+Foxp3+ T regulatory cells. J Immunol. 2008;181:225–234. doi: 10.4049/jimmunol.181.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vang KB, Yang J, Mahmud SA, Burchill MA, Vegoe AL, Farrar MA. IL-2-7, and-15, but not thymic stromal lymphopoeitin, redundantly govern CD4+Foxp3+ regulatory T cell development. J Immunol. 2008;181:3285–3290. doi: 10.4049/jimmunol.181.5.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malek TR, Castro I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity. 2010;33:153–165. doi: 10.1016/j.immuni.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pandey A, Ozaki K, Baumann H, Levin SD, Puel A, Farr AG, et al. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol. 2000;1:59–64. doi: 10.1038/76923. [DOI] [PubMed] [Google Scholar]
- 51.Park LS, Martin U, Garka K, Gliniak B, Di Santo JP, Muller W, et al. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med. 2000;192:659–670. doi: 10.1084/jem.192.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mazzucchelli R, Hixon JA, Spolski R, Chen X, Li WQ, Hall VL, et al. Development of regulatory T cells requires IL-7Rα stimulation by IL-7 or TSLP. Blood. 2008;112:3283–3292. doi: 10.1182/blood-2008-02-137414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watanabe N, Wang YH, Lee HK, Ito T, Cao W, Liu YJ. Hassall's corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436:1181–1185. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- 54.Hanabuchi S, Ito T, Park WR, Watanabe N, Shaw JL, Roman E, et al. Thymic stromal lymphopoietin-activated plasmacytoid dendritic cells induce the generation of FOXP3+ regulatory T cells in human thymus. J Immunol. 2010;184:2999–3007. doi: 10.4049/jimmunol.0804106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, et al. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-β1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marie JC, Liggitt D, Rudensky AY. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-β receptor. Immunity. 2006;25:441–454. doi: 10.1016/j.immuni.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 58.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-β controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455–471. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 59.Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. A critical function for TGF-β signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol. 2008;9:632–640. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 60.Ouyang W, Beckett O, Ma Q, Li MO. Transforming growth factor-β signaling curbs thymic negative selection promoting regulatory T cell development. Immunity. 2010;32:642–653. doi: 10.1016/j.immuni.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sakaguchi N, Takahashi T, Hata H, Nomura T, Tagami T, Yamazaki S, et al. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature. 2003;426:454–460. doi: 10.1038/nature02119. [DOI] [PubMed] [Google Scholar]
- 62.Tanaka S, Maeda S, Hashimoto M, Fujimori C, Ito Y, Teradaira S, et al. Graded attenuation of TCR signaling elicits distinct autoimmune diseases by altering thymic T cell selection and regulatory T cell function. J Immunol. 2010;185:2295–2305. doi: 10.4049/jimmunol.1000848. [DOI] [PubMed] [Google Scholar]
- 63.Fu G, Chen Y, Yu M, Podd A, Schuman J, He Y, et al. Phospholipase C{γ}1 is essential for T cell development, activation, and tolerance. J Exp Med. 2010;207:309–318. doi: 10.1084/jem.20090880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bensinger SJ, Walsh PT, Zhang J, Carroll M, Parsons R, Rathmell JC, et al. Distinct IL-2 receptor signaling pattern in CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5287–5296. doi: 10.4049/jimmunol.172.9.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walsh PT, Buckler JL, Zhang J, Gelman AE, Dalton NM, Taylor DK, et al. PTEN inhibits IL-2 receptor-mediated expansion of CD4+ CD25+ Tregs. J Clin Invest. 2006;116:2521–2531. doi: 10.1172/JCI28057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, et al. T cell receptor signaling controls Foxp3 expression via PI3K Akt mTOR. Proc Natl Acad Sci U S A. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ouyang W, Beckett O, Ma Q, Paik JH, DePinho RA, Li MO. Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nat Immunol. 2010;11:618–627. doi: 10.1038/ni.1884. [DOI] [PubMed] [Google Scholar]
- 69.Ouyang W, Beckett O, Flavell RA, Li MO. An essential role of the Forkhead-box transcription factor Foxo1 in control of T cell homeostasis and tolerance. Immunity. 2009;30:358–371. doi: 10.1016/j.immuni.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kerdiles YM, Stone EL, Beisner DL, McGargill MA, Ch'en IL, Stockmann C, et al. Foxo transcription factors control regulatory T cell development and function. Immunity. 2010;33:890–904. doi: 10.1016/j.immuni.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gupta S, Manicassamy S, Vasu C, Kumar A, Shang W, Sun Z. Differential requirement of PKC-θ in the development and function of natural regulatory T cells. Mol Immunol. 2008;46:213–224. doi: 10.1016/j.molimm.2008.08.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Medoff BD, Sandall BP, Landry A, Nagahama K, Mizoguchi A, Luster AD, et al. Differential requirement for CARMA1 in agonist-selected T-cell development. Eur J Immunol. 2009;39:78–84. doi: 10.1002/eji.200838734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schmidt-Supprian M, Tian J, Grant EP, Pasparakis M, Maehr R, Ovaa H, et al. Differential dependence of CD4+CD25+ regulatory and natural killer-like T cells on signals leading to NF-κB activation. Proc Natl Acad Sci U S A. 2004;101:4566–4571. doi: 10.1073/pnas.0400885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wan YY, Chi H, Xie M, Schneider MD, Flavell RA. The kinase TAK1 integrates antigen and cytokine receptor signaling for T cell development, survival and function. Nat Immunol. 2006;7:851–858. doi: 10.1038/ni1355. [DOI] [PubMed] [Google Scholar]
- 75.Molinero LL, Yang J, Gajewski T, Abraham C, Farrar MA, Alegre ML. CARMA1 controls an early checkpoint in the thymic development of FoxP3+ regulatory T cells. J Immunol. 2009;182:6736–6743. doi: 10.4049/jimmunol.0900498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barnes MJ, Krebs P, Harris N, Eidenschenk C, Gonzalez-Quintial R, Arnold CN, et al. Commitment to the regulatory T cell lineage requires CARMA1 in the thymus but not in the periphery. PLoS Biol. 2009;7:e51. doi: 10.1371/journal.pbio.1000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Long M, Park SG, Strickland I, Hayden MS, Ghosh S. Nuclear factor-κB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity. 2009;31:921–931. doi: 10.1016/j.immuni.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 78.Lee AJ, Wu X, Cheng H, Zhou X, Cheng X, Sun SC. CARMA1 regulation of regulatory T cell development involves modulation of interleukin-2 receptor signaling. J Biol Chem. 2010;285:15696–15703. doi: 10.1074/jbc.M109.095190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ruan Q, Kameswaran V, Tone Y, Li L, Liou HC, Greene MI, et al. Development of Foxp3+ regulatory t cells is driven by the c-Rel enhanceosome. Immunity. 2009;31:932–940. doi: 10.1016/j.immuni.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Soligo M, Camperio C, Caristi S, Scotta C, Porto PD, Costanzo A, et al. CD28 costimulation regulates FOXP3 in a RelA/NF-κB-dependent mechanism. Eur J Immunol. 2011 doi: 10.1002/eji.201040712. [DOI] [PubMed] [Google Scholar]
- 82.Mantel PY, Ouaked N, Ruckert B, Karagiannidis C, Welz R, Blaser K, et al. Molecular mechanisms underlying FOXP3 induction in human T cells. J Immunol. 2006;176:3593–3602. doi: 10.4049/jimmunol.176.6.3593. [DOI] [PubMed] [Google Scholar]
- 83.Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 84.Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, et al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109:4368–4375. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yu A, Zhu L, Altman NH, Malek TR. A low interleukin-2 receptor signaling threshold supports the development and homeostasis of T regulatory cells. Immunity. 2009;30:204–217. doi: 10.1016/j.immuni.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Davidson TS, DiPaolo RJ, Andersson J, Shevach EM. Cutting Edge: IL-2 is essential for TGF-β-mediated induction of Foxp3+ T regulatory cells. J Immunol. 2007;178:4022–4026. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- 87.Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J Exp Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 89.Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA. Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest. 2005;115:1923–1933. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Curotto de Lafaille MA, Lino AC, Kutchukhidze N, Lafaille JJ. CD25− T cells generate CD25+Foxp3+ regulatory T cells by peripheral expansion. J Immunol. 2004;173:7259–7268. doi: 10.4049/jimmunol.173.12.7259. [DOI] [PubMed] [Google Scholar]
- 91.Liang S, Alard P, Zhao Y, Parnell S, Clark SL, Kosiewicz MM. Conversion of CD4+ CD25− cells into CD4+ CD25+ regulatory T cells in vivo requires B7 costimulation, but not the thymus. J Exp Med. 2005;201:127–137. doi: 10.1084/jem.20041201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Izcue A, Hue S, Buonocore S, Arancibia-Carcamo CV, Ahern PP, Iwakura Y, et al. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity. 2008;28:559–570. doi: 10.1016/j.immuni.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Haribhai D, Lin W, Edwards B, Ziegelbauer J, Salzman NH, Carlson MR, et al. A central role for induced regulatory T cells in tolerance induction in experimental colitis. J Immunol. 2009;182:3461–3468. doi: 10.4049/jimmunol.0802535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Barnes MJ, Powrie F. Regulatory T cells reinforce intestinal homeostasis. Immunity. 2009;31:401–411. doi: 10.1016/j.immuni.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 96.Cobbold SP, Castejon R, Adams E, Zelenika D, Graca L, Humm S, et al. Induction of FoxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J Immunol. 2004;172:6003–6010. doi: 10.4049/jimmunol.172.10.6003. [DOI] [PubMed] [Google Scholar]
- 97.Liu VC, Wong LY, Jang T, Shah AH, Park I, Yang X, et al. Tumor evasion of the immune system by converting CD4+CD25− T cells into CD4+CD25+ T regulatory cells: role of tumor-derived TGF-β. J Immunol. 2007;178:2883–2892. doi: 10.4049/jimmunol.178.5.2883. [DOI] [PubMed] [Google Scholar]
- 98.Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008;29:114–126. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 99.Turner MS, Kane LP, Morel PA. Dominant role of antigen dose in CD4+Foxp3+ regulatory T cell induction and expansion. J Immunol. 2009;183:4895–4903. doi: 10.4049/jimmunol.0901459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gottschalk RA, Corse E, Allison JP. TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo. J Exp Med. 2010;207:1701–1711. doi: 10.1084/jem.20091999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Harada Y, Elly C, Ying G, Paik JH, DePinho RA, Liu YC. Transcription factors Foxo3a and Foxo1 couple the E3 ligase Cbl-b to the induction of Foxp3 expression in induced regulatory T cells. J Exp Med. 2010;207:1381–1391. doi: 10.1084/jem.20100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guo F, Iclozan C, Suh WK, Anasetti C, Yu XZ. CD28 controls differentiation of regulatory T cells from naive CD4 T cells. J Immunol. 2008;181:2285–2291. doi: 10.4049/jimmunol.181.4.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim JM, Rudensky A. The role of the transcription factor Foxp3 in the development of regulatory T cells. Immunol Rev. 2006;212:86–98. doi: 10.1111/j.0105-2896.2006.00426.x. [DOI] [PubMed] [Google Scholar]
- 105.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Semple K, Nguyen A, Yu Y, Wang H, Anasetti C, Yu XZ. Strong CD28 costimulation suppresses induction of regulatory T cells from naive precursors through Lck signaling. Blood. 2011;117:3096–3103. doi: 10.1182/blood-2010-08-301275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zheng SG, Wang JH, Stohl W, Kim KS, Gray JD, Horwitz DA. TGF-β requires CTLA-4 early after T cell activation to induce FoxP3 and generate adaptive CD4+CD25+ regulatory cells. J Immunol. 2006;176:3321–3329. doi: 10.4049/jimmunol.176.6.3321. [DOI] [PubMed] [Google Scholar]
- 108.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+CD25− cells to develop suppressive activity: the role of IL-2, TGF-β, and IL-10. J Immunol. 2004;172:5213–5221. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- 110.Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 111.Josefowicz SZ, Wilson CB, Rudensky AY. Cutting edge: TCR stimulation is sufficient for induction of Foxp3 expression in the absence of DNA methyltransferase 1. J Immunol. 2009;182:6648–6652. doi: 10.4049/jimmunol.0803320. [DOI] [PubMed] [Google Scholar]
- 112.Lu L, Wang J, Zhang F, Chai Y, Brand D, Wang X, et al. Role of SMAD and non-SMAD signals in the development of Th17 and regulatory T cells. J Immunol. 2010;184:4295–4306. doi: 10.4049/jimmunol.0903418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schallenberg S, Tsai PY, Riewaldt J, Kretschmer K. Identification of an immediate Foxp3− precursor to Foxp3+ regulatory T cells in peripheral lymphoid organs of nonmanipulated mice. J Exp Med. 2010;207:1393–1407. doi: 10.1084/jem.20100045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-β to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178:2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 115.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 116.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yamazaki S, Dudziak D, Heidkamp GF, Fiorese C, Bonito AJ, Inaba K, et al. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J Immunol. 2008;181:6923–6933. doi: 10.4049/jimmunol.181.10.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Manicassamy S, Reizis B, Ravindran R, Nakaya H, Salazar-Gonzalez RM, Wang YC, et al. Activation of β-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329:849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hill JA, Hall JA, Sun CM, Cai Q, Ghyselinck N, Chambon P, et al. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29:758–770. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mantel PY, Kuipers H, Boyman O, Rhyner C, Ouaked N, Ruckert B, et al. GATA3-driven Th2 responses inhibit TGF-β1-induced FOXP3 expression and the formation of regulatory T cells. PLoS Biol. 2007;5:e329. doi: 10.1371/journal.pbio.0050329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang Y, Su MA, Wan YY. An essential role of the transcription factor GATA-3 for the function of regulatory T cells. Immunity. 2011;35:337–348. doi: 10.1016/j.immuni.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ishikawa H, Tanaka K, Maeda Y, Aiba Y, Hata A, Tsuji NM, et al. Effect of intestinal microbiota on the induction of regulatory CD25+ CD4+ T cells. Clin Exp Immunol. 2008;153:127–135. doi: 10.1111/j.1365-2249.2008.03668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, et al. ATP drives lamina propria TH17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 125.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hall JA, Bouladoux N, Sun CM, Wohlfert EA, Blank RB, Zhu Q, et al. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008;29:637–649. doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 129.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, et al. Transforming growth factor-β induces development of the TH17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 130.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 131.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 132.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, et al. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 134.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, et al. TGF-β-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12:247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Maruyama T, Li J, Vaque JP, Konkel JE, Wang W, Zhang B, et al. Control of the differentiation of regulatory T cells and TH17 cells by the DNA-binding inhibitor Id3. Nat Immunol. 2011;12:86–95. doi: 10.1038/ni.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, et al. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer's patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- 138.Komatsu N, Mariotti-Ferrandiz ME, Wang Y, Malissen B, Waldmann H, Hori S. Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc Natl Acad Sci U S A. 2009;106:1903–1908. doi: 10.1073/pnas.0811556106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Horwitz DA, Zheng SG, Wang J, Gray JD. Critical role of IL-2 and TGF-β in generation, function and stabilization of Foxp3+CD4+ Treg. Eur J Immunol. 2008;38:912–915. doi: 10.1002/eji.200738109. [DOI] [PubMed] [Google Scholar]
- 141.Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, et al. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rudra D, Egawa T, Chong MM, Treuting P, Littman DR, Rudensky AY. Runx-CBFβ complexes control expression of the transcription factor Foxp3 in regulatory T cells. Nat Immunol. 2009;10:1170–1177. doi: 10.1038/ni.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007;5:e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Polansky JK, Kretschmer K, Freyer J, Floess S, Garbe A, Baron U, et al. DNA methylation controls Foxp3 gene expression. Eur J Immunol. 2008;38:1654–1663. doi: 10.1002/eji.200838105. [DOI] [PubMed] [Google Scholar]
- 145.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, et al. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 146.Lin W, Haribhai D, Relland LM, Truong N, Carlson MR, Williams CB, et al. Regulatory T cell development in the absence of functional Foxp3. Nat Immunol. 2007;8:359–368. doi: 10.1038/ni1445. [DOI] [PubMed] [Google Scholar]
- 147.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]