Abstract
Adult stem cells are maintained in a quiescent state, but are able to exit quiescence and rapidly expand and differentiate in response to stress. The quiescent state appears to be necessary for preserving self-renewal of stem cells and a critical factor in resistance of cancer stem cells (CSC) to chemotherapy and targeted therapies. Limited knowledge of quiescence mechanisms has prevented significant advance in targeting of drug resistant quiescent CSC populations in the clinic. Thus improved understanding of the molecular mechanisms of quiescence in adult stem cells is critical for development of molecularly targeted therapies against quiescent CSC in different cancers. Recent studies have provided a better understanding of intrinsic and extrinsic regulatory mechanisms that control stem cell quiescence. It is now appreciated that the p53 gene plays a critical role in regulating stem cell quiescence. Other intrinsic regulatory mechanisms include the FoxO,, HIF-1α and NFATc1 transcription factors, and signaling through ATM and mTOR. Extrinsic microenvironmental regulatory mechanisms include Angiopoietin-1, TGF-β, BMP, TPO, N-Cadherin and integrin adhesion receptors, Wnt/β-catenin signaling and osteopontin. In this article, we review current advances in understanding normal stem cell quiescence, their significance for CSC quiescence and drug resistance, and the potential clinical applications of these findings.
Keywords: Adult stem cells, Quiescence, Cancer stem cells, Stem cell niche, Targeted therapy
BACKGROUND
Adult stem cells are rare populations of cells that are able to regenerate the multiple differentiated cell types of the organ in which they reside and self-renew themselves (1). In contrast to germline stem cell of invertebrates that are constantly cycling (2), mammalian adult stem cells are predominantly in a quiescent, non-dividing, G0 state (3). Hematopoietic stem cells (HSC) are amongst the best studied adult stem cell populations. Quiescence of HSC has been linked to their long-term reconstituting capacity and is critical for long-term sustenance of the stem cell compartment (1). In order to both maintain a supply of mature blood cells throughout the lifetime of an individual without exhausting the HSC pool, most HSC remain quiescent under steady state and only a small number enter the cell cycle (4). However, HSC can exit quiescence and rapidly expand and differentiate to regenerate hematopoiesis in response to stresses such as blood loss (4). Defects in regulation of quiescence can lead to premature exhaustion of the HSC pool, causing hematological failure (3, 5). Stem cell quiescence is also closely associated with protection from myelotoxic insults (5). As with normal tissues, several cancers are also propagated by small populations of cancer stem cells (CSC) (6). Stem cell quiescence is highly relevant to cancer therapy since quiescent CSC are often resistant to both conventional chemotherapy and targeted therapies, and are retained and contribute to relapse following discontinuation of therapy (6). Therefore, improved understanding of mechanisms of stem cell quiescence is important not only for directed manipulation of normal stem cells function, but also for development of approaches to therapeutically target quiescent CSC.
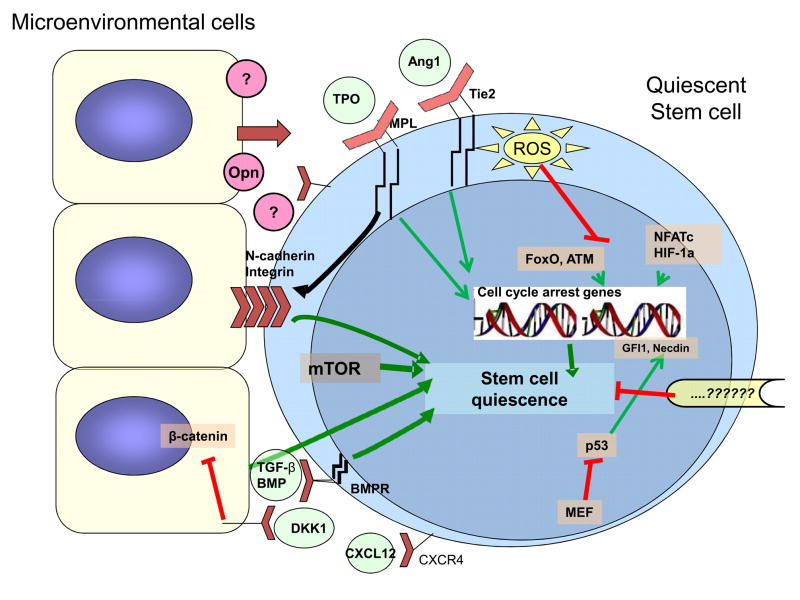
Stem cells quiescence is controlled by both intrinsic regulatory mechanisms and extrinsic signals from the microenvironment as shown in Fig. 1 (7). Several transcription factors play key roles in stem cell fate decisions (8). On the other hand, interactions of stem cells with the niche are critical for the long-term maintenance of HSC quiescence (9). Here, we review recent progress in understanding the mechanisms of quiescence of stem cells and potential applications to cancer treatment.
Figure 1. Pathways to stem cell quiescence.
Stem cell quiescence is controlled by both intrinsic regulatory mechanisms and extrinsic signals from the microenvironment. Several transcription factors play key roles in promoting stem cell quiescence. The transcription factor p53 is a critical regulator of quiescence in the steady state. Reactive oxygen species (ROS) in stem cells regulate expression of the transcription factors FoxO and ATM which in turn act to regulate ROS levels in stem cells and maintain stem cell quiescence. A role for HIF-1α and NFATc in regulating stem cell quiescence has also been shown. Factors inhibiting mTOR also contribute to stem cell quiescence. Interactions of stem cells with microenvironmental supportive cells are critical for the long-term maintenance of HSC quiescence. TGF-β and BMPs produced by microenvironmental cells are important regulators of stem cell quiescence. Interactions of thrombopoietin (TPO) with its receptor MPL and Angiopoietin-1 (Ang-1) with its receptor Tie-2 also promote stem cell quiescence and enhance adhesion to the microenvironment through integrin and cadherin receptors. Osteopontin (Opn) from the microenvironment is also reported to mediate stem cell quiescence. Wnt signaling has complex effects on stem cells but there is evidence that Wnt signaling in the microenvironment plays an essential role for maintaining HSCs in a quiescent state. In future, targeting of molecular mechanisms underlying quiescence may allow sensitization of quiescent cancer stem cells to therapeutic agents.
Intrinsic mechanisms regulating stem cell quiescence
p53 signaling
In addition to its important role in the cellular response to DNA damage, recent studies have shown a critical role for p53 in regulating HSC quiescence in steady state conditions (8, 10). p53 is preferentially expressed in HSC compared to more committed progenitor calls and promotes HSC quiescence (8). The transcription factor MEF/ELF4 modulates p53 expression and facilitates the entry of quiescent HSCs into cell cycle (10). MEFnull HSCs display increased quiescence that is p53 dependent and are resistant to the myelosuppressive effects of chemotherapy and radiation. Although earlier studies suggested a role for the p53 target gene p21, in restricting HSC entry into cell cycle and regulating HSC pool size under stress (11), subsequent studies have indicated that p21 plays a minimal the role in regulating HSC quiescence under steady-state conditions (12). Two other p53 target genes, Gfi-1 and Necdin, have been identified as important regulators of quiescence (8, 13).
Reactive oxygen species, FoxOs and ATM
Reactive oxygen species (ROS) play an important role for regulating stem cell maintenance (14, 15). The FoxO group of human forkhead proteins contains four members, FoxO1, FoxO3a, FoxO4, and FoxO6, with both distinct and overlapping functions. FoxO proteins are activated in response to oxidative stress (16, 17), and up-regulate genes involved in ROS detoxification and cell-cycle arrest (16). HSC from FoxO1, FoxO3, and FoxO4 triple knockout mice exhibit increased levels of ROS, increased cycling, apoptosis, and defective long-term repopulating activity (15). The HSC defect resulting from loss of FoxOs can be rescued by antioxidant administration. ATM, a cell cycle checkpoint regulator activated after DNA damage, also regulates ROS levels in HSC (14). ATM is preferentially expressed in cycling HSC, and ATM-deficient mice show elevated ROS levels, lack quiescent HSC and show progressive BM failure (14). Treatment with antioxidants restores quiescence and BM reconstitutive capacity of ATM−/− HSC.
Hypoxia inducible factor-1α
The transcription factor hypoxia inducible factor-1α (HIF-1α) is stabilized under low oxygen conditions, such as are present in the BM environment (18, 19). HIF-1α levels are elevated in HSC and regulate HSC metabolism (18). HSC from HIF-1α-deficient mice show reduced quiescence and decreased numbers following transplantation, myelosuppression, or aging (19). Overall, these data indicate that precise regulation of HIF-1α levels is required for maintenance of HSC quiescence.
Nuclear factor of activated T cells c1
The hair follicle has also proven to be a good model system for studying stem cell quiescence. Stem cells are localized in the bulge region of the follicle (20, 21). The transcription factor nuclear factor of activated T cells c1 (NFATc1) is preferentially expressed in the bulge relative to proliferative basal cells in the epidermis (20). Pharmacological suppression of NFATc1 or gene ablation reveals a role in regulating HSC quiescence (21). NFATc1 expression is activated by BMP and acts to repress CDK4 transcription. NFATc1 is downregulated when stem cells become activated during hair growth, relieving CDK4 repression and activating proliferation (21).
Negative regulators of mTOR: Fbw7, PTEN, PML
Several reports indicate that negative regulators of mTOR, including Fbxw7 (22), PTEN (23, 24) and PML (25), can maintain stem cell quiescence. Deletion of these genes in mice leads to strikingly similar phenotypes of stem cell hyper-proliferation and subsequent exhaustion, and defective repopulating potential.
Extrinsic mechanisms regulating stem cell quiescence
Stem cells are localized to niches formed by cells that provide a microenvironment that supports their growth and regulates their fate (9, 26). Interaction of stem cells with the niche is crucial for the long-term maintenance of quiescence.
Tie2/Angiopoietin-1
Osteoblasts within the BM provide a niche promoting maintenance of quiescent HSC (27). Osteoblasts are a source of angiopoietin-1 (Ang-1), the ligand for the receptor tyrosine kinase Tie2 specifically expressed in HSCs. Tie2/Ang-1 signaling activates β1-integrin and N-Cadherin in HSC, promoting interactions with extracellular matrix and cellular components of the niche (3). Genetic mouse models indicate that Tie2 interactions maintain quiescence and enhance survival of HSCs by preventing cell division (28). In humans, Ang-1 is expressed in mesenchymal stem cells (29), suggesting that these cells may provide a niche for quiescent human HSCs.
Transforming Growth Factor-β and Bone Morphogenic Proteins
Transforming growth factor-β (TGF-β) and related molecules play important role in maintenance of quiescence. TGF-β is a potent inhibitor of stem cell growth and cycling in vitro (30) - and is hypothesized to be a cardinal regulator of stem cell quiescence in vivo. Disruption of bone morphogenic proteins (BMP) signaling through conditional knock out of Bmpr1a in osteoblasts resulted in increased HSC numbers (31). Conditional ablation of Bmpr1a also activated quiescent hair follicle stem cells to proliferate (32). Quiescent SOX2+ neural stem cells in the subgranular zone are depleted by genetic deletion of Bmpr1a or infusion of the BMP antagonist Noggin (33).
Thrombopoietin
Mice deficient in the MPL receptor or its ligand thrombopoietin (TPO) have fewer HSCs in the BM (34). MPL positive HSCs in close contact with TPO-producing osteoblastic cells at the endosteal surface are quiescent (35, 36). Inhibition of the TPO/MPL interactions with a neutralizing antibody reduced the number of quiescent HSCs. TPO treatment increased the expression of p57Kip2, specifically expressed in the quiescent HSC population (35).
N-Cadherin and integrins
The adhesion molecules N-Cadherin and β1-integrin are required not only for HSC anchoring to the niche, but also in the regulation of HSC cycling (31). N-Cadherin is present at the interface between HSC and osteoblastic cells (31). Tie2/Ang-1 signaling induces β1-integrin and N-Cadherin-dependent HSC adhesion (3). MPL/TPO signaling also up-regulates β1-integrin in HSCs (36). Therefore β1-integrin and N-Cadherin may be key downstream targets of Tie2/Ang-1 and MPL/TPO signaling in HSC.
Osteopontin
Osteopontin (Opn) expressed in osteoblasts negatively regulates HSC number in the BM niche (37). Osteopontin deficient mice show an increase in HSC numbers, suggesting that Opn inhibits HSC proliferation in vivo (38, 39). Normal HSC demonstrate a long-term engraftment defect in an Opn−/− microenvironment (39).
Wnt/β-catenin signaling
Wnt signaling plays a vital role in cellular proliferation, movement, polarity, and in stem cell maintenance (1). Constitutively active nuclear β-catenin signaling reduces quiescence of HSCs and blocks HSC differentiation (40). On the other hand, osteoblast-specific expression of Dickkopf1 (Dkk1), an inhibitor of canonical Wnt signaling, resulted in increased HSC cycling and reduced regenerative capacity (41). Therese findings suggest that Wnt pathway activation in the niche limits HSC proliferation and preserves self-renewal (41). Other studies have shown that microenvironmental β-catenin plays an important role in long-term maintenance of HSC (42). These observations suggest that fine-tuning of Wnt/β-catenin activity in the microenvironment is crucial for maintenance of stem cell quiescence).
CLINICAL TRANSLATIONAL ADVANCES
Malignant stem cells in cancer are characteristically quiescence, and the dormancy of these small populations protects them from elimination following cancer treatment contributing to cancer relapse (1). In several malignancies, including breast and colon cancer, relapse can occur more than a decade after the initial treatment. These late relapses can be explained by the survival and long-term persistence of dormant CSC (6). As with solid tumors, several leukemias such as AML contain heterogeneous cell populations with a small percentage of quiescent leukemia stem cells (LSC) responsible for propagation of the leukemia (6). Recent studies using xenogeneic models indicate that AML LSC are localized to the BM endosteal region are non-cycling and resist elimination by chemotherapy (43). CD34+ cells from CML patients also contain quiescent cells, that are resistant to BCR-ABL tyrosine kinase inhibitors such as Imatinib mesylate (44). Primitive LSC persist in the BM of CML patients in cytogenetic remission on imatinib treatment (44), and stopping treatment frequently leads to disease relapse even in patients in whom BCR-ABL transcripts are no longer detectable by PCR (45). It is likely that overcoming LSC dormancy may be a critical step towards a cure for this and other CSC-driven cancers. Targeting of quiescent CSC is a difficult challenge since most conventional and targeted ant-cancer agents are ineffective in killing this population. There has been increased interest recently to develop approaches based either on activating quiescent CSC inducing their cell cycle entry and increasing their sensitivity to other treatments, or identifying agents that are capable of direct targeting quiescent CSC.
G-CSF
Granulocyte colony-stimulating factor (G-CSF) is used in the clinic to treat neutropenia and to mobilize HSCs to the circulation (46). G-CSF has also been used to enhance the sensitivity of leukemia cells to cytotoxic agents (47). G-CSF treatment induces proteolytic enzyme release in the BM leading to degradation of adhesion molecules, CXCL12 and CXCR4. G-CSF treatment significantly enhanced inhibition of CML LSC by imatinib in vitro. However, a clinical pilot study failed to confirm that combined G-CSF and IM treatment can eliminate LSC in CML patients (48). Recently, G-CSF treatment was shown to efficiently activate dormant human AML stem cells in the endosteal niches, and enhance their elimination by cytarabine without enhancing sensitivity of normal HSCs (43). Thus these protocols may be effective with further improvement and optimization (48).
Interferon
Type I interferons (IFN-α, β) play a critical role in resistance to viral infections and innate and acquired immune responses (49). IFNs also have anti-proliferative properties on many cell types in vitro. However recent observations suggest (50, 51) that IFN-α can stimulate the proliferation of HSC in vivo. How IFN-α signals are perceived differently in HSC compared to other cell types in which IFN-α normally suppress proliferation is unclear. The proliferative effects of IFN on HSC appear to be direct and are transient. Since increased proliferation is only seen in vivo, alterations in niche interactions may play a role. Two recent randomized studies showed greater reduction in BCR-ABL levels when IFN-α is combined with imatinib in the treatment of CML (52, 53). The clinical value of IFN-α in CML treatment may be related to stimulation of quiescent LSC to proliferate, increasing sensitivity to imatinib.
CXC motif receptor-4 antagonists
CXCL12 (stromal cell-derived factor-1, SDF-1) binding to receptor CXC motif receptor-4 (CXCR4) plays an important role in HSC localization to the niche (54). AMD3100 is a bicyclam molecule that selectively and reversibly antagonizes CXCL12-CXCR4 interactions with subsequent dislodgement of HSC from the niche (54). AMD3100 rapidly mobilizes HSC and is approved for stem cell mobilization in combination with G-CSF. AMD3100 also disrupts interaction between AML blasts and the BM stroma, mobilizing blasts to the peripheral blood and sensitizing them to chemotherapy (55). Clinical trials testing AMD3100 as a strategy to sensitize leukemic cells to chemotherapy are underway in patients with AML (54).
Histone deacetylase inhibitors
Histone deacetylase inhibitors (HDACi) have shown promise as a therapy for several cancers (56). In contrast to most other proapoptotic agents that preferentially target dividing cells, HDACi can induce apoptosis in nonproliferating cancer cell lines (56). The combination of HDACi and imatinib induced apoptosis in quiescent CML LSC resistant to elimination with imatinib alone (57). HDACi have pleiotropic effects on cells but potential mechanisms involved in CML LSC inhibition include alteration of gene expression related to LSC self-renewal, survival and microenvironmental interactions. A clinical trial of HDACi in combination with imatinib in patients with CML in cytogenetic remission is underway (58).
Wnt inhibitors
Studies showing a role for Wnt signaling in the microenvironment in maintenance of quiescent HSC (41, 42), suggest that inhibiting Wnt may impair the dormancy of CSC. A small molecule drug ICG001 that selectively inhibits β-catenin binding to the transcriptional cofactor cyclic AMP response element binding protein (CBP) is approved for Phase 1 trial (59, 60). Several polyphenols, such as quercetin, epigallocatechin-3-gallate (EGCG), curcumin and resveratrol have been implicated as inhibitors of Wnt/β-catenin signaling and are being considered for clinical trials, although the specificity of these agents is unclear (60). Therapeutic monoclonal antibodies against Wnt-1 and Wnt-2 have been shown to inhibit Wnt signaling and suppress tumor growth in vivo (60). However when using this approach the potential direct anti-proliferative effects of Wnt inhibition on stem cells also need to be considered.
Conclusion
The resistance of CSC to chemotherapy may be explained by their state of dormancy. Recent studies have improved our understanding of the mechanisms that maintain stem cells in a quiescent state and suggested possible strategies to target these difficult to eliminate populations. Dormant CSC may be activated by targeting the extrinsic or intrinsic mechanisms that maintain them in a quiescent state, potentially rendering them susceptible to targeted or conventional chemotherapy (61). The kinetics of CSC activation and sensitization need to be better understood for optimal design of such combinatorial approaches. Other strategies are aimed towards directly inhibiting survival or self-renewal of quiescent CSC through means such as epigenetic modifications. Identification of molecular mechanisms underlying enhanced survival and drug resistance of quiescent CSC will be very helpful in facilitating development of such strategies in future. (57, 60). Evaluation of treatment approaches also requires development of assays or biomarkers for quiescent CSC that can be used for patient selection and assessment (62). Realization of these objectives may indeed make it possible to target and eliminate quiescent CSC in future enhancing long-term cures for cancer patients.
Acknowledgments
This work was supported by NIH grants R01 HL77847 and R01 CA95684 and a Leukemia and Lymphoma Society Translational Research Grant to Ravi Bhatia,
References
- 1.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 2.Song X, Call GB, Kirilly D, Xie T. Notch signaling controls germline stem cell niche formation in the Drosophila ovary. Development. 2007;134:1071–1080. doi: 10.1242/dev.003392. [DOI] [PubMed] [Google Scholar]
- 3.Arai F, Hirao A, Ohmura M, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Enver T, Heyworth CM, Dexter TM. Do stem cells play dice? Blood. 1998;92:348–351. [PubMed] [Google Scholar]
- 5.Cheshier SH, Morrison SJ, Liao X, Weissman IL. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc Natl Acad Sci USA. 1999;96:3120–3125. doi: 10.1073/pnas.96.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dick JE. Stem cell concepts renew cancer research. Blood. 2008;112:4793–4807. doi: 10.1182/blood-2008-08-077941. [DOI] [PubMed] [Google Scholar]
- 7.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Elf SE, Miyata Y, Sashida G, Liu Y, Huang G, et al. p53 regulates hematopoietic stem cell quiescence. Cell Stem Cell. 2009;4:37–48. doi: 10.1016/j.stem.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- 10.Lacorazza HD, Yamada T, Liu Y, Miyata Y, Sivina M, Nunes J, et al. The transcription factor MEF/ELF4 regulates the quiescence of primitive hematopoietic cells. Cancer Cell. 2006;9:175–87. doi: 10.1016/j.ccr.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 11.Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 12.van Os R, Kamminga LM, Ausema A, Bystrykh LV, Draijer DP, van Pelt K, et al. A Limited role for p21Cip1/Waf1 in maintaining normal hematopoietic stem cell functioning. Stem Cells. 2007;25:836–843. doi: 10.1634/stemcells.2006-0631. [DOI] [PubMed] [Google Scholar]
- 13.Hock H, Hamblen MJ, Rooke HM, Schindler JW, Saleque S, Fujiwara Y, et al. Gfi-1 restricts proliferation and preserves functional integrity of haematopoietic stem cells. Nature. 2004;431:1002–7. doi: 10.1038/nature02994. [DOI] [PubMed] [Google Scholar]
- 14.Ito K, Takubo K, Arai F, Matsuoka S, Takubo K, Hamaguchi I, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 15.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Furukawa-Hibi Y, Yoshida-Araki K, Ohta T, Ikeda K, Motoyama N. FOXO forkhead transcription factors induce G(2)-M checkpoint in response to oxidative stress. J Biol Chem. 2002;277:26729–26732. doi: 10.1074/jbc.C200256200. [DOI] [PubMed] [Google Scholar]
- 17.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 18.Simsek T, Kocabas F, Zheng J, Deberardinis RJ, Mahmoud AI, Olson EN, et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7:380–390. doi: 10.1016/j.stem.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y, et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7:391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, et al. Defining the Epithelial Stem Cell Niche in Skin. Science. 2004;303:359–63. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horsley V, Aliprantis AO, Polak L, Glimcher LH, Fuchs E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell. 2008;132:299–310. doi: 10.1016/j.cell.2007.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson BJ, Jankovic V, Gao J, Buonamici S, Vest A, Lee JM, et al. Control of hematopoietic stem cell quiescence by the E3 ubiquitin ligase Fbw7. J Exp Med. 2008;205:1395–408. doi: 10.1084/jem.20080277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, Grindley JC, Yin T, Jayasinghe S, He XC, Ross JT, et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–522. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- 24.Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia- initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 25.Ito K, Bernardi R, Morotti A, Matsuoka S, Saglio G, Ikeda Y, et al. PML targeting eradicates quiescent leukaemia-initiating cells. Nature. 2008;453:1072–8. doi: 10.1038/nature07016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kopp HG, Avecilla ST, Hooper AT, Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology. 2005;20:349–356. doi: 10.1152/physiol.00025.2005. [DOI] [PubMed] [Google Scholar]
- 27.Walkley CR, Olsen GH, Dworkin S, Fabb SA, Swann J, McArthur GA, et al. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell. 2007;129:1097–1110. doi: 10.1016/j.cell.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puri MC, Bernstein A. Requirement for the TIE family of receptor tyrosine kinases in adult but not fetal hematopoiesis. Proc Natl Acad Sci USA. 2003;100:12753–12758. doi: 10.1073/pnas.2133552100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 30.Blank U, Karlsson G, Karlsson S. Signaling pathways governing stem cell fate. Blood. 2008;111:492–503. doi: 10.1182/blood-2007-07-075168. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 32.Kobielak K, Stokes N, de la Cruz J, Polak L, Fuchs E. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. Proc Natl Acad Sci USA. 2007;104:10063–8. doi: 10.1073/pnas.0703004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mira H, Andreu Z, Suh H, Lie DC, Jessberger S, Consiglio A, et al. Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell Stem Cell. 2010;7:78–89. doi: 10.1016/j.stem.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 34.Kimura S, Roberts AW, Metcalf D, Alexander WS. Hematopoietic stem cell deficiencies in mice lacking c-Mpl, the receptor for thrombopoietin. Proc Natl Acad Sci USA. 1998;95:1195–1200. doi: 10.1073/pnas.95.3.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian H, Buza-Vidas N, Hyland CD, Jensen CT, Antonchuk J, Månsson R, et al. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell. 2007;1:599–600. doi: 10.1016/j.stem.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Yoshihara H, Arai F, Hosokawa K, Hagiwara T, Takubo K, Nakamura Y, et al. Thrombopoietin/MPL Signaling Regulates Hematopoietic Stem Cell Quiescence and Interaction with the Osteoblastic Niche. Cell Stem Cell. 2007;1:599–600. doi: 10.1016/j.stem.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 37.Reinholt FP, Hultenby K, Oldberg A, Heinegard D. Osteopontin: a possible anchor of osteoclasts to bone. Proc Natl Acad Sci USA. 1990;87:4473–4475. doi: 10.1073/pnas.87.12.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stier S, Ko Y, Forkert R, Lutz C, Neuhaus T, Grünewald E, et al. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med. 2005;201:1781–1791. doi: 10.1084/jem.20041992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nilsson SK, Johnston HM, Whitty GA, Williams B, Webb RJ, Denhardt DT, et al. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005;106:1232–9. doi: 10.1182/blood-2004-11-4422. [DOI] [PubMed] [Google Scholar]
- 40.Kirstetter P, Anderson K, Porse BT, Jacobsen SE, Nerlov C. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat Immunol. 2006;7:1048–1056. doi: 10.1038/ni1381. [DOI] [PubMed] [Google Scholar]
- 41.Fleming HE, Janzen V, Lo Celso C, Guo J, Leahy KM, Kronenberg HM, et al. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2:274–283. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nemeth M, Mak KK, Yang Y, Bodine DM. β-Catenin Expression in the Bone Marrow Microenvironment Is Required for Long-Term Maintenance of Primitive Hematopoietic Cells. Stem Cells. 2009;27:1109–1119. doi: 10.1002/stem.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saito Y, Uchida N, Tanaka S, Suzuki N, Tomizawa-Murasawa M, Sone A, et al. Induction of cell cycle entry eliminates human leukemia stem cells in a mouse model of AML. Nat Biotechnol. 2010;28:275–280. doi: 10.1038/nbt.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhatia R, Holtz M, Niu N, Gray R, Snyder DS, Sawyers CL, et al. Persistence of malignant hematopoietic progenitors in chronic myelogenous leukemia patients in complete cytogenetic remission following imatinib mesylate treatment. Blood. 2003;101:4701–7. doi: 10.1182/blood-2002-09-2780. [DOI] [PubMed] [Google Scholar]
- 45.Goldman JM, Green AR, Holyoake T, Jamieson C, Mesa R, Mughal T, et al. Chronic myeloproliferative diseaseswith and without the Ph chromosome: some unresolved issues. Leukemia. 2009;23:1708–1715. doi: 10.1038/leu.2009.142. [DOI] [PubMed] [Google Scholar]
- 46.Morrison SJ, Wright DE, Weissman IL. Cyclophosphamide/granulocyte colony-stimulating factor induces hematopoietic stem cells to proliferate prior to mobilization. Proc Natl Acad Sci USA. 1997;94:1908–1913. doi: 10.1073/pnas.94.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beekman R, Touw IP. G-CSF and its receptor in myeloid malignancy. Blood. 2010;115:5131–6. doi: 10.1182/blood-2010-01-234120. [DOI] [PubMed] [Google Scholar]
- 48.Drummond MW, Heaney N, Kaeda J, Nicolini FE, Clark RE, Wilson G, et al. A pilot study of continuous imatinib vs pulsed imatinib with or without G-CSF in CML patients who have achieved a complete cytogenetic response. Leukemia. 2009;23:1199–1201. doi: 10.1038/leu.2009.43. [DOI] [PubMed] [Google Scholar]
- 49.de Veer MJ, Holko M, Frevel M, Walker E, Der S, Paranjape JM, et al. Functional classification of interferon-stimulated genes identified using microarrays. J Leukoc Biol. 2001;69:912–920. [PubMed] [Google Scholar]
- 50.Sato T, Onai N, Yoshihara H, Arai F, Suda T, Ohteki T. Interferon regulatory factor-2 protects quiescent hematopoietic stem cells from type I interferondependent exhaustion. Nat Med. 2009;15:696–70. doi: 10.1038/nm.1973. [DOI] [PubMed] [Google Scholar]
- 51.Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, et al. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–908. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- 52.Alimena G, Breccia M, Luciano L, Quarantelli F, Diverio D, Izzo B, et al. Imatinib mesylate therapy in chronic myeloid leukemia patients in stable complete cytogenic response after interferon-alpha results in a very high complete molecular response rate. Leuk Res. 2008;32:255–61. doi: 10.1016/j.leukres.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 53.Rousselot P, Huguet F, Rea D, Legros L, Cayuela JM, Maarek O, et al. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood. 2007;109:58–60. doi: 10.1182/blood-2006-03-011239. [DOI] [PubMed] [Google Scholar]
- 54.Konopleva MY, Jordan CT. Leukemia stem cells and microenvironment: biology and therapeutic targeting. J Clin Oncol. 2011;29:591–9. doi: 10.1200/JCO.2010.31.0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nervi B, Ramirez P, Rettig MP, et al. Chemosensitization of AML following mobilization by the CXCR4 antagonist AMD3100. Blood. 2009;113:6206–6214. doi: 10.1182/blood-2008-06-162123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burgess A, Ruefli A, Beamish H, Warrener R, Saunders N, Johnstone R, et al. Histone deacetylase inhibitors specifically kill nonproliferating tumor cells. Oncogene. 2004;23:6693–6701. doi: 10.1038/sj.onc.1207893. [DOI] [PubMed] [Google Scholar]
- 57.Zhang B, Strauss AC, Chu S, Li M, Ho Y, Shiang KD, et al. Effective targeting of quiescent chronic myelogenous leukemia stem cells by histone deacetylase inhibitors in combination with imatinib mesylate. Cancer Cell. 2010;17:427–42. doi: 10.1016/j.ccr.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Glaser KB. HDAC inhibitors: Clinical update and mechanism-based potential. Biochem Pharmacol. 2007;74:659–671. doi: 10.1016/j.bcp.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 59.Emami KH, Nguyen C, Ma H, Kim DH, Jeong KW, Eguchi M, et al. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription. Proc Natl Acad Sci USA. 2004;101:12682–12687. doi: 10.1073/pnas.0404875101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takahashi-Yanaga F, Kahn M. Targeting Wnt signaling: can we safely eradicate cancer stem cells? Clin Cancer Res. 2010;16:3153–3162. doi: 10.1158/1078-0432.CCR-09-2943. [DOI] [PubMed] [Google Scholar]
- 61.Roeder I, Horn M, Glauche I, Hochhaus A, Mueller MC, Loeffler M. Dynamic modeling of imatinib-treated chronic myeloid leukemia: functional insights and clinical implications. Nat Med. 2006;12:1181–1184. doi: 10.1038/nm1487. [DOI] [PubMed] [Google Scholar]
- 62.Li J. Quiescence Regulators for Hematopoietic Stem Cell. Exp Hematol. 2011 Jan 30; doi: 10.1016/j.exphem.2011.01.008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]