Abstract
Purpose.
Antioxidant supplements may reduce age-related macular degeneration (AMD) progression. The macular carotenoids are of particular interest because of their biochemical, optical, and anatomic properties. This classic twin study was designed to determine the heritability of macular pigment (MP) augmentation in response to supplemental lutein (L) and zeaxanthin (Z).
Methods.
A total of 322 healthy female twin volunteers, aged 16–50 years (mean 40 ± 8.7) was enrolled in a prospective, nonrandomized supplement study. Macular pigment optical density (MPOD) measurements using two techniques (2-wavelength fundus autofluorescence [AF] and heterochromatic flicker photometry [HFP]), and serum concentrations of L and Z, were recorded at baseline, and at 3 and 6 months following daily supplementation with 18 mg L and 2.4 mg Z for a study period of 6 months.
Results.
At baseline, mean MPOD was 0.44 density units (SD 0.21, range 0.04–1.25) using HFP, and 0.41 density units (SD 0.15) using AF. Serum L and Z levels were raised significantly from baseline following 3 months' supplementation (mean increase 223% and 633%, respectively, P < 0.0001 for both), with no MPOD increase. After 6 months' supplementation, a small increase in MPOD was seen (mean increase 0.025 ± 0.16, P = 0.02, using HFP). Subdivision of baseline MPOD into quartiles revealed that baseline levels made no difference to the treatment effect. Genetic factors explained 27% (95% confidence interval [CI] 7–45) of the variation in MPOD response. Distribution profiles of macular pigment did not change in response to supplementation.
Conclusions.
MPOD response to supplemental L and Z for a period of 6 months was small (an increase over baseline of 5.7% and 3.7%, measured using HFP and AF, respectively), and was moderately heritable. Further study is indicated to investigate the functional and clinical impact of supplementation with the macular carotenoids.
A classical twin study of 322 twins determined the heritability of macular pigment augmentation in response to 6 months' supplemental lutein and zeaxanthin. There was a small increase in macular pigment optical density, which was moderately heritable; genetic factors explained 27% of variance.
Introduction
Age-related macular degeneration (AMD) is the leading cause of blindness among the elderly in industrialized countries, and oxidative stress is likely to contribute to its pathogenesis.1,2 Macular pigment (MP) represents an accumulation in the central retina of three carotenoids, lutein (L), zeaxanthin (Z), and meso-zeaxanthin (m-Z), which give the macula its characteristic yellow color.3 There is a biologically plausible rationale for the protective role of MP in the retina: it absorbs blue light, thus reducing light-induced oxidative stress, and it has direct antioxidant properties by quenching reactive oxygen species.1,4,5 Furthermore, there is a growing body of evidence that the blue-light filtering properties of this pigment optimize contrast sensitivity and protect against glare disability because of the impact on chromatic aberration and light scatter,6,7 although this has been questioned.8 L and Z cannot be synthesized de novo in mammals, and are entirely of dietary origin, predominantly derived from fruits and vegetables.9 However, m-Z is not found in a typical diet, and is the result of conversion from retinal L.10 Given the potentially beneficial impact of appropriate supplementation on AMD prevalence and progression, there is considerable interest among researchers, clinicians, and the general public regarding the role of supplementation with the macular carotenoids.
Several clinical studies have investigated whether MP density can be modified either through dietary modification (for example, consumption of spinach/corn) or supplementation. Supplement studies in healthy subjects and subjects with retinal degeneration generally have been small (n = 2–108, reviewed by Connolly et al.11), and many have not been randomized, placebo-controlled in design. Their results suggest that there is considerable variability in the MP response to dietary modification/supplementation, ranging from “non-responders” to reports of increases up to 40%.12 Given this variation, the purpose of our study was to determine to what extent variation in response is determined by genes or environment, by establishing a classic twin study to estimate the heritability of MP response to supplemental L and Z.
Methods
We recruited 322 healthy, female twin volunteers, aged 16–50 years (mean 39 ± 8.7 years), from the TwinsUK adult registry held at St. Thomas' Hospital, London,13 which enrolls twins from the general population through local and national media campaigns. Power calculations before starting the study suggested a sample size of 70 monozygotic (MZ) and 70 dizygotic (DZ) twin pairs would detect a heritability change in macular pigment optical density (MPOD) of 30% from baseline; we aimed to recruit 80 MZ and 80 DZ pairs to allow for drop-outs. Informed consent was obtained from each volunteer and, following local ethics committee approval, the research procedures followed the tenets of the Declaration of Helsinki. All subjects were female twins with no ocular pathology (mean ± SD logMAR visual acuity 0.0 ± 0.1 [equivalent to 6/6 Snellen acuity]) or gastrointestinal disease. Details of the study population have been reported previously.14
Zygosity was determined by a standardized questionnaire, which has been shown to be at least 95% accurate.15 In cases when there was any doubt concerning zygosity, DNA analysis of short tandem-repeat polymorphisms using the AmpF1 STR Profiler kit (PE Applied Biosystems, Foster City, CA) was performed. DNA analysis was performed in 68 twin pairs (45%).
All subjects were asked to take a daily MP supplement with food (3 tablets of “Macuvite” [Springfield, Oud-Beijerland, The Netherlands], consisting of 18 mg lutein [in its free form] and 2.4 mg of zeaxanthin [derived from marigold flowers and microalgae]) for 6 months. Subjects were asked to continue their normal diet, and compliance was assessed at 3 and 6 months by pill counts. We calculated the number of supplement tablets taken, as a percentage of the expected number of pills that would have been taken if they had complied with the prescribed dosage.
MP, Dietary, and Serum Carotenoid Measurement
MPOD was measured by one investigator at baseline, and at 3 and 6 months following commencement of supplementation using a psychophysical method (heterochromatic flicker photometry [HFP]), and an imaging method (2-wavelength fundus autofluorescence [AF]). These methods, and their validity and repeatability, have been described previously.14
The apparatus used in our study for HFP measurements was the Maculometer. This instrument uses light emitting diodes, which emit near-monochromatic light.16 The subject views the foveal target at a distance of 330 mm, which subtends a diameter of one degree at the eye. For the parafoveal reference match, where it is assumed there is no MP, the test field is an annulus of 10 degrees (inner diameter) with a width of one degree. The Maculometer uses central fixation for the parafoveal match, when the foveal test field is switched to a dim red spot to provide a fixation target. Thus, the subject always is fixating on the central one-degree field.
HFP is a psychophysical test that uses MP's absorption and spatial distribution characteristics to calculate the optical density of this pigment.17,18 The test field flickers between a light that has a wavelength close to the peak absorption of MP (blue light, λmax = 468 nm) and a longer wavelength reference light, which has a wavelength close to minimum or zero absorption by MP (green light, λmax = 535 nm). It is assumed that the presence of MP decreases the spectral sensitivity of macular photoreceptors to blue light. During this test, the green reference light remains at a fixed luminance and the subject, using a dial, can vary the blue light intensity. The amount of blue light luminance required to achieve matching luminance with the green light will be a measure of MPOD. When the luminances of the blue and green light are matched closely, the subject will perceive minimum flicker. A minimum flicker match is made in the following 2 locations: when the retinal image of the target field lies on the fovea and when the retinal image of the target field lies in the parafovea, where there is minimal or no MP. The logarithm of the ratio of the blue luminosity for the foveal match to the blue luminosity of the parafoveal reference match gives a measure of the MPOD. Following 2 “practice” matches, five foveal readings were obtained, followed by 5 parafoveal readings for each subject at each visit. HFP was performed in both eyes, and the first eye to be tested alternated with each subsequent twin pair tested.
On completion of the HFP test, subjects' eyes were dilated and the AF test for MPOD was carried out in a darkened room. This technique takes advantage of the autofluorescence characteristic of lipofuscin, which is found in the RPE and has been described previously in detail.19 As the MP absorption range (400–550 nm) is within the excitation range of lipofuscin,20 absorption of light by MP before it reaches the RPE layer will cause attenuation of the AF. The principle of this technique is that the amount of light absorption by the MP is related strongly to the amount of MP within the retina and, therefore, can provide a measure of MPOD. Two different wavelengths of light are used to stimulate AF, one that is well absorbed by MP and one that is absorbed minimally by MP, to provide reference values for AF emitted in the absence of MP, as this is not uniform across the field.21 Therefore, a comparison of AF intensity recorded at the 2 different wavelengths allows quantification of MPOD.
A modified confocal scanning laser ophthalmoscope (Heidelberg Engineering, Heidelberg, Germany) was used to obtain high resolution, 20-degree field AF images at 488 nm (blue) and 514 nm (green). The gain of the instrument was fixed at the same intensity for all subjects throughout the entire study. Each day MP measurements were made the instrument was allowed to warm up for 1 hour before a subject was tested. A software program has been developed that creates an MPOD map, generated by using a gray scale index of intensity and by digital subtraction of the AF images taken at the 2 different wavelengths.22 Using the AF method, the MPOD at 0.5 degrees eccentricity was measured. The total MP quantity (proportional to the area under the curve of MP profile, in a10-degree diameter area, centered on fovea) and the quantity of MP in the central area, where MP has the highest concentration (one-degree diameter disc area), also were calculated. As high MPOD interocular symmetry is well established,14 we used readings from subjects' right eyes.
Average daily dietary L and Z intake (mg/day) was evaluated at baseline using a validated food frequency questionnaire (FFQ), developed by the Scottish Collaborative Group,23,24 where subjects were asked to recall their average food consumption of specific foods over the preceding 3 months. The FFQs were scanned and verified at the Medical Research Council Human Nutrition Research, Cambridge, UK. Blood samples were obtained on the same day as MPOD was measured, at baseline, and at 3 months. Serum carotenoid levels were measured using reverse phase high performance liquid chromatography at the Waterford Institute of Technology.11
Data Analysis
Data analysis was performed using the STATA computer statistics package (Version 8 SE; StataCorp, College Station, TX). Wilcoxon signed rank tests were used to test the statistical significance of the change in MPOD between visits. Maximum likelihood modeling, using the Mx program,25 was performed to estimate the heritability of MP response to supplementation.26 This method is based on comparing the covariances of a measured trait between MZ and DZ twins. The observed phenotypic variance can be divided into additive genetic (A), dominant genetic (D), common environmental (C), and unique environmental (E) components. Heritability is defined as the proportion of the phenotypic variation attributable to genetic factors, and is given by the equation, h2=(A+D)/(A+D+C+E). The Akaike Information Criterion (AIC) was used to determine the best fitting model, with the lowest AIC suggesting the best fit.
Many MP studies have divided subjects into “responders” and “non-responders.” For a quantitative trait, this probably is inappropriate, as there likely is to be a range of responses. However, it is of interest to examine whether subjects with lower levels (due to dietary or genetic factors) might have a different response to those with highest levels (who might have reached a saturation threshold). When looking for a change from baseline, as in our study, analyzing the data to check for regression to the mean is necessary. Regression to the mean refers to the widespread statistical phenomenon that those with extreme scores of a biologic measure subject to variation at any measurement time point will, for purely statistical reasons, have a greater chance of less extreme scores at the next time point they are tested. Therefore, a subject who has a below average baseline MPOD is more likely to have a higher score (nearer the population mean) at the following measurement point in time, and vice versa for those with “high” baseline MPOD. This decrease (or increase) can be attributed mistakenly to a treatment effect when it actually has occurred due to chance.27,28 Statistical modeling using analysis of covariance (ANCOVA) was used to examine regression effects.
Results
Of the 322 subjects enrolled 290 completed the 3-month visit (90%) and 264 completed the 6-month visit (82%), with no differences between those completing and not completing the supplement period in terms of baseline MPOD. The mean compliance with L/Z supplementation, measured by pill count, was 89.8% ± 13.5% at 3 months (number of observations = 260) and 90.5% ± 11.9% during the second 3-month interval (number of observations = 240). There were no adverse events associated with the supplements.
The mean dietary consumption of L and Z, before commencing supplementation, was 1.69 ± 1.48 mg/day (range 0.18–11.04). At baseline, MPOD values exhibited a normal distribution using the HFP method of assessment (skew test for normality, P = 0.13) and a near normal distribution using AF (although this was not statistically normal).29 Mean MPOD, MP volume, and serum carotenoid concentration values, at baseline and following supplementation, are given in the Table. There were 83 MZ twin pairs (mean age 39.9 ± 8.76 years) and 78 DZ twin pairs (mean age 40.6 ± 8.46 years) included in the study, and mean baseline MPOD was 0.42 ± 0.21 (measured by HFP) and 0.40 ± 0.15 (AF) for MZ twins, and 0.46 ± 0.21 (HFP) and 0.42 ± 0.15 (AF) for DZ twins, not significantly different (P > 0.05) between zygosities. Mean ± SD baseline serum L was 0.12 ± 0.05 μg/mL, the same for MZ and DZ twins.
Overall, for the entire cohort, serum levels of L and Z showed a significant increase after 3 months of supplementation, with a mean increase of 223% and 633% from baseline for L and Z, respectively (P = < 0.001 for both). However, there was no significant increase in MPOD (HFP method P = 0.75, AF method P = 0.70) following 3 months of supplementation. Following 6 months of supplementation, an increase in MPOD from baseline was seen (mean increase ± SD HFP 0.025 ± 0.16, P = 0.02; AF 0.015 ± 0.058, P < 0.001), which was statistically significant and represented a mean MPOD rise of 5.7% (HFP) and 3.7% (AF, Fig. 1). Analyzing the MP volume results revealed the same trend with no difference in total or central MP volume at 3 months (P = 0.22 total, P = 0.11 central), but a statistically significant increase (1.25% rise in total MP, 1.45% rise in central) after 6 months of supplementation (P = 0.01 total, P < 0.001 central, Fig. 2). There was no change in MP distribution profile, which is heritable.14
Figure 1. .
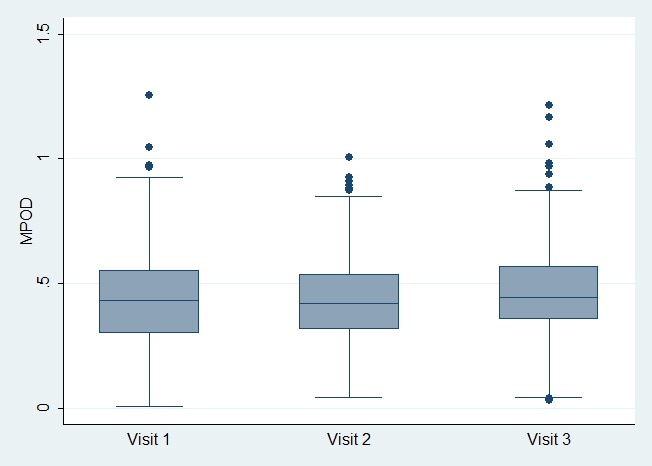
Box plots illustrating macular pigment levels (measured by HFP) at baseline, 3-months (visit 2), and 6-months (visit 3) following L/Z supplementation.
Figure 2. .
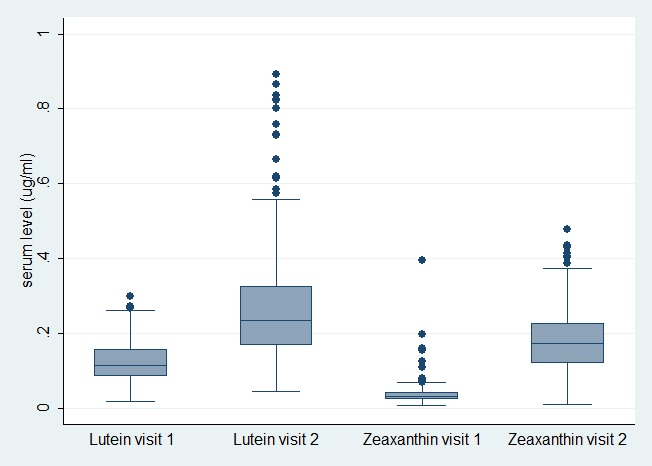
Serum L and Z results at baseline (visit 1) and 3-months (visit 2).
The correlation coefficients within MZ twin pairs for change in MPOD were higher than DZ correlations, although these generally were low at r = 0.32 and r = 0.11 for MZ and DZ twins, respectively, using the AF method, and only r = 0.13 and r = 0.04, respectively, when using the HFP method. Genetic modeling suggested the heritability of MPOD response measured with AF to supplementation was 0.27 (95% confidence interval [CI] 0.07–0.45). The residual variance was explained by environmental effects (0.73, 95% CI 0.55–0.93); this includes measurement error. Modelling using HFP data found no significant genetic influence on MPOD response.
There was no significant difference in supplementation effect between the highest and lowest quartiles of baseline MPOD, after adjusting for regression to the mean (P = 0.629 for quartile 4 vs. 1). The Macuvite capsules used were analyzed in an independent laboratory that quantified the contents of L at 5.28 mg and Z at 0.91 mg per capsule, suggesting the actual daily dosages were 15.9 and 2.7 mg, respectively, similar to the intended doses of 18 mg of L and 2.4 mg of Z. (Hogerhuis, personal communication 2009).
Discussion
We designed a study to investigate heritability of response, in terms of MPOD, to supplementation with its constituent carotenoids (L and Z). Heritability can be defined as the proportion of phenotypic variation attributable to genetic background, and is determined best by a classical twin study with its inherent capacity to assign relative contributions of genes and environment to a given variable following a comparative analysis of MZ and DZ twins. In this context, we report that 27% of variation in response, in terms of MPOD augmentation, is attributable to genetic factors. However, these findings should be interpreted with full appreciation of the modest, yet statistically significant, rise in MPOD observed here, reflected in the wide CIs of the heritability (0.07–0.45). Although MP is entirely of dietary origin, it is neither surprising nor counterintuitive that tissue uptake and/or concentrations of its constituent carotenoids are determined by genetic background, as is the case with vitamin C.29 Indeed, our research group has demonstrated previously heritability estimates of 0.67–0.85 for MPOD in the baseline cross-sectional component of the current study.14
The accumulation of MP in the central retina depends on the journey of its constituent carotenoids from foodstuff to target tissue and, therefore, depends on a wide array of processes, including digestion, absorption, transport in serum, and ultimately, capture by and stabilization within the retina. As a consequence, genetic background could exert its influence on MPOD response to supplementation through any number of mechanisms known to be subject to genetic influence, including lipoprotein and/or xanthophyll-binding protein profile, and concentrations of antioxidant enzymes within the retina.30–32
The possibility that supplementation with the macular carotenoids is beneficial has prompted their widespread use in recent years,33 and is based on the rationale that MP is a filter of short wavelength (blue) visible light at a pre-receptoral level, with consequences for vision and for generation of reactive oxygen intermediates (ROIs) at the central retina. This role might confer protection against AMD development and/or progression, but because of the difficulties inherent in testing such a hypothesis (involving long-term study of the effects of cumulative oxidative damage), current evidence is based largely on cross-sectional studies (with their inherent inability to comment upon cause and effect) and a few clinical trials with small numbers of subjects followed for short periods of time. The extensive literature in this regard recently has been reviewed extensively, and can be summarized as follows: most studies demonstrate a relative lack of MP in association with risk-factors for AMD, and the few reported clinical trials indicated a benefit is association with supplemental L and Z.34 Level 1 evidence that supplementation with antioxidants is beneficial in AMD does exist in the form of the Age Related Eye Disease Study (AREDS),35 but this evidence does not extend to the macular carotenoids, which is the subject of AREDS II.36
In any case, any beneficial effect of supplementation with the macular carotenoids rests on the premise that such supplementation does, indeed, result in augmentation of MP. In this regard, our study has revealed some interesting and clinically important findings. To our knowledge, our study represents the largest investigation of supplemental L and Z in a healthy population, and resulted in a modest, albeit statistically significant, increase in MPOD of only 4–5%, reflected in a mean response of only 0.02 density units, following 6 months of supplementation. Furthermore, this observed MP augmentation of questionable clinical importance was observed despite increases in serum concentrations of L and Z of 233% and 633%, respectively. The similarity of effect in subjects, whether they were in the lowest or highest quartile, suggests the findings are applicable to all subjects, whatever their baseline MPOD, and that the “healthy” population does not have a saturation level. The rise in MPOD (using AF) at 6 months was related only weakly to the serum L levels measured at 3 months (correlation coefficient r = 0.14, P = 0.03).
Previous studies, sometimes following exclusion of “retinal non-responders,” typically have reported larger increases in MP (4%–30%) and in serum concentrations of its constituent carotenoids (400%–1000%) following supplementation with L and/or Z.11 Our 2-fold and 6-fold increase in serum L and Z were approximately half of the 3.7-fold and 13-fold increases in the Lutein Xanthophyll Eye Accumulation (LUXEA) study of 92 subjects, also using free L, which demonstrated a similar, small, increase in MPOD. Although 6 months of MPOD data were not presented, supplementation for 12 months increased MPOD by 14–15%, and graphs presented suggest a linear increase over the 12 months, which can be interpreted as a rise of ∼7% at 6 months).37 The LUNA study, which supplemented 108 patients with features of AMD, found an approximately 4-fold increase in serum L and a mean MPOD increase of 12% following 6 months of supplementation with Ocuvite Lutein, which contains 12 mg L provided as an ester together with co-antioxidants (vitamin C, E, Zn, and selenium).38 However, it should be noted that the control group in this study also showed a raised MPOD, and the analysis did not take this into account. The variable MPOD augmentation with supplementation observed in this and other studies highlights the complexity of the physiology of antioxidants, such as L and Z.
There are several possible explanations of our poorer response. First, our study was comprised of women, and some studies have found lower responses in women compared to men.39–41 Second, there may have been degradation of these compounds where participants were given a three-month supply, a problem identified recently,11 although the capsule concentrations reported here were measured from the same batch at the conclusion of the study. Concentrations of constituent components of many supplements marketed for the purpose of enhancing eye health have been described as inappropriate,42 and disparities between claimed and actual composition of antioxidant supplements is a subject worthy of study. The third reason may relate to bioavailability and optimal formulation of L/Z. The formulation used in our study consisted of free lutein (FloraGlo), and zeaxanthin from marigold and microalgae in a capsule (cellulose, magnesium stearate, Macuvite). It may be that the esterified form of L is more bioavailable.43 It also is known that the bioavailability of L and Z is influenced by interactions with other dietary constituents (e.g., increased bioavailability when associated with lipid matrix, such as in egg yolks).44
The fourth reason may relate to m-Z. We cannot measure relative levels of carotenoids, but m-Z is the dominant carotenoid at the center of the fovea and is the result of conversion of retinal L (but not Z).10 Some individuals might lack the capacity to convert retinal L to m-Z and, therefore, “nonresponders” might not respond to supplemental L at 0.5 degrees (the eccentricity measured and reported here and in most studies), which might be influenced by genetic background. We did not exclude “retinal nonresponders” as other studies have, as we wanted to explore whether response (or lack of it) is determined by genetic factors, and public health measures to recommend generalized supplementation with L and Z must be based on evidence from unselected populations. Approximately 12% of the population has an “atypical” central dip in their spatial profile of MP associated with established risk-factors for AMD, such as smoking and increasing age, and this may reflect an inability to convert retinal L to m-Z.45 We found no change in the MPOD distribution profiles after supplementation with L and Z, but it has been reported that supplementation with all three macular carotenoids (including m-Z) results in augmentation of MPOD in all subjects and normalization of these “atypical” profiles.45,46 Meaningful comment upon the magnitude of response to supplementation with the macular carotenoids requires a placebo-controlled group, which was not included in the design of the current investigation because our primary objective was to determine the heritability of the response to supplementation of L and Z.
The MPOD values from our study population are comparable to other studies of normal subjects47,48 and twins have been shown to be comparable to singletons in complex traits.49 The results were not altered when twin relationships were taken into account, either by randomly dropping one of each twin pair or using generalized estimating equations. Although several large studies have not shown a significant sex difference in MP levels,19,50–52 it should be noted that the mean MPOD in this female, volunteer group is higher than reported in other studies that have shown that females have lower MPOD than males.16,39,53 It is possible that this twin, volunteer group may have healthier lifestyles and diets compared to the general population or other singleton volunteer groups. The average daily L/Z consumption in this volunteer group (1.69 ± 1.48) is comparable to the female volunteers in the study of Nolan et al.54 (1.61 ± 1.06), which used the same FFQ. The normal Western daily diet has been reported to contain approximately 1.3 to 3 mg of L and Z55; however, directly comparing dietary intakes, assessed using different food frequency questionnaires and carotenoid databases, may not be completely accurate.
In conclusion, MPOD response to 6 months of supplementation with L and Z in an unselected, healthy twin volunteer sample was moderately heritable, but in our study the increase in MPOD was small and of questionable clinical significance. The clinical and functional impact of supplementation with the macular carotenoids requires further study.
Table. .
Mean (SD) MPOD, MP Volume, and Serum Carotenoid Measurements at Baseline and 3 and 6 Months following L/Z Supplementation
|
|
Baseline |
3 Months |
6 Months |
| MPOD measured by HFP (DU) | 0.44 (0.21) n = 301 | 0.43 (0.19) n = 251 | 0.47 (0.19) n = 245 |
| MPOD measured by AF (DU) | 0.41 (0.15) n = 321 | 0.40 (0.16) n = 290 | 0.43 (0.16) n = 264 |
| Central MP quantity | 265.9 (92.4) | 259.7 (97.2) | 272.5 (94.0) |
| Total MP quantity | 3788.6 (2059.2) | 3614.1 (1602.5) | 3890.9 (1653.3) |
| Serum L (μg/mL) | 0.13 (0.06) | 0.29 (0.16) | |
| Serum Z (μg/mL) | 0.03 (0.02) | 0.19 (0.09) |
DU, density units.
Acknowledgments
All the twin volunteers and John Marshall, Fred Fitzke, and John Mellerio generously contributed time and expertise to the design and set up of this study.
Footnotes
Supported by Wellcome Trust and by a Research to Prevent Blindness unrestricted grant to University of Minnesota (FJVK).
Disclosure: C.J. Hammond, None; S.H.M. Liew, None; F.J. Van Kuijk, Springfield (F); S. Beatty, None; J.M. Nolan, None; T.D. Spector, None; C.E. Gilbert, None
References
- 1.Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–134 [DOI] [PubMed] [Google Scholar]
- 2.Margrain TH, Boulton M, Marshall J, Sliney DH. Do blue light filters confer protection against age-related macular degeneration? Prog Retin Eye Res. 2004;23:523–531 [DOI] [PubMed] [Google Scholar]
- 3.Bone RA, Landrum JT, Tarsis SL. Preliminary identification of the human macular pigment. Vision Res. 1985;25:1531–1535 [DOI] [PubMed] [Google Scholar]
- 4.Khachik F, Bernstein PS, Garland DL. Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Invest Ophthalmol Vis Sci. 1997;38:1802–1811 [PubMed] [Google Scholar]
- 5.Krinsky NI, Landrum JT, Bone RA. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr. 2003;23:171–201 [DOI] [PubMed] [Google Scholar]
- 6.Stringham JM, Garcia PV, Smith PA, McLin LN, Foutch BK. Macular pigment and visual performance in glare: benefits for photostress recovery, disability glare, and visual discomfort. Invest Ophthalmol Vis Sci. 2011;52:7406–7415 [DOI] [PubMed] [Google Scholar]
- 7.Renzi LM, Hammond BR. The effect of macular pigment on heterochromatic luminance contrast. Exp Eye Res. 2010;91:896–900 [DOI] [PubMed] [Google Scholar]
- 8.McLellan JS, Marcos S, Prieto PM, Burns SA. Imperfect optics may be the eye's defence against chromatic blur. Nature. 2002;417:174–176 [DOI] [PubMed] [Google Scholar]
- 9.Sommerburg O, Keunen JE, Bird AC, van Kuijk FJ. Fruits and vegetables that are sources for lutein and zeaxanthin: the macular pigment in human eyes. Br J Ophthalmol. 1998;82:907–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson EJ, Neuringer M, Russell RM, Schalch W, Snodderly DM. Nutritional manipulation of primate retinas, III: effects of lutein or zeaxanthin supplementation on adipose tissue and retina of xanthophyll-free monkeys. Invest Ophthalmol Vis Sci. 2005;46:692–702 [DOI] [PubMed] [Google Scholar]
- 11.Connolly EE, Beatty S, Loughman J, Howard AN, Louw MS, Nolan JM. Supplementation with all three macular carotenoids; response, stability and safety. Invest Ophthalmol Vis Sci. 2011;52:9207–9217 [DOI] [PubMed] [Google Scholar]
- 12.Landrum JT, Bone RA, Joa H, Kilburn MD, Moore LL, Sprague KE. A one year study of the macular pigment: the effect of 140 days of a lutein supplement. Exp Eye Res. 1997;65:57–62 [DOI] [PubMed] [Google Scholar]
- 13.Boomsma DI. Twin registers in Europe: an overview. Twin Res. 1998;1:34–51 [DOI] [PubMed] [Google Scholar]
- 14.Liew SH, Gilbert CE, Spector TD, et al. Heritability of macular pigment: a twin study. Invest Ophthalmol Vis Sci. 2005;46:4430–4436 [DOI] [PubMed] [Google Scholar]
- 15.Martin NG, Martin PG. The inheritance of scholastric abilities in a sample of twins. I. Ascertainments of the sample and diagnosis of zygosity. Ann Hum Genet. 1975;39:213–218 [DOI] [PubMed] [Google Scholar]
- 16.Mellerio J, Ahmadi-Lari S, van Kuijk F, Pauleikhoff D, Bird A, Marshall J. A portable instrument for measuring macular pigment with central fixation. Curr Eye Res. 2002;25:37–47 [DOI] [PubMed] [Google Scholar]
- 17.Snodderly DM, Auran JD, Delori FC. The macular pigment. II. Spatial distribution in primate retinas. Invest Ophthalmol Vis Sci. 1984;25:674–685 [PubMed] [Google Scholar]
- 18.Bone RA, Landrum JT, Fernandez L, Tarsis SL. Analysis of the macular pigment by HPLC: retinal distribution and age study. Invest Ophthalmol Vis Sci. 1988;29:843–849 [PubMed] [Google Scholar]
- 19.Delori FC, Goger DG, Hammond BR, Snodderly DM, Burns SA. Macular pigment density measured by autofluorescence spectrometry: comparison with reflectometry and heterochromatic flicker photometry. J Opt Soc Am A Opt Image Sci Vis. 2001;18:1212–1230 [DOI] [PubMed] [Google Scholar]
- 20.Snodderly DM, Brown PK, Delori FC, Auran JD. The macular pigment. I. Absorbance spectra, localization, and discrimination from other yellow pigments in primate retinas. Invest Ophthalmol Vis Sci. 1984;25:660–673 [PubMed] [Google Scholar]
- 21.Delori FC, Goger DG, Dorey CK. Age-related accumulation and spatial distribution of lipofuscin in RPE of normal subjects. Invest Ophthalmol Vis Sci. 2001;42:1855–1866 [PubMed] [Google Scholar]
- 22.Wustemeyer H, Moessner A, Jahn C, Wolf S. Macular pigment density in healthy subjects quantified with a modified confocal scanning laser ophthalmoscope. Graefes Arch Clin Exp Ophthalmol. 2003;241:647–651 [DOI] [PubMed] [Google Scholar]
- 23.Bodner CH, Soutar A, New SA, et al. Validation of a food frequency questionnaire for use in a Scottish population: correlation of antioxidant vitamin intakes with biochemical measures. J Hum Nutr Diet. 1998;11:373–380 [Google Scholar]
- 24.Bolton-Smith C, Casey CE, Gey KF, Smith WC, Tunstall-Pedoe H. Antioxidant vitamin intakes assessed using a food-frequency questionnaire: correlation with biochemical status in smokers and non-smokers. Br J Nutr. 1991;65:337–346 [DOI] [PubMed] [Google Scholar]
- 25.Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling, 6th ed. Richmond, VA: Department of Psychiatry, Virginia Commonwealth University; 2004 [Google Scholar]
- 26.Neale MC, Cardon LR. Methodology for Genetic Studies of Twins and Families. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992 [Google Scholar]
- 27.Barnett AG, van der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it. Int J Epidemiol. 2005;34:215–220 [DOI] [PubMed] [Google Scholar]
- 28.Morton V, Torgerson DJ. Regression to the mean: treatment effect without the intervention. J Eval Clin Pract. 2005;11:59–65 [DOI] [PubMed] [Google Scholar]
- 29.Block G, Shaikh N, Jensen CD, Volberg V, Holland N. Serum vitamin C and other biomarkers differ by genotype of phase 2 enzyme genes GSTM1 and GSTT1. Am J Clin Nutr. 2011;94:929–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loane E, Nolan JM, O'Donovan O, Bhosale P, Bernstein PS, Beatty S. Transport and retinal capture of lutein and zeaxanthin with reference to age-related macular degeneration. Surv Ophthalmol. 2008;53:68–81 [DOI] [PubMed] [Google Scholar]
- 31.Loane E, McKay GJ, Nolan JM, Beatty S. Apolipoprotein E genotype is associated with macular pigment optical density. Invest Ophthalmol Vis Sci. 2010;51:2636–2643 [DOI] [PubMed] [Google Scholar]
- 32.Loane E, Nolan JM, McKay GJ, Beatty S. The association between macular pigment optical density and CFH, ARMS2, C2/BF, and C3 genotype. Exp Eye Res. 2011;93:592–598 [DOI] [PubMed] [Google Scholar]
- 33.Loughman J, Nolan JM, Stack J, Beatty S. Online AMD research study for optometrists: current practice in the Republic of Ireland and UK. Optometry Pract. 2011;12:134–144 [Google Scholar]
- 34.Sabour-Pickett S, Nolan JM, Loughman J, Beatty S. A review of the evidence germane to the putative protective role of the macular carotenoids for age-related macular degeneration. Mol Nutr Food Res. 2012;56:270–286 [DOI] [PubMed] [Google Scholar]
- 35.Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coleman H, Chew E. Nutritional supplementation in age-related macular degeneration. Curr Opin Ophthalmol. 2007;18:220–223 [DOI] [PubMed] [Google Scholar]
- 37.Schalch W, Cohn W, Barker FM, et al. Xanthophyll accumulation in the human retina during supplementation with lutein or zeaxanthin – the LUXEA (Lutein Xanthophyll Eye Accumulation) study. Arch Biochem Biophys. 2007;458:128–135 [DOI] [PubMed] [Google Scholar]
- 38.Trieschmann M, Beatty S, Nolan JM, et al. Changes in macular pigment optical density and serum concentrations of its constituent carotenoids following supplemental lutein and zeaxanthin: the LUNA study. Exp Eye Res. 2007;84:718–728 [DOI] [PubMed] [Google Scholar]
- 39.Hammond BR Jr, Curran-Celentano J, Judd S, et al. Sex differences in macular pigment optical density: relation to plasma carotenoid concentrations and dietary patterns. Vision Res. 1996;36:2001–2012 [DOI] [PubMed] [Google Scholar]
- 40.Johnson EJ, Hammond BR, Yeum KJ, et al. Relation among serum and tissue concentrations of lutein and zeaxanthin and macular pigment density. Am J Clin Nutr. 2000;71:1555–1562 [DOI] [PubMed] [Google Scholar]
- 41.Broekmans WM, Berendschot TT, Klöpping-Ketelaars IA, et al. Macular pigment density in relation to serum and adipose tissue concentrations of lutein and serum concentrations of zeaxanthin. Am J Clin Nutr. 2002;76:595–603 [DOI] [PubMed] [Google Scholar]
- 42.Arora S, Musadiq M, Mukherji S, Yang YC. Eye nutrient products for age-related macular degeneration: what do they contain? Eye. 2004;18:470–473 [DOI] [PubMed] [Google Scholar]
- 43.Bowen PE, Herbst-Espinosa SM, Hussain EA, Stacewicz-Sapuntzakis M. Esterification does not impair lutein bioavailability in humans. J Nutr. 2002;132:3668–3673 [DOI] [PubMed] [Google Scholar]
- 44.Handelman GJ, Nightingale ZD, Lichtenstein AH, Schaefer EJ, Blumberg JB. Lutein and zeaxanthin concentrations in plasma after dietary supplementation with egg yolk. Am J Clin Nutr. 1999;70:247–251 [DOI] [PubMed] [Google Scholar]
- 45.Kirby ML, Beatty S, Loane E, et al. A central dip in the macular pigment spatial profile is associated with age and smoking. Invest Ophthalmol Vis Sci. 2010;51:6722–6728 [DOI] [PubMed] [Google Scholar]
- 46.Nolan JM, Akkali MC, Loughman J, Howard AN, Beatty S. Macular carotenoid supplementation in subjects with atypical spatial profiles of macular pigment [ published online ahead of print May 28, 2012]. Exp Eye Res. 2012;101C:9–15 [DOI] [PubMed] [Google Scholar]
- 47.Beatty S, Koh HH, Carden D, Murray IJ. Macular pigment optical density measurement: a novel compact instrument. Ophthalmic Physiol Opt. 2000;20:105–111 [PubMed] [Google Scholar]
- 48.Werner JS, Donnelly SK, Kliegl R. Aging and human macular pigment density. Appended with translations from the work of Max Schultze and Ewald Hering. Vision Res. 1987;27:257–268 [DOI] [PubMed] [Google Scholar]
- 49.Andrew T, Hart DJ, Snieder H, de Lange M, Spector TD, MacGregor AJ. Are twins and singletons comparable? A study of disease-related and lifestyle characteristics in adult women. Twin Res. 2001;4:464–477 [DOI] [PubMed] [Google Scholar]
- 50.Berendschot TT, Broekmans WM, Klöpping-Ketelaars IA, Kardinaal AF, Van Poppel G, Van Norren D. Lens aging in relation to nutritional determinants and possible risk factors for age-related cataract. Arch Ophthalmol. 2002;120:1732–1737 [DOI] [PubMed] [Google Scholar]
- 51.Ciulla TA, Curran-Celantano J, Cooper DA, et al. Macular pigment optical density in a midwestern sample. Ophthalmology. 2001;108:730–737 [DOI] [PubMed] [Google Scholar]
- 52.Curran-Celentano J, Hammond BR Jr, Ciulla TA, Cooper DA, Pratt LM, Danis RB. Relation between dietary intake, serum concentrations, and retinal concentrations of lutein and zeaxanthin in adults in a Midwest population. Am J Clin Nutr. 2001;74:796–802 [DOI] [PubMed] [Google Scholar]
- 53.Hammond BR Jr, Caruso-Avery M. Macular pigment optical density in a Southwestern sample. Invest Ophthalmol Vis Sci. 2000;41:1492–1497 [PubMed] [Google Scholar]
- 54.Nolan J, O'Donovan O, Kavanagh H, et al. Macular pigment and percentage of body fat. Invest Ophthalmol Vis Sci. 2004;45:3940–3950 [DOI] [PubMed] [Google Scholar]
- 55.Landrum JT, Bone RA. Lutein, zeaxanthin, and the macular pigment. Arch Biochem Biophys. 2001;385:28–40 [DOI] [PubMed] [Google Scholar]


