Abstract
Purpose.
We investigated the effects on refractive development and ocular growth of 2-zone concentric lenses with different negative powers in each of the optical zones, in normal and myopic eyes in young chicks.
Methods.
Monocular defocusing lenses were worn for 10–15 days from 12 days of age. Two 2-zone concentric lens types combining −5 and −10 diopter (D) powers, with center zone diameters ranging from 4.5–7.5 mm were tested. One group of chickens wore 2-zone negative lenses from 12 days of age for 10 days, without any previous lens treatment. A second group of 12-day-old chickens were treated initially with −10 D single vision (SV) lenses for 5 days to induce myopia, and then for another 10 days with 2-zone lenses, when the zone of lower power served as a positive addition.
Results.
With the 2-zone negative lens treatment alone, the magnitude of on-axis–induced myopia fell between that expected for two negative powers presented in SV lens format, while for eyes first made myopic by pretreatment with −10 SV lenses, the 2-zone negative lenses caused regression of the induced myopia due to inhibitory effects on axial ocular growth, with the greatest effects observed in eyes with higher baseline myopia.
Conclusions.
Our results provided further evidence for a role of the peripheral retina in ocular growth regulation. They also lent weight to the idea of using concentric multifocal contact lenses to appropriately manipulate peripheral retinal defocus as one approach to controlling human myopia progression.
The effects of 2-zone concentric lenses with different negative powers in each of the optical zones, in both normal and myopic eyes were studied, as an attempt to simulate the ocular optical conditions in which concentric multifocal contact lenses are applied for myopia control in humans.
Introduction
The prevalence of myopia is increasing rapidly worldwide, with the fastest increases in East Asian populations.1 In addition, the age at onset of myopia is decreasing, with earlier onset linked to more rapid progression, older age of stabilization, and higher prevalence of pathological myopia.2 Thus, myopia can no longer be considered as a benign condition, requiring only simple optical corrections for the purpose of eliminating distance blur. Rather, myopia should be viewed as a progressive condition carrying a potential risk of irreversible vision loss, with early intervention aimed at controlling its progression being a priority in its management.
Emmetropization is now generally accepted to be an active process rather than a byproduct of abnormal genetics, with supporting evidence coming from a variety of animal models.3 Of relevance to both myopia and our study, sustained retinal hyperopic defocus, imposed using optical lenses, is known to be a reliable stimulus to increased eye growth, leading to myopia in a variety of animals. On the other hand, imposed myopic defocus either is without effect or slows eye growth.4–7 Two examples of hyperopic defocus in human eyes offer plausible links to the finding of defocus-induced myopia in animal studies. First, relatively prolate eye shapes, which are encountered more commonly among myopic eyes, result in relative hyperopic defocus at peripheral retinal locations.8,9 Second, lags of accommodation, which are reported to be increased in myopes, impose hyperopic defocus on the central retina.10,11 Eyes undergoing myopia progression, whether or not they are myopic, also are reported to show greater relative peripheral hyperopia, and progressing myopes, larger lags of accommodation.9,12,13
The optical defocus studies in animals have renewed interest in the possibility that myopia progression in humans can be slowed through optical intervention. Furthermore, a number of recent studies in humans have reported inhibitory effects on myopia progression of bifocal and multifocal soft contact lenses (SCL)14,15 and orthokeratology.16,17 These accumulating, promising data contrast with equivocal results in earlier studies testing spectacle bifocal and progressive addition lenses (PAL) as treatments to slow myopia progression.18–22 Although confirmatory large scale, appropriately controlled studies of these contact lens treatments are yet to be undertaken, differences in the retinal experience of defocus offer plausible explanations for the different treatment outcomes. The myopia controlling effects of traditional bifocal and PAL spectacles are limited to reducing accommodative lags and, thus, the amount of hyperopic defocus experienced by the central retina, and this effect is contingent on the spectacles being used correctly. While the above contact lens treatments may also reduce accommodative lags, reductions in or overcorrection of peripheral retinal hyperopia, as reported in human myopia, also may contribute to their myopia control effect. It also is noteworthy that the bifocal spectacle lens study yielding the strongest myopia control effect made use of high-set executive bifocal lenses, which presumably significantly altered the defocus experience of the superior peripheral retina, in addition to any effect on accommodative lag.22
Despite the increasing interest in optical treatments for myopia, and at a more general level, understanding on how the multifocal optical environment influences refractive development, animal studies using multifocal lenses or conditions so far have been limited to only a small number of studies in chicks.23–25 When myopic defocus and hyperopic defocus are set in competition, as with Fresnel lenses and dual-focus cone imaging systems, myopic defocus dominates over hyperopic defocus in terms of effects on eye growth, although the latter presentation modality, which uses a closed, artificial visual environment, has no potential for translation into treatments for human myopia.
In a recent study in chicks, we tested a 2-zone concentric lens design more similar to the soft contact lens designs showing promising anti-myopia effects in humans.26 In contrast to the effects of the Fresnel lenses and dual-focus cone systems, for which multifocality is imposed on the same or nearby local retinal regions, our 2-zone lens design allows retinal eccentricity-dependent differences in optical defocus to be imposed. Note, however, that the lenses were tested on normal eyes and incorporated only one power, either positive or negative, with plano power in the other optical zone, to examine the effects of localized optical defocus. An unexpected and intriguing finding with the 2-zone positive lenses was that they slowed eye growth more than with single vision lenses of the same positive power.
We fitted 2-zone lenses incorporating negative powers in both optical zones to both eyes of previously untreated chicks and eyes that had been made myopic, to simulate the conditions experienced when multifocal soft contact lenses are used to treat myopia. Our study sought to obtain further insight into the relative contributions of peripheral and central retinal regions to emmetropization. Of relevance to human myopia control, we also asked the question of whether treatments aimed at central or peripheral retinal regions alone are sufficient to slow myopia progression.
Methods
Animals
White-Leghorn hatchling chicks, obtained from a commercial hatchery (Privett Hatchery, Portales, NM), were used in this study. They were reared in a normal diurnal, photopic white lighting environment (12 hours on/12 hours off), with food and water freely available. A total of 126 birds were used in this study. All animal care and treatments in this study conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Experimental protocols were approved by the Animal Care and Use Committee of the University of California-Berkeley.
Lens Designs
All lenses had an overall optical diameter of 10 mm and total diameter of 12.2 mm. Two 2-zone concentric lens types combined −10 and −5 diopter (D) powers in the following ways: (1) −10 D center/−5 D periphery (peripheral add) and (2) −5 D center/−10 D periphery (central add). Four central zone diameters (CZD) were tested, ranging from 4.5–7.5 mm in 1 mm increments. Single vision (SV) −10 D and plano lenses were included as control treatments.
The lenses were attached using Velcro support rings, one half glued to the feathers around the eyes and the other half used to mount the lenses. The vertex distance for the attached lenses, measured by high frequency ultrasonography, was 3.3 mm.26 The vertex distance of the lenses, the size of the entrance pupil of the eye, and the dimensions of the central and peripheral zones all influence the pattern of retinal defocus imposed by the 2-zone lenses.27,28 With the eye in primary gaze position, only limited central and peripheral retinal regions would have experienced unifocal (single vision) defocus; the experience of the remaining, paracentral retinal region would have been influenced by the central and peripheral lens zones. Table 1 summarizes the estimated dimensions of these visual field zones for the various 2-zone lens designs.29 Note that these calculations assume the eye to be in the primary gaze position. Under natural experimental conditions, the unifocal central fields would have been less than the estimates in Table 1 due to eye movements.
Table 1. .
CZD and Peripheral Optical Zone Diameters (PZD) of the Four 2-Zone Lens Designs Used and Corresponding Dimensions of the Unifocal Central Visual Field, and Annular Multifocal Paracentral and Unifocal Peripheral Visual Fields
|
CZD (mm) |
PZD (mm) |
Unifocal Central Field (deg)* |
Multifocal Paracentral Hemi-Field (deg)* |
Unifocal Peripheral Hemi-Field (deg)* |
| 4.5 | 2.75 | 12 | 35 | 13 |
| 5.5 | 2.25 | 34 | 27 | 10 |
| 6.5 | 1.75 | 54 | 19 | 8 |
| 7.5 | 1.25 | 72 | 14 | 4 |
Calculations based on anterior chamber depth (ACD) of 1.56 mm and vertex distance of 3.3 mm (means for 185 chickens tested), and estimated entrance pupil diameter of 4 mm, which is 0.2 mm in front of the iris based on an assumed total corneal power of 96 D.29
Treatment Assignments
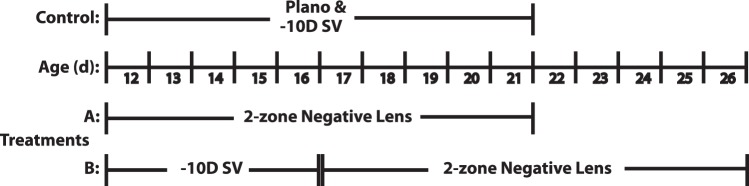
Chickens were assigned randomly to one of four treatment groups, which were exposed to different monocular lens treatment regimens, as summarized schematically in Figure 1. In one group (Fig. 1A), two-zone negative lenses were applied to 12-day-old chickens without any previous lens treatment and worn for 10 days. Two control groups wore single vision lenses, either plano or −10 D (−10 SV) for the same period. In a fourth group (Fig. 1B), 12-day-old chickens were treated first for 5 days with −10 SV lenses, which were then replaced with 2-zone lenses, the latter being left in place for another 10 days. Seven chickens were included in each treatment group. Lenses were cleaned and inspected at least 3 times daily to ensure that their optical centers remained approximately aligned with the ocular pupil centers of the chickens, thereby minimizing the confounding effect of lens decentration.
Figure 1. .
Schematic summary of the two lens treatment paradigms (A, B) used in this study. SV lenses were included either as a comparison control treatment (A), or as an initial myopia-inducing treatment (B).
Measurements
Immediately before the start of lens treatments, baseline refractive errors and axial ocular dimensions were measured, using static retinoscopy and high frequency A-scan ultrasonography, respectively, under gaseous anesthesia (1.5% isoflurane in oxygen). Ultrasonography measurements were repeated every other day and retinoscopy was repeated every 5 days. Refractive errors were measured on-axis (centrally) as well as 30 degrees off-axis nasally and temporally.
Statistical Analyses
Because there was no significant increase in astigmatism at the two off-axis compared to on-axis locations, either before or after the lens treatments, central (C, on-axis) and peripheral (P) refractive errors are represented as spherical equivalent refractive errors (SER; averages between the refractions for two principal meridians). Off-axis refractive errors are expressed as relative peripheral refractive errors (RPR), representing the difference between the peripheral and central values (i.e., P − C). Because changes in vitreous chamber depth (VCD) and choroidal thickness (CT) largely account for the changes in central refractive error, only these biometric changes are shown. Also, as no group-related differences in untreated contralateral (fellow) eyes were found, only changes over the treatment period in treated eyes are reported as primary outcome measures of treatment effects. Additionally, for paradigm B, as there was no significant difference between the refractive error changes recorded 5 and 10 days after switching to the 2-zone lenses, only the 5 day data were used in statistical comparisons with the groups subjected to paradigm A, to avoid any confounding effect of age.
Data analysis protocols were similar to those used in our previous, closely-related study.26 In brief, the normality of the distributions of changes in refractive error and axial dimensions first were verified and then factorial ANOVAs performed using STATA (STATA Corp., College Station, TX), on changes in the central SERs and VCDs over the treatment period, as well as endpoint RPR. For central SER and VCD data, the effects of two factors, lens type and CZD, were explored, as well as the interaction between these two factors. The RPR data were subjected to a factorial ANOVA, with change in central (on-axis) SER, lens type, CZD, and the interaction term between lens type and CZD as factors. Differences between groups were assessed by 2-sample t-tests, using the Hochberg step-up procedure to keep the family-wise error rate for the entire set of tests equal to 0.05. Box plots are used to show the results graphically.
Results
Effects of 2-Zone Negative Lenses on Previously Untreated Eyes (Fig. 1, Treatment A)
Effects of Lens Type and CZD on Central (On-Axis) Refractive Errors (SERs).
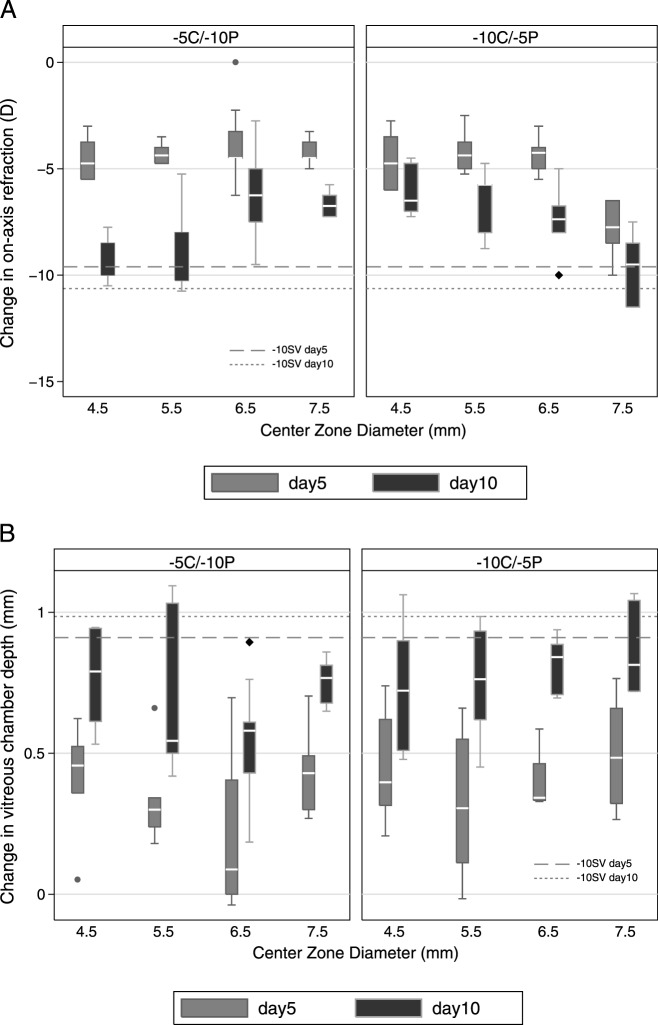
Changes in the central SERs over the treatment period are summarized by lens type and CZD in Table 2, and are also shown graphically in Figure 2 (top panel). With the two SV control treatments, there was minimal change in refractive error with the plano lens, and with the −10 SV lens, compensation to the imposed defocus was nearly complete after just 5 days of treatment (−9.61 ± 1.25 D) comparing to that at day 10 (−10.63 ± 0.70 D).
Table 2. .
Changes from Baseline in Central Spherical Equivalent Refractive Errors Recorded on Treatment Days 5 and 10 (Mean ± SD, D), Organized by Lens Type and CZD (n = 7 in Each Cell)
|
CZD (mm) |
Lens Type |
|||
|
–5C/–10P |
–10C/–5P |
|||
|
Day 5 |
Day 10 |
Day 5 |
Day 10 |
|
| 4.5 | −4.54 ± 0.99 | −9.17 ± 1.07 | −4.63 ± 1.32 | −6.08 ± 1.18 |
| 5.5 | −4.29 ± 0.49 | −8.67 ± 1.95 | −4.25 ± 0.79 | −6.46 ± 1.55 |
| 6.5 | −3.81 ± 1.86 | −6.03 ± 2.22 | −4.33 ± 0.86 | −7.42 ± 1.68 |
| 7.5 | −4.25 ± 0.63 | −6.67 ± 0.61 | −7.83 ± 1.33 | −9.67 ± 1.63 |
Equivalent values for the −10 SV lens treatment are −9.61 ± 1.25 D for day 5 and −10.63 ± 0.70 D for day 10. Values for the plano lens treatment are −0.37 ± 0.24 D for day 5 and −0.54 ± 0.74 D for day 10. Lenses were fitted to previously untreated 12-day-old chicks.
Figure 2. .
Box plots of changes from baseline in central spherical refractive error (top panel), and corresponding changes of vitreous chamber depth (bottom panel) over the treatment period for (A) the −5C/−10P lens type, (B) −10C/−5P lens type, with varying CZD (on X-axis) ranged from 4.5–7.5 mm. Dashed reference lines: mean changes induced by −10 SV lens. Whisker length: the shorter of 1.5 times the interquartile range and the distance to the extreme.
As with the −10 SV lens, all 2-zone lens designs induced significant myopic shifts in central SERs compared to values recorded with the plano lens. Significant changes were evident by day 5, and increased further by day 10, although all values were significantly less than those recorded with the −10 SV lens, except for the largest CZD −10C/−5P lens, which induced similar changes to the −10 SV lens (−9.67 ± 1.63 vs. −10.63 ± 0.70; P = 0.11, day 10; P < 0.05 for other designs and both time points). The changes in SER also showed increased variability across the treatment period with the 2-zone lenses, opposite of the trend with the −10D SV lens.
While lens type did not influence significantly the changes in central SERs, induced myopic shifts in refraction increased in magnitude with increasing lens area devoted to −10 D power for both 2-zone lens types, reached statistical significance for the day 10 data (P = 0.003 and <0.0001 for −5C/−10P and −10C/−5P designs, respectively). For the 2-zone lenses, the percentage of myopia development from days 5–10 was also affected significantly by the lens type (F1,8 = 8.97, P = 0.005), implying that the later changes were, in fact, affected by the lens type.
Relative Peripheral Refractive (RPR) Change Induced by 2-Zone Negative Lenses.
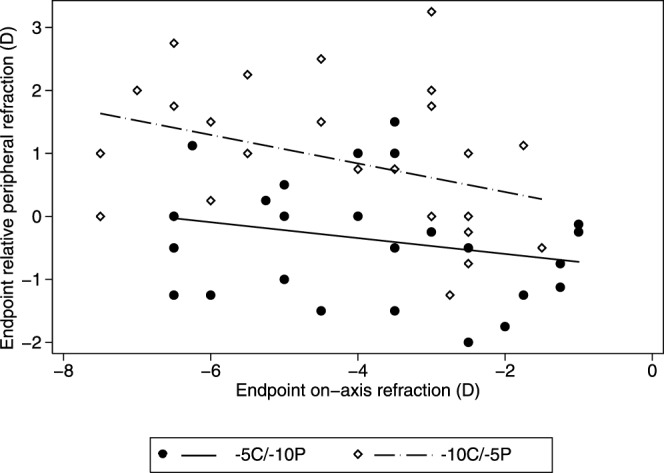
Since there was no evidence of nasal-temporal asymmetries in RPR changes, either for the 2-zone lenses or the −10 SV lens, the values derived for the nasal and temporal fields were averaged for use in analyses of the influences of lens type and CZD. Figure 3 shows endpoint RPRs plotted against endpoint on-axis refractive errors for both 2-zone lens types. The overall trend is for RPR to become more hyperopic with increasing on-axis myopia (F1,8 = 5.75, P = 0.02), although the two lens types induced very different patterns of RPR. Specifically and as expected, exposing the peripheral retina to higher amounts of imposed hyperopic defocus than the central retina, for example with the −5C/−10P lens type, resulted in more myopic RPRs, compared to the changes induced with the −10C/−5P lens type. This design-dependent difference is reflected in the large gap between the regression lines derived for the two lens types and was confirmed statistically (F1,8 = 31.82, P < 0.0001). Although CZD alone did not affect RPR significantly (F3,8 = 2.43, P = 0.08), its interaction with lens type was significant (F3,8 = 3.83, P = 0.02).
Figure 3. .
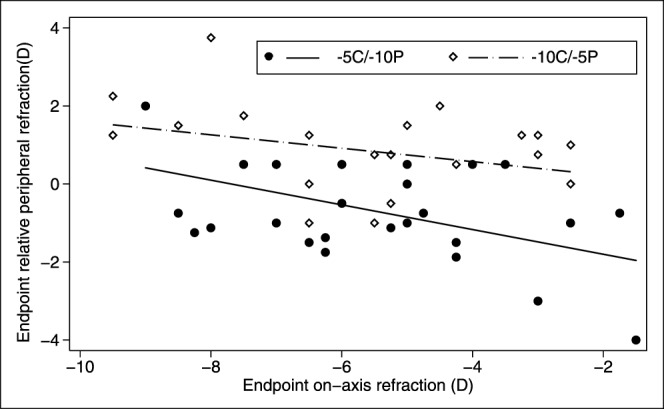
Scatter plots of endpoint relative RPR as a function of endpoint on-axis refractive errors for the two lens types. Solid circle: −5C/−10P. Open diamond: −10C/−5P. The mean RPR for eyes wearing −10 SV and plano lenses were +1.58 ± 0.44 D and −0.09 ± 0.83 D, respectively.
Effects of 2-Zone Lenses Design on Central (On-Axis) Axial Ocular Dimensions.
Induced changes in VCD are summarized by lens type and CZD in Table 3 and Figure 2 (bottom panel). For the −10 SV lens, the required compensatory increase in VCD was accomplished largely over the first 5 days of treatment, consistent with the pattern of change in central SER, and thus changes from baseline, measured on days 5 and 10, are similar (0.91 ± 0.21 mm for day 5, and 0.99 ± 0.22 mm for day 10). Increased elongation of the vitreous chamber also accounts largely for central SER changes with the 2-zone lenses, with changes in these two parameters being correlated significantly (R2 = 0.56 and 0.30 for days 5 and 10, respectively, P < 0.0001). As with the changes in SERs, significant elongation already was evident by treatment day 5 for both 2-zone lens types and the various CZDs, with the exception of the 6.5 mm CZD, −5C/−10P lens, although all treatment groups showed greater elongation by day 10. The induced VCD changes were also significantly less than those induced by −10 SV lens at both time points (P < 0.05 for all groups) for all but one lens design, the largest CZD −10C/−5P lens, for which the changes were indistinguishable by day 10 from those recorded with the −10 SV lens. The dose-response relationship between VCD elongation and CZD was not significant for either lens type, but approached significance for the −5C/−10P lens (P = 0.06). All 2-zone lens groups as well as the −10 SV lens group exhibited significant choroid thinning on treatment day 5 (P = 0.01, treated compared to fellow eye), but these changes were not sustained and no group recorded significant choroidal thinning on day 10 (P = 0.46).
Table 3. .
Changes from Baseline in Vitreous Chamber Depth Recorded on Treatment Days 5 and 10 (Mean ± SD, mm), Organized by Lens Type and CZD (n = 7 in Each Cell)
|
CZD (mm) |
Lens Type |
|||
|
–5C/–10P |
–10C/–5P |
|||
|
Day 5 |
Day 10 |
Day 5 |
Day 10 |
|
| 4.5 | 0.41 ± 0.20 | 0.77 ± 0.17 | 0.45 ± 0.20 | 0.73 ± 0.23 |
| 5.5 | 0.34 ± 0.17 | 0.69 ± 0.29 | 0.32 ± 0.26 | 0.75 ± 0.21 |
| 6.5 | 0.20 ± 0.26 | 0.55 ± 0.21 | 0.40 ± 0.10 | 0.82 ± 0.10 |
| 7.5 | 0.44 ± 0.16 | 0.76 ± 0.08 | 0.50 ± 0.19 | 0.86 ± 0.16 |
Equivalent values for the −10 SV lens treatment are 0.91 ± 0.21 mm for day 5 and 0.99 ± 0.22 mm for day 10. Values for the plano lens treatment are 0.18 ± 0.09 mm for day 5 and 0.41 ± 0.12 for day 10, the latter values being similar to the normal growth changes in the untreated fellow eyes (0.24 ± 0.15 mm for day 5 and 0.45 ± 0.11 for day 10). Lenses were fitted to previously untreated 12-day-old chicks.
Effects of 2-Zone Negative Lenses on Already Myopic Eyes (Pre-Treated with −10 SV Lenses; Fig. 1, Treatment B)
Effects of Lens Type and CZD on Central (On-Axis) Refractive Errors.
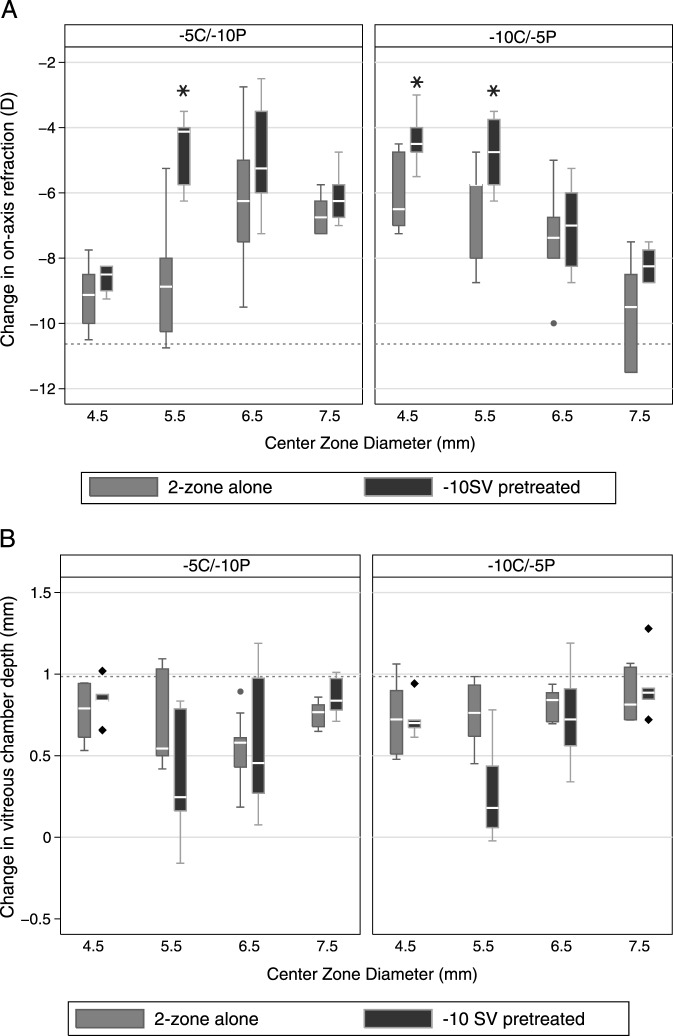
Data collected at the end of the 5-day, −10 SV lens treatment period confirmed that all treated eyes had become highly myopic; the mean change in central SER was −9.61 ± 1.25 D. This induced myopia showed regression when the −10 SV lenses were replaced at this time with one of the 2-zone lenses. Irrespective of their design, the reduction in myopia achieved statistical significance after 5 and 10 days of exposure to the 2-zone lenses (P < 0.05 for all). These data are summarized by lens type and CZD in Table 4. The reduction in induced myopia was affected significantly by CZD (F3,8 = 13.56, P < 0.0001), which interacted significantly with lens type (F3,8 = 21.18, P < 0.0001), although lens type alone did not affect the outcome significantly (F1,8 = 0.72, P = 0.4).
Table 4. .
Changes from Baseline in Central (On-Axis) Spherical Equivalent Refractive Errors after 10 Days of Lens Wear (Mean ± SD, D), Organized by Lens Type and CZD
|
CZD (mm) |
Lens Type |
|||
|
–5C/–10P |
–10C/–5P |
|||
|
2-Zone Alone |
–10 SV Pretreatment |
2-Zone Alone |
–10 SV Pretreatment |
|
| 4.5 | −9.17 ± 1.07 | −8.56 ± 0.45 | −6.08 ± 1.18 | −4.36 ± 0.79* |
| 5.5 | −8.67 ± 1.95 | −4.63 ± 1.10* | −6.46 ± 1.55 | −4.79 ± 1.08* |
| 6.5 | −6.03 ± 2.22 | −4.98 ± 1.61 | −7.42 ± 1.68 | −6.98 ± 1.24 |
| 7.5 | −6.67 ± 0.61 | −5.80 ± 1.86 | −9.67 ± 1.63 | −8.21 ± 0.53 |
Eyes wore either a 2-zone lens for 10 days (2-zone alone) or for 5 days as a replacement for a −10 SV lens worn for 5 days.
* Significant differences between groups.
Further analysis of the myopia regression patterns revealed three interesting trends. First, the magnitude of the reduction in myopia with the 2-zone lenses in place was associated positively with the initial level of induced myopia (F1,8 = 53.86, P < 0.0001), that is the higher the initial myopia, the larger the subsequent reduction in myopia. Second, the refractive errors recorded 5 and 10 days after switching to 2-zone lenses were not significantly different, implying that the myopia control effect was achieved within the first 5 days, after which the lenses served to stabilize refractive errors (Table 5, top row). Third, endpoint refractions generally were less myopic for eyes in which a −10 SV lens was switched with a 2-zone lens compared to the refractive errors of eyes wearing the same 2-zone lens for the entire treatment period (Fig. 4, top panel), with these differences reaching statistical significance for the 5.5 mm CZD, −5C/−10P lens, as well as 4.5 and 5.5 mm CZD, −10C/−5P lenses (P < 0.05 for all).
Table 5.
Changes from Baseline in Central (Top Row) and Peripheral Spherical Equivalent Refractive Errors (Bottom Row) of Treated Eyes, Recorded 5 and 10 Days after a −10 SV Lens Worn for 5 Days was Switched to a 2-Zone Lens (Mean ± SD, Days), Organized by Lens Type and CZD
|
CZD (mm) |
Lens Type |
|||
|
-–5C/–10P |
–10C/–5P |
|||
|
–10 SV Pretreatment 2-Zone, 5 Days |
–10 SV Pretreatment 2-Zone, 10 Days |
–10 SV Pretreatment 2-Zone, 5 Days |
–10 SV Pretreatment 2-Zone, 10 Days |
|
| 4.5 | −8.56 ± 0.45 | −8.86 ± 0.70 | −4.36 ± 0.79 | −4.73 ± 0.89 |
| 0.84 ± 0.13 | 0.89 ± 0.17 | 0.72 ± 0.10 | 0.75 ± 0.10 | |
| 5.5 | −4.63 ± 1.10 | −5.13 ± 1.41 | −4.79 ± 1.08 | −5.02 ± 0.44 |
| 0.35 ± 0.39 | 0.39 ± 0.30 | 0.27 ± 0.29 | 0.33 ± 0.21 | |
| 6.5 | −4.98 ± 1.61 | −4.72 ± 2.54 | −6.98 ± 1.24 | −6.75 ± 1.57 |
| 0.56 ± 0.38 | 0.55 ± 0.44 | 0.72 ± 0.27 | 0.74 ± 0.30 | |
| 7.5 | −5.80 ± 1.86 | −6.45 ± 1.24 | −8.21 ± 0.53 | −8.84 ± 1.01 |
| 0.86 ± 0.12 | 0.93 ± 0.10 | 0.92 ± 0.19 | 0.95 ± 0.24 | |
Figure 4. .
Box plots of changes from baseline in central refractive errors (top panel), and corresponding changes in vitreous chamber depth (bottom panel) after (A) 2-zone lens treatment for 10 days, and (B) −10 SV lens pretreatment for 5 days followed by 2-zone lens treatment for 5 days. Dashed reference lines: mean changes induced by −10 SV lens before replacement with a 2-zone lens. Whisker length: the shorter of 1.5 times the interquartile range and the distance to the extreme.
RPR Change Induced by 2-Zone Negative Lenses.
There was no significant difference between the RPRs recorded 5 and 10 days after switching from a −10 SV lens to a 2-zone lens. These data are summarized in Table 5. The day 5 data were analyzed further statistically. While there was greater variability in the RPRs of eyes switched from a −10 SV lens to a 2-zone lens than in the RPRs of eyes wearing only a 2-zone lens, lens type remained highly significant as a determinant of RPRs (F1,8 = 28.40, P < 0.0001, Fig. 5), and the influence of CZD and its interaction with lens type also reached statistical significance (F3,8 = 2.98, P = 0.04 and F3,8 = 2.80, P = 0.05, respectively). Here, as in eyes wearing 2-zone lenses for the entire treatment period, those with greater on-axis myopia before their −10 SV lenses were replaced tended to show greater relative peripheral hyperopia after 5 days of 2-zone lens wear; however this association did not reach statistical significance (F1,8 = 2.53, P = 0.12).
Figure 5. .
Scatter plots of endpoint relative RPR as a function of endpoint on-axis refractive errors for eyes wearing a 2-zone lens for 5 days after pretreatment with a −10 SV lens for 5 days. Solid circle: −5C/−10P. Open diamond: −10C/−5P (bottom panel). The mean RPR induced by −10 SV lens worn for 10 days was 1.58 ± 0.44 D.
Effects of 2-Zone Lenses Design on Central (On-Axis) Axial Ocular Dimensions.
The changes in VCD underlying the refractive changes described above, are summarized in Table 6 and shown graphically in Figure 4 (bottom panel). The early regression in myopia seen after −10 SV lenses were replaced with 2-zone lenses can be attributed to reductions in VCD (−0.14 ± 0.26 mm), coupled to significant choroidal thickening (0.14 ± 0.14 mm, P = 0.0001). These changes were evident at the first day 5 measurement time point after the lenses were switched, and both changes in choroidal thickness and VCD correlated well with the reduction in induced myopia (R2 = 0.59, P < 0.0001). After a further 5 days (day 10 of 2-zone lens treatment), the choroids had returned to baseline thickness values (P > 0.54 for all). VCDs also elongated, rather than shrinking, over the latter period, although the changes over this period were less than the changes in the untreated fellow eyes. As with the changes in refractive error during the 2-zone lens treatment period, changes in VCD were influenced significantly by the VCD recorded at the beginning of this period, that is the end of the −10 SV lens treatment period (F1,8 = 7.0, P = 0.01), as well as CZD (F3,8 = 8.70, P = 0.0001), with significant interaction between lens type and CZD (F3.8 = 3.38, P = 0.03). The effect of lens type alone was not significant (F1,8 = 0.54, P = 0.5). There was a further parallel with the refractive error data in that for the 5.5 mm CZD, −10C/−5P lens, the change in VCD was significantly less than that induced by the same 2-zone lens worn for the entire treatment period (P = 0.01). Data collected with the 5.5 mm CZD, −5C/−10P lens showed a similar trend although the difference is of borderline significance (P = 0.045).
Table 6. .
Changes from Baseline in VCD of Treated Eyes (Mean ± SD, D), Organized by Lens Type and CZD
|
CZD (mm) |
Lens Type |
|||
|
–5C/–10P |
–10C/–5P |
|||
|
2-Zone Alone |
−10 SV Pretreatment 2 Zone, 5 D (2-Zone, 10d–5d) |
2-Zone Alone |
−10 SV Pretreatment 2 Zone, 5 D (2-Zone, 10d–5d) |
|
| 4.5 | 0.77 ± 0.17 | 0.84 ± 0.13 | 0.73 ± 0.23 | 0.72 ± 0.10 |
| (0.15 ± 0.17) | (0.13 ± 0.10) | |||
| 5.5 | 0.69 ± 0.29 | 0.35 ± 0.39* | 0.75 ± 0.21 | 0.27 ± 0.29* |
| (0.19 ± 0.30) | (0.16 ± 0.21) | |||
| 6.5 | 0.55 ± 0.21 | 0.56 ± 0.38 | 0.82 ± 0.10 | 0.72 ± 0.27 |
| (0.16 ± 0.44) | (0.12 ± 0.30) | |||
| 7.5 | 0.76 ± 0.08 | 0.86 ± 0.12 | 0.86 ± 0.16 | 0.92 ± 0.19 |
| (0.17 ± 0.10) | (0.13 ± 0.24) | |||
Eyes wore either a 2-zone lens as a replacement for a −10 SV lens or for the entire treatment period (2-zone alone; data transposed from Table 3 for comparison). VCD change after an additional 5 days of 2-zone lens treatment is shown in parentheses. Mean VCD growth in untreated fellow eyes from days 10–15 is 0.21 ± 0.14 mm.
P < 0.05 comparing to 2-zone alone group.
Discussion
Our current study was an extension of our recently reported study, in which 2-zone lenses combining zones of either positive or negative power with a plano power zone were used to titrate differentially the optical defocus experience of central and peripheral retinal regions.26 Our current study made use of two different experimental paradigms of 2-zone lenses that incorporated negative powers, differing in magnitude (−5 or −10 D), in central and peripheral zones. Thus, when the lenses were fitted to previously untreated eyes, the first of the experimental paradigms tested, the visual experience of all retinal regions was altered, albeit differently for central and peripheral regions according to the magnitude of imposed hyperopic defocus. These 2-zone lenses induced less myopia than observed with single vision −10 D lenses, although more than expected for single vision −5 D lenses, with changes in peripheral refractions tending to reflect more directly the imposed defocus. In a second experimental paradigm, intended to simulate the conditions imposed by multifocal contact lenses used for human myopia control in recent pilot studies, the same lenses were substituted for single vision −10 D lenses, which had been left in place for 5 days first to induce myopia. The net effect of them switching to the 2-zone lenses was to impose myopic defocus of approximately −5 D in the retinal regions onto which the zones of lower negative power projected, and elsewhere, to approximately correct the induced myopia. Curiously, this substitution resulted in regression of the previously induced myopia to levels generally less than seen with the 2-zone lenses worn for the entire period, suggesting that different processes are at play in determining endpoint refractive errors in these two cases. In the following discussion, we examined our results further and speculated on their significance for the clinical management of myopia.
In eyes exposed to only the 2-zone negative lenses, both lens types induced VCD elongation and, thus, myopia, although the changes were smaller than seen with −10 SV lenses. While the central (on-axis) endpoint refractions after 10 days of lens wear tended to lie between results expected for each of two optical powers presented on their own, these data also showed a “dose effect,” in that the amount of induced myopia was associated positively with the area of the −10 D zone. Interestingly, this dose-response effect was not evident at the earlier day 5 measurement time point, when the magnitude of induced myopia was similar, around −4.3 D, for most of the lenses. This early pattern is consistent with eyes responding in an all-or-none way, that is at the same rate, to the imposed hyperopic defocus, irrespective of the area-average defocus, consistent with results from an earlier study.30 At this time, the amount of induced myopia compensated approximately for the lower of the two optical powers incorporated in the 2-zone lenses and, thereafter, the hyperopic experience of eyes would have been biased regionally and lens type-dependent, limited to more central or peripheral retinal regions for the −10C/−5P and −5C/−10P lenses, respectively. Thus, for these regions, the stimulus for further compensatory ocular growth remained. In contrast, in the regions exposed to the lower amount of hyperopic defocus, further enhanced growth would have resulted in overcompensation, that is relative myopia, which in turn is expected to generate a competing inhibitory growth signal. The endpoint on-axis refractions recorded at day 10 are consistent with competing myopic and hyperopic signals, requiring only that eye movements beneath the lenses to be sufficient to expose much of the retina to both signals. Even if the central retina is not exposed directly to competing defocus stimuli, altered growth of adjacent mid-peripheral regions may alter its location passively (mechanically) and, thus, the central refraction. This model also explains why the −5C/−10P lenses with the smallest (4.5 and 5.5 mm) central zones and the −10C/−5P lens with the largest (7.5 mm) CZD induced more myopia than the other 2-zone lens designs, although it does not explain why significantly greater myopia also was observed at day 5 with the latter lens, compared to that observed with the other lens/CZD combinations.
Consistent with the findings of our earlier 2-zone lens study, we observed in eyes exposed only to 2-zone lenses, an overall weak but statistically significant negative correlation between central (on-axis) and peripheral refraction changes, in which increasing central myopia was coupled to increasing relative peripheral hyperopia. We interpreted this observation as evidence of an underlying eye shape regulator. However the change of RPR cannot be considered as merely a byproduct of altered on-axis growth, as there were significant lens design-dependent differences in observed peripheral refraction patterns (Fig. 2). For example, for the −5C/−10P groups, RPRs were mostly myopic, even for eyes recording the highest amounts of central myopia, suggesting active local compensation to the stronger hyperopic defocus imposed on the peripheral retina. The converse is true for −10C/−5P lens groups; here RPRs mostly were hyperopic, even in eyes recording modest amounts of central myopia. These lens type-dependent differences in RPR patterns strongly argue for active, local regulation of ocular growth by the peripheral retina, as suggested by a previous study using simpler hemifield lens designs.31 This result also necessarily implies that the peripheral retina is capable of decoding the sign of optical defocus. Of important translational value are parallel findings from primates, showing that, as in the chick, the peripheral retina not only has a major role in central emmetropization,32 but also is capable of localized emmetropization,33 although to date, the role of peripheral hyperopia as a stimulus for myopia development and progression has been challenged in more recent studies.34,35
The second experimental paradigm applied in our study represents an attempt to address a more clinically relevant question related to patients seeking myopia control interventions, who typically are already myopic. Specifically, in making eyes myopic before fitting the 2-zone lenses, we sought to simulate the situation created by concentric bifocal contact lens corrections for myopia, in which the zone of lower negative power in each design serves as a positive addition. Interestingly, all eyes showed significant reductions in myopia as well as VCD after the 2-zone lenses were substituted for the −10 SV lenses, irrespective of the lens type and CZDs. These results are consistent with the “adds” of the 2-zone lenses imposing myopic defocus, which is predicted to inhibit further ocular elongation, and in chicks also promote choroidal thickening, as observed. Perhaps not surprisingly, the total amount of myopia reduction was also affected significantly by the level of myopia (induced by −10 SV lenses), present at the beginning of the 2-zone lens treatment period, as it also would have determined the size of the add (imposed myopia).
While relative peripheral hyperopia was a consistent finding in −10 SV lens-treated eyes, the RPRs resolved into two distinctive patterns after the replacement 2-zone lenses had been worn for 5 days. Specifically, compared to the changes induced by the −10C/−5P lenses, the relative change in peripheral refraction from −10 SV lens-treated level was much smaller with the −5C/−10P lenses. More specifically, the reduction of myopia was greater centrally than in the periphery with −5C/−10P lenses, resulting in a relative peripheral myopia. This result is consistent with the altered defocus experience when the latter lenses were used, the central but not the peripheral retina experiencing a significant change in lens-imposed defocus. Furthermore, while the reduction in central (on-axis) myopia was affected significantly by the amount of induced myopia, the RPRs were not affected by either the baseline or the endpoint central refractive errors but were affected strongly by the lens type. These findings support our earlier conclusion that the peripheral retina of the chicken eye is capable of decoding and responding to local defocus stimuli, independent of the central retina.
It is noteworthy that when −10 SV lenses were replaced by 2-zone lenses, the central endpoint refractions at the end of the 2-zone lens treatment period were not only reduced relative to the myopic refractions induced by −10 SV lens, but also were less myopic than those recorded in eyes exposed only to 2-zone lenses, that is worn for the total 10-day treatment period. These differences reached statistical significance for the 5.5 mm CZD and both lens types, as well as the 4.5 mm CZD, −10C/−5P lens. For eyes exposed only to 2-zone lenses, the mean central endpoint refractions for both lens types and the 2 intermediate-sized CZDs were close to −5 D, that is approximately matching the lower of the two negative powers incorporated into these lenses. The mean endpoint refractions for the same lenses fitted to already myopic eyes were lower in the case of the 5.5 mm CZD, −5C/−10P lens as well as −10C/−5P lenses with CZDs smaller than 6.5 mm, is equivalent to under-compensation relative to the lower of the 2 negative powers. A similar bias towards relative hyperopia was described in our earlier study in which the refractive error changes induced by our +5/plano 2-zone lenses were more hyperopic than those induced by a +5 SV lens.26 The surprising, albeit consistent, strong inhibitory growth responses underlying these various results are presumed to reflect complex interactions between growth modulatory signals generated by central and peripheral retinal regions, although we cannot easily eliminate additional effects of intermittent exposure to relative or absolute myopic defocus, and interactions between the imposed multifocal optical environment and the higher order aberrations (HOAs) of the eye. First, although we made every effort to keep the 2-zone lenses well centered, eye movements behind the lenses would have expanded the retinal areas intermittently exposed to each type of defocus. In earlier studies encompassing many different defocus paradigms in chicks, it has been shown that the effects of myopic defocus are very enduring,36 and thus likely to dominate when exposures to both types of defocus are similar. As noted above, the central refraction likely is influenced passively by adjacent mid-peripheral regions. Second, interactions between imposed and naturally occurring HOAs may alter the target plane of best focus for emmetropization, as suggested in clinical studies involving concentric bifocal contact lenses as well as orthokeratology lenses.37 However, this mechanism is unlikely to have a dominant role in the effects induced by the 2-zone negative lenses, since reductions in myopic endpoint refractions were found with both lens types, which are expected to have opposite effects on spherical aberration. Nonetheless, because of the high relevance of these unresolved issues to clinical attempts to control myopia progression, their further study is warranted.
Our findings of significant changes in refractive errors with 2-zone negative lenses, both centrally (on-axis) and peripherally, attributable to ocular growth changes in the case of on-axis changes, confirmed the critical contributions of peripheral optics and peripheral retina to emmetropization and myopia control. They also lent weight to recent clinical observations suggesting that appropriately designed concentric multifocal contact lenses can control myopia progression and argued for further clinical studies of these lenses.
Footnotes
Supported by NIH K12 EY 017296 (YL) and NIH R01 EY12392 (CW).
Disclosure: Y. Liu, None; C. Wildsoet, None
References
- 1.Saw SM, Tong L, Chua WH, et al. Incidence and progression of myopia in Singaporean school children. Invest Ophthalmol Vis Sci. 2005;46:51–57 [DOI] [PubMed] [Google Scholar]
- 2.Vitale S, Sperduto RD, Ferris FL 3rd. Increased prevalence of myopia in the United States between 1971–1972 and 1999–2004. Arch Ophthalmol. 2009;127:1632–1639 [DOI] [PubMed] [Google Scholar]
- 3.Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–468 [DOI] [PubMed] [Google Scholar]
- 4.Wallman J, Gottlieb MD, Rajaram V, Fugate-Wentzek LA. Local retinal regions control local eye growth and myopia. Science. 1987;237:73–77 [DOI] [PubMed] [Google Scholar]
- 5.Wildsoet C, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res. 1995;35:1175–1194 [DOI] [PubMed] [Google Scholar]
- 6.McBrien NA, Gentle A, Cottriall C. Optical correction of induced axial myopia in the tree shrew: implications for emmetropization. Optom Vis Sci. 1999;76:419–427 [DOI] [PubMed] [Google Scholar]
- 7.Smith EL 3rd, Hung LF. The role of optical defocus in regulating refractive development in infant monkeys. Vision Res. 1999;39:1415–1435 [DOI] [PubMed] [Google Scholar]
- 8.Charman WN, Radhakrishnan H. Peripheral refraction and the development of refractive error: a review. Ophthalmic Physiol Opt. 2010;30:321–338 [DOI] [PubMed] [Google Scholar]
- 9.Mutti DO, Hayes JR, Mitchell GL, et al. Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Invest Ophthalmol Vis Sci. 2007;48:2510–2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gwiazda J, Bauer J, Thorn F, Held R. A dynamic relationship between myopia and blur-driven accommodation in school-aged children. Vision Res. 1995;35:1299–1304 [DOI] [PubMed] [Google Scholar]
- 11.Charman WN. Near vision, lags of accommodation and myopia. Ophthalmic Physiol Opt. 1999;19:126–133 [DOI] [PubMed] [Google Scholar]
- 12.Mutti DO, Mitchell GL, Hayes JR, et al. Accommodative lag before and after the onset of myopia. Invest Ophthalmol Vis Sci. 2006;47:837–846 [DOI] [PubMed] [Google Scholar]
- 13.Gwiazda J, Thorn F, Held R. Accommodation, accommodative convergence, and response AC/A ratios before and at the onset of myopia in children. Optom Vis Sci. 2005;82:273–278 [DOI] [PubMed] [Google Scholar]
- 14.Aller TA, Laure A, Wildsoet C. Results of a one-year prospective clinical trial (CONTROL) of the use of bifocal soft contact lenses to control myopia progression. Ophthalmic Physiol Opt. 2006;26:8–9 [Google Scholar]
- 15.Aller TA, Wildsoet C. Bifocal soft contact lenses as a possible myopia control treatment: a case report involving identical twins. Clin Exp Optom. 2008;91:394–399 [DOI] [PubMed] [Google Scholar]
- 16.Cho P, Cheung SW, Edwards M. The longitudinal orthokeratology research in children (LORIC) in Hong Kong: a pilot study on refractive changes and myopic control. Curr Eye Res. 2005;30:71–80 [DOI] [PubMed] [Google Scholar]
- 17.Walline JJ, Jones LA, Sinnott LT. Corneal reshaping and myopia progression. Br J Ophthalmol. 2009;93:1181–1185 [DOI] [PubMed] [Google Scholar]
- 18.Gwiazda J, Hyman L, Hussein M, et al. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Invest Ophthalmol Vis Sci. 2003;44:1492–1500 [DOI] [PubMed] [Google Scholar]
- 19.Fulk GW, Cyert LA. Can bifocals slow myopia progression? J Am Optom Assoc. 1996;67:749–754 [PubMed] [Google Scholar]
- 20.Fulk GW, Cyert LA, Parker DE. A randomized trial of the effect of single-vision vs. bifocal lenses on myopia progression in children with esophoria. Optom Vis Sci. 2000;77:395–401 [DOI] [PubMed] [Google Scholar]
- 21.Edwards MH, Li RW, Lam CS, Lew JK, Yu BS. The Hong Kong progressive lens myopia control study: study design and main findings. Invest Ophthalmol Vis Sci. 2002;43:2852–2858 [PubMed] [Google Scholar]
- 22.Cheng D, Schmid KL, Woo GC, Drobe B. Randomized trial of effect of bifocal and prismatic bifocal spectacles on myopic progression: two-year results. Arch Ophthalmol. 2010;128:12–19 [DOI] [PubMed] [Google Scholar]
- 23.Tse DY, Lam CS, Guggenheim JA, et al. Simultaneous defocus integration during refractive development. Invest Ophthalmol Vis Sci. 2007;48:5352–5359 [DOI] [PubMed] [Google Scholar]
- 24.Diether S, Wildsoet CF. Stimulus requirements for the decoding of myopic and hyperopic defocus under single and competing defocus conditions in the chicken. Invest Ophthalmol Vis Sci. 2005;46:2242–2252 [DOI] [PubMed] [Google Scholar]
- 25.Wildsoet C, Collins M. Competing defocus stimuli of opposite sign produce opposite effects in eyes with intact and sectioned optic nerves in the chick. Invest Ophthalmol Vis Sci. 2000;41:S738 [Google Scholar]
- 26.Liu Y, Wildsoet C. The effect of two-zone concentric bifocal spectacle lenses on refractive error development and eye growth in young chicks. Invest Ophthalmol Vis Sci. 2011;52:1078–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carkeet A. Field restriction and vignetting in contact lenses with opaque peripheries. Clin Exp Optom. 1998;81:151–158 [DOI] [PubMed] [Google Scholar]
- 28.Smith EL 3rd, Hung LF, Huang J. Relative peripheral hyperopic defocus alters central refractive development in infant monkeys. Vision Res. 2009;49:2386–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irving EL, Sivak JG, Curry TA, Callender MG. Chick eye optics: zero to fourteen days. J Comp Physiol A. 1996;179:185–194 [DOI] [PubMed] [Google Scholar]
- 30.Wildsoet C, Wallman J. Is the rate of lens compensation proportional to the degree of defocus. Invest Ophthalmol Vis Sci. 1997;38:21529331279 [Google Scholar]
- 31.Diether S, Schaeffel F. Local changes in eye growth induced by imposed local refractive error despite active accommodation. Vision Res. 1997;37:659–668 [DOI] [PubMed] [Google Scholar]
- 32.Smith EL 3rd, Kee CS, Ramamirtham R, Qiao-Grider Y, Hung LF. Peripheral vision can influence eye growth and refractive development in infant monkeys. Invest Ophthalmol Vis Sci. 2005;46:3965–3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith EL 3rd, Hung LF, Huang J, Blasdel TL, Humbird TL, Bockhorst KH. Effects of optical defocus on refractive development in monkeys: evidence for local, regionally selective mechanisms. Invest Ophthalmol Vis Sci. 2010;51:3864–3873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sng CC, Lin XY, Gazzard G, et al. Change in peripheral refraction over time in Singapore Chinese children. Invest Ophthalmol Vis Sci. 2011;52:7880–7887 [DOI] [PubMed] [Google Scholar]
- 35.Mutti DO, Sinnott LT, Mitchell GL, et al. Relative peripheral refractive error and the risk of onset and progression of myopia in children. Invest Ophthalmol Vis Sci. 2011;52:199–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu X, Winawer JA, Wallman J. Potency of myopic defocus in spectacle lens compensation. Invest Ophthalmol Vis Sci. 2003;44:2818–2827 [DOI] [PubMed] [Google Scholar]
- 37.Tarrant J, Severson H, Wildsoet CF. Accommodation in emmetropic and myopic young adults wearing bifocal soft contact lenses. Ophthalmic Physiol Opt. 2008;28:62–72 [DOI] [PMC free article] [PubMed] [Google Scholar]