Abstract
Background
Sarcopenia is strongly associated with an inadequate intake of dietary protein. Dietary protein supplementation boosts muscle-protein synthesis and increases muscle mass in the elderly. This study tested whether adding a protein-rich food, ricotta cheese, to the habitual diet increased total appendicular skeletal muscle mass and strength in elderly people.
Methods
Participants (n = 40), were sarcopenic elderly men and women over 60 years of age. Two comparison groups were formed at random and followed for 3 months: the intervention group received 210 g/day of ricotta cheese plus the habitual diet, while the control group followed the habitual diet with no additional intervention. Total appendicular skeletal muscle (TASM) was assessed by dual-energy X-ray absorptiometry, while strength was measured using a handheld dynamometer at baseline and after the intervention period. The primary outcomes were the percentage of relative change in TASM and strength.
Results
The percentage of relative change in TASM was not significant between the groups after the intervention period. Muscle strength improved in the intervention group, but showed only a tendency towards significance (P = 0.06). Secondary analysis showed that the men in the intervention group gained 270 g in TASM compared to those in the control group, and improved their fasting insulin levels (P = 0.05), muscle strength, lean body mass in the arms, and body weight variables.
Conclusion
The results of this study indicate that a nutritional intervention using a high-quality protein food, specifically ricotta cheese, in order to increase the amount of protein intake might not be regarded as fully promising in elderly men and women with sarcopenia. However, the gender effects on muscle strength, lean tissue in the arms, homeostatic assessment of insulin resistance, and body weight detected in this study suggest that additional research is needed on elderly male subjects with sarcopenia.
Keywords: nutritional intervention, protein-rich food, ricotta cheese, sarcopenia, elderly
Introduction
Sarcopenia is defined as the age-associated loss of skeletal muscle mass and function.1 Several studies have shown that sarcopenia might result from low levels of testosterone or vitamin D, and low chronic inflammation.2–6 Recently, an inadequate intake of dietary protein has been suggested as an additional contributing factor to the development of sarcopenia.7 Because sarcopenia is a multifactorial2–6 disorder, no effective treatment for it has yet been established. However, protein and essential amino acid supplementation have been emphasized in treating sarcopenia,8–10 and it has been suggested that dietary protein may be a modifiable risk factor for sarcopenia in older adults.7 A daily intake of 1.2 g/kg to 1.5 g/kg of protein has been reported to prevent sarcopenia.11
The rationale behind protein, essential amino acid, or dietary protein supplementation is based on the hypothesis that such supplementation will boost muscle-protein synthesis.12 With respect to dietary protein supplementation, Symons et al13,14 reported an increase in skeletal muscle-protein synthesis after ingestion of certain foods, such as meat (lean beef), in both young and elderly subjects.12,13 Milk is another important source of high-quality dietary protein, one that efficiently stimulates muscle-protein synthesis in both younger15 and older adults.16 It seems that an increase in protein synthesis through foods translates into an increase in whole-body lean mass in young people.17 To the best of our knowledge, there is no evidence of studies of this issue in the elderly. Finding ways to incorporate protein-rich foods that are not high in calories, fat, and cholesterol into the diet and then translating this into an increase in whole-body lean mass can be challenging.
Some studies in the elderly have tested the effect on muscle mass and strength of exercise combined with ingesting animal-based foods such as regular dairy products, like eggs and milk, but results are controversial.18,19 It is clear that both milk and exercise seem to be effective in improving muscle mass and function in older people with an inflammatory disease.19 However, the effect on body composition parameters of adding protein-rich foods to the habitual diet, particularly on regional lean tissue, muscle mass, and strength in sarcopenic elderly subjects, has not been explored. Ricotta cheese is an important source of high-quality dietary protein and is a low-calorie dairy product that the elderly accept well. Recent commentaries and research have suggested that moderately increasing dietary protein intake may enhance muscle-protein anabolism.12 Therefore, this study tested whether adding a protein-rich food, ricotta cheese, to the habitual diet increased total appendicular skeletal muscle mass and strength in sarcopenic elderly people.
Methods
Participants
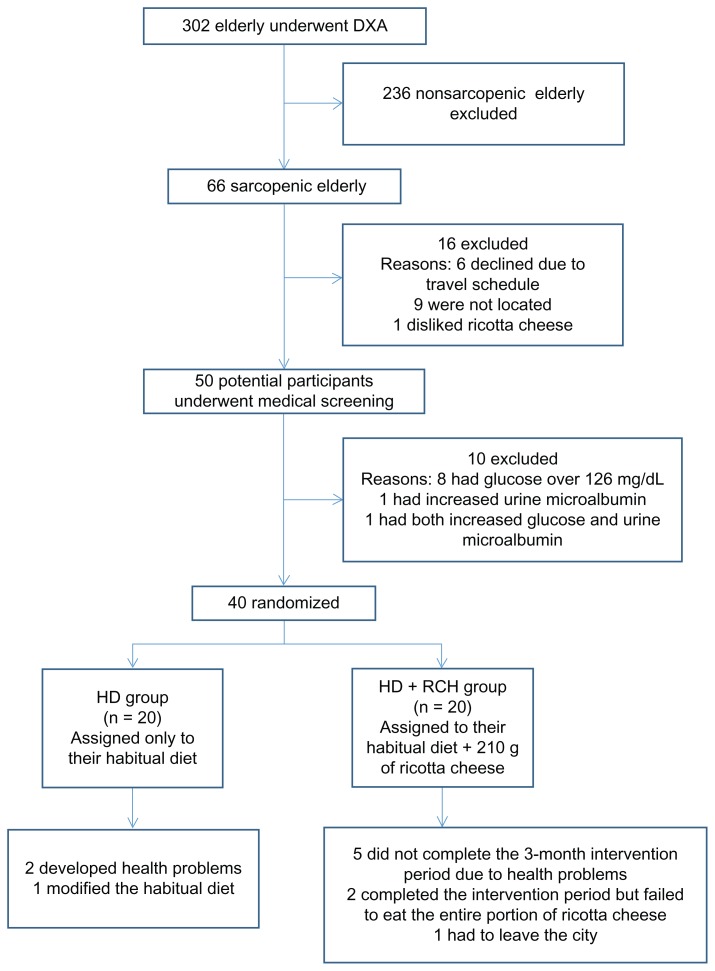
Three hundred and two physically independent and apparently healthy elderly subjects from Hermosillo, Sonora, Mexico were recruited through home visits and phone calls. All volunteers underwent dual-energy X-ray absorptiometry (DXA) measurements, and sarcopenia was diagnosed by the residuals method.20 From this sample, 66 elderly men and women were classified as sarcopenic and deemed potential participants for the nutritional intervention trial. All 66 potential participants underwent medical screening in order to exclude any individual with impaired kidney function. Other eligibility requirements included age (≥60 years), physical independence, being free of type-2 diabetes, and having no kidney or liver disease. After medical screening, 26 participants were excluded due to one of the following additional reasons: glucose above 126 g/dL, microalbuminuria, refusal to eat ricotta cheese, gastrointestinal problems related to consuming dairy products, or refusal to participate.
Ethics
The study was reviewed and approved by the Ethics Committee of the Research Center for Food and Development. All volunteers received a complete explanation of the protocol and then gave their written informed consent to participate.
Baseline and follow-up measurements
After the diagnosis of sarcopenia, the following procedures were performed to determine final eligibility. A medical examination was conducted that included several lab tests: hemoglobin was measured with HemoCue™ (HemoCue AB, Ängelholm, Sweden) and fasting glucose by GOD-PAP, lipid profile (TL100), and a hepatic profile (including serum glutamic oxaloacetic transaminase, serum glutamic pyruvic transaminase [AS147], and alkaline phosphatase [AL1200]) by Merck Vitalab (Selectra Analyze; Frankfurt, Germany). Kidney function was evaluated for creatinine, uric acid, and urea blood levels (measured by Randox™ techniques; Randox Laboratories, Oceanside, CA), and by the glomerular filtration rate (assessed using the Cockcroft-Gault formula21 and by NycoCard™ u-albumin test [Axis Shield NycoCard™, Dundee, UK]). All measurements were taken again at the end of the intervention study to discern possible adverse effects of the additional protein intake on kidney function. The anabolic effect of adding dietary protein supplementation (ricotta cheese) was assessed by insulin-like growth factor 1 (IGF-1; ALPCO Diagnostics, Salem, NH) and fasting insulin (DRG Instruments GmbH, Marburg, Germany), both measured by enzyme-linked immunosorbent assay, using ALPCO™ (cat no. 22-IGFHU-E01 and cat. EIA2935 DRG, respectively). Finally, insulin resistance was assessed using the homeostatic model assessment of insulin resistance (HOMA-IR).22
Body weight was measured using a digital electronic scale (AND FV-150 KA1; A&D Co. Ltd, Tokyo, Japan), with subjects barefoot and lightly dressed. Standing height was recorded using a Holtain stadiometer (Holtain Ltd, Dyfed, UK). Body mass index was calculated by dividing body weight in kilograms by the square of the height as measured by the stadiometer. All anthropometric measures were assessed using the standardized International Society for the Advancement of Kinanthropometry protocol.23
Total appendicular skeletal muscle (TASM) and other body composition components, as well as total body mass, were measured by DXA using a GE Lunar DPX-MD+ (Lunar Radiation Corp, Madison, WI), as reported previously.24 TASM was calculated as the sum of bone-free lean tissue in the arms and legs. The value was derived from DXA scans; the DXA was calibrated daily, following the manufacturer’s guidelines.
Muscle strength was measured with a Takei Smedley handgrip dynamometer (Takei Scientific Instruments Co, Niigata, Japan) according to the manufacturer’s recommendations.
Experimental design
This study was a randomized controlled clinical trial. Forty subjects were randomly assigned 1:1 to the intervention or control group. Participants in the intervention group added 210 g of ricotta cheese to their habitual diet (RCH + HD), while the other group consumed only their habitual diet (HD). Subjects in each group were followed during a 3-month intervention during which they were asked to perform their normal daily physical activities and follow their habitual diet. In an effort to ensure adherence to the protocol, subjects in both groups were visited three times a week at their homes. In addition, recommendations on how to combine the ricotta cheese with their habitual diet were given to the subjects in the RCH + HD group. The elapsed time between baseline measurements and the beginning of the intervention trial was 2 weeks, and follow-up measurements began after the last day of the intervention (3 months of follow-up; September to November, 2011). Data were collected at the Research Center for Food and Development; assessment personnel were blinded to participants’ assigned conditions.
Intervention
The ricotta cheese supplementation consisted of 210 g per day. The total portion was previously divided, weighed, and packed (70 g/serving). Participants were asked to eat the ricotta cheese with their habitual foods at breakfast, lunch, and dinner, record the amount of ricotta cheese left in the plastic containers, and consume the same amount of food or meals as they typically ingested. The 70 g of ricotta cheese was weighed using a compact CS2000 OHAUS scale (OHAUS Corporation, Pine Brook, NJ) and packed in labeled containers, using proper techniques for food handling. The plastic containers were transported at appropriate temperatures and delivered to the corresponding subject’s home three times a week. According to the results of bromatologic and high-performance liquid chromatography analyses, the amount of 210 g/day of ricotta cheese provided 15.7 g of protein (including essential amino acids at 8.6 g/day), 18.4 g of fat, and 10.4 g of carbohydrates. The total extra calories provided were 267 kcal/day. The amount of ricotta cheese given to subjects was based on the reported anabolic effect of using 15 g of whey protein (including 6.7 g of essential amino acids) on skeletal muscle in healthy older subjects.25 All intervention team members praised participants for their success throughout the intervention trial. As mentioned above, strategies to encourage adherence and improve retention were undertaken.
Habitual diet group
The subjects in the HD (control) group were asked to maintain their usual eating patterns. Participants in both groups (RCH + HD, HD) were treated under similar conditions during the intervention trial and were asked to maintain their normal physical activity and eating patterns; they were not allowed to engage in any intentional effort to gain or lose weight for the ensuing 3 months.
Primary outcomes: total appendicular skeletal muscle and muscle strength
In this study, the primary response variables were the percentages of relative change in total appendicular skeletal muscle and muscle strength. The percentage of relative change for these two variables was calculated as follows: [(follow-up value – baseline value)/baseline value] × 100.26 The effect of ricotta cheese on IGF-1, insulin levels, and all other variables was assessed by the same procedure described for the primary response variables.
Statistical methods
Sample size was based on information from a paper published by Dillon et al9 assuming a two-tailed test with alpha set at 0.05, a mean difference of 1.8 kg, and a similar standard deviation of 2.8 kg of lean body mass; 40 participants (20 per group) were estimated to provide 80% power to detect differences in kilograms of lean body mass between the two randomized groups (with similar proportions of men and women). Balance in the randomized groups (RCH + HD versus HD) was confirmed by evaluating intergroup differences using an independent t-test. Similarly, differences in the aforementioned intergroup outcomes were analyzed using an independent t-test under the intention-to-treat strategy. A P-value of ≤0.05 indicated statistical significance. Normality was verified graphically. All analyses were performed using NCSS 2007 software (NCSS, Kaysville, UT).
Results
The intervention sample consisted of 23 women and 17 men with a mean age of 76 ± 5.4 years and a mean body weight of 68.3 ± 9.6 kg. The mean value for body mass index was 26.3 ± 3.8 kg/m2. Forty elderly sarcopenic men and women were successfully randomized; the results of this process are summarized in Table 1. As expected, there were no significant differences in age, anthropometric measurements, body composition parameters, muscle strength, or the results of a series of laboratory tests between groups at baseline. Figure 1 shows the flow of the participants during the trial. Only 12 volunteers in the RCH + HD group and 17 in the HD group successfully completed the protocol; however, all subjects were included in the statistical analysis using the intention-to-treat approach.
Table 1.
Descriptive characteristics of subjects in the RCH + HD and HD groups
| RCH + HD Group (n = 20) | HD Group (n = 20) | P-value | |
|---|---|---|---|
| Sex | |||
| Male, n | 8 | 9 | – |
| Female, n | 12 | 11 | – |
| Age, years | 75.4 ± 5.0 | 76.7 ± 5.8 | 0.45 |
| Body weight, kg | 68.7 ± 8.6 | 67.8 ± 10.9 | 0.78 |
| Height, cm | 161.4 ± 8.7 | 161.3 ± 8.9 | 0.99 |
| BMI, kg/m2 | 26.5 ± 4.0 | 26.1 ± 3.7 | 0.71 |
| Body fat, % | 41.9 ± 9.4 | 41.3 ± 10.1 | 0.83 |
| Lean tissue in arm, kg | 4.1 ± 0.8 | 3.9 ± 0.8 | 0.51 |
| Lean tissue in leg, kg | 11.6 ± 2.1 | 11.4 ± 2.0 | 0.80 |
| TASM, kg | 15.7 ± 3.0 | 15.3 ± 2.8 | 0.70 |
| Lean body mass, kg | 37.1 ± 6.3 | 36.9 ± 6.4 | 0.89 |
| Bone mineral content, kg | 2.5 ± 0.5 | 2.4 ± 0.5 | 0.66 |
| Strength, kg | 22.0 ± 6.1 | 22.7 ± 6.4 | 0.73 |
| Glucose, mg/dL | 95.8 ± 11.6 | 97.7 ± 11.2 | 0.60 |
| Insulin, μUI/L | 9.9 ± 3.8 | 9.5 ± 6.2 | 0.79 |
| HOMA-IR | 2.4 ± 1.2 | 2.3 ± 1.8 | 0.89 |
| GFR | 58.2 ± 20.4 | 58.3 ± 16.1 | 0.98 |
| Total cholesterol, mg/dL | 200.7 ± 45.1 | 209.2 ± 43.4 | 0.54 |
| Triglycerides, mg/dL | 137.4 ± 53.6 | 130.4 ± 59.5 | 0.69 |
| IGF-1, μg/L | 53.5 ± 36.8 | 57.7 ± 27.3 | 0.68 |
Note: Data are presented as means ± SD.
Abbreviations: TASM, total appendicular skeletal muscle; BMI, body mass index; HOMA-IR, homeostatic model assessment of insulin resistance; GFR, glomerular filtration rate; IGF-1, insulin-like growth factor 1; HD, habitual diet; RCH, ricotta cheese.
Figure 1.
Participant flow during the trial.
Abbreviations: DXA, dual-energy X-ray absorptiometry; HD, habitual diet; RCH, ricotta cheese.
Outcomes and assessment
Primary outcomes: percentage of relative change in TASM and strength
Table 2 shows the effect of the additional intake of 210 g/day of ricotta cheese on TASM and muscle strength compared to the HD group. The percentage of relative change in TASM was not significantly different between the RCH + HD and HD groups after the 3-month follow-up period. With respect to the muscle-strength variable, the RCH + HD group improved with a positive percentage of relative change that showed a clear tendency towards significance (P = 0.06).
Table 2.
Relative changes associated with the additional intake of 210 g of ricotta cheese in sarcopenic elderly
| RCH + HD group (n = 20) | HD group (n = 20) | P-value | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Baseline | Follow-up | % relative change | Baseline | Follow-up | % relative change | ||
| TASM, kg | 15.7 ± 2.9 | 16.0 ± 3.1 | 2.2 | 15.3 ± 2.8 | 15.5 ± 2.8 | 1.5 | 0.54 |
| Strength, kg | 22.0 ± 6.1 | 22.1 ± 6.6 | 0.9 | 22.7 ± 6.4 | 22.0 ± 6.8 | −3.5 | 0.06 |
| Body fat, kg | 27.3 ± 7.8 | 26.8 ± 7.8 | −2.3 | 26.7 ± 9.1 | 25.7 ± 8.6 | −3.7 | 0.46 |
| Truncal fat, kg | 16.4 ± 4.1 | 16.1 ± 4.1 | −2.4 | 15.9 ± 6.0 | 15.4 ± 5.1 | 1.5 | 0.52 |
| Lean body mass, arms, kg | 4.1 ± 0.8 | 4.2 ± 0.9 | 2.3 | 3.9 ± 0.8 | 4.0 ± 0.8 | 1.4 | 0.60 |
| Lean body mass, legs, kg | 11.6 ± 2.1 | 11.8 ± 2.2 | 2.3 | 11.4 ± 2.0 | 11.6 ± 2.0 | 1.6 | 0.60 |
| Lean body mass, kg | 37.1 ± 6.3 | 37.9 ± 6.5 | 2.0 | 36.8 ± 6.4 | 37.6 ± 6.4 | 2.1 | 0.95 |
| Total body mass, kg | 66.9 ± 8.3 | 67.1 ± 9.0 | 0.2 | 66.0 ± 10.6 | 65.7 ± 10.2 | −0.4 | 0.46 |
| Body weight, kg | 68.7 ± 8.5 | 68.8 ± 9.1 | 0.1 | 67.8 ± 10.9 | 67.4 ± 10.4 | −0.6 | 0.31 |
| IGF-1, μg/L | 50.0 ± 34.3 | 59.6 ± 42.7 | 38.8 | 57.7 ± 27.3 | 64.9 ± 42.2 | 13.6 | 0.34 |
| Insulin, μUI/L | 9.6 ± 3.6 | 9.3 ± 3.2 | −0.8 | 9.5 ± 6.2 | 8.7 ± 2.4 | 0.6 | 0.82 |
| HOMA-IR | 2.4 ± 1.1 | 2.2 ± 1.0 | −2.4 | 2.3 ± 1.8 | 2.1 ± 0.8 | −1.8 | 0.92 |
| Glucose, mg/dL | 96.0 ± 11.9 | 94.2 ± 12.1 | −1.6 | 97.7 ± 11.2 | 94.2 ± 14.9 | −3.0 | 0.71 |
| Hemoglobin, g/dL | 13.6 ± 1.8 | 14.0 ± 1.5 | 3.7 | 13.1 ± 1.4 | 13.3 ± 1.7 | 1.8 | 0.36 |
| Total cholesterol, mg/dL | 200.6 ± 46.4 | 197.3 ± 54.6 | −1.3 | 209.3 ± 43.4 | 209.7 ± 48.5 | 0.3 | 0.71 |
| Triglycerides, mg/dL | 141.1 ± 52.5 | 129.5 ± 71.2 | −8.5 | 130.4 ± 59.5 | 120.2 ± 56.4 | −3.2 | 0.58 |
| Creatinine, mg/dL | 1.1 ± 0.3 | 1.0 ± 0.3 | 3.4 | 1.0 ± 0.3 | 0.8 ± 0.2 | −14.8 | 0.23 |
| Uric acid, mg/dL | 4.6 ± 1.8 | 3.9 ± 1.2 | −8.9 | 4.4 ± 1.3 | 4.1 ± 1.2 | 3.9 | 0.59 |
| Urea, mg/dL | 35.5 ± 11.0 | 34.3 ± 11.9 | −1.0 | 35.3 ± 8.7 | 33.1 ± 10.8 | −4.2 | 0.73 |
| GFR | 57.4 ± 20.6 | 62.1 ± 21.9 | 19.7 | 58.3 ± 16.1 | 81.8 ± 57.2 | 42.2 | 0.37 |
| Microalbumin, mg/g of creatinine | 7.0 ± 6.3 | 6.7 ± 6.2 | 7.2 | 8.4 ± 7.6 | 9.6 ± 10.0 | 19.3 | 0.61 |
Note: Values are presented as means ± SD.
Abbreviations: TASM, total appendicular skeletal muscle; IGF-1, insulin-like growth factor 1; HOMA-IR, homeostatic model assessment of insulin resistance; GFR, glomerular filtration rate; HD, habitual diet; RCH, ricotta cheese.
Adverse effects
The percentage of relative change in total cholesterol, triglycerides, and liver enzymes, and the percentage relative change in kidney function markers were not statistically different between groups. Finally, none of the subjects in the RCH + HD group developed microalbuminuria based on the results of the u-albumin test. Twenty-five percent of the women in the RCH + HD group referred to early satiety after consuming the ricotta cheese.
Secondary analysis
Tables 2 and 3 show the results of the secondary analysis, including the percentage of relative change in insulin levels and IGF-1. The percentage of relative change in IGF-1 was positive in both groups, while the percentage of relative change in fasting insulin levels was negative only in RCH + HD. However, these changes were not statistically significant. The results of the percentage of relative change in anthropometric, other body composition, and biochemical variables are shown in Table 2.
Table 3.
Effect of gender on relative changes associated with the additional intake of 210 g of ricotta cheese
| RCH + HD group | HD group | P | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Baseline | Follow-up | % relative change | Baseline | Follow-up | % relative change | ||
| Men | |||||||
| TASM (kg) | 18.8 ± 4.4 | 19.3 ± 1.6 | 2.7 | 18.0 ± 1.2 | 18.2 ± 1.2 | 1.2 | 0.42 |
| Strength (kg) | 27.4 ± 5.4 | 28.1 ± 6.1 | 3.2 | 28.4 ± 4.4 | 28.3 ± 4.60 | −0.3 | 0.17 |
| Body fat (kg) | 21.9 ± 4.1 | 22.0 ± 4.9 | 0.1 | 22.9 ± 9.5 | 21.9 ± 8.4 | −3.9 | 0.27 |
| Truncal fat, kg | 14.6 ± 2.4 | 14.8 ± 3.0 | 0.9 | 15.1 ± 6.8 | 14.5 ± 6.0 | −2.8 | 0.44 |
| Lean body mass, arms, kg | 4.9 ± 0.5 | 5.1 ± 0.6 | 4.7 | 4.7 ± 0.4 | 4.8 ± 0.4 | 1.3 | 0.12 |
| Lean body mass, legs, kg | 13.8 ±1.2 | 14.1 ± 1.2 | 1.9 | 13.3 ± 1.0 | 13.4 ± 0.9 | 1.3 | 0.74 |
| Lean body mass, kg | 43.7 ± 2.1 | 44.8 ± 2.8 | 2.5 | 43.2 ± 2.7 | 44.0 ± 2.6 | 1.9 | 0.60 |
| Total body mass, kg | 68.5 ± 4.5 | 69.7 ± 6.2 | 1.7 | 68.9 ± 11.7 | 68.7 ± 10.4 | −0.1 | 0.26 |
| Body weight, kg | 70.0 ± 5.1 | 71.2 ± 6.2 | 1.6 | 70.6 ± 12.1 | 70.2 ± 10.8 | −0.4 | 0.15 |
| Glucose (mg/dL) | 99.3 ± 14.4 | 97.5 ± 11.0 | −1.3 | 99.5 ± 9.4 | 93.3 ± 7.8 | −5.7 | 0.32 |
| IGF-1 (μg/L) | 60.4 ± 20.0 | 78.8 ± 44.8 | 23.4 | 60.5 ± 23.3 | 69.8 ± 41.5 | 12.9 | 0.70 |
| Insulin (μUI/L) | 9.9 ± 3.9 | 8.5 ± 2.1 | −10.1 | 7.7 ± 1.2 | 8.1 ± 1.4 | 5.0 | 0.05 |
| HOMA-IR | 2.5 ± 1.3 | 2.1 ± 0.7 | −11.1 | 1.9 ± 0.4 | 1.9 ± 0.4 | −0.8 | 0.27 |
| Women | |||||||
| TASM (kg) | 13.6 ± 1.4 | 13.9 ± 1.5 | 2.0 | 13.1 ± 1.2 | 13.3 ± 1.3 | 1.7 | 0.87 |
| Strength (kg) | 18.3 ± 2.9 | 18.2 ± 3.0 | −0.3 | 18.0 ± 3.2 | 16.9 ± 2.8 | −6.1 | 0.10 |
| Body fat (kg) | 31.0 ± 7.6 | 30.0 ± 7.9 | −3.9 | 29.9 ± 7.8 | 28.8 ± 7.7 | −3.5 | 0.82 |
| Abdominal fat, kg | 17.7 ± 4.6 | 17.0 ± 4.6 | −4.6 | 16.7 ± 5.5 | 16.2 ± 4.4 | 4.9 | 0.34 |
| Lean body mass, arms, kg | 3.5 ± 0.5 | 3.5 ± 0.4 | 0.7 | 3.2 ± 0.3 | 3.3 ± 0.3 | 1.2 | 0.72 |
| Lean body mass, legs, kg | 10.1 ± 1.0 | 10.3 ± 1.2 | 2.5 | 9.9 ± 0.9 | 10.1 ± 1.0 | 1.8 | 0.70 |
| Lean body mass, kg | 32.8 ± 3.7 | 33.3 ± 3.5 | 1.7 | 31.7 ± 2.4 | 32.4 ± 2.7 | 2.2 | 0.71 |
| Total body mass, kg | 65.9 ± 10.2 | 65.4 ± 10.4 | −0.7 | 63.6 ± 9.6 | 63.2 ± 9.8 | −0.6 | 0.87 |
| Body weight, kg | 67.8 ± 10.3 | 67.2 ± 10.6 | −0.8 | 65.5 ± 9.7 | 65.1 ± 10.0 | −0.8 | 0.92 |
| Glucose (mg/dL) | 94.1 ± 10.3 | 92.3 ± 12.7 | −1.9 | 96.1 ± 12.7 | 95.0 ± 19.2 | −0.8 | 0.84 |
| IGF-1 (μg/L) | 44.0 ± 40.0 | 48.5 ± 38.9 | 47.9 | 55.4 ± 31.2 | 60.8 ± 44.2 | 14.1 | 0.42 |
| Insulin (μUI/L) | 9.4 ± 3.6 | 9.8 ± 3.7 | 4.7 | 10.9 ± 8.2 | 9.2 ± 2.9 | −3.0 | 0.41 |
| HOMA-IR | 2.3 ± 1.0 | 2.3 ± 1.2 | 2.5 | 2.7 ± 2.4 | 2.2 ± 1.0 | −2.5 | 0.66 |
Note: Values are presented as means ± SD.
Abbreviations: TASM, total appendicular skeletal muscle; IGF-1, insulin-like growth factor 1; HOMA-IR, homeostatic model assessment of insulin resistance; HD, habitual diet; RCH, ricotta cheese.
Analysis by gender showed that the men in the RCH + HD group increased their body weight by approximately 1.6 kg and gained 490 g of TASM, while those in the HD group gained only 220 g of TASM and lost body weight. In contrast, the women in the RCH + HD and HD groups gained 260 g and 220 g, respectively. The women in both groups lost approximately 800 g of body weight. Muscle strength, lean body mass in the arms, and body weight also improved in the men in this group, though only a tendency was seen in the percentage of relative change in these variables, whereas in the women in the intervention group only the muscle strength variable showed a clear tendency towards significance (Table 3).
A significant effect of gender on some physiological variables was found. For instance, fasting insulin levels in the men in the RCH + HD group decreased by 10.1% (from 9.9% to 8.5%), while the men in the HD group showed an increase of 5.1% (from 7.7% to 8.1%). The percentage of relative change in fasting insulin levels was statistically significant in the men in the intervention group; also, HOMA-IR values decreased by 11.1% in the men in the RCH + HD group, while in the HD group they decreased by only 0.8%, though this change was not significant. IGF-1 values for the men in the RCH + HD group increased twofold, compared to those in the HD group after the 3-month follow-up period: 23.4% (from 60.4 to 78.8 μg/L) and 12.9% (from 60.5 to 69.8 μg/L), respectively. However, the difference in the percentage of relative change in IGF-1 values between groups (10.5%) was not statistically significant. In neither group was the percentage of relative change in kidney function markers and liver enzymes between the male subgroups significant.
Treatment adherence
During the intervention trial, eight subjects in the RCH + HD group dropped out for the following reasons: five did not complete the 3-month intervention period due to personal health issues unrelated to consuming ricotta cheese; two completed the intervention period but failed to eat the entire portion of cheese; and one had to leave the city. Three subjects in the HD group dropped out: two developed health problems, and one modified the habitual diet. However, to avoid bias, all subjects, including those who dropped out of the protocol, were measured twice (baseline and after 3 months of follow-up) and were included in the intention-to-treat analysis.
Discussion
The results of this study indicate that adding 210 g of ricotta cheese, or 15.7 g of high-quality protein supplement (containing 8.6 g essential amino acids) did not enhance TASM in free-living sarcopenic elderly male and female subjects. However, other physiological effects beyond the significant gain in total appendicular skeletal muscle in sarcopenic elderly men were found.
Similar results have been shown by other studies. Recently, Carlsson et al27 evaluated the effect of a high-intensity functional exercise program and a timed protein-enriched drink on muscle mass in 177 people aged 65 to 99 with severe physical or cognitive impairments, and living in residential care facilities. His results showed that after a three-month high-intensity functional exercise program, muscle mass did not increase. Furthermore, the intake of a protein-enriched drink immediately after the exercise did not induce any additional effect on muscle mass. Iglay et al18 concluded that consumption of diets that contained moderately higher protein did not differentially affect body composition and skeletal muscle fiber size in response to resistance training in older people.
Paddon-Jones et al10 showed that both 15 g of essential amino acids and 15 g of whey protein stimulated muscle-protein synthesis in healthy elderly individuals (65–79 years). As we stated before, we provided 15.7 g of protein (whey proteins) through the ricotta cheese. Maybe the amount of protein intake in sarcopenic elderly should be higher. Early results published by Bos et al28 and Chevalier et al29 concerning malnourished and frail elderly subjects point out that an increase in the total amount of dietary protein from 0.5 to 2.0 g/kg/day produced greater rates of whole-body protein synthesis and improved nitrogen balance.
With respect to the other primary response variable, handgrip strength increased in the RCH + HD group compared to the HD group; indeed, this variable showed a clear tendency towards significance (P = 0.06; Table 2). It is important to clarify that the sample size was calculated to determine differences in lean body mass, not in muscle strength. The latter does not depend solely on muscle mass, and the relationship between strength and muscle mass is not linear.30,31 This could explain the findings in the present study.
Reversing strength loss is of great clinical importance in the geriatric population, because several studies have shown that the risk of falling is significantly higher in subjects with reduced muscle strength.32–37 Other intervention studies using nutritional supplements (11 g of essential amino acids, plus arginine) have reported a significant gain in muscle strength in both male and female elderly subjects.38 According to results reported by other studies, it seems that the amount, form of administration,25 and sample size are other key factors that impact muscle strength in sarcopenic elderly people – factors that must be taken into account in future research.
The major finding of this study was that sarcopenic elderly men experienced some physiological effects beyond the significant gain in total appendicular skeletal muscle after a 3-month nutrition intervention trial based on adding 210 g of ricotta cheese to their habitual diet. The men in the RCH + HD group increased TASM by 270 g compared to those in the HD group after 3 months of follow-up. This finding is important when considered in relation to the average loss of skeletal muscle in elderly adults. Recently, Alemán et al39 reported an average loss of TASM in healthy elderly men of 1.9 kg after 5 years of follow-up. Our findings indicate that the loss of skeletal muscle in elderly men could be reversed by adding protein-rich foods, such as ricotta cheese, if the subject prefers natural foods to specifically designed nutritional supplements. Meat increases protein synthesis in both elderly and young subjects,13,14 and milk improves both protein synthesis and muscle mass in young people.15,16 In fact, the men in the RCH + HD group gained 270 g of TASM more, in relation to those in the HD group, and improved their fasting insulin levels (P = 0.05). Table 3 shows the improvements in muscle strength, lean body mass in the arms, and body weight variables for the men in the intervention group.
With respect to fasting insulin levels, Solerte et al8 recently reported a significant decrease in serum insulin after 6 months, and more consistently after 18 months, of intervention with oral nutritional supplementation in the form of a special mixture of amino acids. The recovery of anabolic conditions such as decreased insulin levels, and hence the enhancement of insulin sensitivity through protein-rich foods, may potentially induce the beneficial effect of restoring muscle mass, thus reversing sarcopenia in elderly men.
It is important to mention that the sample size was not calculated to detect differences by gender. Furthermore, drop-out rates (unrelated to the nutritional intervention) were similar in the men and women in both groups. The fact that the failure to consume the entire portion of ricotta cheese occurred only in women indicates that men adhered better to treatment in this study. The results mentioned above highlight a possible effect of gender on the response variables used in this study. We are aware that the amount of 210 g of ricotta cheese used was large, and that some people would find it difficult to consume such an amount of this food on a routine basis. However, it might be an option, if the elderly people involved preferred natural foods to specifically designed nutritional supplements. Moreover, ricotta cheese is a low-fat, low-carbohydrate, low-caloric food that can be incorporated as a component in numerous recipes.
The results of the present study should be interpreted in the context of its limitations. As mentioned above, the primary limitation was that the sample size was calculated based on gains in lean body mass in women and not TASM. In addition, the response variable of muscle strength was not considered in this procedure, though the percentage of relative change in muscle strength in the total intervention group and in the men in the intervention group did show a tendency. Second, not all subjects met the protocol of the intervention trial, though the research staff made every effort to maintain treatment compliance. Nevertheless, statistical analysis was carried out considering the total sample, in order to comply with the recommendations for clinical trials. According to the results of other intervention studies whose authors found a significant gain in muscle mass and strength, it seems that the form of administration (in supplements versus in foods) is a key factor in augmenting muscle mass and strength in elderly people with or without sarcopenia. It also appears that the time of administration is an important factor (with meals versus between meals) in enhancing muscle mass and strength. Finally, we cannot deny that both the ingestion of total protein (including the protein provided by ricotta cheese) and the calories in the diet were insufficient to enhance protein synthesis and translate it into a greater gain in muscle mass and strength in our subjects. Furthermore, the effect of gender on the response variables should be considered in future studies using protein-rich foods.
Conclusion
Nutritional intervention with 210 g of ricotta cheese in addition to the subjects’ usual diet failed to significantly reverse the loss of total appendicular skeletal muscle in free-living sarcopenic elderly men and women, though there was a tendency to gain muscle strength in both men and women in the intervention group. The amount of 210 g of ricotta cheese in addition to the subjects’ usual diet increased total appendicular skeletal muscle and was accompanied by significant decreases in fasting insulin levels only in the men in the intervention group. Muscle strength, lean body mass in the arms, and body weight improved in the men in the intervention group. We are aware that the amount of ricotta cheese used in this protocol was large, and that it would be difficult for some people to routinely consume this amount; however, this product could be an option for elderly people who prefer natural foods to specifically designed nutritional supplements. According to the results of this study, then, a nutritional intervention using a high-quality protein food, specifically ricotta cheese, in order to increase the amount of protein intake might therefore not be regarded as fully promising in elderly men and women with sarcopenia. However, the effect of gender on muscle strength, lean tissue in the arms, HOMA-IR, and body weight clearly suggests that more studies are needed on elderly men with sarcopenia.
Acknowledgments
This project was funded by the Institute of Nutrition and Health, Kellogg’s, and CONACYT, Mexico (S0008-2010-1-140157). We are grateful to the study participants and to Ana Cristina Gallegos Aguilar, Daniel David Robles Ochoa, Bertha Isabel Pacheco Moreno, Orlando Tortoledo Ortiz, Diana Josefina Mendoza Bermudez, and José Antonio Ponce Martínez for their technical assistance. Special acknowledgement is given to José Rogelio Ramos Enríquez, manager of LACIUS, Universidad de Sonora, for his participation in the laboratory analyses.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Fielding RA, Vellas B, Evans W, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boirie Y. Physiopathological mechanism of sarcopenia. J Nutr Health Aging. 2009;13:717–723. doi: 10.1007/s12603-009-0203-x. [DOI] [PubMed] [Google Scholar]
- 3.Morley JE. Sarcopenia in the elderly. Fam Pract. 2012;29:i44–i48. doi: 10.1093/fampra/cmr063. [DOI] [PubMed] [Google Scholar]
- 4.Marcell TJ. Sarcopenia: causes, consequences, and preventions. J Gerontol A Biol Sci Med Sci. 2003;58:M911–M916. doi: 10.1093/gerona/58.10.m911. [DOI] [PubMed] [Google Scholar]
- 5.Rolland Y, Czerwinski S, Abellan Van, Kan G, et al. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging. 2008;12:433–450. doi: 10.1007/BF02982704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roubenoff R, Hughes VA. Sarcopenia: current concepts. J Gerontol A Biol Sci Med Sci. 2000;55:M716–M724. doi: 10.1093/gerona/55.12.m716. [DOI] [PubMed] [Google Scholar]
- 7.Houston DK, Nicklas BJ, Ding J, et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr. 2008;87:150–155. doi: 10.1093/ajcn/87.1.150. [DOI] [PubMed] [Google Scholar]
- 8.Solerte SB, Gazzaruso C, Bonacasa R, et al. Nutritional supplements with oral amino acid mixtures increases whole-body lean mass and insulin sensitivity in elderly subjects with sarcopenia. Am J Cardiol. 2008;101:69E–77E. doi: 10.1016/j.amjcard.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Dillon E, Sheffield-Moore M, Paddon-Jones D, et al. Amino acid supplementation increases lean body mass, basal muscle protein synthesis, and insulin-like growth factor-I expression in older women. J Clin Endocrinol Metab. 2009;94:1630–1637. doi: 10.1210/jc.2008-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paddon-Jones D, Sheffield-Moore M, Katsanos CS, Zhang XJ, Wolfe RR. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Exp Gerontol. 2006;41:215–219. doi: 10.1016/j.exger.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Morley JE. Sarcopenia: diagnosis and treatment. J Nutr Health Aging. 2008;12:452–456. doi: 10.1007/BF02982705. [DOI] [PubMed] [Google Scholar]
- 12.Stout JR. Nutrition intervention in sarcopenia. Sarcopenia Proceedings Book. [Accessed 2 January, 2010]. Available from: http://search-ebooks.eu/nutrition-intervention-in-sarcopenia-25868874.
- 13.Symons TB, Sheffield-Moore M, Wolfe RR, Paddon-Jones D. A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J Am Diet Assoc. 2009;109:1582–1586. doi: 10.1016/j.jada.2009.06.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Symons TB, Schutzler SE, Cocke TL, Chinkes DL, Wolfe RR, Paddon-Jones D. Aging does not impair the anabolic response to a protein-rich meal. Am J Clin Nutr. 2007;86:451–456. doi: 10.1093/ajcn/86.2.451. [DOI] [PubMed] [Google Scholar]
- 15.Wilkinson SB, Tarnopolsky MA, Macdonald MJ, Macdonald JR, Armstrong D, Phillips SM. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am J Clin Nutr. 2007;85:1031–1040. doi: 10.1093/ajcn/85.4.1031. [DOI] [PubMed] [Google Scholar]
- 16.Phillips SM, Tang JE, Moore DR. The role of milk- and soy-based protein in support of muscle protein synthesis and muscle protein accretion in young and elderly persons. J Am Coll Nutr. 2009;28:343–354. doi: 10.1080/07315724.2009.10718096. [DOI] [PubMed] [Google Scholar]
- 17.Phillips SM, Hartman JW, Wilkinson SB. Dietary protein to support anabolism with resistance exercise in young men. J Am Coll Nutr. 2005;24(Suppl 2):134S–139S. doi: 10.1080/07315724.2005.10719454. [DOI] [PubMed] [Google Scholar]
- 18.Iglay HB, Apolzan JW, Gerrard DE, Eash JK, Anderson JC, Campbell WW. Moderately increased protein intake predominantly from egg sources does not influence whole body, regional, or muscle composition responses to resistance training in older people. J Nutr Health Aging. 2009;13:108–114. doi: 10.1007/s12603-009-0016-y. [DOI] [PubMed] [Google Scholar]
- 19.Björkman MP, Pilvi TK, Kekkonen RA, Korpela R, Tilvis RS. Similar effects of leucine rich and regular dairy products on muscle mass and functions of older polymyalgia rheumatica patients: a randomized crossover trial. J Nutr Health Aging. 2011;15:462–467. doi: 10.1007/s12603-010-0276-6. [DOI] [PubMed] [Google Scholar]
- 20.Delmonico MJ, Harris TB, Lee JS, et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc. 2007;55:769–774. doi: 10.1111/j.1532-5415.2007.01140.x. [DOI] [PubMed] [Google Scholar]
- 21.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 22.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetología. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 23.Norton K, Whittingham N, Carter L, Kerr D, Gore C, Marfell-Jones M. Measurement techniques in anthropometry. In: Norton K, Olds T, editors. Antropométrica: A Textbook of Body Measurement for Sports and Health Courses. Sydney: University of New South Wales Press; 1996. pp. 25–75. [Google Scholar]
- 24.Aleman-Mateo H, Lee SY, Javed F, et al. Elderly Mexicans have less muscle and greater total and truncal fat compared to African-Americans and Caucasians with the same BMI. J Nutr Health Aging. 2009;13:919–923. doi: 10.1007/s12603-009-0252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katsanos CS, Chinkes DL, Paddon-Jones D, Zhang XJ, Aarsland A, Wolfe RR. Whey protein ingestion in elderly results in greater muscle protein accrual than ingestion of its constituent essential amino acid content. Nutr Res. 2008;28:651–658. doi: 10.1016/j.nutres.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119:526.e9–e17. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 27.Carlsson M, Littbrand H, Gustafson Y, et al. Effects of high-intensity exercise and protein supplement on muscle mass in ADL dependent older people with and without malnutrition: a randomized controlled trial. J Nutr Health Aging. 2011;15:554–560. doi: 10.1007/s12603-011-0017-5. [DOI] [PubMed] [Google Scholar]
- 28.Bos C, Benamouzig R, Bruhat A, et al. Nutritional status after short-term dietary supplementation in hospitalized malnourished geriatric patients. Clin Nutr. 2001;20:225–233. doi: 10.1054/clnu.2000.0387. [DOI] [PubMed] [Google Scholar]
- 29.Chevalier S, Gougeon R, Nayar K, et al. Frailty amplifies the effects of aging on protein metabolism: Role of protein intake. Am J Clin Nutr. 2003;78:422–429. doi: 10.1093/ajcn/78.3.422. [DOI] [PubMed] [Google Scholar]
- 30.Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 31.Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159:413–421. doi: 10.1093/aje/kwh058. [DOI] [PubMed] [Google Scholar]
- 32.Lord SR, Menz HB, Tiedemann A. A physiological profile approach to falls risk assessment and prevention. Phys Ther. 2003;83:237–252. [PubMed] [Google Scholar]
- 33.de Rekeneire N, Visser M, Peila R, et al. Is a fall just a fall: correlates of falling in healthy older persons. The health, aging and body composition study. J Am Geriatr Soc. 2003;51:841–846. doi: 10.1046/j.1365-2389.2003.51267.x. [DOI] [PubMed] [Google Scholar]
- 34.Moreland JD, Richardson JA, Goldsmith CH, Clase CM. Muscle weakness and falls in older adults: a systematic review and meta-analysis. J Am Ger Soc. 2004;52:1121–1129. doi: 10.1111/j.1532-5415.2004.52310.x. [DOI] [PubMed] [Google Scholar]
- 35.Rao SS. Prevention of falls in older patients. Am Fam Physician. 2005;72:81–88. [PubMed] [Google Scholar]
- 36.Rubenstein LZ. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing. 2006;35(Suppl 2):ii37–ii41. doi: 10.1093/ageing/afl084. [DOI] [PubMed] [Google Scholar]
- 37.Skelton DA, Kennedy J, Rutherford OM. Explosive power and asymmetry in leg muscle function in frequent fallers and non-fallers aged over 65. Age Ageing. 2002;31:119–125. doi: 10.1093/ageing/31.2.119. [DOI] [PubMed] [Google Scholar]
- 38.Børsheim E, Bui QU, Tissier S, Kobayashi H, Ferrando AA, Wolfe RR. Effect of amino acid supplementation on muscle mass, strength and physical function in elderly. Clin Nutr. 2008;27:189–195. doi: 10.1016/j.clnu.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alemán H, Esparza J, Ramirez FA, Astiazaran H, Payette H. Longitudinal evidence on the association between interleukin-6 and c-reactive protein with the loss of total appendicular skeletal muscle in free-living older men and women. Age Ageing. 2011;40:469–475. doi: 10.1093/ageing/afr040. [DOI] [PubMed] [Google Scholar]