Abstract
Heavy prenatal alcohol exposure can cause alterations to the developing brain. The resulting neurobehavioral deficits seen following this exposure are wide-ranging and potentially devastating and, therefore, are of significant concern to individuals, families, communities, and society. These effects occur on a continuum, and qualitatively similar neuropsychological and behavioral features are seen across the spectrum of effect. The term fetal alcohol spectrum disorders (FASD) has been used to emphasize the continuous nature of the outcomes of prenatal alcohol exposure, with fetal alcohol syndrome (FAS) representing one point on the spectrum. This paper will provide a comprehensive review of the neuropsychological and behavioral effects of heavy prenatal alcohol exposure, including a discussion of the emerging neurobehavioral profile. Supporting studies of lower levels of exposure, brain-behavior associations, and animal model systems will be included when appropriate.
Keywords: fetal alcohol syndrome (FAS), fetal alcohol spectrum disorders (FASD), neuropsychological outcome, behavior, neurobehavioral profile, behavioral teratology
Of the many potential outcomes of prenatal alcohol exposure, alterations to the developing brain and resulting neurobehavioral deficits are of the most devastating. The term fetal alcohol spectrum disorders (FASD) has been used to emphasize the continuous nature of the effects of prenatal alcohol exposure, with the fetal alcohol syndrome (FAS) at the more severe end of the spectrum. Exposure to alcohol in utero is associated with cognitive impairment in various neuropsychological domains, including overall intellectual performance, executive function, learning and memory, language, visual-spatial ability, motor function, attention, and activity levels as well as behavioral problems including adaptive dysfunction, academic difficulties, and increased rates of psychiatric disorders. This paper will provide a comprehensive review of the neuropsychological effects of heavy prenatal alcohol exposure. Supporting studies of lower levels of exposure, brain-behavior associations, and animal model systems will be included when appropriate. Related topics, including structural (Lebel et al., 2011) and functional (Coles et al., 2011) neuroimaging and diffusion tensor imaging (Wozniak et al., 2011) as well as interventions (Kodituwakku et al., 2011), also are included in this issue.
Diagnostic Terminology
The term FAS, or fetal alcohol syndrome, is used throughout this paper to refer to patients or subjects who meet the diagnostic criteria for FAS, as defined by the Institute of Medicine (IOM; Stratton, Howe, & Battaglia, 1996), including modifications suggested by Hoyme et al. (Hoyme et al., 2005). The term FASD, or fetal alcohol spectrum disorders, is used to refer to the larger group of patients who are affected by prenatal alcohol exposure and who may or may not meet diagnostic criteria for FAS (Bertrand et al., 2004). Individuals encompassed under this non-diagnostic umbrella term are those with diagnoses of FAS, partial FAS, or alcohol-related neurodevelopmental disorder (ARND) (Bertrand, et al., 2004; Hoyme, et al., 2005), as well as individuals affected by prenatal alcohol exposure who may not have any diagnosis.
One of the biggest challenges in understanding the considerable variability of neurobehavioral outcomes of prenatal alcohol exposure involves identifying the dose and pattern of alcohol consumption as well as developmental timing of exposure. In general, the amount of alcohol consumed is correlated with the severity of outcome (e.g., Sood et al., 2001; Streissguth, Sampson, & Barr, 1989). However, pattern of alcohol exposure can often moderate these effects, with binge-like exposures resulting in more severe deficits than chronic exposure (Bailey et al., 2004; Bonthius, Goodlett, & West, 1988). Timing of exposure is also important. Alcohol exposure during different periods of fetal development can greatly influence the pattern and severity of structural and functional abnormalities (Guerri, Bazinet, & Riley, 2009). Unfortunately, this level of detail is often difficult to document, particularly in retrospectively recruited samples, and individual studies provide varying degrees of detail concerning levels and patterns of exposure. However, although criteria used to delineate heavy prenatal alcohol exposure may be inconsistent across studies, these samples generally include children of women who meet criteria for alcohol abuse and dependence (e.g., Coles et al., 1991; Mattson, Riley, Gramling, Delis, & Jones, 1998; Mattson et al., 2010). Prospectively recruited samples, by design, allow for a greater precision when describing dose, pattern, and timing effects of prenatal alcohol exposure, but exposure levels are generally lower than in retrospective samples. Lower levels of exposure are generally defined as one to three drinks per week (e.g., Fried & Watkinson, 1988; Jacobson, Jacobson, Sokol, Martier, & Ager, 1993; Willford, Richardson, Leech, & Day, 2004). Different criteria for moderate to low exposure are included when available. These issues highlight the complexity of conducting research on prenatal alcohol exposure and the many risk factors and moderating variables that contribute to the considerable range of phenotypes presented by individuals with FASD.
General Intelligence
One of the most common neurocognitive findings among those exposed to alcohol during pregnancy is diminished intellectual capacity. The majority of individuals diagnosed with FAS are not intellectually disabled (defined as overall IQ score < 70 and adaptive disability), and intellectual disability is not a necessary criterion for the diagnosis of FAS. However, FAS is considered one of the leading identifiable causes of mental retardation (i.e., intellectual disability, Abel & Sokol, 1987; Pulsifer, 1996). Many affected individuals exhibit impaired intellectual abilities, even in the absence of facial features (i.e., smooth philtrum, short palpebral fissures, and thin vermillion) and growth retardation (Dalen, Bruaroy, Wentzel-Larsen, & Laegreid, 2009; Mattson, Riley, Gramling, Delis, & Jones, 1997), although children with a diagnosis of FAS tend to have more severe impairments than those who were exposed prenatally to alcohol but do not have sufficient dysmorphic features for a diagnosis (Mattson, et al., 1997). One study indicated that children with FAS have mean IQ scores significantly lower than those with partial FAS and ARND, who do not differ significantly from one another (Chasnoff, Wells, Telford, Schmidt, & Messer, 2010), and another reported a significant relation between general cognitive function and degree of dysmorphic features and growth deficiency (Ervalahti et al., 2007). The average IQ estimate of individuals with heavy prenatal alcohol exposure is 70 for those with FAS (Streissguth et al., 1991) and 80 for nondysmorphic individuals (Mattson, et al., 1997). Although a clinically significant difference between verbal and nonverbal intelligence scores is often present, the direction of this difference is not consistent (for review, see Mattson & Riley, 1998). Furthermore, IQ score is significantly correlated with psychopathology; children with moderate and severe intellectual disability experienced greater psychiatric disturbance, and IQ scores below 50 indicated poor psychiatric outcome (Steinhausen, Willms, & Spohr, 1994).
Much less research has examined intellectual abilities among individuals with lower levels of alcohol exposure, and results have been conflicting. Early studies found moderate levels of prenatal alcohol exposure to be associated with decreases in intelligence scores in young children (Fried & Watkinson, 1988; Streissguth, Barr, & Sampson, 1990; Streissguth, Barr, Sampson, Darby, & Martin, 1989). A more recent study found that moderate alcohol exposure during first and second trimesters significantly affected intellectual ability, but only among African American children (Willford, Leech, & Day, 2006). However, another study reported that moderate levels of consumption are not associated with verbal, performance, or overall IQ scores in childhood (Alati et al., 2008). Although a weak effect of binge drinking (4 or more units of alcohol per occasion) was found, it was attenuated when mother’s educational attainment was taken into account. The discrepancies among studies with lower levels of exposure highlight the need for additional research and importance of considering a variety of factors when evaluating studies.
Executive Function
Great interest has been shown in understanding higher-order cognitive processes in developmental psychopathologies, including FASD. Executive functions are related to frontal-subcortical circuits involving projections from the frontal lobes to the basal ganglia and thalamic nuclei (Cummings, 1993), which have been found to be vulnerable to prenatal alcohol exposure (Fryer et al., 2007; Mattson et al., 1996). Executive functions have been widely defined as “the ability to maintain an appropriate problem-solving set for attainment of a future goal” (pp. 201–202, Welsh & Pennington, 1988). This complex construct encompasses a variety of cognitive abilities, such as planning, response inhibition, and working memory, and involves the integration of more basic processes such as attention, memory, sensation, perception, and motor activity (Pennington & Ozonoff, 1996).
The Behavior Rating Inventory of Executive Functioning (BRIEF) is a parent report of executive function in children (Gioia, Isquith, Guy, & Kenworthy, 2000). It includes eight subscales and two summary indices. Several studies have evaluated children with FASD using the BRIEF, however, many of these have not included a comparison group of children. In one study without controls, children with FASD had scores in the clinical range (> 1.5 standard deviation from the population average) on all subscales, with greatest difficulty on the inhibitory control, working memory, and problem-solving subscales (Rasmussen, McAuley, & Andrew, 2007), while another study revealed abnormal elevation on all but two subscales (Rasmussen, Horne, & Witol, 2006). In a separate investigation, these executive function deficits (using the two BRIEF summary indices) were predictive of poorer social skills and greater problem behaviors (Schonfeld, Paley, Frankel, & O'Connor, 2006). Because these studies did not include a comparison group it is difficult to conclude whether the magnitude of the deficits persists when compared to a demographically matched control group. A later investigation of 43 adolescents (age 13–18 years) did include a comparison group and replicated these effects, revealing that children with prenatal alcohol exposure demonstrate poorer executive functioning than typically developing controls for all subscales and summary indices of the BRIEF (McGee, Fryer, Bjorkquist, Mattson, & Riley, 2008). However, parents of the children with FASD in this study were instructed to recruit another child similar to their own but without prenatal alcohol exposure. These unexposed children comprised the comparison group; as a result, the group may not have been representative of the population of typically developing children. While more research is needed in this area, these parent-report findings are further supported by neuropsychological data, which indicate that alcohol-exposed children are delayed on executive function tasks, including measures of problem-solving, planning, concept formation and conceptual set shifting, verbal and nonverbal fluency, response inhibition, and working memory, as detailed below.
Problem-Solving and Planning
Various tower tasks have been used to demonstrate deficits in the domains of problem-solving and planning. In these studies, alcohol-exposed children display increased perseverations on incorrect strategies, increased rule violations, fewer passed items overall, and decreased initial time planning a strategy to complete each problem than typically-developing controls (Aragon, Kalberg, et al., 2008; Green, Mihic, Nikkel, et al., 2009; Kodituwakku, Handmaker, Cutler, Weathersby, & Handmaker, 1995; Mattson, Goodman, Caine, Delis, & Riley, 1999).
Concept Formation and Set-Shifting
Difficulties forming and identifying abstract concepts and shifting to new conceptual categories have also been reported. Alcohol-exposed children make more errors and complete fewer categories compared to controls on the Wisconsin Card Sorting Test (WCST), a task that requires abstract reasoning and the ability to shift cognitive strategies in response to feedback (Carmichael Olson, Feldman, Streissguth, Sampson, & Bookstein, 1998; Coles et al., 1997; Kodituwakku, et al., 1995; McGee, Schonfeld, Roebuck-Spencer, Riley, & Mattson, 2008; Vaurio, Riley, & Mattson, 2008). In one study using the WCST, children with FAS tended to perform more poorly than exposed children without FAS (McGee, Schonfeld, et al., 2008), yet in another study, differences among groups of alcohol-exposed children—FAS, partial FAS, and ARND—were not significant (Chasnoff, et al., 2010). Similarly, in a study using the California Card Sorting Test of the Delis-Kaplan Executive Functioning System (D-KEFS), children with and without a diagnosis of FAS completed fewer sorts than control children and received fewer points for their description of their sorts (McGee, Schonfeld, et al., 2008). Not only were alcohol-exposed children less able to generate concepts independently, they were less able to recognize categories when cued by the examiner. Furthermore, on the California Word Context Test of the D-KEFS, a test that assesses concept formation and reasoning in the verbal domain, children with prenatal exposure to alcohol needed more sentences to form a correct response and made more set loss errors (Mattson & Riley, 1999). Combined, these findings suggest that individuals with histories of prenatal alcohol exposure have difficulties forming and shifting concepts and thinking analytically, which in turn, impair their problem-solving abilities.
Fluency
Another area of executive function that is compromised by prenatal alcohol exposure is fluency. Children exposed to alcohol during gestation demonstrate deficits on both traditional and set-shifting measures of verbal and nonverbal fluency. In one study, deficits on higher-order switching tasks were not accounted for by traditional fluency tasks, and deficits persisted when IQ was controlled statistically (Schonfeld, Mattson, Lang, Delis, & Riley, 2001). Within the verbal domain, four studies have demonstrated that although deficits are noted in both letter and category fluency, deficits are greater on letter fluency tasks (Kodituwakku, Adnams, et al., 2006; Mattson & Riley, 1999; Rasmussen & Bisanz, 2009; Vaurio, et al., 2008). Similar findings were also reported within a sample of Native American Indian children: compared to controls, alcohol-affected children were impaired in letter fluency but unimpaired on category fluency (Aragon, Kalberg, et al., 2008).
Findings within the nonverbal domain have been less clear. While one study documented impaired design fluency amongst alcohol-exposed children compared to controls (Schonfeld, et al., 2001), another found that children with FASD did not display deficits relative to normative data (Rasmussen & Bisanz, 2009). This discrepancy in findings may be related to the two study designs. In the first study, scores of alcohol-exposed children were compared to a typically developing control group matched on demographic characteristics including age, sex, and race, whereas in the second study, they were compared to the normative mean (scaled score = 10), possibly attenuating group differences.
Inhibitory Control
Response inhibition is another area of weakness in alcohol-exposed children. On measures of inhibitory control, such as the Stroop Test, children with histories of heavy prenatal exposure to alcohol make more errors than typically developing children, particularly on the switching and interference conditions (Connor, Sampson, Bookstein, Barr, & Streissguth, 2000; Mattson, et al., 1999). A study using event-related potentials to examine response inhibition processing found that children with FAS and partial FAS behaviorally inhibited responses as well as control groups on a Go/No-Go task; however, the level of neural activation was greater in children prenatally exposed to alcohol, suggesting greater cognitive effort (Burden et al., 2009). Similar findings have been described in a study using fMRI during a Go/No-Go task (Fryer, Tapert, et al., 2007) (for further discussion, see Coles, et al., this issue). Poor inhibitory ability may be related to impaired theory of mind, as poor performance on theory of mind measures has been found to be correlated with a task of inhibition control in children with prenatal alcohol exposure (Rasmussen, Wyper, & Talwar, 2009).
Working Memory
Alcohol-exposed children show deficits in the ability to hold and manipulate information in working memory (Green, Mihic, Nikkel, et al., 2009; Kodituwakku, et al., 1995). A frequently used measure of working memory that has been found to be sensitive to prenatal alcohol effects is the backwards condition of the digit span subtest of the Wechsler Intelligence Scales for Children (WISC). Children with heavy prenatal alcohol exposure recall fewer digits on this condition than typically developing controls (Aragon, Kalberg, et al., 2008; Carmichael Olson, et al., 1998; O'Hare et al., 2009). Furthermore, of all the WISC subtests, prenatal alcohol consumption was found to be most correlated with the digit span subtest as well as the arithmetic subtest, which also requires manipulation of information in working memory (Streissguth, et al., 1990). In addition to verbal working memory, children with FASD also struggle with visual-spatial working memory. In one study, these children committed more errors and demonstrated poorer use of strategy on a computerized task of spatial working memory (Green, Mihic, Nikkel, et al., 2009). In another study, children and adults with FASD provided fewer correct responses, greater incorrect responses and non-responses, and longer latencies during correct responses on an experimental visual-spatial n-back task (Malisza et al., 2005). Although less work has been done in this area, it appears that working memory abilities are impaired in alcohol-exposed children above and beyond global intellectual deficits. One investigation demonstrated that the association between prenatal alcohol exposure and performance on working memory measures remained significant even after IQ was statistically controlled (Burden, Jacobson, Sokol, & Jacobson, 2005). Understanding of these impairments is particularly significant because these processes often underlie other executive control and attention skills (Burden, Jacobson, Sokol, et al., 2005).
Learning and Memory
Animal research has established that the hippocampus is particularly sensitive to the teratogenic effects of prenatal alcohol exposure (e.g., Bonthius & West, 1990; Livy, Miller, Maier, & West, 2003; Maier & West, 2001). While neuroimaging data in humans have been less conclusive, some studies have shown vulnerability of the hippocampus to prenatal alcohol insult (e.g., Autti-Rämö et al., 2002; Willoughby, Sheard, Nash, & Rovet, 2008). Not surprisingly, a number of clinical studies have reported learning and memory deficits in children with heavy prenatal alcohol exposure. These impairments range across specific aspects of learning and memory, including verbal and nonverbal skills.
Verbal Learning and Memory
Alcohol-exposed children display deficits in both learning and recall of verbal information (Mattson, Riley, Delis, Stern, & Jones, 1996; Mattson & Roebuck, 2002). They learn fewer words on the learning trials of the California Verbal Learning Test-Children’s Version (CVLT-C) and have greater difficulty recalling them on both free and recognition recall trials (Crocker, Vaurio, Riley, & Mattson, 2011; Mattson, Riley, Delis, et al., 1996). These deficits are present in both children with and without the physical features of FAS (Mattson, et al., 1998; Mattson & Roebuck, 2002). Interestingly, when the number of words initially learned was controlled, alcohol-exposed children displayed retention rates that were similar to typically developing controls (Mattson, et al., 1998; Mattson & Roebuck, 2002). These findings have been replicated in independent samples of children (Kaemingk, Mulvaney, & Tanner Halverson, 2003; Willoughby, et al., 2008) and adults (Coles, Lynch, Kable, Johnson, & Goldstein, 2010), and in a study of light to moderate levels of alcohol exposure (Willford, et al., 2004). Of note, implicit learning strategies on tasks like the CVLT-C may positively influence the ability of alcohol-exposed children to retain verbal information, because spared retention was not detected on another task of word list learning without an implicit learning strategy (Roebuck-Spencer & Mattson, 2004).
When given verbal tasks that involve a story rather than word list, children with FASD exhibit superior memory during both immediate and delayed recall compared to their performance on word list tasks. In one study, performance on story recall and word-list recall were compared in alcohol-exposed children (Pei, Rinaldi, Rasmussen, Massey, & Massey, 2008). In comparison to the word-list task, subjects recalled more information on the story task but also recounted more inaccurate information. However, intrusions were only penalized on the word-list task, which may have affected the scores on these tasks. Because no control group was used, it is unclear in this study whether memory for stories in prenatal alcohol exposure is impaired compared to typically developing peers. Other studies, however, have reported deficits in this domain in children with heavy levels of exposure relative to comparison children (Willoughby, et al., 2008). In light to moderate levels of exposure, prenatal alcohol exposure predicted poor memory for stories at 10 years of age (Richardson, Ryan, Willford, Day, & Goldschmidt, 2002) but not 14 years (Willford, et al., 2004) within the same cohort of children. These findings suggest that these abilities might improve with age, at least in less affected children.
Nonverbal Learning and Memory
In the nonverbal domain, children with prenatal alcohol exposure also display learning and recall deficits: a lower rate of learning across acquisition trials and less recall of information after a delay period. However, it is less clear whether retention of nonverbal material is intact or impaired because results are mixed when initial learning is taken into account (Aragon, Kalberg, et al., 2008; Kaemingk, et al., 2003; Mattson & Roebuck, 2002). Differences may be due to task characteristics or length of delay, as described below.
Within the nonverbal domain, limited research has addressed visual-spatial memory, and current data reveal inconsistent results. One study described performance on a virtual Morris water maze task that is sensitive to hippocampal damage. Children and adolescents with histories of prenatal alcohol exposure exhibited poorer performance on the hidden and probe trials but not cued-navigation trials, suggesting that these place-learning deficits were not attributable to visual-motor or motivation deficits (Hamilton, Kodituwakku, Sutherland, & Savage, 2003). In addition, these findings were consistent with those found in the animal literature (e.g., Johnson & Goodlett, 2002). A second study used the Memory for 16 Objects test, a task also sensitive to hippocampal damage. In this study, children with FAS remembered the same average number of objects on immediate recall as control subjects but fewer objects in delayed recall (Uecker & Nadel, 1996). In a subsequent study with the same subjects, deficits were noted in spatial but not object recall (Uecker & Nadel, 1998). Because retention was not addressed directly in either of these studies, it is unclear whether the nature of the deficits was related to encoding or memory difficulties. Additional studies have also shown impaired spatial recall in alcohol-exposed children compared to controls (Aragon, Kalberg, et al., 2008) and a negative correlation between alcohol exposure and memory for nonverbal figures. In contrast, another research group found greater deficits in object relative to spatial recall in one study (Rasmussen, et al., 2006), but no significant differences in spatial compared to object recall at either immediate or delayed time intervals in another study (Pei, et al., 2008). Discrepancies may be due to differences in visual memory task design, including the objects participants are asked to remember (familiar everyday items vs. faces) and time delay intervals.
Furthermore, children with FASD have poor spatial recall on the Rey-Osterrieth Complex Figure task relative to controls (Willoughby, et al., 2008). Because learning was not assessed in this study, it is difficult to conclude whether impairments are due to learning or memory deficits. A separate study found that light-to-moderate levels of prenatal alcohol exposure predicted poorer performance on a different design memory task (Richardson, et al., 2002). Additional studies have demonstrated that when performance on visual perception and verbal memory tasks are taken into account with statistical analysis, group differences in spatial memory are no longer apparent, suggesting that impairments in spatial memory may be explained by deficits in lower order processes (Kaemingk & Halverson, 2000).
Language
Research on the effects of prenatal alcohol exposure on language skills has been mixed. Case reports suggest that prenatal alcohol exposure is associated with speech and language disturbances, ranging from an absence of comprehensible speech to mild dysarthria or lisping (Abel, 1990). Furthermore, indications of maternal problem drinking may be related to poor receptive language functioning (Russell, Czarnecki, Cowan, McPherson, & Mudar, 1991). Retrospective group studies of language functioning in this population also have revealed language deficits. Impairments include word comprehension (Conry, 1990; LaDue, Streissguth, & Randels, 1992; Mattson, et al., 1998), naming ability (Mattson, et al., 1998), articulation (Becker, Warr-Leeper, & Leeper, 1990), grammatical and semantic abilities (Becker, et al., 1990), pragmatics (Abkarian, 1992), and expressive and receptive skills (Aragon, Coriale, et al., 2008; Carney & Chermak, 1991; Janzen, Nanson, & Block, 1995; McGee, Bjorkquist, Riley, & Mattson, 2009). Population studies with individuals in South Africa and Italy further reveal that exposed individuals have impaired grammar comprehension skills (Kodituwakku, Coriale, et al., 2006).
While retrospective studies of language have shown fairly clear and consistent results, prospective studies have been more equivocal. Phonological processing deficits at age 14 years were related to prenatal alcohol exposure levels in a long-term prospective study (Streissguth, Barr, et al., 1994). Another series of prospective studies found lower levels of alcohol exposure to be associated with significantly lower language comprehension and expression in children at 13-months (Gusella & Fried, 1984), 2 years (Fried & Watkinson, 1988), and 3 years (Fried & Watkinson, 1990). However, within the same cohort of children, deficits in language abilities were not detected later at 4 (Fried & Watkinson, 1990), 5, and 6 years of age (Fried, O'Connell, & Watkinson, 1992), and another prospective study found neither expressive nor receptive language impairments in children ages 1, 2, and 3 years (Greene, Ernhart, Martier, Sokol, & Ager, 1990). A more recent study also found no association between low levels of alcohol exposure and parent reports of language delay at age 2 years (O'Leary, Zubrick, Taylor, Dixon, & Bower, 2009). A possible explanation for these discrepancies may be due to varying levels of alcohol exposure. Many of these prospective studies included samples of children who were exposed predominantly to low levels of alcohol; it may be that language deficits are more common in children with heavy prenatal alcohol exposure. It may also be that language deficits are secondary to overall intellectual deficits (McGee, Bjorkquist, Riley, & Mattson, 2009) but further study is needed to clarify this relationship.
Beyond assessing language skills in standardized contexts, recent research has focused on understanding how children exposed to alcohol prenatally use language to achieve communicative goals and conceptualize their social environment. Findings suggest that in social interactions, children with FASD struggle to balance linguistic and social-cognitive task demands in order to produce contextually integrated discourse (Coggins, Olswang, Carmichael Olson, & Timler, 2003). They provide insufficient organization and information for listeners in narratives (Coggins, Timler, & Olswang, 2007) and, according to caregiver reports, fail to consider the perspective of the listener during interaction (Timler, Olswang, & Coggins, 2005). Using narrative analysis, one study examined semantic elaboration and strategic use of linguistic references to identify concepts in a group of children prenatally exposed to alcohol. Both normal controls and alcohol-exposed children varied in their extent of semantic elaboration in narrative telling, but children with FASD were significantly more likely to use ambiguous references and inappropriately distinguish concepts in their stories (Thorne, Coggins, Carmichael Olson, & Astley, 2007).
Visual-spatial Ability
Although research is limited in this domain, deficits in visual-spatial perception and construction have been reported in children with histories of prenatal alcohol exposure. As previously discussed, prenatal exposure to alcohol has been found to be associated with abnormal development of hippocampal structure and functioning (e.g., Autti-Rämö, et al., 2002; Barnes & Walker, 1981; Berman & Hannigan, 2000; Riikonen, Salonen, Partanen, & Verho, 1999). Given that behavioral research with animals has shown that visual-spatial abilities depend on medial temporal lobe and hippocampal functioning (e.g., Morris, Garrud, Rawlins, & O'Keefe, 1982; O'Keefe & Dostrovsky, 1971), it is reasonable to expect visual-spatial abilities to be compromised in prenatally exposed individuals.
Multiple studies document impairments in simple visual-spatial construction on tests like the Beery-Buktenica Developmental Test of Visual Motor Integration, which requires individuals to copy drawings of geometric forms (Aronson & Hagberg, 1998; Chiodo, Janisse, Delaney-Black, Sokol, & Hannigan, 2009; Conry, 1990; Janzen, et al., 1995; Jirikowic, Carmichael Olson, & Kartin, 2008; Korkman, Autti-Rämö, Koivulehto, & Granström, 1998; Mattson, et al., 1998; Uecker & Nadel, 1996). Performance on more complex visual-spatial tasks has been reported only rarely. On a clock-drawing task, although children with FAS were able to remember the essential features of a clock, they disregarded details like spacing between numbers. These observations, combined with reported simple visual-spatial construction deficits, suggest that alcohol-exposed individuals may demonstrate a form of constructional apraxia (Uecker & Nadel, 1996). In one very small study of four boys with fetal alcohol effects, none of the subjects were able to successfully model the construction of a simple symmetrical block structure when shown a video of a peer building the same configuration (Meyer, 1998). Additional research has shown that children with prenatal alcohol exposure differentially process specific features of visual stimuli. On a hierarchical processing task consisting of large symbols (the global feature) made up of smaller symbols (the local feature), alcohol-exposed children had significantly greater difficulty processing local features compared to global features (Mattson, Gramling, Delis, Jones, & Riley, 1996).
Impairments in visual-spatial perception may account for some of the spatial memory deficits described above (e.g., Kaemingk & Halverson, 2000; Uecker & Nadel, 1996), as group differences on measures of spatial location are reduced once visual perception performance is statistically controlled (Kaemingk & Halverson, 2000). These results imply that visual-spatial perception deficits in children with FASD may influence spatial memory, but do not completely account for those impairments.
Motor Function
Many studies of FASD suggest an association between alcohol exposure and poor motor performance. The earliest reports by Jones and Smith (Jones & Smith, 1973) described poor hand/eye coordination, weak grasp, tremors, and balance and gait difficulties. More recent studies have found that children prenatally exposed to heavy levels of alcohol exhibit impairment of both fine and gross motor skills. Young children with FAS show clinically important developmental delays in fine but not gross motor skills (Kalberg et al., 2006). Other findings of motor impairment in this clinical population include postural instability (Roebuck, Simmons, Richardson, Mattson, & Riley, 1998), atypical gait (Marcus, 1987), delayed motor reaction timing (Green, Mihic, Nikkel, et al., 2009; Simmons, Thomas, Levy, & Riley, 2010; Simmons, Wass, Thomas, & Riley, 2002; Wass, Simmons, Thomas, & Riley, 2002), impaired fine-motor speed and coordination (Chiodo, et al., 2009; Jirikowic, Carmichael Olson, et al., 2008; Mattson, et al., 1998), increased motor timing variability (Simmons, Levy, Riley, Madra, & Mattson, 2009), poor hand/eye coordination (Adnams et al., 2001), poor bimanual coordination (Roebuck-Spencer, Mattson, Marion, Brown, & Riley, 2004), dysfunctional force regulation (Simmons et al., 2011), atypical trajectories in goal-directed arm movements (Domellof, Fagard, Jacquet, & Ronnqvist, 2010), impaired oculomotor control (Green, Mihic, Brien, et al., 2009), poor sensory processing and sensorimotor performance (Jirikowic, Carmichael Olson, et al., 2008), and weak grasp (Conry, 1990). However, one study found that children with FASD exhibit deficits in static postural control (Kooistra et al., 2009), which is in contrast to a previous study (Roebuck, Simmons, Mattson, & Riley, 1998) reporting that these children perform as well as controls in stable environmental conditions. Another found no differences in gross motor functioning in FAS children (Adnams, et al., 2001). Inconsistencies amongst these studies may be attributed to differences in the comprehensiveness of assessment of postural instability and insensitivity of the Griffith’s Locomotor subscale to detecting more subtle motor deficits in this population.
These findings are not surprising considering the teratogenic effects of alcohol to brain regions associated with motor functioning such as the cerebellum and basal ganglia. Animal research has found that cerebellar neurons are sensitive to alcohol-induced damage (Goodlett, Pearlman, & Lundahl, 1998; Hamre & West, 1993; Thomas, Goodlett, & West, 1998), and neuroimaging research has documented significant volume and size reductions in the cerebellum and caudate nucleus of the basal ganglia (Archibald et al., 2001) (for detail, see Lebel et al., this issue). The causes of motor impairment observed in individuals with FASD are not limited to brain dysfunction. In addition to central nervous system abnormalities, peripheral motor nerve damage is also evident. Children prenatally exposed to alcohol show increased motor delay variability (Simmons, et al., 2009) that is likely attributable to atypical muscle development (David & Subramaniam, 2005), reduced motor neurons (Bradley, Beaman, Moore, & Heaton, 1997; Heaton & Bradley, 1995), poor peripheral nerve myelination (Zoeller, Butnariu, Fletcher, & Riley, 1994), and slowed nerve conductivity (de los Angeles Avaria et al., 2004) that are associated with prenatal alcohol exposure. Furthermore, skeletal malformations of the hands and feet (e.g., tetraectrodactyly and camptodactyly) (Church, Eldis, Blakley, & Bawle, 1997; Herrmann, Pallister, & Optiz, 1980) and delayed skeletal maturity (Naidoo, Norval, Swanevelder, & Lombard, 2006) are evident in these individuals and may contribute to poor performance on motor tasks.
While the aforementioned studies used samples that included both young children and adolescents, subsequent research focusing specifically on motor function in older individuals are equivocal about whether the observed deficits persist into adolescence and adulthood. Young children with histories of heavy prenatal alcohol exposure exhibit slower motor reaction time on both simple and choice reaction time tasks (Simmons, et al., 2002). However, in a subsequent study with adolescent subjects, group differences were not observed (Simmons, Thomas, Levy, & Riley, 2006). Another study investigating fine motor coordination and balance in two adult populations with FASD, including a prospective longitudinal sample of adults who were exposed to varying levels of alcohol, reported results to the contrary. Data revealed that adults with FASD perform worse than controls on tests of fine motor control and balance and that the dose-dependent effects of alcohol on motor coordination during childhood continued to be apparent in adulthood among individuals previously diagnosed with FAS or ARND (Connor, Sampson, Streissguth, Bookstein, & Barr, 2006). It is possible that the discrepancies between studies are because each examined different aspects of motor function. It is also possible that some types of motor impairment may represent developmental delays that eventually normalize with age, while others may be long lasting impairments in function. Additional research will help to discern the extent of motor impairment throughout the lifespan.
Attention and Activity Levels
Hyperactivity and attention deficits are frequently observed in individuals with heavy prenatal alcohol exposure. Early reports on FAS describe these children to be tremulous, hyperactive, and irritable (Hanson, Jones, & Smith, 1976). Affected children show deficits in attention on neuropsychological tasks of vigilance, reaction time, and information processing (Burden, Jacobson, & Jacobson, 2005; Jacobson, Jacobson, & Sokol, 1994; Jacobson, et al., 1993; Streissguth et al., 1986; Streissguth et al., 1984; Streissguth, Sampson, et al., 1994). Parent (Janzen, et al., 1995; Mattson & Riley, 2000; Nash et al., 2006) and teacher (Aragon, Coriale, et al., 2008; Brown et al., 1991; Carmichael Olson, Sampson, Barr, Streissguth, & Bookstein, 1992) reports of attention difficulties are also common. More than 60% of children exposed to alcohol in utero exhibit deficits in attention (LaDue, et al., 1992), and they demonstrate a significantly higher rate of attention-deficit/hyperactivity disorder (ADHD) (Fryer, McGee, Matt, Riley, & Mattson, 2007) and hyperkinetic disorders (Steinhausen, Willms, & Spohr, 1993) than typically developing children. Like children with ADHD, children prenatally exposed to alcohol show impairments investing, organizing, and maintaining attention as well as inhibiting impulsive responses (Nanson & Hiscock, 1990). Recent studies have focused on the comparison between FASD and ADHD, particularly in the domain of attention. These studies are described in detail in the following section.
Among children with histories of heavy prenatal alcohol exposure, deficits in attention are not global. Research suggests that prenatal alcohol exposure leads to differential deficits in visual and auditory attention; however, these findings have not been entirely consistent. Two studies revealed greater impairment in visual sustained attention in children and adolescents with prenatal alcohol exposure (Coles, Platzman, Lynch, & Freides, 2002; Mattson, Calarco, & Lang, 2006). In contrast, another study described more severe deficits in the auditory modality in adults (Connor, Streissguth, Sampson, Bookstein, & Barr, 1999). Discrepancies may relate to the tasks used or to the age of the subjects in these studies. Additional studies of attention in adults may provide clarification in this domain.
Academic Impairments
Children with FASD also have difficulties with academic function. Studies demonstrate deficits in both verbal (i.e., reading and spelling) and mathematical domains (Carmichael Olson, et al., 1992; Coles, et al., 1991; Howell, Lynch, Platzman, Smith, & Coles, 2006; Mattson, et al., 1998; Rasmussen & Bisanz, 2010; Streissguth, Barr, et al., 1994; Streissguth, et al., 1990), and these deficits persist even after controlling for IQ (Goldschmidt, Richardson, Stoffer, Geva, & Day, 1996). Mathematics has emerged as a specific area of weakness; however, little is known about the nature of these deficits. Alcohol-exposed children consistently perform lower than controls on measures of global mathematics achievement (Mattson, et al., 1998; Streissguth, Barr, et al., 1994), and there is evidence suggesting that individuals with FASD may have particular deficits in basic numerical processing skills such as cognitive estimation (Jacobson, Dodge, Burden, Klorman, & Jacobson, 2010; Kopera-Frye, Dehaene, & Streissguth, 1996; Meintjes et al., 2010). Furthermore, recent neuroimaging studies support neuropsychological findings and have shown that children with FASD show abnormalities in regions thought to be important in mathematical processing, such as left and right parietal regions and the medial frontal gyrus (Lebel, Rasmussen, Wyper, Andrew, & Beaulieu, 2010; Santhanam, Li, Hu, Lynch, & Coles, 2009) (for detail, see papers by Coles et al. and Wozniak et al., this issue). One study that evaluated the effects of dose and timing of alcohol exposure on academic ability reported that the relationship between prenatal alcohol exposure and mathematics was best characterized by a linear dose-response relationship, whereas the relationships between alcohol-exposure and the verbal academic domains were better modeled as threshold effects (Goldschmidt, et al., 1996).
Clinical and Behavioral Features
In addition to the neuropsychological deficits already described, children with FASD are likely to present with a wide range of maladaptive and clinically significant behavioral characteristics. Studies of individuals with FASD reveal marked deficits in parent and self-reports of behavior (Coles, Platzman, Brown, Smith, & Falek, 1997; Coles, Platzman, & Lynch, 1999; Mattson & Riley, 2000; Nash, et al., 2006; Sood, et al., 2001; Steinhausen, Willms, Metzke, & Spohr, 2003). In one study, children with histories of heavy prenatal alcohol exposure were compared to an IQ matched sample of controls using the Child Behavior Checklist (Mattson & Riley, 2000). Children in the alcohol-exposed group had significantly more parent-reported behavioral and emotional disturbances than controls on five of the eight measure subscales. As a group, children with prenatal alcohol exposure had clinically significant scores on several externalizing behavior domains, such as the social problems, attention problems, and aggressive behavior scales of the measure. When examined individually, 90.1% of alcohol-exposed children in the sample had profiles with problem scores in the clinical range, whereas only 27% of controls had any clinically elevated scores. In this study, internalizing problem behaviors were also elevated in comparison to the control group, but the differences were not as large when compared with externalizing behavior domains. However, prenatal alcohol exposure has been associated with negative affect and increased risk for major depressive disorder in childhood (Fryer, McGee, et al., 2007; O'Connor, 2001; O'Connor & Paley, 2006; O'Connor et al., 2002). Thus, further examination of the association between FASD and internalizing behaviors is necessary to conclude whether one domain is relatively more affected than another.
Other studies have identified a dose-response effect between prenatal alcohol exposure and behavioral problems (Sood, et al., 2001), and recent studies have begun to tease apart the effects of environmental factors from prenatal alcohol exposure when evaluating the association with behavioral difficulties in affected children (D'Onofrio et al., 2007; Hill, Lowers, Locke-Wellman, & Shen, 2000; O'Connor & Paley, 2006; Rodriguez et al., 2009; Staroselsky et al., 2009). Some findings suggest that maternal psychopathology may be a better predictor of internalizing problems in children with FASD but that alcohol exposure is more directly related to externalizing problems (Staroselsky, et al., 2009). However, other studies fail to find strong associations between prenatal alcohol exposure and externalizing difficulties once environmental factors are taken into account (D'Onofrio, et al., 2007; Hill, et al., 2000; Rodriguez, et al., 2009). D’Onofrio and colleagues suggest that childhood conduct problems might be related to an environmentally mediated causal effect of prenatal alcohol exposure whereas impulsivity and attention difficulties might be accounted for by other factors that are correlated with maternal drinking during pregnancy (D'Onofrio, et al., 2007). Differences in findings might be related to varied sample sizes and exposure levels between studies as well as discrepancies in methodologies used.
Increased levels of secondary disabilities and psychiatric diagnoses are other common consequences of heavy prenatal alcohol exposure (Burd, Klug, Martsolf, & Kerbeshian, 2003; Famy, Streissguth, & Unis, 1998; Fryer, McGee, et al., 2007; Lynch, Coles, Corley, & Falek, 2003; O'Connor, 2001; O'Connor & Kasari, 2000; O'Connor & Paley, 2006; O'Connor, et al., 2002; Roebuck, Mattson, & Riley, 1999; Steinhausen & Spohr, 1998; Streissguth, Barr, Kogan, & Bookstein, 1996). As mentioned, children with FASD often have increased rates of mood disturbance. One study used structural equation modeling to construct a model of the relationship between prenatal alcohol exposure and childhood depression (O'Connor & Paley, 2006). This model considered both pre- and postnatal factors, and data suggested that higher levels of prenatal alcohol exposure are related to increased negative affect and depressive symptoms but that this relationship is mediated by mother-child interactions that occur over time, such as lower levels of emotional support and decreased expressions of positive affect from mothers. Because externalizing behaviors are also elevated in children with FASD, it is not surprising that rates of oppositional defiant disorder, conduct disorder, and ADHD are elevated (Burd, et al., 2003; D'Onofrio, et al., 2007; Disney, Iacono, McGue, Tully, & Legrand, 2008; Fryer, McGee, et al., 2007; Steinhausen & Spohr, 1998; Steinhausen, et al., 1993). One study indicated that within FASD, male participants were significantly more likely than female participants (86% compared to 29%) to be diagnosed with ADHD (Herman, Acosta, & Chang, 2008). In the greater population of children with ADHD, the ratio of boys to girls is estimated at 2 to 1 (Merikangas et al., 2010), although higher ratios (4:1) have been reported (Cantwell, 1996).
Other studies suggest that children with prenatal alcohol exposure are more likely to be rated as having delinquent behavior (Roebuck, et al., 1999) and exhibit impaired moral decision-making abilities (Schonfeld, Mattson, & Riley, 2005). One study demonstrated that low IQ of children with FASD predicted lower moral maturity relative to their non-exposed peers and that these children exhibit a specific deficit in moral value judgments in their relationships with others. In this same sample of children, delinquency was higher in the FASD group, and half of non-dysmorphic alcohol-exposed children studied had probable conduct disorder (Schonfeld, et al., 2005). Other studies have demonstrated that children with prenatal alcohol exposure are more likely (94.4% vs. 72.2%) to lie about their behavior and are more skilled lie-tellers at a younger age then typically developing controls (Rasmussen, Talwar, Loomes, & Andrew, 2008).
Evidence suggests that the behavioral difficulties and psychopathology of children with FASD persist into adulthood (Barr et al., 2006; Famy, et al., 1998; Spohr, Willms, & Steinhausen, 2007) and often result in adverse life outcomes such as substance abuse problems (Alati et al., 2006; Alati, et al., 2008; Baer, Barr, Bookstein, Sampson, & Streissguth, 1998; Baer, Sampson, Barr, Connor, & Streissguth, 2003) and trouble with the law (Fast, Conry, & Loock, 1999; Streissguth et al., 2004). In one cohort of prospectively identified subjects, prenatal alcohol exposure was associated with alcohol-problems at 21-years of age, and these effects remained even after controlling for the effects of family history of alcohol use disorders, other prenatal exposures, and other environmental factors such as postnatal parental use of other drugs (Baer, et al., 2003). These findings are supported by similar studies (Alati, et al., 2006; Alati, et al., 2008) and demonstrate that the behavioral effects of prenatal alcohol exposure are persistent and extend beyond childhood into adolescence and early adulthood. Furthermore, the host of clinical difficulties associated with prenatal alcohol exposure, such as impulsivity, mood disorder, and substance abuse, place affected individuals at high risk for suicide, and research suggests that individuals with FASD have an increase in lifetime suicide attempts relative to the general population (Baldwin, 2007; O'Malley & Huggins, 2005; Streissguth, et al., 1996). In one account, 43% of adults with FASD reported suicide threats and 23% reported a history of suicide attempts throughout the lifetime (Streissguth, et al., 1996).
The increase in adverse life outcomes in adults with prenatal alcohol exposure may also relate to deficits in adaptive functioning, which have been demonstrated in the domains of communication, daily living skills, and socialization (Crocker, Vaurio, Riley, & Mattson, 2009; Jirikowic, Kartin, & Carmichael Olson, 2008; Thomas, Kelly, Mattson, & Riley, 1998; Whaley, O'Connor, & Gunderson, 2001). Convergent evidence suggests that socialization may be the most affected domain within adaptive functioning (Crocker, et al., 2009; McGee, Bjorkquist, Price, Mattson, & Riley, 2009; McGee, Fryer, et al., 2008; Thomas, Kelly, et al., 1998; Whaley, et al., 2001). More specifically, socialization abilities of children with FASD fail to improve with increasing age (Crocker, et al., 2009; Thomas, Kelly, et al., 1998; Whaley, et al., 2001), suggesting an arrest in development of these skills rather than a delay. A similar arrest in development within the communication domain was documented in an investigation that compared children with FASD to children with ADHD and controls on adaptive ability (Crocker, et al., 2009). Thus, children with prenatal alcohol exposure are likely to have increasing difficulty meeting the greater demands in social and communication function as they become teenagers and adults. Importantly, poor social skills observed in children with FASD are associated with executive dysfunction, and problem solving and planning deficits appear to contribute to interpersonal difficulty in this population (Schonfeld, et al., 2006).
Recent interest also has been shown in understanding sensory processing in children with FASD and the impact difficulties in this domain might have on behavioral problems in this population. Sensory processing is defined as the ability to integrate neurological processing of sensory input and appropriate behavioral responses and deficits are thought to negatively impact everyday functions, including behavior (Cosbey, Johnston, & Dunn, 2010). Such deficits have been documented in alcohol-affected children and are associated with other neurobehavioral impairments (Franklin, Deitz, Jirikowic, & Astley, 2008) and decreased adaptive and academic function (Carr, Agnihotri, & Keightley, 2010; Jirikowic, Carmichael Olson, et al., 2008). One study examined the occurrence of sensory processing difficulties in children with partial FAS, ARND, and alcohol-exposed children that did not meet any formal alcohol-related diagnosis (Carr, et al., 2010). Though all subjects demonstrated sensory processing deficits, results suggested differences in severity of impairment among groups. In addition, the relationships between sensory processing difficulties and adaptive function and IQ differed depending on alcohol-related diagnosis, highlighting the importance of nontraditional assessment tools in order to fully understand the full range of deficits associated with prenatal alcohol exposure.
Neurobehavioral Profile
The extant data, summarized previously, along with studies demonstrating similarities between individuals with FASD and controls, can be used to construct a neuropsychological profile of strengths and weaknesses useful for the identification of affected individuals as well as specific domains to target for clinical intervention. While the definition of this profile is far from complete and the possibility that multiple profiles will be described is likely, thus far, the profile of children with FASD is characterized by general deficits in intellectual ability (e.g., Mattson, et al., 1997) and relative deficits in executive function (e.g., Kodituwakku, Adnams, et al., 2006; Kodituwakku, et al., 1995; Mattson, et al., 1999; Vaurio, et al., 2008), visual attention (e.g., Coles, et al., 2002; Mattson, et al., 2006), verbal (e.g., Mattson, Riley, Delis, et al., 1996; Mattson, et al., 1998) and nonverbal (e.g., Kaemingk, et al., 2003; Mattson & Roebuck, 2002) learning, motor function (e.g., Roebuck, Simmons, Mattson, et al., 1998), social skills (e.g., Schonfeld, et al., 2006), externalizing behaviors (e.g., Mattson & Riley, 2000) and adaptive function (e.g., Crocker, et al., 2009; Thomas, Kelly, et al., 1998; Whaley, et al., 2001). Relative strengths or lack of impairment are present in auditory attention (e.g., Coles, et al., 2002; Mattson, et al., 2006), retention of verbal information (e.g., Mattson, Riley, Delis, et al., 1996; Mattson, et al., 1998), and basic language function (McGee, Bjorkquist, Riley, et al., 2009). One recent investigation used latent profile analysis to statistically test the presence of a neurobehavioral profile of prenatal alcohol exposure. Findings demonstrated that there was a distinct profile of function for children with heavy prenatal alcohol exposure and that this resulting profile could accurately distinguish alcohol-exposed children from controls (Mattson, et al., 2010).
Given the heterogeneity of outcomes, overlap in presentation with other clinical groups, and the lack of a definitive physical or biological marker with which to identify all children who have been prenatally exposed to alcohol, identification of affected children is often difficult, especially without confirmed maternal history of alcohol exposure. Focusing on domains that are particularly affected and continuing to develop the neuropsychological profile will assist in determining the expected pattern of deficits in children with prenatal alcohol exposure. A focus of more recent neuropsychological investigations is to compare individuals with prenatal alcohol exposure to those who manifest clinically similar behaviors in order to clarify the specificity of deficits to prenatal alcohol exposure and determine a pattern of performance that might distinguish the groups. Thus far, this literature has focused on comparisons between children with FASD and non-exposed children with ADHD as well as between children with FASD and non-exposed children with low IQ scores.
Comparisons with ADHD
As stated, although children with FASD are at increased risk for ADHD, studies that have evaluated both clinical groups suggest that they can be characterized by separate neurobehavioral profiles. There is a small but growing literature aimed at understanding the specificity of deficits seen in children with heavy prenatal alcohol exposure through direct comparison with children with ADHD on specific cognitive and behavioral domains (Burden et al., 2010; Coffin, Baroody, Schneider, & O'Neill, 2005; Coles, Platzman, Raskind-Hood, et al., 1997; Crocker, et al., 2009; Crocker, et al., 2011; Greenbaum, Stevens, Nash, Koren, & Rovet, 2009; Jacobson, et al., 2010; Kooistra, Crawford, Gibbard, Ramage, & Kaplan, 2010; Kooistra, et al., 2009; Nanson & Hiscock, 1990; Nash, et al., 2006; Vaurio, et al., 2008). Results from these investigations suggest that the two clinical groups are similar on parent reports of attention (Nanson & Hiscock, 1990), communication and socialization aspects of adaptive function (Crocker, et al., 2009), and performance on the Wisconsin Card Sorting Test (Vaurio, et al., 2008). However, children with FASD and ADHD also have been distinguished on several cognitive domains, as follows.
In the domain of attention, three studies have documented differences in the nature of the attention impairments seen in these two clinical groups. Coles and colleagues (Coles, Platzman, Raskind-Hood, et al., 1997) demonstrated unique attention profiles between these two clinical groups by assessing attention using the four factor model proposed by Mirsky (Mirsky, Anthony, Duncan, Ahearn, & Kellam, 1991): Focus, Sustain, Encode, and Shift. While children with ADHD had more difficulty in the Focus and Sustain components, children with FASD were more impaired in the Encode and Shift domains. These results indicate that ADHD is characterized by difficulties focusing and sustaining attention, whereas FASD is associated with deficits in shifting attention, encoding of information, and flexibility in problem solving. More recent studies using a Go/No-Go response inhibition task indicate that children with ADHD, regardless of prenatal alcohol exposure, exhibited poorer inhibitory control but only children with ADHD without prenatal alcohol exposure showed impaired neural processing, suggesting that the attention deficits in ADHD associated with prenatal alcohol exposure are qualitatively different than those in ADHD alone (Burden, et al., 2010). Furthermore, in comparison to children with ADHD but without prenatal alcohol exposure, children with ADHD and prenatal alcohol exposure are more variable in their responding at different event rates (Kooistra, et al., 2010).
Furthermore, differences in attention profiles between children with prenatal alcohol exposure with or without a diagnosis of ADHD have been found to vary depending on gender. In a study investigating the effect of ADHD diagnosis on a sample of boys and girls with prenatal alcohol exposure, girls with FASD and ADHD showed significantly impaired ability to sustain attention and encode information compared to other girls with FASD without a diagnosis of ADHD, while boys with FASD and ADHD were significantly better than their non-ADHD counterparts in sustaining attention (Herman, et al., 2008).
Studies of other cognitive abilities have revealed further differences between these groups. In one study of executive function, different patterns of deficit were revealed: although the groups were similar on the Wisconsin Card Sorting Test, only children with FASD displayed overall deficits on letter fluency and a relative weakness on the Trail Making Test–B versus the Trail Making Test–A (Vaurio, et al., 2008). In a study of verbal learning and memory using the CVLT-C, verbal learning was affected in both groups, although in different ways; performance of alcohol-exposed children appears to reflect inefficient encoding of verbal material, whereas performance of children with ADHD is better characterized by a deficit in retrieval of learned material (Crocker, et al., 2011). Children with FASD and ADHD have also been distinguished using measures of mathematics and numerical processing (Jacobson, et al., 2010). Results of this study suggest that mathematics difficulty in children with ADHD might be more related to impairments in general cognitive abilities important for proficient academic achievement whereas children with FASD display a specific impairment in basic numerical processing abilities, such as the ability to mentally represent and manipulate numbers and quantities.
Motor competence and balance control also appear to be differentially affected between children with ADHD and FASD. Although both clinical groups of children show similar levels of impairment on tasks targeting complex motor skills and static balance, children with ADHD are more likely than children with FASD to show severe impairment on basic cerebellar motor control functions (Kooistra, et al., 2009). These findings suggest that children with ADHD exhibit problems with both basic and complex motor and balance skills, while children with FASD have relatively intact basic motor skills but struggle to integrate these functions into coordinated, complex motor skills.
Tasks tapping more basic cerebellar functions also have been able to discriminate children with FASD and ADHD. In one study, children with FASD failed to learn a classically conditioned eyeblink response, producing longer latencies and poorly timed responses to the conditioning stimulus. Children with ADHD, on the other hand, were impaired on measures of adaptively timed responses but showed learning of the conditioned eyeblink response that was similar to controls (Coffin, et al., 2005). In other studies of motor abilities, children with ADHD were more likely to be clinically impaired on measures of postural stability than were children with FASD (Kooistra, et al., 2009).
Finally, the distinct behavioral phenotypes of children with FASD and ADHD have been evaluated and findings suggest that parent-reported behavioral measures are useful in detecting differences in clinical presentation between the two groups. On measures of adaptive function, only children with FASD display arrested development of socialization and communication skills with abilities failing to improve with increasing age. Although children with ADHD demonstrate impaired abilities on these domains, their difficulties appear to be characterized by a developmental delay with skills improving at a rate that is similar to controls (Crocker, et al., 2009). Children with FASD also demonstrate weaker daily living skills (Crocker, et al., 2009), social cognition, and facial emotion processing ability (Greenbaum, et al., 2009) than do children with ADHD and typically developing controls. See Figure 1 for a visual representation of the patterns of impairments in FASD and ADHD populations.
Figure 1.
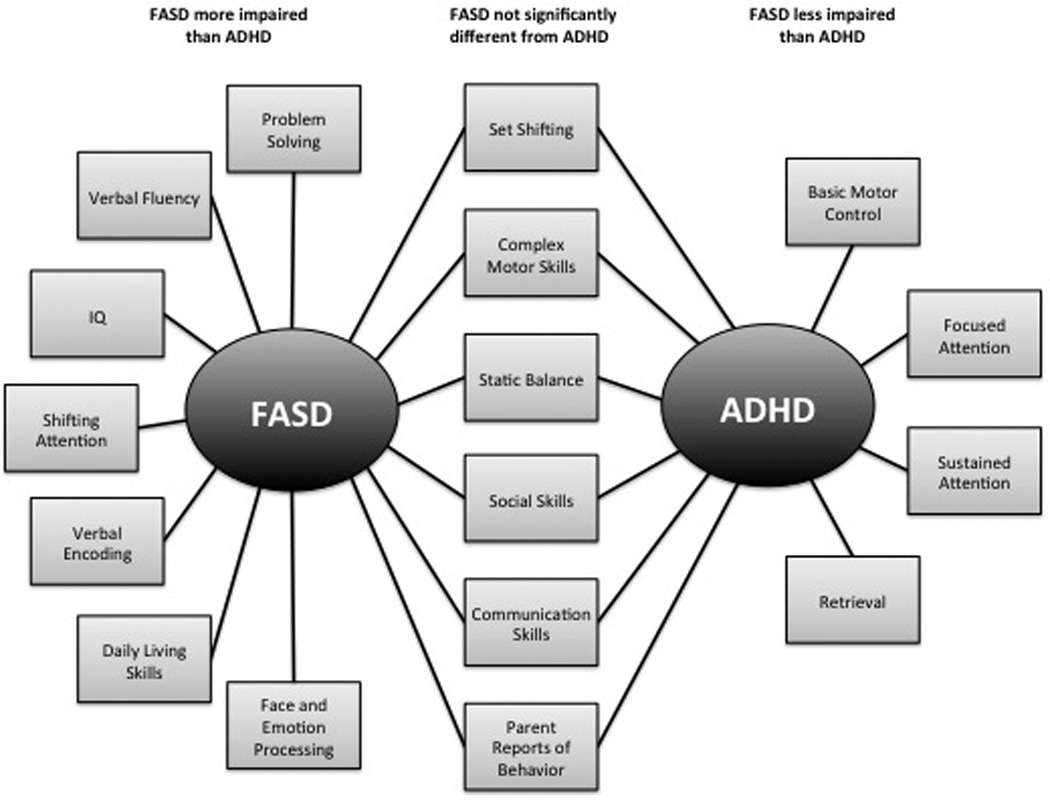
Patterns of neuropsychological impairments in children with FASD and ADHD. Note that domains listed do not reflect absolute impairments (i.e., when compared to non-exposed, typically developing controls) but rather, relative impairment on domains on which the two clinical groups have been directly compared. See text and Table 1 for details and related references.
Comparisons with IQ-Matched Samples
When children with heavy prenatal alcohol exposure are compared to children without histories of alcohol exposure but who have similar IQ scores, similarities and differences in their neurobehavioral presentation are also noted. Both groups of children show similarities on measures of expressive and receptive language ability (McGee, Bjorkquist, Riley, et al., 2009), sustained attention, and retention of verbal material (Vaurio, Riley, & Mattson, 2011). However, alcohol-affected subjects are more impaired than IQ-matched controls on measures of externalizing behavior (Mattson & Riley, 2000), adaptive skills (Thomas, Kelly, et al., 1998; Whaley, et al., 2001), and verbal learning (Coles, et al., 2010; Mattson, Riley, Delis, et al., 1996; Vaurio, et al., 2011). Parent reports of attention have indicated that externalizing behavior is more affected in alcohol-exposed children than in IQ-matched samples, but results of internalizing behavior are mixed (Mattson & Riley, 2000; Vaurio, et al., 2011), indicating that further study is needed.
Conclusion
Individuals exposed to alcohol during pregnancy exhibit a wide range of long-lasting impairments in neuropsychological and behavioral domains. Deficits include diminished intellectual function, poor learning and memory, impaired executive and visual-spatial function, delayed motor and language development, and attention difficulties. In addition, these children present with increased internalizing and externalizing behavior problems, poor academic achievement, and high rates of comorbid psychiatric disorders. Despite some inconsistencies, the extensive neuropsychological literature on children prenatally exposed to alcohol during pregnancy illustrates a pattern in which affected children perform relatively well on simple tasks but show greater impairment on more complex tasks that require processing of complex information and greater involvement of executive functioning (Kodituwakku, 2007). Recent efforts have begun to examine the specificity of these deficits through development of a neuropsychological profile of prenatal alcohol exposure that will aid in distinguishing affected individuals from other clinical populations. Continuing research comparing alcohol-exposed children to similar clinical groups will assist in the refinement this profile. Furthermore, greater understanding of the neurobehavioral impairments in children with prenatal alcohol exposure will help tailor intervention programs aimed at improving outcomes for this population.
Table 1.
Summary of Neuropsychological Findings reported in Individuals with fetal alcohol spectrum disorders (FASD) when compared to typically developing children, children without alcohol exposure but with a diagnosis of attention-deficit/hyperactivity disorder (ADHD) and children without prenatal alcohol exposure but with low IQ scores. See text for details.
| Cognitive domain |
Compared to typically developing controls | Compared to children with ADHD |
Compared to low IQ controls |
|---|---|---|---|
| Global deficits | The average IQ score of individuals with FAS is approximately 70 (Streissguth, et al., 1991). Individuals with prenatal alcohol exposure who lack facial dysmorphia do not have as severe intellectual impairment as those with dysmorphic features, but they still exhibit lower IQ scores compared to nonexposed peers (Chasnoff, et al., 2010; Mattson, et al., 1997). General cognitive function is significantly associated with the degree of facial dysmorphia and growth deficiency. The average IQ of nondysmorphic individuals is approximately 80 (Mattson, et al., 1997). | Children with FASD exhibit decreased IQ scores compared to children with ADHD but without prenatal alcohol exposure. (e.g., Crocker, et al., 2011; Vaurio, et al., 2008). | |
| Executive function | Deficits have been noted on several executive abilities including planning (Aragon, Kalberg, et al., 2008; Green, Mihic, Nikkel, et al., 2009; Kodituwakku, et al., 1995; Mattson, et al., 1999), set shifting (Carmichael Olson, et al., 1998; Coles, Platzman, Raskind-Hood, et al., 1997; Kodituwakku, et al., 1995; McGee, Schonfeld, et al., 2008; Vaurio, et al., 2008), fluency (Aragon, Kalberg, et al., 2008; Kodituwakku, Adnams, et al., 2006; Mattson & Riley, 1999; Schonfeld, et al., 2001; Vaurio, et al., 2008), response inhibition (Burden, et al., 2009; Connor, et al., 2000; Mattson, et al., 1999) and working memory (Green, Mihic, Nikkel, et al., 2009; Kodituwakku, et al., 1995). | Groups perform similarly on set shifting measures, such as the Wisconsin Card Sorting Test but only children with FASD display overall deficits on letter fluency and a relative weakness on the Trail Making Test–B versus the Trail Making Test–A. (Vaurio, et al., 2008). | When compared directly to an IQ matched comparison group, children with FASD perform similarly on measures of set shifting and verbal fluency (Vaurio, et al., 2011). However, deficits in nonverbal fluency persist when IQ is statistically controlled (Schonfeld, et al., 2001). |
| Verbal learning and memory | Alcohol-exposed children show impaired initial learning but spared retention of verbal information (Kaemingk, et al., 2003; Mattson, Riley, Delis, et al., 1996; Mattson, et al., 1998; Mattson & Roebuck, 2002; Willford, et al., 2004; Willoughby, et al., 2008). Verbal recall deficits are accounted for by difficulties encoding information rather than retention of information already learned. However, spared retention may be due to implicit learning strategies of some word list tests like the CVLT-C (Roebuck-Spencer & Mattson, 2004). | Although verbal learning is impaired in both groups, children with FASD appear to have greater difficulties encoding information, whereas children with ADHD have deficits retrieving already learned material (Crocker, et al., 2011). | Both groups of children show similar retention of verbal material that is encoded (Vaurio, et al., 2011). However, deficits in some aspects of verbal learning and memory continue to persist even after IQ is controlled (Coles, et al., 2010) and when children with FASD are compared to IQ-matched controls (Mattson, Riley, Delis, et al., 1996; Vaurio, et al., 2011). |
| Nonverbal learning and memory | Impaired learning and memory of nonverbal information are apparent; however, findings about whether retention of nonverbal material is intact or impaired are inconsistent (Aragon, Kalberg, et al., 2008; Kaemingk, et al., 2003; Mattson & Roebuck, 2002). Some research suggests that spatial recall is impaired (Hamilton, et al., 2003; Uecker & Nadel, 1998), while others report that it is intact (Kaemingk & Halverson, 2000; Rasmussen, et al., 2006). | N/A1 | Deficits in visual learning and recall persist even after IQ is controlled (Coles, et al., 2010; Kaemingk, et al., 2003). |
| Language | Retrospective studies show speech production deficits and difficulty with both expressive and receptive language skills (Aragon, Coriale, et al., 2008; Carney & Chermak, 1991; Janzen, et al., 1995; McGee, Bjorkquist, Riley, et al., 2009), however, results from prospective studies of low to moderate alcohol exposure are mixed (O'Leary, et al., 2009; Streissguth, Barr, et al., 1994) | N/A | Both groups of children show similarities on measures of expressive and receptive language ability (McGee, Bjorkquist, Riley, et al., 2009) |
| Visual-spatial ability | Children with FASD show deficits on visual-motor tasks (Aronson & Hagberg, 1998; Chiodo, et al., 2009; Conry, 1990; Janzen, et al., 1995; Jirikowic, Carmichael Olson, et al., 2008; Korkman, et al., 1998; Mattson, et al., 1998; Anne Uecker & Nadel, 1996) and on specific aspects of visual processing (Mattson, Gramling, et al., 1996). Deficits in visual-spatial perception (Kaemingk & Halverson, 2000) and motor ability (Janzen, et al., 1995) may account for difficulties in these domains. | N/A | In one investigation, children with FASD performed similarly to IQ matched controls on the Beery-Butenica Developmental Test of Visual Motor Integration (Vaurio, et al., 2011). |
| Motor function | Deficits in fine and gross motor abilities have been documented in FASD (Chiodo, et al., 2009; Green, Mihic, Nikkel, et al., 2009; Jirikowic, Carmichael Olson, et al., 2008; Mattson, et al., 1998; Simmons, et al., 2010; Simmons, et al., 2002; Wass, et al., 2002). However, some findings may be related to task demands (Adnams, et al., 2001). In addition, the trajectory of these abilities is unclear as some studies report that deficits persist with age (Connor, et al., 2006), while others fail to find differences in samples of adolescents (Simmons, et al., 2006). | Motor competence and balance control are differentially affected between children with ADHD and FASD. Both clinical groups of children show similar levels of impairment on tasks targeting complex motor skills and static balance, but children with ADHD are more likely than children with FASD to show severe impairment on basic cerebellar motor control functions (Kooistra, et al., 2009). | Children perform similarly to IQ matched controls on measures of fine motor skills (Vaurio, et al., 2011) |
| Attention and hyperactivity | Children with prenatal alcohol exposure show deficits in attention on neuropsychological tasks of vigilance, reaction time, and information processing (Burden, Jacobson, & Jacobson, 2005; Jacobson, et al., 1994; Jacobson, et al., 1993; Streissguth, et al., 1986; Streissguth, et al., 1984; Streissguth, Sampson, et al., 1994). Parent (Janzen, et al., 1995; Mattson & Riley, 2000; Nash, et al., 2006) and teacher (Aragon, Coriale, et al., 2008; Brown, et al., 1991; Carmichael Olson, et al., 1992) reports of attention difficulties are common. Visual sustained attention appears to be more impaired than auditory attention (Coles, et al., 2002; Mattson, et al., 2006); however findings have not been entirely consistent (Connor, et al., 1999). | Compared to children with ADHD, children with FASD show deficits shifting attention, encoding information, and problem-solving, while those with ADHD have more difficulties focusing and sustaining attention (Coles, Platzman, Raskind-Hood, et al., 1997) and controlling motor skills and static balance (Kooistra, et al., 2009). | Alcohol-exposed children do not show significant impairment on sustained attention above and beyond that explained by IQ (Coles, et al., 2002; Vaurio, et al., 2011). |
| Psychopathology and secondary disabilities | Children with FASD are likely to be classified as hyperactive, impulsive, disruptive, or delinquent and have increased rates of internalizing and externalizing behavior disorders (Mattson & Riley, 2000; Nash, et al., 2006). Alcohol-exposed children also have elevated rates of comorbid psychiatric disorders (Fryer, McGee, et al., 2007; O'Connor & Paley, 2006), sensory processing difficulties (Carr, et al., 2010; Jirikowic, Carmichael Olson, et al., 2008), and deficits in adaptive (Crocker, et al., 2009; Jirikowic, Kartin, et al., 2008; Thomas, Kelly, et al., 1998; Whaley, et al., 2001) and academic (Carmichael Olson, et al., 1992; Coles, et al., 1991; Howell, et al., 2006; Mattson, et al., 1998; Streissguth, Barr, et al., 1994; Streissguth, et al., 1990) functions. | Children with FASD and ADHD are similar on parent reports of attention and behavior (Coles, Platzman, Raskind-Hood, et al., 1997). However, on measures of adaptive function, only children with FASD display arrested development of socialization and communication skills with abilities failing to improve with increasing age. (Crocker, et al., 2009). Children with FASD also demonstrate weaker daily living skills (Crocker, et al., 2009), social cognition, and facial emotion processing ability (Greenbaum, et al., 2009) than do children with ADHD and typically developing controls. | Alcohol-affected subjects are more impaired than IQ-matched controls on measures of externalizing behavior (Mattson & Riley, 2000) and adaptive skills (Thomas, Kelly, et al., 1998; Whaley, et al., 2001) |
N/A: Research in this area not yet available.
Acknowledgements
The authors thank the members of the Center for Behavioral Teratology and the families who graciously participate in our studies. Preparation of this paper supported by NIAAA Grants R01 AA019605, R01 AA010417, U01 AA014834, T32 AA013525, and F31 AA020142.
Footnotes
Disclosure
The authors have no financial relationship to the organization that sponsored the research.
References
- Abel EL. Fetal Alcohol Syndrome. Oradell, New Jersey: Medical Economics Company, Inc.; 1990. [Google Scholar]
- Abel EL, Sokol RJ. Incidence of fetal alcohol syndrome and economic impact of FAS-related anomalies. Drug and Alcohol Dependence. 1987;19:51–70. doi: 10.1016/0376-8716(87)90087-1. [DOI] [PubMed] [Google Scholar]
- Abkarian GG. Communication effects of prenatal alcohol exposure. Journal of Communication Disorders. 1992;25:221–240. doi: 10.1016/0021-9924(92)90017-q. [DOI] [PubMed] [Google Scholar]
- Adnams CM, Kodituwakku PW, Hay A, Molteno CD, Viljoen D, May PA. Patterns of cognitive-motor development in children with fetal alcohol syndrome from a community in South Africa. Alcoholism: Clinical and Experimental Research. 2001;25(4):557–562. [PubMed] [Google Scholar]
- Alati R, Al Mamun A, Williams GM, O'Callaghan M, Najman JM, Bor W. In utero alcohol exposure and prediction of alcohol disorders in early adulthood: A birth cohort study. Archives of General Psychiatry. 2006;63(9):1009–1016. doi: 10.1001/archpsyc.63.9.1009. [DOI] [PubMed] [Google Scholar]
- Alati R, Clavarino A, Najman JM, O'Callaghan M, Bor W, Mamun AA, et al. The developmental origin of adolescent alcohol use: Findings from the Mater University Study of Pregnancy and its outcomes. Drug and Alcohol Dependence. 2008:136–143. doi: 10.1016/j.drugalcdep.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Aragon AS, Coriale G, Fiorentino D, Kalberg WO, Buckley D, Phillip Gossage J, et al. Neuropsychological characteristics of Italian children with fetal alcohol spectrum disorders. Alcoholism: Clinical and Experimental Research. 2008;32(11):1909–1919. doi: 10.1111/j.1530-0277.2008.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragon AS, Kalberg WO, Buckley D, Barela-Scott LM, Tabachnick BG, May PA. Neuropsychological study of FASD in a sample of American Indian children: Processing simple versus complex information. Alcoholism: Clinical and Experimental Research. 2008;32(12):2136–2148. doi: 10.1111/j.1530-0277.2008.00802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Developmental Medicine and Child Neurology. 2001;43(3):148–154. [PubMed] [Google Scholar]
- Aronson M, Hagberg B. Neuropsychological disorders in children exposed to alcohol during pregnancy: A follow-up study of 24 children to alcoholic mothers in Goteborg, Sweden. Alcoholism: Clinical and Experimental Research. 1998;22(2):321–324. doi: 10.1111/j.1530-0277.1998.tb03655.x. [DOI] [PubMed] [Google Scholar]
- Autti-Rämö I, Autti T, Korkman M, Kettunen S, Salonen O, Valanne L. MRI findings in children with school problems who had been exposed prenatally to alcohol. Developmental Medicine and Child Neurology. 2002;44(2):98–106. doi: 10.1017/s0012162201001748. [DOI] [PubMed] [Google Scholar]
- Baer JS, Barr HM, Bookstein FL, Sampson PD, Streissguth AP. Prenatal alcohol exposure and family history of alcoholism in the etiology of adolescent alcohol problems. Journal of Studies on Alcohol. 1998;59(5):533–543. doi: 10.15288/jsa.1998.59.533. [DOI] [PubMed] [Google Scholar]
- Baer JS, Sampson PD, Barr HM, Connor PD, Streissguth AP. 21- year longitudinal analysis of the effects of prenatal alcohol exposure on young adult drinking. Archives of General Psychiatry. 2003;60(4):377–385. doi: 10.1001/archpsyc.60.4.377. [DOI] [PubMed] [Google Scholar]
- Bailey BN, Delaney-Black V, Covington CY, Ager J, Janisse J, Hannigan JH, et al. Prenatal exposure to binge drinking and cognitive and behavioral outcomes at age 7 years. American Journal of Obstetrics and Gynecology. 2004;191(3):1037–1043. doi: 10.1016/j.ajog.2004.05.048. [DOI] [PubMed] [Google Scholar]
- Baldwin MR. Fetal alcohol spectrum disorders and suicidality in a healthcare setting. International Journal of Circumpolar Health. 2007;66:54–60. [PubMed] [Google Scholar]
- Barnes DE, Walker DW. Prenatal ethanol exposure permanently reduces the number of pyramidal neurons in rat hippocampus. Developmental Brain Research. 1981;1(3):333–340. doi: 10.1016/0165-3806(81)90071-7. [DOI] [PubMed] [Google Scholar]
- Barr HM, Bookstein FL, O'Malley KD, Connor PD, Huggins JE, Streissguth AP. Binge drinking during pregnancy as a predictor of psychiatric disorders on the structured clinical interview for DSM-IV in young adult offspring. American Journal of Psychiatry. 2006;163(6):1061–1065. doi: 10.1176/ajp.2006.163.6.1061. [DOI] [PubMed] [Google Scholar]
- Becker M, Warr-Leeper GA, Leeper HA. Fetal alcohol syndrome: A description of oral motor, articulatory, short-term memory, grammatical, and semantic abilities. Journal of Communication Disorders. 1990;23:97–124. doi: 10.1016/0021-9924(90)90016-r. [DOI] [PubMed] [Google Scholar]
- Berman RF, Hannigan JH. Effects of prenatal alcohol exposure on the hippocampus: Spatial behavior, electrophysiology, and neuroanatomy. Hippocampus. 2000;10(1):94–110. doi: 10.1002/(SICI)1098-1063(2000)10:1<94::AID-HIPO11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Bertrand J, Floyd RL, Weber MK, O'Connor M, Riley EP, Johnson KA, et al. National Task Force on FAS/FAE: Guidelines for Referral and Diagnosis. Atlanta, GA: Centers for Disease Control and Prevention; 2004. [Google Scholar]
- Bonthius DJ, Goodlett CR, West JR. Blood alcohol concentration and severity of microencephaly in neonatal rats depend on the pattern of alcohol administration. Alcohol. 1988;5:209–214. doi: 10.1016/0741-8329(88)90054-7. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, West JR. Alcohol-induced neuronal loss in developing rats: Increased brain damage with binge exposure. Alcoholism: Clinical and Experimental Research. 1990;14(1):107–118. doi: 10.1111/j.1530-0277.1990.tb00455.x. [DOI] [PubMed] [Google Scholar]
- Bradley DM, Beaman FD, Moore DB, Heaton MB. Ethanol influences on the chick embryo spinal cord motor system. II. Effects of neuromuscular blockade and period of exposure. Journal of Neurobiology. 1997;32(7):684–694. doi: 10.1002/(sici)1097-4695(19970620)32:7<684::aid-neu4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Brown RT, Coles CD, Smith IE, Platzman KA, Silverstein J, Erickson S, et al. Effects of prenatal alcohol exposure at school age. II. Attention and behavior. Neurotoxicology and Teratology. 1991;13(4):369–376. doi: 10.1016/0892-0362(91)90085-b. [DOI] [PubMed] [Google Scholar]
- Burd L, Klug MG, Martsolf JT, Kerbeshian J. Fetal alcohol syndrome: Neuropsychiatric phenomics. Neurotoxicology and Teratology. 2003;25(6):697–705. doi: 10.1016/j.ntt.2003.07.014. [DOI] [PubMed] [Google Scholar]
- Burden MJ, Andrew C, Saint-Amour D, Meintjes EM, Molteno CD, Hoyme HE, et al. The effects of fetal alcohol syndrome on response execution and inhibition: An event-related potential study. Alcoholism: Clinical and Experimental Research. 2009;33(11):1994–2004. doi: 10.1111/j.1530-0277.2009.01038.x. [DOI] [PubMed] [Google Scholar]
- Burden MJ, Jacobson JL, Westerlund A, Lundahl LH, Morrison A, Dodge NC, et al. An event-related potential study of response inhibition in ADHD with and without prenatal alcohol exposure. Alcoholism: Clinical and Experimental Research. 2010;34(4):617–627. doi: 10.1111/j.1530-0277.2009.01130.x. [DOI] [PubMed] [Google Scholar]
- Burden MJ, Jacobson SW, Jacobson JL. Relation of prenatal alcohol exposure to cognitive processing speed and efficiency in childhood. Alcoholism: Clinical and Experimental Research. 2005;29(8):1473–1483. doi: 10.1097/01.alc.0000175036.34076.a0. [DOI] [PubMed] [Google Scholar]
- Burden MJ, Jacobson SW, Sokol RJ, Jacobson JL. Effects of prenatal alcohol exposure on attention and working memory at 7.5 years of age. Alcoholism: Clinical and Experimental Research. 2005;29(3):443–452. doi: 10.1097/01.alc.0000156125.50577.ec. [DOI] [PubMed] [Google Scholar]
- Cantwell DP. Attention deficit disorder: A review of the past 10 years. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35(8):978–987. doi: 10.1097/00004583-199608000-00008. [DOI] [PubMed] [Google Scholar]
- Carmichael Olson H, Feldman JJ, Streissguth AP, Sampson PD, Bookstein FL. Neuropsychological deficits in adolescents with fetal alcohol syndrome: Clinical findings. Alcoholism: Clinical and Experimental Research. 1998;22(9):1998–2012. [PubMed] [Google Scholar]
- Carmichael Olson H, Sampson PD, Barr H, Streissguth AP, Bookstein FL. Prenatal exposure to alcohol and school problems in late childhood: A longitudinal prospective study. Development and Psychopathology. 1992;4:341–359. [Google Scholar]
- Carney LJ, Chermak GD. Performance of American Indian children with fetal alcohol syndrome on the test of language development. Journal of Communication Disorders. 1991;24:123–134. doi: 10.1016/0021-9924(91)90016-c. [DOI] [PubMed] [Google Scholar]
- Carr JL, Agnihotri S, Keightley M. Sensory processing and adaptive behavior deficits of children across the fetal alcohol spectrum disorder continuum. Alcoholism: Clinical and Experimental Research. 2010;34(6):1022–1032. doi: 10.1111/j.1530-0277.2010.01177.x. [DOI] [PubMed] [Google Scholar]
- Chasnoff IJ, Wells AM, Telford E, Schmidt C, Messer G. Neurodevelopmental functioning in children with FAS, pFAS, and ARND. Journal of Developmental and Behavioral Pediatrics. 2010;31(3):192–201. doi: 10.1097/DBP.0b013e3181d5a4e2. [DOI] [PubMed] [Google Scholar]
- Chiodo LM, Janisse J, Delaney-Black V, Sokol RJ, Hannigan JH. A metric of maternal prenatal risk drinking predicts neurobehavioral outcomes in preschool children. Alcoholism: Clinical and Experimental Research. 2009;33(4):634–644. doi: 10.1111/j.1530-0277.2008.00878.x. [DOI] [PubMed] [Google Scholar]
- Church MW, Eldis F, Blakley BW, Bawle EV. Hearing, language, speech, vestibular, and dentofacial disorders in fetal alcohol syndrome. Alcoholism: Clinical and Experimental Research. 1997;21(2):227–237. [PubMed] [Google Scholar]
- Coffin JM, Baroody S, Schneider K, O'Neill J. Impaired cerebellar learning in children with prenatal alcohol exposure: A comparative study of eyeblink conditioning in children with ADHD and dyslexia. Cortex. 2005;41(3):389–398. doi: 10.1016/s0010-9452(08)70275-2. [DOI] [PubMed] [Google Scholar]
- Coggins TE, Olswang LB, Carmichael Olson H, Timler GR. On becoming socially competent communicators: The challenge for children with fetal alcohol exposure. International Review of Research in Mental Retardation. 2003;27:121–150. [Google Scholar]
- Coggins TE, Timler GR, Olswang LB. A state of double jeopardy: Impact of prenatal alcohol exposure and adverse environments on the social communicative abilities of school-age children with fetal alcohol spectrum disorder. Language, Speech, and Hearing Services in Schools. 2007;38(2):117–127. doi: 10.1044/0161-1461(2007/012). [DOI] [PubMed] [Google Scholar]
- Coles CD, Brown RT, Smith IE, Platzman KA, Erickson S, Falek A. Effects of prenatal alcohol exposure at school age. I. Physical and cognitive development. Neurotoxicology and Teratology. 1991;13(4):357–367. doi: 10.1016/0892-0362(91)90084-a. [DOI] [PubMed] [Google Scholar]
- Coles CD, Lynch ME, Kable JA, Johnson KC, Goldstein FC. Verbal and nonverbal memory in adults prenatally exposed to alcohol. Alcoholism: Clinical and Experimental Research. 2010;34(5):897–906. doi: 10.1111/j.1530-0277.2010.01162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Brown RT, Smith IE, Falek A. Behavior and emotional problems at school age in alcohol-affected children. Alcoholism: Clinical and Experimental Research. 1997;21(3 Supplement):116A. [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Lynch ME. Behavior problems reported by alcohol-affected adolescents and caregivers. Alcoholism: Clinical and Experimental Research. 1999;23(5 Supplement):107A. [Google Scholar]
- Coles CD, Platzman KA, Lynch ME, Freides D. Auditory and visual sustained attention in adolescents prenatally exposed to alcohol. Alcoholism: Clinical and Experimental Research. 2002;26(2):263–271. [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Raskind-Hood CL, Brown RT, Falek A, Smith IE. A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcoholism: Clinical and Experimental Research. 1997;21(1):150–161. [PubMed] [Google Scholar]
- Connor PD, Sampson PD, Bookstein FL, Barr HM, Streissguth AP. Direct and indirect effects of prenatal alcohol damage on executive function. Developmental Neuropsychology. 2000;18(3):331–354. doi: 10.1207/S1532694204Connor. [DOI] [PubMed] [Google Scholar]
- Connor PD, Sampson PD, Streissguth AP, Bookstein FL, Barr HM. Effects of prenatal alcohol exposure on fine motor coordination and balance: A study of two adult samples. Neuropsychologia. 2006;44(5):744–751. doi: 10.1016/j.neuropsychologia.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Connor PD, Streissguth AP, Sampson PD, Bookstein FL, Barr HM. Individual differences in auditory and visual attention among fetal alcohol-affected adults. Alcoholism: Clinical and Experimental Research. 1999;23(8):1395–1402. [PubMed] [Google Scholar]
- Conry J. Neuropsychological deficits in fetal alcohol syndrome and fetal alcohol effects. Alcoholism: Clinical and Experimental Research. 1990;14(5):650–655. doi: 10.1111/j.1530-0277.1990.tb01222.x. [DOI] [PubMed] [Google Scholar]
- Cosbey J, Johnston SS, Dunn ML. Sensory processing disorders and social participation. American Journal of Occupational Therapy. 2010;64(3):462–473. doi: 10.5014/ajot.2010.09076. [DOI] [PubMed] [Google Scholar]
- Crocker N, Vaurio L, Riley EP, Mattson SN. Comparison of adaptive behavior in children with heavy prenatal alcohol exposure or attention-deficit/hyperactivity disorder. Alcoholism: Clinical and Experimental Research. 2009;33(11):2015–2023. doi: 10.1111/j.1530-0277.2009.01040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker N, Vaurio L, Riley EP, Mattson SN. Comparison of verbal learning and memory in children with heavy prenatal alcohol exposure or attention-deficit/hyperactivity disorder. Alcoholism: Clinical and Experimental Research. 2011 doi: 10.1111/j.1530-0277.2011.01444.x. in press. Published online before print March 18, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human behavior. Archives of Neurology. 1993;50(8):873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- D'Onofrio BM, Van Hulle CA, Waldman ID, Rodgers JL, Rathouz PJ, Lahey BB. Causal inferences regarding prenatal alcohol exposure and childhood externalizing problems. Archives of General Psychiatry. 2007;64(11):1296–1304. doi: 10.1001/archpsyc.64.11.1296. [DOI] [PubMed] [Google Scholar]
- Dalen K, Bruaroy S, Wentzel-Larsen T, Laegreid LM. Cognitive functioning in children prenatally exposed to alcohol and psychotropic drugs. Neuropediatrics. 2009;40(4):162–167. doi: 10.1055/s-0029-1243176. [DOI] [PubMed] [Google Scholar]
- David P, Subramaniam K. Prenatal alcohol exposure and early postnatal changes in the developing nerve-muscle system. Birth Defects Research Part A: Clinical and Molecular Teratology. 2005;73(11):897–903. doi: 10.1002/bdra.20190. [DOI] [PubMed] [Google Scholar]
- de los Angeles Avaria M, Mills JL, Kleinsteuber K, Aros S, Conley MR, Cox C, et al. Peripheral nerve conduction abnormalities in children exposed to alcohol in utero. Journal of Pediatrics. 2004;144(3):338–343. doi: 10.1016/j.jpeds.2003.11.028. [DOI] [PubMed] [Google Scholar]
- Disney ER, Iacono W, McGue M, Tully E, Legrand L. Strengthening the case: Prenatal alcohol exposure is associated with increased risk for conduct disorder. Pediatrics. 2008;122(6):e1225–e1230. doi: 10.1542/peds.2008-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domellof E, Fagard J, Jacquet AY, Ronnqvist L. Goal-directed arm movements in children with fetal alcohol syndrome: a kinematic approach. European Journal of Neurology. 2010;18(2):312–320. doi: 10.1111/j.1468-1331.2010.03142.x. [DOI] [PubMed] [Google Scholar]
- Ervalahti N, Korkman M, Fagerlund A, Autti-Ramo I, Loimu L, Hoyme HE. Relationship between dysmorphic features and general cognitive function in children with fetal alcohol spectrum disorders. American Journal of Medical Genetics Part A. 2007;143A(24):2916–2923. doi: 10.1002/ajmg.a.32009. [DOI] [PubMed] [Google Scholar]
- Famy C, Streissguth AP, Unis AS. Mental illness in adults with fetal alcohol syndrome or fetal alcohol effects. American Journal of Psychiatry. 1998;155(4):552–554. doi: 10.1176/ajp.155.4.552. [DOI] [PubMed] [Google Scholar]
- Fast DK, Conry J, Loock CA. Identifying fetal alcohol syndrome among youth in the criminal justice system. Journal of Developmental and Behavioral Pediatrics. 1999;20(5):370–372. doi: 10.1097/00004703-199910000-00012. [DOI] [PubMed] [Google Scholar]
- Franklin L, Deitz J, Jirikowic T, Astley S. Children with fetal alcohol spectrum disorders: problem behaviors and sensory processing. American Journal of Occupational Therapy. 2008;62(3):265–273. doi: 10.5014/ajot.62.3.265. [DOI] [PubMed] [Google Scholar]
- Fried PA, O'Connell CM, Watkinson B. 60- and 72-month follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol: Cognitive and language assessment. Journal of Developmental and Behavioral Pediatrics. 1992;13(6):383–391. [PubMed] [Google Scholar]
- Fried PA, Watkinson B. 12- and 24-month neurobehavioural follow-up of children prenatally exposed to marihuana, cigarettes and alcohol. Neurotoxicology and Teratology. 1988;10(4):305–313. doi: 10.1016/0892-0362(88)90032-3. [DOI] [PubMed] [Google Scholar]
- Fried PA, Watkinson B. 36- and 48-month neurobehavioral follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol. Journal of Developmental and Behavioral Pediatrics. 1990;11(2):49–58. [PubMed] [Google Scholar]
- Fryer SL, McGee CL, Matt GE, Riley EP, Mattson SN. Evaluation of psychopathological conditions in children with heavy prenatal alcohol exposure. Pediatrics. 2007;119:e733–e741. doi: 10.1542/peds.2006-1606. [DOI] [PubMed] [Google Scholar]
- Fryer SL, Tapert SF, Mattson SN, Paulus MP, Spadoni AD, Riley EP. Prenatal alcohol exposure affects frontal-striatal BOLD response during inhibitory control. Alcoholism: Clinical and Experimental Research. 2007;31(8):1415–1424. doi: 10.1111/j.1530-0277.2007.00443.x. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior Rating Inventory of Executive Function: Professional Manual. Odessa, FL: Psychological Assessment Resources, Inc.; 2000. [Google Scholar]
- Goldschmidt L, Richardson GA, Stoffer DS, Geva D, Day NL. Prenatal alcohol exposure and academic achievement at age six: A nonlinear fit. Alcoholism: Clinical and Experimental Research. 1996;20(4):763–770. doi: 10.1111/j.1530-0277.1996.tb01684.x. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Pearlman AD, Lundahl KR. Binge neonatal alcohol intubations induce dose-dependent loss of Purkinje cells. Neurotoxicology and Teratology. 1998;20(3):285–292. doi: 10.1016/s0892-0362(97)00102-5. [DOI] [PubMed] [Google Scholar]
- Green CR, Mihic AM, Brien DC, Armstrong IT, Nikkel SM, Stade BC, et al. Oculomotor control in children with fetal alcohol spectrum disorders assessed using a mobile eye-tracking laboratory. European Journal of Neuroscience. 2009;29(6):1302–1309. doi: 10.1111/j.1460-9568.2009.06668.x. [DOI] [PubMed] [Google Scholar]
- Green CR, Mihic AM, Nikkel SM, Stade BC, Rasmussen C, Munoz DP, et al. Executive function deficits in children with fetal alcohol spectrum disorders (FASD) measured using the Cambridge Neuropsychological Tests Automated Battery (CANTAB) Journal of Child Psychology and Psychiatry. 2009;50(6):688–697. doi: 10.1111/j.1469-7610.2008.01990.x. [DOI] [PubMed] [Google Scholar]
- Greenbaum RL, Stevens SA, Nash K, Koren G, Rovet J. Social cognitive and emotion processing abilities of children with fetal alcohol spectrum disorders: A comparison with attention deficit hyperactivity disorder. Alcoholism: Clinical and Experimental Research. 2009;33(10):1656–1670. doi: 10.1111/j.1530-0277.2009.01003.x. [DOI] [PubMed] [Google Scholar]
- Greene T, Ernhart CB, Martier SS, Sokol RJ, Ager JW., Jr Prenatal alcohol exposure and language development. Alcoholism: Clinical and Experimental Research. 1990;14(6):937–945. doi: 10.1111/j.1530-0277.1990.tb01842.x. [DOI] [PubMed] [Google Scholar]
- Guerri C, Bazinet A, Riley EP. Foetal alcohol spectrum disorders and alterations in brain and behaviour. Alcohol and Alcoholism. 2009;44(2):108–114. doi: 10.1093/alcalc/agn105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusella JL, Fried PA. Effects of maternal social drinking and smoking on offspring at 13 months. Neurobehavioral Toxicology and Teratology. 1984;6(1):13–17. [PubMed] [Google Scholar]
- Hamilton DA, Kodituwakku P, Sutherland RJ, Savage DD. Children with fetal alcohol syndrome are impaired at place learning but not cued-navigation in a virtual Morris water task. Behavioural Brain Research. 2003;143(1):85–94. doi: 10.1016/s0166-4328(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Hamre KM, West JR. The effects of the timing of ethanol exposure during the brain growth spurt on the number of cerebellar Purkinje and granule cell nuclear profiles. Alcoholism: Clinical and Experimental Research. 1993;17(3):610–622. doi: 10.1111/j.1530-0277.1993.tb00808.x. [DOI] [PubMed] [Google Scholar]
- Hanson JW, Jones KL, Smith DW. Fetal alcohol syndrome: Experience with 41 patients. Journal of the American Medical Association. 1976;235(14):1458–1460. [PubMed] [Google Scholar]
- Heaton MB, Bradley DM. Ethanol influences on the chick embryo spinal cord motor system: Analyses of motoneuron cell death, motility, and target trophic factor activity and in vitro analyses of neurotoxicity and trophic factor neuroprotection. Journal of Neurobiology. 1995;26(1):47–61. doi: 10.1002/neu.480260105. [DOI] [PubMed] [Google Scholar]
- Herman LE, Acosta MC, Chang PN. Gender and attention deficits in children diagnosed with a fetal alcohol spectrum disorder. The Canadian Journal of Clinical Pharmacology. 2008;15(3):e411–e419. [PubMed] [Google Scholar]
- Herrmann J, Pallister PD, Optiz JM. Tetraectrodactyly and other skeletal manifestations in the fetal alcohol syndrome. European Journal of Pediatrics. 1980;133:221–226. doi: 10.1007/BF00496080. [DOI] [PubMed] [Google Scholar]
- Hill SY, Lowers L, Locke-Wellman J, Shen S. Maternal smoking and drinking during pregnancy and the risk for child and adolescent psychiatric disorders. Journal of Studies on Alcohol. 2000;61(5):661–668. doi: 10.15288/jsa.2000.61.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell KK, Lynch ME, Platzman KA, Smith GH, Coles CD. Prenatal alcohol exposure and ability, academic achievement, and school functioning in adolescence: A longitudinal follow-up. Journal of Pediatric Psychology. 2006;31(1):116–126. doi: 10.1093/jpepsy/jsj029. [DOI] [PubMed] [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, et al. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: Clarification of the 1996 Institute of Medicine criteria. Pediatrics. 2005;115(1):39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JL, Dodge NC, Burden MJ, Klorman R, Jacobson SW. Number Processing in Adolescents With Prenatal Alcohol Exposure and ADHD: Differences in the Neurobehavioral Phenotype. Alcoholism: Clinical and Experimental Research. 2011;35(3):431–442. doi: 10.1111/j.1530-0277.2010.01360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ. Effects of fetal alcohol exposure on infant reaction time. Alcoholism: Clinical and Experimental Research. 1994;18(5):1125–1132. doi: 10.1111/j.1530-0277.1994.tb00092.x. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Ager JW. Prenatal alcohol exposure and infant information processing ability. Child Development. 1993;64(6):1706–1721. [PubMed] [Google Scholar]
- Janzen LA, Nanson JL, Block GW. Neuropsychological evaluation of preschoolers with fetal alcohol syndrome. Neurotoxicology and Teratology. 1995;17(3):273–279. doi: 10.1016/0892-0362(94)00063-j. [DOI] [PubMed] [Google Scholar]
- Jirikowic T, Carmichael Olson H, Kartin D. Sensory processing, school performance, and adaptive behavior of young school-age children with fetal alcohol spectrum disorders. Physical & Occupational Therapy in Pediatrics. 2008;28(2):117–136. doi: 10.1080/01942630802031800. [DOI] [PubMed] [Google Scholar]
- Jirikowic T, Kartin D, Carmichael Olson H. Children with fetal alcohol spectrum disorders: A descriptive profile of adaptive function. Canadian Journal of Occupational Therapy. 2008;75(4):238–248. doi: 10.1177/000841740807500411. [DOI] [PubMed] [Google Scholar]
- Johnson TB, Goodlett CR. Selective and enduring deficits in spatial learning after limited neonatal binge alcohol exposure in male rats. Alcoholism: Clinical and Experimental Research. 2002;26(1):83–93. [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;2(7836):999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Kaemingk KL, Halverson PT. Spatial memory following prenatal alcohol exposure: More than a material specific memory deficit. Child Neuropsychology. 2000;6(2):115–128. doi: 10.1076/chin.6.2.115.7058. [DOI] [PubMed] [Google Scholar]
- Kaemingk KL, Mulvaney S, Tanner Halverson P. Learning following prenatal alcohol exposure: Performance on verbal and visual multitrial tasks. Archives of Clinical Neuropsychology. 2003;18(1):33–47. [PubMed] [Google Scholar]
- Kalberg WO, Provost B, Tollison SJ, Tabachnick BG, Robinson LK, Hoyme HE, et al. Comparison of motor delays in young children with fetal alcohol syndrome to those with prenatal alcohol exposure and with no prenatal alcohol exposure. Alcoholism: Clinical and Experimental Research. 2006;30(12):2037–2045. doi: 10.1111/j.1530-0277.2006.00250.x. [DOI] [PubMed] [Google Scholar]
- Kodituwakku P, Coriale G, Fiorentino D, Aragón AS, Kalberg WO, Buckley D, et al. Neurobehavioral characteristics of children with fetal alcohol spectrum disorders in communities from Italy: Preliminary results. Alcoholism: Clinical and Experimental Research. 2006;30(9):1551–1561. doi: 10.1111/j.1530-0277.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW. Defining the behavioral phenotype in children with fetal alcohol spectrum disorders: A review. Neuroscience and Biobehavioral Reviews. 2007;31(2):192–201. doi: 10.1016/j.neubiorev.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW, Adnams CM, Hay A, Kitching AE, Burger E, Kalberg WO, et al. Letter and category fluency in children with fetal alcohol syndrome from a community in South Africa. Journal of Studies on Alcohol. 2006;67(4):502–509. doi: 10.15288/jsa.2006.67.502. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW, Handmaker NS, Cutler SK, Weathersby EK, Handmaker SD. Specific impairments in self-regulation in children exposed to alcohol prenatally. Alcoholism: Clinical and Experimental Research. 1995;19(6):1558–1564. doi: 10.1111/j.1530-0277.1995.tb01024.x. [DOI] [PubMed] [Google Scholar]
- Kooistra L, Crawford S, Gibbard B, Ramage B, Kaplan BJ. Differentiating attention deficits in children with fetal alcohol spectrum disorder or attention-deficit-hyperactivity disorder. Developmental Medicine and Child Neurology. 2010;52(2):205–211. doi: 10.1111/j.1469-8749.2009.03352.x. [DOI] [PubMed] [Google Scholar]
- Kooistra L, Ramage B, Crawford S, Cantell M, Wormsbecker S, Gibbard B, et al. Can attention deficit hyperactivity disorder and fetal alcohol spectrum disorder be differentiated by motor and balance deficits? Human Movement Science. 2009;28(4):529–542. doi: 10.1016/j.humov.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Kopera-Frye K, Dehaene S, Streissguth AP. Impairments of number processing induced by prenatal alcohol exposure. Neuropsychologia. 1996;34(12):1187–1196. doi: 10.1016/0028-3932(96)00043-7. [DOI] [PubMed] [Google Scholar]
- Korkman M, Autti-Rämö I, Koivulehto H, Granström M-L. Neuropsychological effects at early school age of fetal alcohol exposure of varying duration. Child Neuropsychology. 1998;4(3):199–212. [Google Scholar]
- LaDue RA, Streissguth AP, Randels SP. Clinical considerations pertaining to adolescents and adults with fetal alcohol syndrome. In: Sonderegger TB, editor. Perinatal Substance Abuse: Research Findings and Clinical Implications. Baltimore: The Johns Hopkins University Press; 1992. pp. 104–131. [Google Scholar]
- Lebel C, Rasmussen C, Wyper K, Andrew G, Beaulieu C. Brain microstructure is related to math ability in children with fetal alcohol spectrum disorder. Alcoholism: Clinical and Experimental Research. 2010;34(2):354–363. doi: 10.1111/j.1530-0277.2009.01097.x. [DOI] [PubMed] [Google Scholar]
- Livy DJ, Miller EK, Maier SE, West JR. Fetal alcohol exposure and temporal vulnerability: Effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicology and Teratology. 2003;25(4):447–458. doi: 10.1016/s0892-0362(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Lynch ME, Coles CD, Corley T, Falek A. Examining delinquency in adolescents differentially prenatally exposed to alcohol: The role of proximal and distal risk factors. Journal of Studies on Alcohol. 2003;64(5):678–686. doi: 10.15288/jsa.2003.64.678. [DOI] [PubMed] [Google Scholar]
- Maier SE, West JR. Regional differences in cell loss associated with binge-like alcohol exposure during the first two trimesters equivalent in the rat. Alcohol. 2001;23(1):49–57. doi: 10.1016/s0741-8329(00)00133-6. [DOI] [PubMed] [Google Scholar]
- Malisza KL, Allman A-A, Shiloff D, Jakobson L, Longstaffe S, Chudley AE. Evaluation of spatial working memory function in children and adults with fetal alcohol spectrum disorders: A functional magnetic resonance imaging study. Pediatric Research. 2005;58(6):1150–1157. doi: 10.1203/01.pdr.0000185479.92484.a1. [DOI] [PubMed] [Google Scholar]
- Marcus JC. Neurological findings in the fetal alcohol syndrome. Neuropediatrics. 1987;18(3):158–160. doi: 10.1055/s-2008-1052471. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Calarco KE, Lang AR. Focused and shifting attention in children with heavy prenatal alcohol exposure. Neuropsychology. 2006;20(3):361–369. doi: 10.1037/0894-4105.20.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Goodman AM, Caine C, Delis DC, Riley EP. Executive functioning in children with heavy prenatal alcohol exposure. Alcoholism: Clinical and Experimental Research. 1999;23(11):1808–1815. [PubMed] [Google Scholar]
- Mattson SN, Gramling L, Delis D, Jones KL, Riley EP. Global-local processing in children prenatally exposed to alcohol. Child Neuropsychology. 1996;2(3):165–175. [Google Scholar]
- Mattson SN, Riley EP. A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcoholism: Clinical and Experimental Research. 1998;22(2):279–294. doi: 10.1111/j.1530-0277.1998.tb03651.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP. Implicit and explicit memory functioning in children with heavy prenatal alcohol exposure. Journal of the International Neuropsychological Society. 1999;5(5):462–471. doi: 10.1017/s1355617799555082. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP. Parent ratings of behavior in children with heavy prenatal alcohol exposure and IQ-matched controls. Alcoholism: Clinical and Experimental Research. 2000;24(2):226–231. [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Delis DC, Stern C, Jones KL. Verbal learning and memory in children with fetal alcohol syndrome. Alcoholism: Clinical and Experimental Research. 1996;20(5):810–816. doi: 10.1111/j.1530-0277.1996.tb05256.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling LJ, Delis DC, Jones KL. Heavy prenatal alcohol exposure with or without physical features of fetal alcohol syndrome leads to IQ deficits. Journal of Pediatrics. 1997;131(5):718–721. doi: 10.1016/s0022-3476(97)70099-4. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling LJ, Delis DC, Jones KL. Neuropsychological comparison of alcohol-exposed children with or without physical features of fetal alcohol syndrome. Neuropsychology. 1998;12(1):146–153. doi: 10.1037//0894-4105.12.1.146. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Sowell ER, Jernigan TL, Sobel DF, Jones KL. A decrease in the size of the basal ganglia in children with fetal alcohol syndrome. Alcoholism: Clinical and Experimental Research. 1996;20(6):1088–1093. doi: 10.1111/j.1530-0277.1996.tb01951.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Roebuck TM. Acquisition and retention of verbal and nonverbal information in children with heavy prenatal alcohol exposure. Alcoholism: Clinical and Experimental Research. 2002;26(6):875–882. [PubMed] [Google Scholar]
- Mattson SN, Roesch SC, Fagerlund A, Autti-Ramo I, Jones KL, May PA, et al. Toward a neurobehavioral profile of fetal alcohol spectrum disorders. Alcoholism: Clinical and Experimental Research. 2010;34(9):1640–1650. doi: 10.1111/j.1530-0277.2010.01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee CL, Bjorkquist OA, Price JM, Mattson SN, Riley EP. Social information processing skills in children with histories of heavy prenatal alcohol exposure. Journal of Abnormal Child Psychology. 2009;37(6):817–830. doi: 10.1007/s10802-009-9313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee CL, Bjorkquist OA, Riley EP, Mattson SN. Impaired language performance in young children with heavy prenatal alcohol exposure. Neurotoxicology and Teratology. 2009;31(2):71–75. doi: 10.1016/j.ntt.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee CL, Fryer SL, Bjorkquist OA, Mattson SN, Riley EP. Deficits in social problem solving in adolescents with prenatal exposure to alcohol. The American Journal of Drug and Alcohol Abuse. 2008;34(4):423–431. doi: 10.1080/00952990802122630. [DOI] [PubMed] [Google Scholar]
- McGee CL, Schonfeld AM, Roebuck-Spencer TM, Riley EP, Mattson SN. Children with heavy prenatal alcohol exposure demonstrate deficits on multiple measures of concept formation. Alcoholism: Clinical and Experimental Research. 2008;32(8):1388–1397. doi: 10.1111/j.1530-0277.2008.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meintjes EM, Jacobson JL, Molteno CD, Gatenby JC, Warton C, Cannistraci CJ, et al. An FMRI study of number processing in children with fetal alcohol syndrome. Alcoholism: Clinical and Experimental Research. 2010;34(8):1450–1464. doi: 10.1111/j.1530-0277.2010.01230.x. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Brody D, Fisher PW, Bourdon K, Koretz DS. Prevalence and treatment of mental disorders among US children in the 2001–2004 NHANES. Pediatrics. 2010;125(1):75–81. doi: 10.1542/peds.2008-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MJ. Perceptual differences in fetal alcohol effect boys performing a modeling task. Perceptual and Motor Skills. 1998;87(3 Pt 1):784–786. doi: 10.2466/pms.1998.87.3.784. [DOI] [PubMed] [Google Scholar]
- Mirsky AF, Anthony BJ, Duncan CC, Ahearn MB, Kellam SG. Analysis of the elements of attention: A neuropsychological approach. Neuropsychology Review. 1991;2(2):109–145. doi: 10.1007/BF01109051. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Garrud P, Rawlins JNP, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Naidoo S, Norval G, Swanevelder S, Lombard C. Foetal alcohol syndrome: A dental and skeletal age analysis of patients and controls. European Journal of Orthodontics. 2006;28:247–253. doi: 10.1093/ejo/cji109. [DOI] [PubMed] [Google Scholar]
- Nanson JL, Hiscock M. Attention deficits in children exposed to alcohol prenatally. Alcoholism: Clinical and Experimental Research. 1990;14(5):656–661. doi: 10.1111/j.1530-0277.1990.tb01223.x. [DOI] [PubMed] [Google Scholar]
- Nash K, Rovet J, Greenbaum R, Fantus E, Nulman I, Koren G. Identifying the behavioural phenotype in fetal alcohol spectrum disorder: Sensitivity, specificity and screening potential. Archives of Women's Mental Health. 2006;9(4):181–186. doi: 10.1007/s00737-006-0130-3. [DOI] [PubMed] [Google Scholar]
- O'Connor MJ. Prenatal alcohol exposure and infant negative affect as precursors of depressive features in children. Infant Mental Health Journal. 2001;22(3):291–299. [Google Scholar]
- O'Connor MJ, Kasari C. Prenatal alcohol exposure and depressive features in children. Alcoholism: Clinical and Experimental Research. 2000;24(7):1084–1092. [PubMed] [Google Scholar]
- O'Connor MJ, Paley B. The relationship of prenatal alcohol exposure and the postnatal environment to child depressive symptoms. Journal of Pediatric Psychology. 2006;31(1):50–64. doi: 10.1093/jpepsy/jsj021. [DOI] [PubMed] [Google Scholar]
- O'Connor MJ, Shah B, Whaley S, Cronin P, Gunderson B, Graham J. Psychiatric illness in a clinical sample of children with prenatal alcohol exposure. The American Journal of Drug and Alcohol Abuse. 2002;28(4):743–754. doi: 10.1081/ada-120015880. [DOI] [PubMed] [Google Scholar]
- O'Hare ED, Lu LH, Houston SM, Bookheimer SY, Mattson SN, O'Connor MJ, et al. Altered frontal-parietal functioning during verbal working memory in children and adolescents with heavy prenatal alcohol exposure. Human Brain Mapping. 2009;30(10):3200–3208. doi: 10.1002/hbm.20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Research. 1971;34(1):171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O'Leary C, Zubrick SR, Taylor CL, Dixon G, Bower C. Prenatal alcohol exposure and language delay in 2-year-old children: The importance of dose and timing on risk. Pediatrics. 2009;123(2):547–554. doi: 10.1542/peds.2008-0459. [DOI] [PubMed] [Google Scholar]
- O'Malley K, Huggins J. Suicidality in adolescents and adults with fetal alcohol spectrum disorders. Canadian Journal of Psychiatry. 2005;50(2):125. doi: 10.1177/070674370505000211. [DOI] [PubMed] [Google Scholar]
- Pei JR, Rinaldi CM, Rasmussen C, Massey V, Massey D. Memory patterns of acquisition and retention of verbal and nonverbal information in children with fetal alcohol spectrum disorders. The Canadian Journal of Clinical Pharmacology. 2008;15(1):e44–e56. [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1996;37(1):51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Pulsifer MB. The neuropsychology of mental retardation. Journal of the International Neuropsychological Society. 1996;2(2):159–176. doi: 10.1017/s1355617700001016. [DOI] [PubMed] [Google Scholar]
- Rasmussen C, Bisanz J. Executive functioning in children with fetal alcohol spectrum disorders: Profiles and age-related differences. Child Neuropsychology. 2009;15(3):201–215. doi: 10.1080/09297040802385400. [DOI] [PubMed] [Google Scholar]
- Rasmussen C, Bisanz J. The Relation Between Mathematics and Working Memory in Young Children With Fetal Alcohol Spectrum Disorders. The Journal of Special Education. 2010 Published online before print January 8, 2010. [Google Scholar]
- Rasmussen C, Horne K, Witol A. Neurobehavioral functioning in children with fetal alcohol spectrum disorder. Child Neuropsychology. 2006;12:453–468. doi: 10.1080/09297040600646854. [DOI] [PubMed] [Google Scholar]
- Rasmussen C, McAuley R, Andrew G. Parental ratings of children with fetal alcohol spectrum disorder on the Behavior Rating Inventory of Executive Functioning (BRIEF) Journal of FAS International. 2007;5 Retrieved from http://www.motherisk.org/FAR/econtent_commonDetail.jsp?econtent_id=134. [Google Scholar]
- Rasmussen C, Talwar V, Loomes C, Andrew G. Brief report: Lie-telling in children with fetal alcohol spectrum disorder. Journal of Pediatric Psychology. 2008;33(2):220–226. doi: 10.1093/jpepsy/jsm069. [DOI] [PubMed] [Google Scholar]
- Rasmussen C, Wyper K, Talwar V. The relation between theory of mind and executive functions in children with fetal alcohol spectrum disorders. The Canadian Journal of Clinical Pharmacology (Journal Canadien de Pharmacologie Clinique) 2009;16(2):e370–e380. [PubMed] [Google Scholar]
- Richardson GA, Ryan C, Willford J, Day NL, Goldschmidt L. Prenatal alcohol and marijuana exposure: Effects on neuropsychological outcomes at 10 years. Neurotoxicology and Teratology. 2002;24(3):309–320. doi: 10.1016/s0892-0362(02)00193-9. [DOI] [PubMed] [Google Scholar]
- Riikonen R, Salonen I, Partanen K, Verho S. Brain perfusion SPECT and MRI in foetal alcohol syndrome. Developmental Medicine and Child Neurology. 1999;41(10):652–659. doi: 10.1017/s0012162299001358. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Olsen J, Kotimaa AJ, Kaakinen M, Moilanen I, Henriksen TB, et al. Is prenatal alcohol exposure related to inattention and hyperactivity symptoms in children? Disentangling the effects of social adversity. Journal of Child Psychology and Psychiatry. 2009;50(9):1073–1083. doi: 10.1111/j.1469-7610.2009.02071.x. [DOI] [PubMed] [Google Scholar]
- Roebuck TM, Mattson SN, Riley EP. Behavioral and psychosocial profiles of alcohol-exposed children. Alcoholism: Clinical and Experimental Research. 1999;23(6):1070–1076. [PubMed] [Google Scholar]
- Roebuck TM, Simmons RW, Mattson SN, Riley EP. Prenatal exposure to alcohol affects the ability to maintain postural balance. Alcoholism: Clinical and Experimental Research. 1998;22(1):252–258. [PubMed] [Google Scholar]
- Roebuck TM, Simmons RW, Richardson C, Mattson SN, Riley EP. Neuromuscular responses to disturbance of balance in children with prenatal exposure to alcohol. Alcoholism: Clinical and Experimental Research. 1998;22(9):1992–1997. [PubMed] [Google Scholar]
- Roebuck-Spencer TM, Mattson SN. Implicit strategy affects learning in children with heavy prenatal alcohol exposure. Alcoholism: Clinical and Experimental Research. 2004;28(9):1424–1431. doi: 10.1097/01.alc.0000139826.25247.5b. [DOI] [PubMed] [Google Scholar]
- Roebuck-Spencer TM, Mattson SN, Marion SD, Brown WS, Riley EP. Bimanual coordination in alcohol-exposed children: Role of the corpus callosum. Journal of the International Neuropsychological Society. 2004;10(4):536–548. doi: 10.1017/S1355617704104116. [DOI] [PubMed] [Google Scholar]
- Russell M, Czarnecki DM, Cowan R, McPherson E, Mudar PJ. Measures of maternal alcohol use as predictors of development in early childhood. Alcoholism: Clinical and Experimental Research. 1991;15(6):991–1000. doi: 10.1111/j.1530-0277.1991.tb05200.x. [DOI] [PubMed] [Google Scholar]
- Santhanam P, Li Z, Hu X, Lynch ME, Coles CD. Effects of prenatal alcohol exposure on brain activation during an arithmetic task: An fMRI study. Alcoholism: Clinical and Experimental Research. 2009;33(11):1901–1908. doi: 10.1111/j.1530-0277.2009.01028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonfeld AM, Mattson SN, Lang AR, Delis DC, Riley EP. Verbal and nonverbal fluency in children with heavy prenatal alcohol exposure. Journal of Studies on Alcohol. 2001;62(2):239–246. doi: 10.15288/jsa.2001.62.239. [DOI] [PubMed] [Google Scholar]
- Schonfeld AM, Mattson SN, Riley EP. Moral maturity and deliquency after prenatal alcohol exposure. Journal of Studies on Alcohol. 2005;66(4):545–555. doi: 10.15288/jsa.2005.66.545. [DOI] [PubMed] [Google Scholar]
- Schonfeld AM, Paley B, Frankel F, O'Connor MJ. Executive functioning predicts social skills following prenatal alcohol exposure. Child Neuropsychology. 2006;12(6):439–452. doi: 10.1080/09297040600611338. [DOI] [PubMed] [Google Scholar]
- Simmons RW, Levy SS, Riley EP, Madra NM, Mattson SN. Central and peripheral timing variability in children with heavy prenatal alcohol exposure. Alcoholism: Clinical and Experimental Research. 2009;33(3):400–407. doi: 10.1111/j.1530-0277.2008.00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons RW, Nguyen TT, Levy SS, Thomas JD, Riley EP, Mattson SN. Regulation of Isometric Force in Children with Heavy Prenatal Alcohol Exposure. Manuscript submitted for publication. 2011 [Google Scholar]
- Simmons RW, Thomas JD, Levy SS, Riley EP. Motor response selection in children with fetal alcohol spectrum disorders. Neurotoxicology and Teratology. 2006;28(2):278–285. doi: 10.1016/j.ntt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Simmons RW, Thomas JD, Levy SS, Riley EP. Motor response programming and movement time in children with heavy prenatal alcohol exposure. Alcohol. 2010;44(4):371–378. doi: 10.1016/j.alcohol.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons RW, Wass T, Thomas JD, Riley EP. Fractionated simple and choice reaction time in children with prenatal exposure to alcohol. Alcoholism: Clinical and Experimental Research. 2002;26(9):1412–1419. doi: 10.1097/01.ALC.0000030563.14827.29. [DOI] [PubMed] [Google Scholar]
- Sood B, Delaney-Black V, Covington C, Nordstrom-Klee B, Ager J, Templin T, et al. Prenatal alcohol exposure and childhood behavior at age 6 to 7 years: I. Dose-response effect. Pediatrics. 2001;108(2):e34–e42. doi: 10.1542/peds.108.2.e34. [DOI] [PubMed] [Google Scholar]
- Spohr H-L, Willms J, Steinhausen H-C. Fetal alcohol spectrum disorders in young adulthood. The Journal of Pediatrics. 2007;150(2):175–179. doi: 10.1016/j.jpeds.2006.11.044. [DOI] [PubMed] [Google Scholar]
- Staroselsky A, Fantus E, Sussman R, Sandor P, Koren G, Nulman I. Both parental psychopathology and prenatal maternal alcohol dependency can predict the behavioral phenotype in children. Pediatric Drugs. 2009;11(1):22–25. doi: 10.2165/0148581-200911010-00009. [DOI] [PubMed] [Google Scholar]
- Steinhausen H-C, Spohr H-L. Long-term outcome of children with fetal alcohol syndrome: Psychopathology, behavior and intelligence. Alcoholism: Clinical and Experimental Research. 1998;22(2):334–338. doi: 10.1111/j.1530-0277.1998.tb03657.x. [DOI] [PubMed] [Google Scholar]
- Steinhausen H-C, Willms J, Metzke CW, Spohr H-L. Behavioural phenotype in foetal alcohol syndrome and foetal alcohol effects. Developmental Medicine and Child Neurology. 2003;45(3):179–182. doi: 10.1017/s0012162203000343. [DOI] [PubMed] [Google Scholar]
- Steinhausen H-C, Willms J, Spohr H-L. Long-term psychopathological and cognitive outcome of children with fetal alcohol syndrome. Journal of the American Academy of Child and Adolescent Psychiatry. 1993;32(5):990–994. doi: 10.1097/00004583-199309000-00016. [DOI] [PubMed] [Google Scholar]
- Steinhausen H-C, Willms J, Spohr H-L. Correlates of psychopathology and intelligence in children with fetal alcohol syndrome. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1994;35(2):323–331. doi: 10.1111/j.1469-7610.1994.tb01165.x. [DOI] [PubMed] [Google Scholar]
- Stratton K, Howe C, Battaglia F. Fetal alcohol syndrome: Diagnosis, epidemiology, prevention, and treatment. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Streissguth AP, Aase JM, Clarren SK, Randels SP, LaDue RA, Smith DF. Fetal alcohol syndrome in adolescents and adults. Journal of the American Medical Association. 1991;265(15):1961–1967. [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Kogan J, Bookstein FL. Final report: Understanding the occurrence of secondary disabilities in clients with fetal alcohol syndrome (FAS) and fetal alcohol effects (FAE) Seattle, WA: University of Washington Publication Services; 1996. [Google Scholar]
- Streissguth AP, Barr HM, Olson HC, Sampson PD, Bookstein FL, Burgess DM. Drinking during pregnancy decreases word attack and arithmetic scores on standardized tests: Adolescent data from a population-based prospective study. Alcoholism: Clinical and Experimental Research. 1994;18(2):248–254. doi: 10.1111/j.1530-0277.1994.tb00009.x. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Sampson PD. Moderate prenatal alcohol exposure: Effects on child IQ and learning problems at age 7 1/2 years. Alcoholism: Clinical and Experimental Research. 1990;14(5):662–669. doi: 10.1111/j.1530-0277.1990.tb01224.x. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Sampson PD, Darby BL, Martin DC. IQ at age 4 in relation to maternal alcohol use and smoking during pregnancy. Developmental Psychology. 1989;25(1):3–11. [Google Scholar]
- Streissguth AP, Barr HM, Sampson PD, Parrish-Johnson JC, Kirchner GL, Martin DC. Attention, distraction and reaction time at age 7 years and prenatal alcohol exposure. Neurobehavioral Toxicology and Teratology. 1986;8(6):717–725. [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O'Malley K, Young JK. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. Journal of Developmental and Behavioral Pediatrics. 2004;25(4):228–238. doi: 10.1097/00004703-200408000-00002. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Martin DC, Barr HM, Sandman BM, Kirchner GL, Darby BL. Intrauterine alcohol and nicotine exposure: Attention and reaction time in 4-year-old children. Developmental Psychology. 1984;20(4):533–541. [Google Scholar]
- Streissguth AP, Sampson PD, Barr HM. Neurobehavioral dose-response effects of prenatal alcohol exposure from infancy to adulthood. Annals of the New York Academy of Sciences. 1989;562:145–158. doi: 10.1111/j.1749-6632.1989.tb21013.x. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Sampson PD, Olson HC, Bookstein FL, Barr HM, Scott M, et al. Maternal drinking during pregnancy: Attention and short-term memory in 14-year-old offspring -- a longitudinal prospective study. Alcoholism: Clinical and Experimental Research. 1994;18(1):202–218. doi: 10.1111/j.1530-0277.1994.tb00904.x. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Goodlett CR, West JR. Alcohol-induced Purkinje cell loss depends on developmental timing of alcohol exposure and correlates with motor performance. Developmental Brain Research. 1998;105(2):159–166. doi: 10.1016/s0165-3806(97)00164-8. [DOI] [PubMed] [Google Scholar]
- Thomas SE, Kelly SJ, Mattson SN, Riley EP. Comparison of social abilities of children with fetal alcohol syndrome to those of children with similar IQ scores and normal controls. Alcoholism: Clinical and Experimental Research. 1998;22(2):528–533. [PubMed] [Google Scholar]
- Thorne JC, Coggins TE, Carmichael Olson H, Astley SJ. Exploring the utility of narrative analysis in diagnostic decision making: Picture-bound reference, elaboration, and fetal alcohol spectrum disorders. Journal of Speech, Language, and Hearing Research. 2007;50(2):459–474. doi: 10.1044/1092-4388(2007/032). [DOI] [PubMed] [Google Scholar]
- Timler GR, Olswang LB, Coggins TE. "Do I know what I need to do?" A social communication intervention for children with complex clinical profiles. Language, Speech, and Hearing Services in Schools. 2005;36(1):73–85. doi: 10.1044/0161-1461(2005/007). [DOI] [PubMed] [Google Scholar]
- Uecker A, Nadel L. Spatial locations gone awry: Object and spatial memory deficits in children with fetal alcohol syndrome. Neuropsychologia. 1996;34(3):209–223. doi: 10.1016/0028-3932(95)00096-8. [DOI] [PubMed] [Google Scholar]
- Uecker A, Nadel L. Spatial but not object memory impairments in children with fetal alcohol syndrome. American Journal on Mental Retardation. 1998;103(1):12–18. doi: 10.1352/0895-8017(1998)103<0012:SBNOMI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Vaurio L, Riley EP, Mattson SN. Differences in executive functioning in children with heavy prenatal alcohol exposure or attention-deficit/hyperactivity disorder. Journal of the International Neuropsychological Society. 2008;14:119–129. doi: 10.1017/S1355617708080144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaurio L, Riley EP, Mattson SN. Comparison of neuropsychological performance in children with heavy prenatal alcohol exposure and an IQ-matched comparison group. Journal of the International Neuropsychological Society. 2011 doi: 10.1017/S1355617711000063. in press. Published online before print February 26, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wass TS, Simmons RW, Thomas JD, Riley EP. Timing accuracy and variability in children with prenatal exposure to alcohol. Alcoholism: Clinical and Experimental Research. 2002;26(12):1887–1896. doi: 10.1097/01.ALC.0000042221.73478.4F. [DOI] [PubMed] [Google Scholar]
- Welsh MC, Pennington BF. Assessing frontal lobe functioning in children: Views from developmental psychology. Developmental Neuropsychology. 1988;4(3):199–230. [Google Scholar]
- Whaley SE, O'Connor MJ, Gunderson B. Comparison of the adaptive functioning of children prenatally exposed to alcohol to a nonexposed clinical sample. Alcoholism: Clinical and Experimental Research. 2001;25(7):1018–1024. [PubMed] [Google Scholar]
- Willford JA, Leech SL, Day NL. Moderate prenatal alcohol exposure and cognitive status of children at age 10. Alcoholism: Clinical and Experimental Research. 2006;30(6):1051–1059. doi: 10.1111/j.1530-0277.2006.00119.x. [DOI] [PubMed] [Google Scholar]
- Willford JA, Richardson GA, Leech SL, Day NL. Verbal and visuospatial learning and memory function in children with moderate prenatal alcohol exposure. Alcoholism: Clinical and Experimental Research. 2004;28(3):497–507. doi: 10.1097/01.alc.0000117868.97486.2d. [DOI] [PubMed] [Google Scholar]
- Willoughby KA, Sheard ED, Nash K, Rovet J. Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. Journal of the International Neuropsychological Society. 2008;14(6):1022–1033. doi: 10.1017/S1355617708081368. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Butnariu OV, Fletcher DL, Riley EP. Limited postnatal ethanol exposure permanently alters the expression of mRNAS encoding myelin basic protein and myelin-associated glycoprotein in cerebellum. Alcoholism: Clinical and Experimental Research. 1994;18(4):909–916. doi: 10.1111/j.1530-0277.1994.tb00059.x. [DOI] [PubMed] [Google Scholar]


