Abstract
We have shown reduced density and altered kinetics in slowly activating K+ currents (IKs) in epicardial border zone (EBZ) cells (IZs) of the 5-day-infarcted canine heart (Jiang M, Cabo, C, Yao J-A, Boyden PA, and Tseng G-N. Cardiovasc Res 48: 34–43, 2000). β-Adrenergic stimulation with isoproterenol increases IKs in normal cells (NZs). In this study, we used a voltage-clamp protocol with an external solution to isolate IKs from contaminating currents to determine the effects of 1 µM isoproterenol on IKs in IZs and NZs. Under our recording conditions, 10 µM azimilide-sensitive currents were stimulated with isoproterenol to compare responsiveness of IKs to isoproterenol in the two cell groups. IKs tail density was reduced 67% in IZs (group I, n = 26) compared with NZs (n = 24, P < 0.05). Isoproterenol-stimulated azimilide-sensitive tail currents were increased 1.72 ± 0.2-fold in NZs and 2.2 ± 0.3-fold in IZs (P > 0.05). In 33% of IZs (group II, n = 13), native currents showed no tail currents; however, isoproterenol-stimulated azimilide-sensitive currents were voltage dependent, fast activating, and large in amplitude compared with group I IZs, similar to “lone” KCNQ1 currents. Using short clamp pulses, we also found an increase in sustained currents sensitive to tetraethylammonium chloride (TEA) and no change in C-9356-sensitive currents in IZs with little or no transient outward current. In some IZs where IKs is downregulated, the effect of isoproterenol on IKs was similar to that on IKs in NZs. In others, the existence of lone KCNQ1-type currents, which are sensitive to β-adrenergic stimulation, is consistent with our findings of an increased KCNQ1-to-KCNE1 mRNA ratio (Jiang et al.). Accompanying altered IKs in IZs are an enhanced TEA-sensitive current and a normal C-9356-sensitive current.
Keywords: epicardial border zone, delayed rectifier, tetraethylammonium, C-9356, isoproterenol
voltage-gated K+ channels are highly responsive to their environment, with many studies using models of acquired heart disease describing highly remodeled K+ currents. Most studies have noted a significant downregulation of the transient outward current (Ito), the K+ current important for early action potential repolarization. Fewer studies have reported on the density and function of the important components of the delayed rectifier currents [rapidly and slowly activating K+ currents (IKr and IKs)]. In the chronic atrioventricular block model, reduced IKs and coordinated downregulation of KCNQ1 and KCNE1 have been described (22, 24). In a feline model of hypertrophy in the healed heart after myocardial infarction, IKr is downregulated (28), whereas in canine cells from the left ventricular midwall injured by coronary microembolizations, IKr is increased and KCNE2 is decreased (11).
In a canine pacing-induced failing heart model, IKs is downregulated but IKr is not (16). E-4031-sensitive currents are increased in some subendocardial Purkinje cells that survive in the infarcted heart (21). Finally, in cells that have survived in the epicardial border zone (EBZ) of the 5-day-infarcted heart (IZs), we found that IKr and IKs were reduced in density but that mRNA changes in specific α- and β-subunits of these currents were not consistent with these changes in function (10). When the changes in delayed rectifier currents were incorporated into our computer model of IZs (4), we noted that IKr and IKs provide little current for repolarization of IZs. However, there are no data on β-adrenergic-stimulated IKs in IZs. Perhaps there is a change in other K+ currents that do not normally contribute to canine repolarization. Thus, in this study, we sought to identify the nature and function of several K+ currents in response to short and long voltage-clamp pulses in IZs of the 5-day-infarcted canine heart. Furthermore, we determined how IKs in IZs is affected by exposure to the β-adrenergic receptor agonist isoproterenol.
METHODS
Animal preparation
This investigation conforms to the National Institutes of Health Guide for the Care and Use of Laboratory Animals [DHHS Publication No. (NIH) 85-23, 1996], and all protocols were approved by the Institution’s Animal Care and Use Committee.
Healthy male mongrel dogs (12–15 kg, 2–3 yr old) were used in these studies. Under isoflurane (30 mg/kg) anesthesia, myocardial infarction was produced by a two-step total occlusion of the left coronary artery using procedure described by Harris (9). At 5 days after occlusion, hearts (n = 35) were used to disperse cells from the EBZ (17). Epicardial slices from normal noninfarcted hearts were used for preparation of normal cells (NZs).
Myocyte preparation
Single Ca2+-tolerant cells were dispersed using a modification of our previously described method (17). Slices were rinsed twice in a Ca2+-free solution (in mM: 115 NaCl, 5 KCl, 35 sucrose, 10 dextrose, 10 HEPES, and 4 taurine, pH 6.95) to remove blood. The solution was triturated in 20 ml of enzyme-containing solution [collagenase type II (Worthington Biochemical) at 0.38 mg/ml and protease type XIV (Sigma Chemical) at 0.05 mg/ml] at 36–37°C for 20 min; then it was decanted and discarded. The second trituration without the protease was discarded after 30 min. The next six to seven triturations were each done for 15 min. Each time, the solution was centrifuged at 500 rpm for 3 min for collection of the supernatant and dispersed cells. Resuspension solution was changed every 30 min for solutions containing increasing concentrations of Ca2+. With this procedure, the viable cell yield was ~30–40%. Only rod-shaped NZs with staircase ends, clear cross-striations, and surface membranes free from blebs were used. IZs had a ruffled appearance with somewhat irregular cross-striations. Additionally, small dark droplets were apparent in IZs and were used to identify myocytes that had survived in the EBZ (17).
Electrophysiological studies
An aliquot of cells was transferred onto a polylysine-coated glass coverslip placed at the bottom of a 0.5-ml tissue chamber that had been mounted on the stage of an inverted microscope (Diaphot, Nikon, Tokyo, Japan). Myocytes were continuously superfused (2–3 ml/min) with Tyrode solution containing (in mM) 140 NaCl, 5.4 KCl, 2.0 MgCl2, 1.8 CaCl2, 10 HEPES, and 10 dextrose (pH 7.4) at 30.0–31.0°C. Cell capacitance was 177 ± 6.2 and 208 ± 8.4 pF for NZs and IZs, respectively.
In the first set of studies, short clamp pulses were used to determine the nature of currents remaining in IZs in the absence of Ito. To measure the outward currents, patch pipettes with resistances of 1–1.5 MΩ when filled with the internal solution (in mM: 140 KCl, 1 MgCl2, 10 EGTA, 5 MgATP, 5 creatine phosphate, 0.2 GTP, and 10 HEPES, with pH adjusted to 7.2 with KOH) were used. For recording, cells were superfused with an Na+-free solution (in mM: 144 N-methyl-D-glucamine chloride, 5.4 KCl, 1 MgCl2, 2.5 CaCl2, 0.5 CdCl2, and 10 HEPES 10, pH 7.4) at 30–31°C. Na+ currents were suppressed with an Na+-free solution, and L-type Ca2+ current (ICa,L) was blocked with 0.5 mM Cd2 + . Membrane currents associated with Na+/Ca2 + exchange were eliminated because of the absence of external Na+. Currents were elicited by 200-ms voltage steps to transmembrane potentials (Vt) of −50 and +60 mV from a holding potential of −60 mV at 0.1 Hz after a 10-ms prepulse to −90 mV. I200 was taken as the amplitude of the current at the end of the test pulse relative to zero-current level (7). Currents sensitive to tetraethylammonium chloride (TEA; 5 mM) or to a compound specific for Kv1.5 currents in canine atria, C-9356 (8), were determined in cells of each group and compared.
A different set of conditions was used to measure current elicited by long clamp pulses, i.e., IKs. Pipettes with resistances of 1−1.5 MΩ when filled with an internal solution containing (in mM) 125 K-aspartate, 20 KCl, 1 MgCl2, 5 HEPES, 5 K-ATP, and 10 EGTA, with pH adjusted to 7.3 with KOH, were used. After formation of the gigaohm seal, the stray capacitance was electronically nulled, the cell membrane under the pipette tip was ruptured, and then, by a brief increase in suction, the whole cell recording configuration was formed. A period of 5 min was allowed for intracellular dialysis to begin before the solution was switched to the external recording solution (132 mM NaCl, 4 mM KCl, 2 mM CaCl2, 1.2 mM MgSO4, 20 mM HEPES, 11.1 mM dextrose, 5 µM nisoldipine, 1 µM dofetilide, and 100 µM DIDS, with pH adjusted to 7.3 with NaOH). Currents were elicited using a series of 5-s pulses to various test potentials from holding potentials of −50 mV, followed by repolarization to −20 mV for recording of tail current. Data collection was begun 10 min after membrane rupture and completed within 30 min to minimize “run down” of IKs (10).
In some experiments, currents elicited in NZs and IZs were determined and compared as currents blocked by 10 µM azimilide. Azimilide has been shown to inhibit IKs via a binding site on the KCNQ1 protein (3). At this concentration and under our recording conditions, azimilide has little or no effect on other currents (27).
Data acquisition and analysis
Clamp protocol generation and data acquisition were controlled by Clampex (version 8) via an analog-to-digital converter (Digidata 1200, Axon Instruments). Transmembrane currents were obtained using Axopatch 200 amplifiers (Axon Instruments), low-pass filtered at 2 kHz, digitized at 0.1 kHz, and sampled online to a computer hard disk. Clampfit of pClamp (version 8.1) was used for amplitude measurement and curve fitting.
Drugs
Azimilide, dofetilide, and nisoldipine were first prepared as stock solutions and then added to the test solution as required. DIDS (Sigma) was prepared daily as a 10 mM stock solution, dissolved in water, and added to the test solution for a final concentration of 100 µM. DIDS-containing solutions were protected from light to prevent degradation. Isoproterenol (Sigma) stock solution was made on the day of the experiment (1 mg/ml) and added to the test solution for a final concentration of 1 µM. TEA (Sigma) was dissolved in double-distilled water for stock solution. C-9356 was a gift from Cardiome Pharma (Vancouver, BC, Canada). The stock solution of C-9356 (20 mM) was made in DMSO; final bath concentration of DMSO was 0.05%.
Statistics
Values are means ± SE. Statistical analysis was performed using SigmaStat Student’s t-test and paired t-test. P < 0.05 was significant.
RESULTS
Effect of TEA on I200
The time course of the effects of 5 mM TEA in NZs and IZs is shown in Fig. 1A. TEA inhibits outward currents of NZs and IZs. A typical current-voltage (I-V) curve of I200 in the absence and presence of TEA and the TEA-sensitive current is plotted in Fig. 1B. We found smaller TEA-sensitive I200 in NZs: 0.06 ± 0.02 pA/pF in NZs (n = 10) vs. 0.32 ± 0.07 pA/pF in IZs (n = 12) at +40 mV (P < 0.05). Percent inhibition of I200 by TEA was significantly larger at +40 mV in IZs than in NZs: 22.1 ± 3.9% vs. 4.5 ± 1.7% (P < 0.05; Fig. 1C). Thus, in IZs with reduced or absent Ito (17), there is a TEA-sensitive increase in plateau outward current.
Fig. 1.
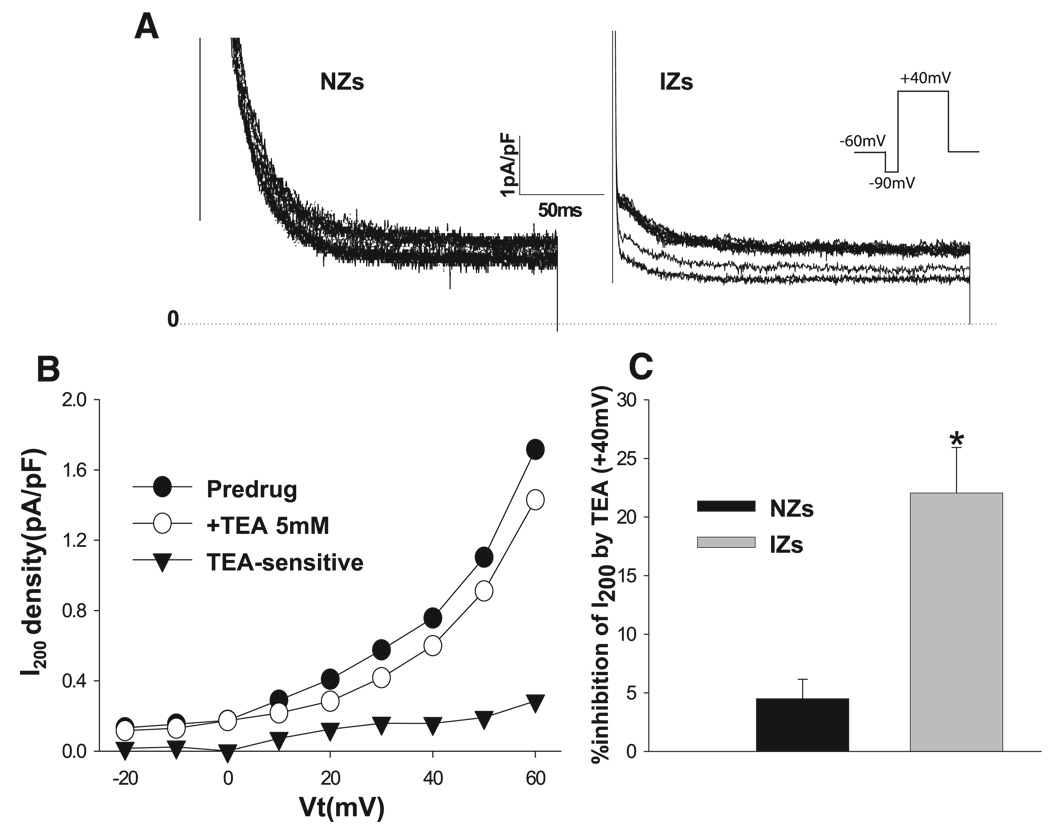
Effects of tetraethylammonium chloride (TEA, 5 mM) on currents elicited by 200-ms voltage steps from −50 to +60 mV at 0.1 Hz after a 10-ms prepulse to −90 mV (I200) in normal cells (NZs) and surviving cells in the epicardial border zone of a 5-day-infarcted heart (IZs). A: typical recording of the time course of the effect of 5 mM TEA on outward currents elicited with a holding potential of −60 mV and a transmembrane potential (Vt) of +40 mV for 200 ms in NZs and IZs. NZ records have been enlarged/truncated to emphasize the effect on sustained currents. Dotted line, zero-current level. B: typical current-voltage (I-V) plot of I200 before TEA (Predrug), with 5 mM TEA, and TEA-sensitive currents in an IZ. I200 was measured as the amplitude of the current at the end of the test pulse relative to zero-current level. C: percent inhibition of I200 by TEA at +40 mV in NZs (n = 12) and IZs (n = 10). *P < 0.05 vs. NZs.
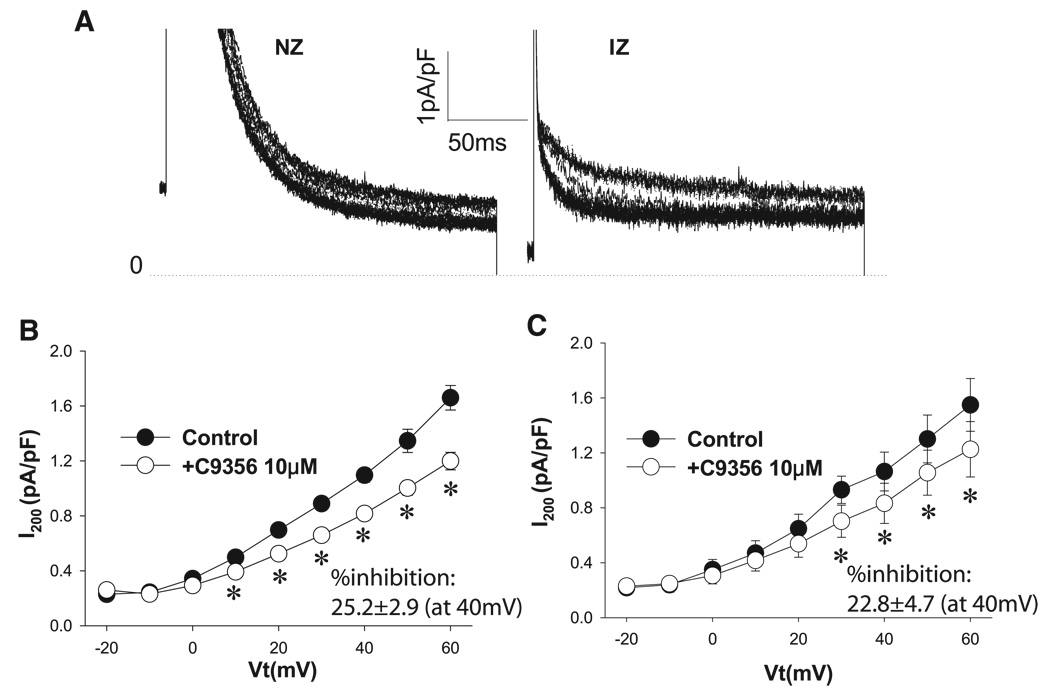
Effect of the Kv1.5 blocker C-9356 on I200
Because TEA inhibited I200 in IZs (see Effect of TEA on I200), we considered whether other components that may not be expected on the basis of normal electrophysiology might contribute to I200, for example, the atrium-specific current IKur, which presumably is due to Kv1.5. Thus we determined and compared the effects of a drug that blocks Kv1.5 selectively (at 10 µM), with minor or no effects on Kv3.1, Kv2.1, or Kv4.2 currents (8). We used 10 µM C-9356 in our study of currents in NZs and IZs in the absence of TEA. Figure 2A shows the time course of the effect of C-9356 as it equilibrates in an NZ and an IZ. Note the effect on I200 in each cell type. Average I-V plots of the effect of C-9356 on I200 are shown in Fig. 2, B and C, for cells that showed a response. The percent inhibition by C-9356 at +40 mV did not differ between the two cell groups (25.2 ± 2.9% and 22.8 ± 4.7% in NZ and IZ, respectively, P > 0.05). Thus there is no change in C-9356-sensitive currents in the greatly remodeled IZ.
Fig. 2.
Effects of C-9356 (10 µM) on I200 in NZs and IZs. A: typical recording of the time course of the effect of 10 µM C-9356 on outward currents in an NZ and an IZ. See Fig. 1 legend for protocol. NZ records have been enlarged/truncated to emphasize the effect on sustained currents. Dotted line, zero-current level. B: effect of C-9356 on I-V relation of I200 in NZs. C-9356 (10 µM) significantly inhibited I200 in NZs. *P < 0.05 vs. control. C: effect of C-9356 on I-V relation of I200 in IZs. Similar to its effect on NZs, C-9356 significantly inhibited I200 in IZs. *P < 0.05 vs. control.
Effects of isoproterenol on IKs
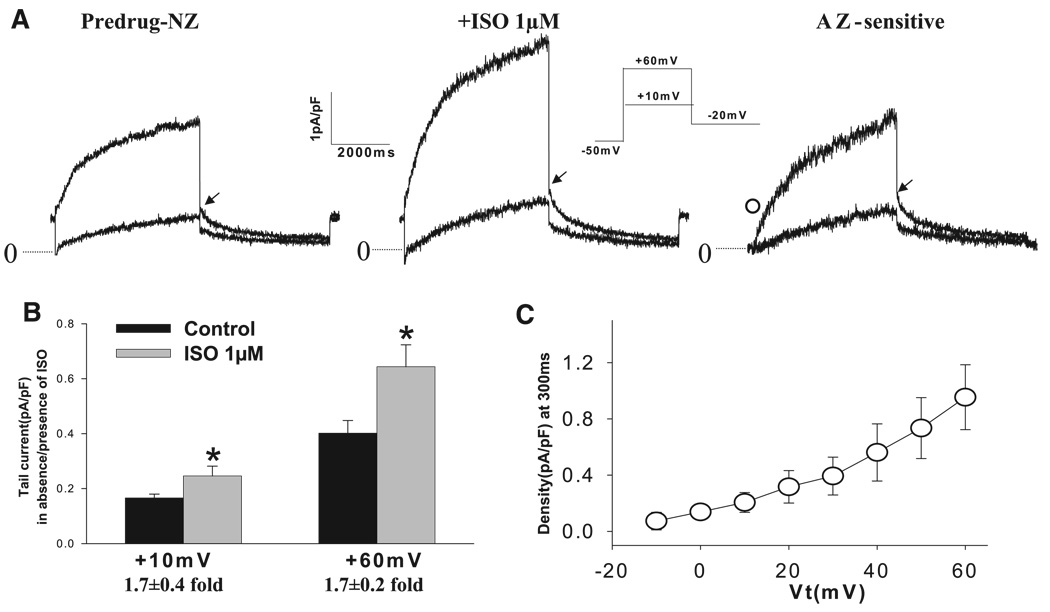
Increased TEA-sensitive sustained currents in IZs with short clamp pulses could be due to IKs that is altered (10), because KCNQ1 channels are TEA sensitive (14). Therefore, in this set of studies, we elicited currents with long clamp pulses in IZs and NZs in the absence or presence of β-adrenergic stimulation. Recording conditions (see methods) were designed to eliminate contaminating currents that could occur as we tested the effects of 1 µM isoproterenol in both cell types. Figure 3A shows the currents elicited in the absence and presence of isoproterenol and the corresponding azimilide-sensitive currents for a typical NZ. Depolarization pulses (to +10 and +60 mV) elicited time-dependent outward currents that continued to increase during the 5-s pulses. Subsequent repolarization was accompanied by slowly decaying tail currents. Isoproterenol produced a marked increase in IKs tail density (from 0.38 ± 0.06 to 0.64 ± 0.08 pA/pF at +60 mV, P < 0.05), representing an average 1.7 ± 0.2-fold effect in NZs (n = 15; Fig. 3B). Average I-V plots of time-dependent azimilide-sensitive currents during test pulses in NZs are shown in Fig. 3C. Under these conditions, isoproterenol-stimulated azimilide-sensitive currents represent iso-proterenol-enhanced IKs, because other azimilide-sensitive currents are blocked (e.g., IKr by 1 µM dofetilide).
Fig. 3.
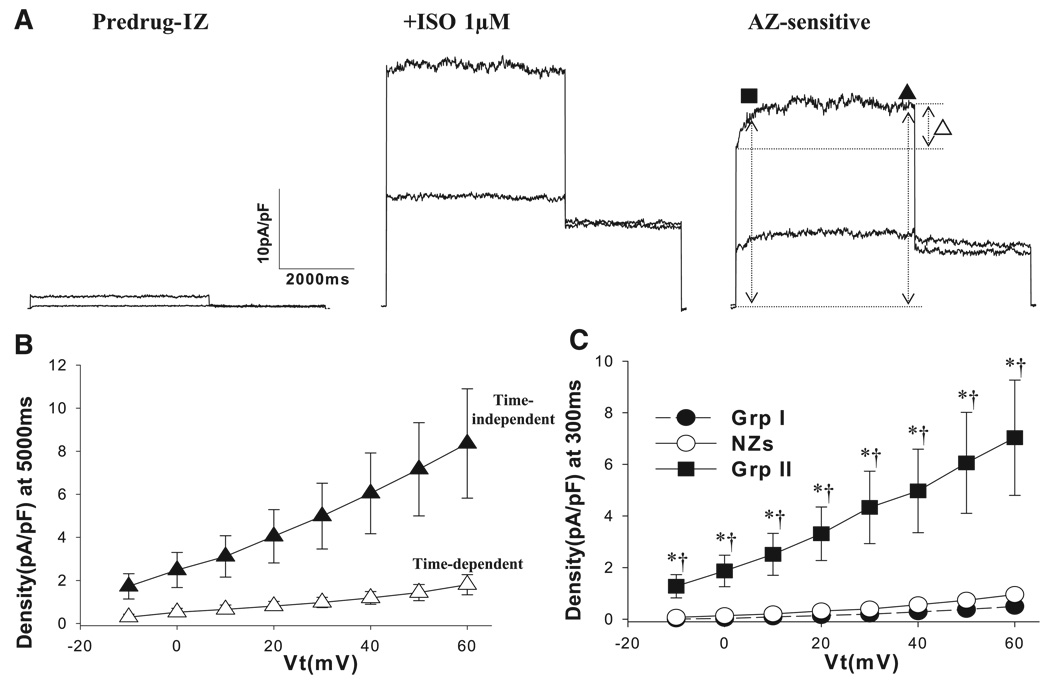
Effects of isoproterenol (1 µM, Iso) stimulation on slowly activating K+ current (IKs) in NZs. A: representative traces of IKs in control (Predrug) and with isoproterenol (1 µM) and typical azimilide (AZ)-sensitive currents. IKs was isolated in the presence of nisoldipine (2 µM), dofetilide (1 µM), and DIDS (100 µM) to block Ca2+ current, rapidly activating K+ current (IKr), and Cl− current, respectively. Currents were elicited from a holding potential of −50 mV to Vt of +10 and +60 mV for 5 s, followed by repolarization to −20 mV for recording of tail currents (inset). Time interval was 30 s. Dotted lines, zero-current level. IKs tail currents were measured as peak tail currents (arrow) upon repolarization on the basis of zero-current level. ○, AZ-sensitive current at 300 ms of activation (in pA/pF). B: in NZs (n = 15), IKs tail density was significantly increased with isoproterenol stimulation. *P < 0.05 vs. control. At +60 mV, the effect was 1.7 ± 0.2-fold. C: average I-V relation of time-dependent AZ-sensitive currents (n = 9) at 300 ms of activation in the presence of 1 µM isoproterenol.
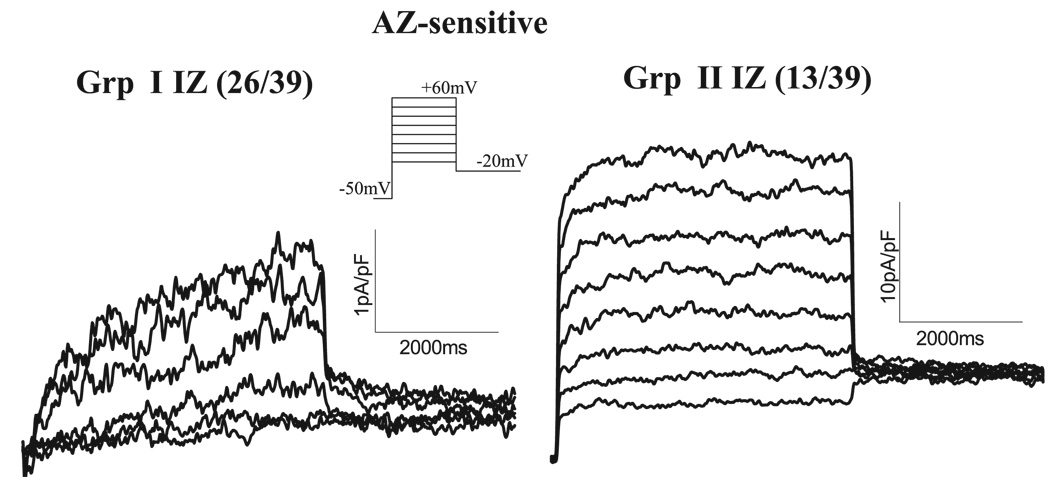
In IZs, we observed two contrasting phenotypes of isoproterenol-stimulated azimilide-sensitive currents (Fig. 4): group I and group II IZs. In group I, IKs is downregulated, and isoproterenol affected IKs by producing a slowly activating current. This response was observed in 67% of IZs tested. In group II, isoproterenol-stimulated azimilide-sensitive currents were voltage dependent, fast activating, large in amplitude, and showed no apparent tail currents. In 33% of IZs tested, we observed the group II phenotype. In 4 of 33 (12%) NZs tested, we saw a small current that was similar to this phenotype.
Fig. 4.
Two types of AZ-sensitive currents (IKs) were observed in the presence of isoproterenol in IZs. Currents were elicited from a holding potential of −50 mV to a Vt of −10 to +60 mV for 5 s, followed by repolarization to −20 mV (inset). Two types of phenotypic responses were observed in IZs: group I (Grp I, 26 of 39 IZs) and group II (Grp II, 13 of 39 IZs).
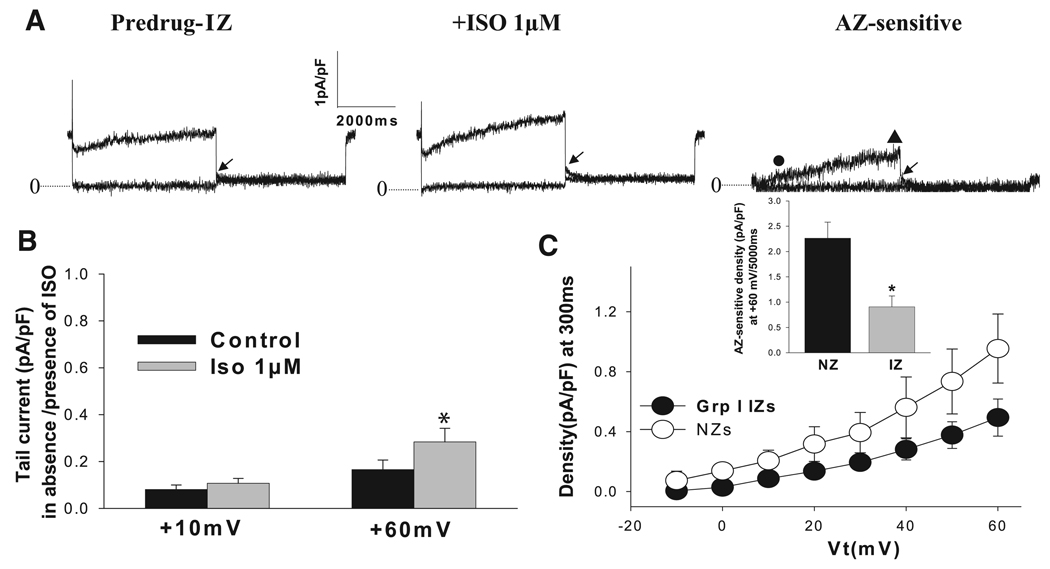
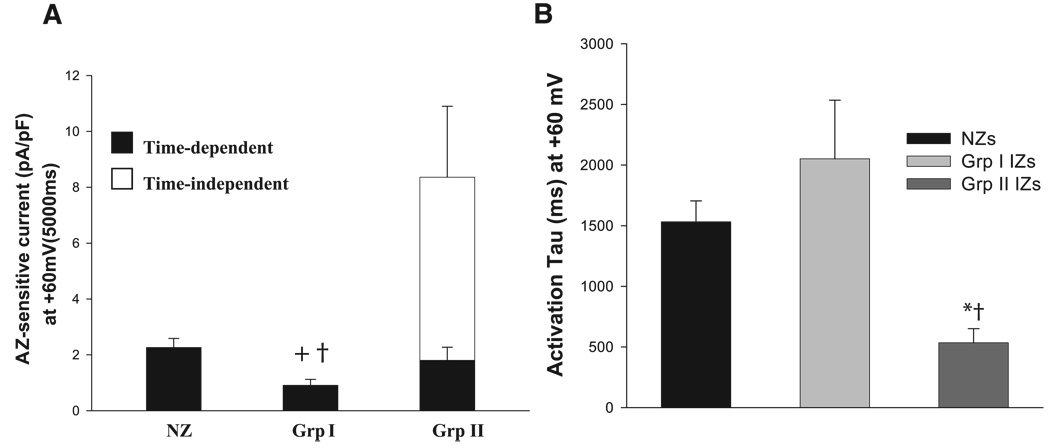
In group I IZS, where IKs is downregulated in the absence of drug (Fig. 5A), the effect of isoproterenol on IKs was similar to that in NZs; i.e., there was no difference in the “fold” effect (P > 0.05; Fig. 5B). Azimilide-sensitive currents in group I IZs were not different from those in NZs at short (300-ms) intervals (Fig. 5C) but were significantly decreased in IZs at longer intervals (Fig. 5C, inset). In group II IZs (Fig. 6), currents in the absence of isoproterenol were small (Fig. 6A), and azimilide-sensitive currents were time independent and time dependent (Fig. 6B). Sensitive currents at 300 and 5,000 ms were strikingly increased over NZs and group I IZs (Figs. 6C and Fig. 7A). Finally, the activation of azimilide-sensitive currents was rapid in group II IZs compared with group I IZs and NZs (P < 0.05; Fig. 7B).
Fig. 5.
Effects of isoproterenol on IKs in group I IZs. A: representative traces of IKs in control (Predrug) and with isoproterenol (1 µM) and AZ-sensitive currents. See Fig. 3 legend for recording conditions and protocols. Dotted lines, zero-current level; arrow, IKs tail currents measured as peak tail currents; ●, AZ-sensitive current at 300 ms (in pA/pF); ▲, AZ-sensitive current at 5,000 ms at +60 mV (in pA/pF). B: in group I IZs (n = 19), although IKs tail density was reduced vs. NZ values, it was significantly increased with isoproterenol. *P < 0.05 vs. control. At +60 mV, effect was 2.2 ± 0.3-fold, which was not different from NZs (P > 0.05). C: average I-V relation of time-dependent AZ-sensitive currents at 300 ms in the presence of 1 µM isoproterenol in group I IZs (n = 14). Inset: maximal currents at 5,000 ms at +60 mV were reduced in group I IZs. *P < 0.05 vs. NZs.
Fig. 6.
Effects of isoproterenol on IKs in group II IZs. A: representative traces of K+ current (IK) in control (Predrug) and with isoproterenol and AZ-sensitive currents. See Fig. 3 legend for recording conditions and protocols. Note marked difference in the genotype of this isoproterenol-stimulated AZ-sensitive current. B: average I-V relations of AZ-sensitive time-independent currents in group II IZs (n = 13). For comparison, time-dependent component is plotted (△). ▲, AZ-sensitive current at 5,000 ms at +60 mV (in pA/pF); ■, AZ-sensitive current at 300 ms (in pA/pF). C: average I-V relation of AZ-sensitive currents at 300 ms of activation in group II IZs (n = 13) and I-V relations of NZs and group I IZs. Currents were significantly larger in group II IZs than in NZs and group I IZs. *P < 0.05 vs. NZs. †P < 0.05 vs. group I IZs.
Fig. 7.
Effects of isoproterenol on activation of IKs in NZs, group I IZs, and group II IZs. A: summary of AZ-sensitive current density at the end of the depolarizing pulse (+60 mV). In NZs and group I IZs, there was only a time-dependent component. In group II IZs, there was a large time-independent component. Note similarity of time-dependent current in group II IZs and NZs. Time-dependent current was smaller in group I IZs than in NZs and group II IZs. +P < 0.05 vs. NZs. †P < 0.05 vs. group II IZs. B: activation time constants (tau) at +60 mV for NZs, group I IZs, and group II IZs. Currents during depolarization pulses at +60 mV were fit with a single-exponential function. NZ and group I IZ currents activated more slowly than group II IZ currents. *P < 0.05 vs. NZ. †P < 0.05 vs. group I IZ.
DISCUSSION
We have demonstrated that I200 is more sensitive to TEA in IZs than in NZs, although I200 in NZs and IZs shows a similar response to the Kv1.5 blocker C-9356. The effects of β-adrenergic stimulation on K+ current were similar in NZs and IZs; the exception is a new phenotype of adrenergic-mediated currents, the group II IZs.
Our previous reports of the remodeling of ion channels in cells that survive in the 5-day-infarcted heart (IZs) showed that Ito is absent (17), Ca2+ current is reduced (1), and K+ current is altered (10). In the latter study (10), we found altered kinetics as well as amplitude in K+ currents in IZs. Because of these changes, action potential repolarization in IZs is critically dependent on remaining currents. The nature of these currents is not totally known. In this study, we hypothesized that there may be a change in other K+ currents that do not normally contribute to canine repolarization. We tested for the existence of IKur or a rapidly activating outward current similar to the current that is key to atrial cell repolarization. Kv1.5 protein is thought to underlie this current in canine atrial cells (8). We previously showed pronounced structural remodeling (lateralization) of Kv1.5 protein in cells that survive in the EBZ (19). However, in the present study, we report no difference in C-9356-sensitive currents between NZs and IZs. In contrast to a previous report (8), we found small C-9356-sensitive currents in normal canine ventricular cells, suggesting that this current may contribute to ventricular repolarization under normal conditions.
To our surprise, we show an upregulation of TEA-sensitive currents in IZs. The molecular nature of this upregulated TEA-sensitive current is unknown, but, on the basis of its TEA sensitivity, this current most probably corresponds to an up-regulated Kv2.1 current, which in mouse ventricle underlies Ito,slow2 (25).
IKs. We considered that an upregulated TEA-sensitive current could be due to an altered IKs (10), because TEA blocks slowly activating K+ channels (14). However, when clamp studies were designed to isolate and study IKs in NZs and IZs, we found no difference in currents (in the presence of isoproterenol) at the end of short intervals in most IZs (Fig. 5C). We found that in IZs where IKs was reduced, isoproterenol affected the current as it did in NZs (Figs. 3B and 5B). In a previous study, we showed that the responsiveness of ICa,L to β-adrenergic stimulation was reduced in these 5-day-infarcted myocytes (2). In contrast to those results, we report here that the fold effect of β-adrenergic stimulation on IKs did not differ between NZs and IZs, although IKs was smaller in group I IZs. Our knowledge that ICa,L and K+ current are differentially regulated by cAMP-dependent protein kinase cascade in the newborn canine epicardial cell (5) and under special cases of atrial fibrillation (6) suggests preferential paths of activation of different ion channels by specific and different pools of cAMP in canine cells.
In a large group of IZs (group II), we found a “different” type of azimilide-sensitive current in the presence of isoproterenol (Fig. 6). Although single-cell PCR was not completed in each of these group II IZs, our previous mRNA studies showed that mRNA of KCNQ1 returns to its control level, whereas that of KCNE1 remains reduced in the EBZ of the 5-day-infarcted heart (10). If these changes in mRNA lead to corresponding changes in subunit protein levels, we might expect an effect on kinetics of K+ current in cells surviving in the infarcted heart, because KCNE1 subunits are decreased relative to KCNQ1 subunits. Studies designed to determine the molecular basis for PKA regulation of IKs have shown that expression of KCNE1 is required with KCNQ1, and the KCNQ1 NH2-terminal residue is critical for this action (13, 15). Recently, it has been observed that lone KCNQ1 (Kv-LQT1) can be a target for cAMP (26), suggesting that adrenergic stimulation may modulate this K+ current at two (or more) sites: KCNE1 and KCNQ1. We suggest that because KCNQ1 mRNA is not different in cells from EBZ of the 5-day-infarcted heart and KCNE1 is markedly reduced, IZs have ionic currents with a structural component that is solely KCNQ1 proteins. Such “lone” KCNQ1 currents would resemble those of group II cells in Fig. 6. Interestingly, during development of the canine epicardial cell, similar-appearing K+ currents change phenotype as KCNE1 is expressed and IKs increases (12). Alternatively, group II IZs have a K+ current complex that is formed by KCNQ1 aligned with another member of the KCNE family, such as KCNE3. Recently, KCNE3 has been shown to convert the slow voltage-dependent activation of K+ current to a voltage-dependent rapidly activating current (18, 20, 23).
In summary, for some IZs where IKs is downregulated, isoproterenol affects IKs as it affects IKs in NZs. In other IZs, lone KCNQ1-type currents, which are sensitive to β-adrenergic stimulation, exist and are consistent with our findings of an increased KCNQ1-to-KCNE1 mRNA ratio determined in our previous work (10). Accompanying these altered IKs in IZs are an enhanced TEA-sensitive current and a normal C-9356-sensitive current. Heterogeneity in IZ K+ current phenotypes induced by isoproterenol will contribute to diverse adrenergic responses of EBZ fibers.
Acknowledgments
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-66140.
REFERENCES
- 1.Aggarwal R, Boyden PA. Diminished calcium and barium currents in myocytes surviving in the epicardial border zone of the 5 day infarcted canine heart. Circ Res. 1995;77:1180–1191. doi: 10.1161/01.res.77.6.1180. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal R, Boyden PA. Altered pharmacologic responsiveness of reduced L-type calcium currents in myocytes surviving in the infarcted heart. J Cardiovasc Electrophysiol. 1996;7:20–35. doi: 10.1111/j.1540-8167.1996.tb00457.x. [DOI] [PubMed] [Google Scholar]
- 3.Busch AE, Busch GL, Ford E, Suessbrich H, Lang HJ, Greger R, Kunzelmann K, Attali B, Stuhmer W. The role of the IsK protein in the specific pharmacological properties of the IKs channel complex. Br J Pharmacol. 1997;122:187–189. doi: 10.1038/sj.bjp.0701434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cabo C, Boyden PA. Electrical remodeling of the epicardial border zone in the canine infarcted heart: a computational analysis. Am J Physiol Heart Circ Physiol. 2003;284:H372–H384. doi: 10.1152/ajpheart.00512.2002. [DOI] [PubMed] [Google Scholar]
- 5.Charpentier F, Liu Q, Rosen MR, Robinson RB. Age-related differences in β-adrenergic regulation of repolarization in canine epicardial myocytes. Am J Physiol Heart Circ Physiol. 1996;271:H1174–H1181. doi: 10.1152/ajpheart.1996.271.3.H1174. [DOI] [PubMed] [Google Scholar]
- 6.Chen YH, Xu SJ, Bendahhou S, Wang XL, Wang Y, Xu W, Jin H, Sun H, Su X, Zhuang Q, Yang Y, Li Y, Liu Y, Xu H, Li X, Ma N, Mou C, Chen Z, Barhanin J, Huang W. KCNQ1 gain of function mutation in familial atrial fibrillation. Science. 2003;299:251–254. doi: 10.1126/science.1077771. [DOI] [PubMed] [Google Scholar]
- 7.Dun W, Chandra P, Danilo P, Jr, Rosen MR, Boyden PA. Chronic atrial fibrillation does not further decrease outward currents. It increases them. Am J Physiol Heart Circ Physiol. 2003;285:H1378–H1384. doi: 10.1152/ajpheart.00137.2003. [DOI] [PubMed] [Google Scholar]
- 8.Fedida D, Eldstrom J, Hesketh JC, Lamorgese M, Castel L, Steele DF, Van Wagoner DR. Kv1.5 is an important component of repolarizing K current in canine atrial myocytes. Circ Res. 2003;93:744–751. doi: 10.1161/01.RES.0000096362.60730.AE. [DOI] [PubMed] [Google Scholar]
- 9.Harris AS. Delayed development of ventricular ectopic rhythms following experimental coronary occlusion. Circulation. 1950;1:1318–1328. doi: 10.1161/01.cir.1.6.1318. [DOI] [PubMed] [Google Scholar]
- 10.Jiang M, Cabo C, Yao JA, Boyden PA, Tseng GN. Delayed rectifier K currents have reduced amplitudes and altered kinetics in myocytes from infarcted canine ventricle. Cardiovasc Res. 2000;48:34–43. doi: 10.1016/s0008-6363(00)00159-0. [DOI] [PubMed] [Google Scholar]
- 11.Jiang M, Zhang M, Tang DG, Clemo HF, Liu J, Holwitt D, Kasirajan V, Pond A, Wettwer E, Tseng GN. KCNE2 protein is expressed in ventricles of different species and changes in its expression contribute to electrical remodeling in diseased hearts. Circulation. 2004;109:1783–1788. doi: 10.1161/01.CIR.0000124225.43852.50. [DOI] [PubMed] [Google Scholar]
- 12.Krishnamurthy G, Patberg K, Obreztchikova MN, Rybin AV, Rosen MR. Developmental evolution of the delayed rectifier current in IKs in canine heart appears dependent on the β-subunit minK. Heart Rhythm. 2004;1:704–711. doi: 10.1016/j.hrthm.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Kurokawa J, Chen L, Kass RS. Requirement of subunit expression for cAMP-mediated regulation of a heart potassium channel. Proc Natl Acad Sci USA. 2003;100:2122–2127. doi: 10.1073/pnas.0434935100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurokawa J, Motoike HK, Kass RS. TEA sensitive KCNQ1 constructs reveal pore-independent access to KCNE1 in assembled IKs channels. J Gen Physiol. 2001;117:43–52. doi: 10.1085/jgp.117.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurokawa J, Motoike HK, Rao J, Kass RS. Regulatory actions of the A kinase anchoring protein Yotiao on a heart potassium channel downstream of PKA phosphorylation. Proc Natl Acad Sci USA. 2004;101:16374–16378. doi: 10.1073/pnas.0405583101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li D, Melnyk P, Feng J, Wang Z, Petrecca K, Shrier A, Nattel S. Effects of experimental heart failure on atrial cellular and ionic electrophysiology. Circulation. 2000;101:2631–2638. doi: 10.1161/01.cir.101.22.2631. [DOI] [PubMed] [Google Scholar]
- 17.Lue WM, Boyden PA. Abnormal electrical properties of myocytes from chronically infarcted canine heart. Alterations in Vmax and the transient outward current. Circulation. 1992;85:1175–1188. doi: 10.1161/01.cir.85.3.1175. [DOI] [PubMed] [Google Scholar]
- 18.Lundquist A, Mandefield L, Vanoye C, Rogers C, Donahue B, Chang P, Drinkwater D, Murray KT, George AL., Jr Expression of multiple KCNE genes in human heart may enable variable modulation of IKs. J Mol Cell Cardiol. 2005;38:277–287. doi: 10.1016/j.yjmcc.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Mays DJ, Boyden PA, Tamkun MM. Redistribution of the Kv1.5 K+ channel protein on the surface of myocytes from the epicardial border zone of the infarcted canine heart. CV Pathobio. 1997;2:79–87. [Google Scholar]
- 20.Melman Y, Krummerman A, McDonald TV. KCNE regulation of KvLQT1 channels: structure-function correlates. Trends Cardiovasc Med. 2002;12:182–185. doi: 10.1016/s1050-1738(02)00158-5. [DOI] [PubMed] [Google Scholar]
- 21.Pinto JM, Boyden PA. Reduced inward rectifying and increased E-4031-sensitive K+ current density in arrhythmogenic subendocardial Purkinje myocytes from the infarcted heart. J Cardiovasc Electrophysiol. 1998;9:299–311. doi: 10.1111/j.1540-8167.1998.tb00915.x. [DOI] [PubMed] [Google Scholar]
- 22.Ramakers C, Vos MA, Doevendans P, Schoenmakers M, Wu YS, Scicchitano S, Iodice A, Thomas GP, Antzelevitch C, Dumaine R. Coordinated down regulation of KCNQ1 and KCNE1 expression contributes to reduction of IKs in canine hypertrophied hearts. Cardiovasc Res. 2003;57:486–496. doi: 10.1016/s0008-6363(02)00717-4. [DOI] [PubMed] [Google Scholar]
- 23.Schroeder BC, Waldegger S, Fehr S, Bleich M, Warth R, Greger R, Jentsch TJ. A constitutively open potassium channel formed by KCNQ1 and KCNE3. Nature. 2000;403:196–199. doi: 10.1038/35003200. [DOI] [PubMed] [Google Scholar]
- 24.Volders PG, Sipido KR, Vos MA, Spatjens RLHMG, Leunissen J, Carmeliet E, Wellens HJJ. Downregulation of delayed rectifier K currents in dogs with chronic complete atrioventricular block and acquired torsades de pointes. Circulation. 1999;100:2455–2461. doi: 10.1161/01.cir.100.24.2455. [DOI] [PubMed] [Google Scholar]
- 25.Xu H, Barry DM, Li H, Brunet S, Guo W, Nerbonne JM. Attenuation of the slow component of delayed rectification, action potential prolongation, and triggered activity in mice expressing a dominant-negative Kv2 α-subunit. Circ Res. 1999;85:623–633. doi: 10.1161/01.res.85.7.623. [DOI] [PubMed] [Google Scholar]
- 26.Yang T, Kanki H, Roden DM. Phosphorylation of the IKs channel complex inhibits drug block: novel mechanism underlying variable antiarrhythmic drug actions. Circulation. 2003;108:132–134. doi: 10.1161/01.CIR.0000082708.86266.B8. [DOI] [PubMed] [Google Scholar]
- 27.Yao JA, Tseng GN. Azimilide (NE-10064) can prolong or shorten the action potential duration in canine ventricular myocytes: dependence on blockade of K,Ca and Na channels. J Cardiovasc Electrophysiol. 1997;8:184–198. doi: 10.1111/j.1540-8167.1997.tb00780.x. [DOI] [PubMed] [Google Scholar]
- 28.Yuan F, Pinto JMB, Hong Z, Myerburg R, Bassett AL. Abnormal delayed rectifier potassium current (IK) in feline left ventricular myocytes adjacent to healed myocardial infarct (Abstract) Circulation. 1995;92:1–158. [Google Scholar]