Abstract
Background
Inflammation induced by either endotoxin or vaccination has previously been shown to impair endothelium-dependent vasodilation (EDV) in healthy young individuals. However, the vascular effects of these two mechanisms of inducing inflammation have not been compared in the same individuals.
Methods
Twelve young healthy males were studied at the same time of the day on three occasions in a random order; on one occasion 4 hours following an endotoxin injection (Escherichia coli endotoxin, 20 IU/kg), on another occasion 8 hours following vaccination against Salmonella typhi, and on a third occasion 4 hours following a saline control injection. EDV and endothelium-independent vasodilation (EIDV) were evaluated by local infusions of acetylcholine and sodium nitroprusside in the brachial artery, and forearm blood flow was measured with venous occlusion plethysmography. The augmentation index was determined by pulse wave analysis as an index of pulse wave reflection.
Results
Both endotoxin and vaccination impaired EDV to a similar degree compared with the saline control (P = 0.005 and P = 0.014, respectively). EIDV was not significantly affected by inflammation. Endotoxin, but not vaccination, increased body temperature and circulating levels of intracellular adhesion molecule-1 and interleukin-6. Augmentation index was not affected by the interventions.
Conclusion
Despite the fact that endotoxin induced a more pronounced degree of inflammation than vaccination, both inflammatory challenges impaired EDV to a similar degree, supporting the view that different inflammatory stimuli could induce harmful effects on the vasculature.
Keywords: endothelium, endotoxin, vaccination, vasodilation, inflammation
Introduction
During the last decade it has been highlighted that inflammation plays a major role in the development of atherosclerosis and its major consequences, ie, myocardial infarction and stroke.1 Elevated levels of C-reactive protein and other markers of low-grade systemic inflammation have been shown to predict cardiovascular events in prospective studies,2–4 and vascular-derived inflammatory mediators, such as intercellular adhesion molecule (ICAM)-1, have also been shown to be powerful cardiovascular risk factors.5
Endothelial dysfunction is one of the earliest features of development of atherosclerosis and has been shown to precede vascular stenosis6 and to be able to predict a deleterious outcome in atherosclerotic patients.7,8 The degree of endothelium-dependent vasodilation (EDV), as a measure of endothelial function, has been associated with increased levels of C-reactive protein,9 as well as with high ICAM-1 levels,10 and impairment in EDV has repeatedly been demonstrated in chronic inflammatory disorders, such as rheumatoid arthritis, vasculitis, and systemic lupus.11–15
Two different models have recently been presented to evaluate the acute effects of inflammation on EDV in humans. The first of these use vaccination with Salmonella typhi as the challenge to induce systemic inflammation,16 while the other uses a bolus injection of Escherichia coli endotoxin (lipopolysaccharide) as the stimulus.17 Both of these approaches induce a reproducible systemic inflammation and a parallel reduction in EDV, as evaluated by the blood flow response to infused acetylcholine in the brachial artery. Thus, it seems as if both acute and chronic inflammation are associated with impaired EDV.
Pulse wave analysis with evaluation of the timing and amplitude of the reflected waves, as well as direct measures of aortic pulse wave velocity, have gained popularity as ways to determine aortic stiffness. Pulse wave reflection and pulse wave velocity measurements have both been shown to predict mortality and cardiovascular events.18,19 It has recently been suggested that aortic stiffness, apart from morphological features, also involves a certain degree of nitric oxide dependency. 20 Recently, a study reported that inflammation induced by vaccination affected arterial stiffness, as evaluated by pulse wave analysis and pulse wave velocity.21
The aim of the present study was to compare two inflammatory challenges, ie, endotoxin and vaccination, in healthy subjects both in terms of their ability to induce inflammation, as evaluated by interleukin (IL)-6 and ICAM-1 levels, as well as their effect on EDV and pulse wave reflection in healthy individuals.
Methods and materials
Study population
The study was conducted in 12 healthy male volunteers aged 20–30 years. The participants were nonsmokers, had no history of cardiovascular or other serious disorders, and were not taking any regular medication. All subjects were told to refrain from intake of anti-inflammatory drugs from 2 weeks prior to the investigations.
Study design
The study was conducted with a three-way, randomized, open, crossover design. On one occasion the effects of vaccination were evaluated, on another occasion the effects of a lipopolysaccharide bolus were investigated, and on a third occasion a bolus of saline was given as a control. The three interventions were given in a randomized order with 2–3 weeks in between the investigations. Because vaccination was given intramuscularly and the lipopolysaccharide intravenously, no blinding of the order of interventions was performed. The study was approved by the local ethics committee and each participant gave their informed consent.
For the vaccination, S. typhi capsular lipopolysaccharide vaccine 0.025 mg (Typherix®, GlaxoSmithKline, Rixensart, Belgium) was injected intramuscularly. Evaluation of inflammatory markers and EDV were performed 8 hours following vaccination in accordance with a previously published protocol. 16 When subjects were challenged with E. coli endotoxin (lipopolysaccharide, 20 IU/kg, national reference endotoxin, US Pharmacopeia Convention Inc, Rockville, MD), the dose was given as an intravenous bolus over 5 minutes, and measurements of inflammation and EDV were performed after 4 hours in accordance with a previously published protocol.17 The timing of the evaluation of the challenges (8 hours and 4 hours, respectively) were based on previous data in order to perform the measurements when the inflammatory responses were at their peak. On the saline control occasion, the same procedure was used as during the lipopolysaccharide challenges. The timing of the challenges was adjusted so that the evaluations of inflammatory markers and EDV took place at the same time of the day for all interventions (3 pm to 4 pm) in order to avoid bias by diurnal rhythms. The subjects were fasted on the study days to avoid the effects of food.
Methods
Invasive forearm technique
Forearm blood flow was measured by venous occlusion plethysmography before and at the end of the different dosages of the two vasodilators. A mercury in-silastic strain-gauge was placed on the upper third of the forearm, which rested comfortably slightly above the level of the heart. The strain-gauge was connected to a calibrated plethysmograph. Venous occlusion was achieved by a blood pressure cuff applied proximal to the elbow and inflated to 50 mmHg by a rapid cuff inflator. Evaluations of forearm blood flow in both arms were made by calculations of the mean of at least five consecutive recordings. Blood samples were collected from the brachial artery before the forearm blood flow measurements were performed.
After evaluation of resting forearm blood flow, local intra-arterial drug infusions were given over 5 minutes for each dose, with a 20-minute washout period between drugs. The dosages were infused at a rate of 6, 12, 25, and 50 μg per minute for acetylcholine (Clin-Alpha, Laufelingen, Switzerland) to evaluate EDV and 5 and 10 μg per minute for sodium nitroprusside (Nitropress®, Abbott Laboratories, Maidenhead, UK) to evaluate endothelium-independent vasodilation (EIDV). The dosages of these drugs had been chosen to result in forearm blood flow on the steep part of the dose-response curve. The drugs were given in a random order at a maximal rate of 1 mL/min.
In the present study, only data for the vasodilatory procedure are presented, both as absolute numbers of forearm blood flow and as the relative change in forearm blood flow from baseline. We considered the latter approach to be our main readout of the study. Thus, EDV was defined as forearm blood flow during infusion of 50 μg/min of acetylcholine minus resting forearm blood flow divided by resting forearm blood flow. EIDV was defined as forearm blood flow during infusion of 10 μg/min of sodium nitroprusside minus resting forearm blood flow divided by resting forearm blood flow.
Pulse wave analysis
For assessment of the pulse wave, a micromanometer tipped probe (Sphygmocor, Pulse Wave Medical Ltd, Sydney, Australia) was applied to the surface of the skin overlying the radial artery, and the peripheral radial pulse wave was continuously recorded. For accurate recordings, the micromanometer must be applied with light pressure to flatten the vessel walls so that transmural forces within the vessel are perpendicular to the arterial surface. The mean values of at least 10 pulse waves were used for analyses. The maximal systolic peak and the reflected waves were identified by calculations of the first and second derivative of the different parts of the pulse curve, and a transfer function supplied by the manufacturer converted the peripheral pulse curve to a central pulse curve. The ratio between the amplitude of the first reflected wave in systole and the primary systolic amplitude denoted the augmentation index (P2/P1). Also, the time from the start of the systolic part of the curve to the peak of the first reflected wave in systole was calculated.
Inflammatory markers
Circulating levels of soluble ICAM-1 and high sensitive interleukin-6 were measured by commercially available kits (R and D systems, Minneapolis, MN) on serum that had been frozen at −70°C.
Statistical analysis
Skewed variables, such as interleukin-6, were log-transformed to obtain a normal distribution. Differences between the treatment periods were evaluated with analysis of variance for repeated measurements. The Bonferroni correction was used for post hoc analysis. Two-tailed P values are given, with P < 0.05 regarded as statistically significant.
Results
Hemodynamics, inflammation, and body temperature
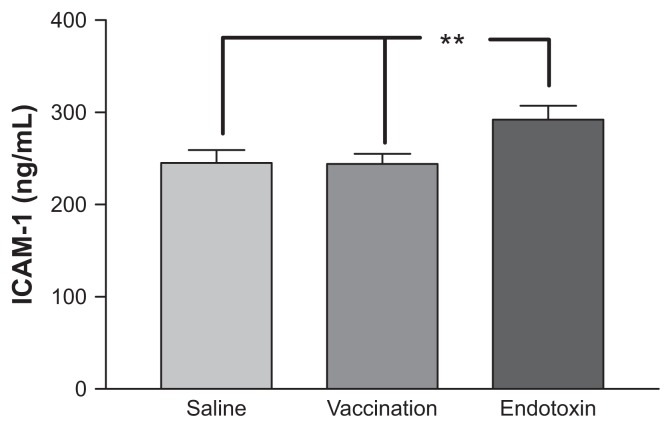
Compared with the saline control experiments, vaccination did not induce any changes in blood pressure, heart rate, or body temperature. Endotoxin injection, on the other hand, induced significant increments in heart rate and body temperature compared with both the saline control experiments and with vaccination (P < 0.001 for both). Blood pressure tended to be reduced by endotoxin, but this reduction was not significant (see Table 1). Endotoxin injection, but not vaccination, induced significant increments in the two markers of inflammation measured, ie, ICAM-1 and interleukin-1 (P < 0.0001 versus saline for both markers, see Figure 1 and Table 1). No subject reported any adverse events during the vaccine and saline protocols, but endotoxemia generally induced flu-like symptoms that resolved after 8–10 hours.
Table 1.
Means (standard deviations) for systolic blood pressure, diastolic blood pressure, heart rate, pulse wave indices, and body temperature during the three interventions of endotoxin injection, vaccination, and saline control injection
| Saline | Endotoxin | Vaccination | |
|---|---|---|---|
| Systolic blood pressure (mmHg) | 115 (5.4) | 113 (9.8) | 115 (12) |
| Diastolic blood pressure (mmHg) | 59 (5.9) | 55 (5.2) | 60 (7.3) |
| Heart rate (beats/min) | 59 (7.7) | 75 (7.8)a | 58 (8.2) |
| Body temperature (°C) | 36.4 (0.3) | 37.4 (0.3)a | 36.5 (0.3) |
| Aortic AIx (%) | 96 (10) | 94 (9.3) | 99 (9.2) |
| Time to first reflection (msec) | 195 (27) | 176 (24)b | 203 (23) |
| IL-6 (pg/mL) | 1.2 (0.5–5.2) | 19.5 (2.1–28.5)a | 1.7 (0.4–4.4) |
Notes:
P < 0.0001 versus saline and vaccination;
P < 0.05 versus saline only; interleukin-1 is given as median and range (n = 12).
Abbreviations: AIx, augmentation index; IL-6, interleukin-6.
Figure 1.
ICAM-1 levels following the three interventions (vaccine, endotoxin or saline control).
Notes: Means and standard errors of the mean are given (n = 12); ICAM-1 levels are given in ng/mL; **P < 0.01.
Abbreviation: ICAM, intracellular adhesion molecule.
Effects on EDV and EIDV
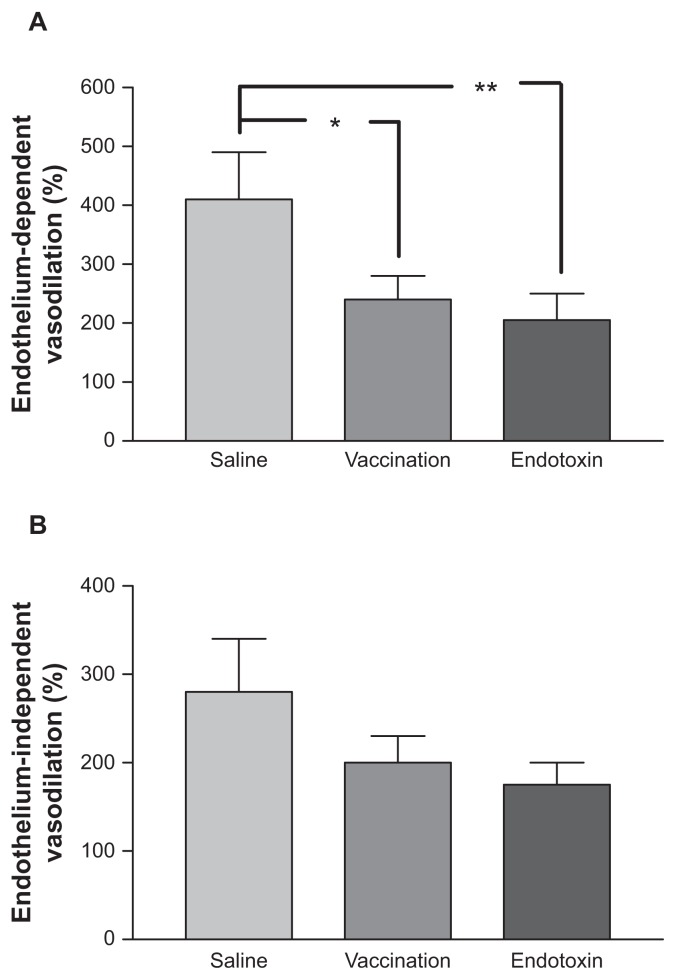
When EDV was evaluated as the percentage change from baseline, a significant difference was seen between the interventions (P = 0.010). As shown in Figure 2, EDV was significantly lower during both endotoxin injection and vaccination when compared with the saline control experiment (P = 0.014 for vaccine versus saline and P = 0.005). No difference was seen regarding EDV between the two inflammatory states (P = 0.63). When evaluated at the individual level, 10 of 12 subjects showed a reduction in EDV, both when given endotoxin and during the vaccine exposure, compared with the saline control value. As can be seen in Figure 2, a similar tendency as found for EDV was also seen for EIDV, although less pronounced. The differences between the interventions did not reach statistical significance (P = 0.067).
Figure 2.
Endothelium-dependent vasodilation (A) and endothelium-independent vasodilation (B) following the three interventions (vaccine, endotoxin, or saline control).
Notes: Means and standard errors of the mean are given (n = 12); vasodilation is given as a percentage of resting forearm blood flow; *P < 0.05; **P < 0.01.
Effects on resting forearm blood flow and vasodilatation in absolute numbers
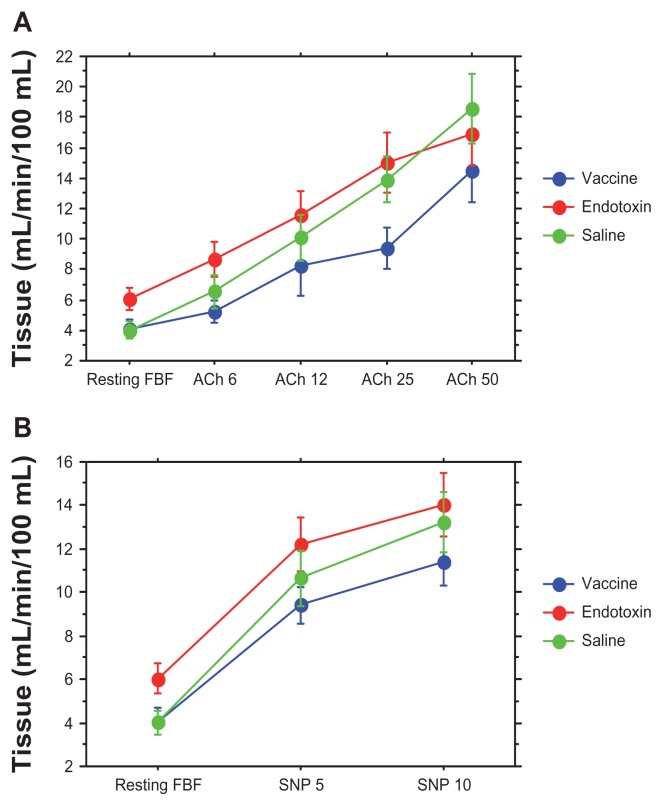
Figure 3 shows that vaccination and endotoxin induced different effects on resting baseline forearm blood flow. While the vaccination had no effect, endotoxin induced a profound increase in baseline forearm blood flow compared with both the saline control experiment and vaccination (P = 0.021 and P = 0.022, respectively). When an intervention has a profound effect on resting forearm blood flow, the effects of the intervention on vasodilation induced by acetylcholine or sodium nitroprusside cannot be evaluated in absolute numbers, but must be related to the baseline forearm blood flow as in the calculations of EDV and EIDV used above. The flatter doseresponse curve seen following vaccination as compared with the saline control was shifted upwards following endotoxin exposure (Figure 3). Forearm blood flow in the contralateral arm did not change significantly during vasodilation with acetylcholine or sodium nitroprusside, and therefore no correction for contralateral arm forearm blood flow was performed.
Figure 3.
Vasodilation following local infusion of acetylcholine at increasing dosages (A) and sodium nitroprusside (B) in the brachial artery following the three interventions (vaccine, endotoxin, or saline control). The endotoxin dose resulted in a significant increased resting forearm blood flow compared with saline vaccination (P < 0.05).
Notes: Dosages are in μg/min; means and standard errors of the mean are given; forearm blood flow on the Y axis is given in mL/min/100 mL tissue (n = 12).
Abbreviations: ACh, acetylcholine; FBF, forearm blood flow; SNP, sodium nitroprusside.
Effects on pulse wave refection
Neither endotoxin nor vaccination changed the augmentation index compared with saline (see Table 1). However, time to the first reflected wave was reduced by endotoxin, but not vaccination, when compared with saline (P = 0.010).
Discussion
The present study showed that two different challenges inducing acute inflammation, endotoxin injection and vaccination for S. typhi, both impaired EDV to a similar degree.
This was seen despite the fact that only endotoxin increased body temperature. Activation of the inflammatory markers, ICAM-1 and interleukin-6, induced a hyperdynamic circulation with increased resting blood flow. Thus, using a head-to-head comparison between endotoxin and vaccination, the major novel finding in the present study was that although endotoxin induced a markedly more pronounced degree of inflammation compared with vaccination, both inflammatory stimuli induced similar impairments in EDV. However, the augmentation index was not affected by the inflammatory stimuli.
The present study confirms a previous report that endotoxin injection in healthy subjects induces a transient reduction in EDV, in parallel with an increase in baseline forearm blood flow and a rise in body temperature.17 In that study, endotoxin exposure did not change the effects of the nitric oxide blocker, L-NMMA, on forearm blood flow, suggesting that the basal vasodilation induced by the endotoxin is mediated by substances other than nitric oxide. Thus, the seeming paradox that endotoxin induced a reduced response to the muscarinic receptor agonist, acetylcholine, a known stimulus of nitric oxide release from the endothelium, in parallel with an increased vasodilation in the basal state, could readily be explained by these observations.
The mechanism by which endotoxin impairs EDV is not known, but experimental studies have shown that endotoxin can inhibit muscarinic receptor-mediated signal transduction. 22 It has also recently been shown that high doses of vitamin C given locally in the forearm can reverse impairment in EDV caused by endotoxin,23 supporting the view that endotoxin could induce reactive oxygen species that might neutralize the vasodilatory action of nitric oxide. However, because neither L-NMMA or vitamin C were given in the present study, no further conclusions regarding the mechanisms behind the endotoxin effect could be drawn.
Moreover, the present study confirms previous reports that vaccination against S. typhi induced impaired EDV.16 It has furthermore been shown that pretreatment with aspirin could reverse this effect of vaccination in parallel with a reduction in the interleukin-1 receptor antagonist, a marker of interleukin-1 activation.24 In light of previous findings that local infusion of certain cytokines, tumor necrosis factor-alpha and interleukin-1, in the dorsal hand vein could induce locally impaired EDV,25 the action of aspirin in this model suggests a role for the proinflammatory cytokines in the impairment in EDV caused by inflammatory stimuli.
In the present study, endotoxin induced a substantial increment in interleukin-1, a general marker of inflammation, as expected. However, this was not seen when subjects were challenged with vaccination. We used the same protocol regarding the timing of the vaccination and evaluation of effects and the same vaccine that was used in a previous study reporting that vaccination against S. typhi induced impaired EDV.16 However, in that study, it was reported that white blood cell count, interleukin-6, and interleukin-1 receptor antagonist levels increased 8 hours following vaccination while no effects were seen on interleukin-1 and tumor necrosis factor-alpha. The reason for the increase in interleukin-6 levels in the previous study, which was not seen in the present study, is unclear. However, it is clear that endotoxin induced a substantially more pronounced inflammatory state than vaccination, despite both interventions impairing EDV to a similar extent in the present study.
Endotoxin also induced a substantial increment in ICAM-1, a known marker of vascular inflammation, as expected. This response was lacking when subjects were challenged with vaccination. Given that a relationship between EDV and ICAM-1 levels has been found in healthy subjects,10 the present data suggest that the two different actions of the endothelium, ie, vasodilation by nitric oxide synthesis and production of the adhesion molecule ICAM-1, could be disassociated following vaccination.
Given that both endotoxin administration and vaccination induced reproducible reductions in EDV, both of these inflammatory models could serve as tools to investigate the links between inflammation and endothelial dysfunction, which is the earliest step in atherogenesis. These models could also evaluate the anti-inflammatory and vasoprotective properties of different drugs, as in a recently published study, where 4 days of pretreatment with the lipid-lowering drug simvastatin was able to reverse impairment in EDV caused by endotoxin.26
The present study could not reproduce the recently published data from Vlachopoulos et al, who showed that the augmentation index was reduced by a vaccination challenge, although very similar protocols were used.20 They also reported pulse wave velocity to be increased following vaccination.
In the present study, pulse wave velocity was not measured directly, but no effect of vaccination on the time to the first reflected wave was seen following vaccination. This would be expected if pulse wave velocity has increased. Endotoxin, on the other hand, did reduce this time index. However, this finding should be interpreted with caution because a reduction in the time to the first systolic peak due to a more rapid ejection from the left ventricle, in addition to increased pulse wave velocity, could also reduce the time to the first reflected wave.
In conclusion, despite the fact that endotoxin induced a more pronounced degree of inflammation compared with vaccination, both inflammatory challenges impaired EDV to a similar degree, supporting the view that different inflammatory stimuli may have harmful effects on the vasculature. However, the augmentation index was not affected by the inflammatory challenges.
Acknowledgment
The authors acknowledge the helpful advice of Dr Michael Wolzt regarding preparation and administration of the endotoxin.
Footnotes
Disclosure
JH, AJ, and EH are employed full-time or part-time by AstraZeneca R and D, Sweden.
References
- 1.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Liuzzo G, Biassuci LM, Gallimore JR, et al. The prognostic value of C-reactive protein and serum amyloid A protein in severe unstable angina. N Engl J Med. 1994;331:417–424. doi: 10.1056/NEJM199408183310701. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Cushman M, Stampfer MJ, et al. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation. 1998;97:425–428. doi: 10.1161/01.cir.97.5.425. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Rifai N, Stampfer MJ, et al. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Hennekens CH, Roitman-Johnson B, et al. Plasma concentration of soluble intercellular adhesion molecule 1 and the risk of future myocardial infarction in apparently healthy men. Lancet. 1998;351:88–92. doi: 10.1016/S0140-6736(97)09032-6. [DOI] [PubMed] [Google Scholar]
- 6.Werns SW, Walton JA, Hsaia HH, et al. Evidence of endothelial dysfunction in angiographically normal coronary arteries of patients with coronary artery disease. Circulation. 1989;79:287–291. doi: 10.1161/01.cir.79.2.287. [DOI] [PubMed] [Google Scholar]
- 7.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 8.Perticone F, Ceravalo R, Puija A, et al. Prognostic significance of endothelial dysfuntion in hyptertensive patients. Circulation. 2001;104:191–196. doi: 10.1161/01.cir.104.2.191. [DOI] [PubMed] [Google Scholar]
- 9.Fischlscherer S, Rosenberger G, Walter DH, et al. Elevated C-reactive protein and impaired vasoreactivity in patients with coronary artery disease. Circulation. 2000;102:1000–1006. doi: 10.1161/01.cir.102.9.1000. [DOI] [PubMed] [Google Scholar]
- 10.Holmlund A, Hulthe J, Millgard J, Sarabi M, Kahan T, Lind L. Soluble intercellular adhesion molecule-1 is related to endothelial vasodilatory function in healthy individuals. Atherosclerosis. 2002;165:271–276. doi: 10.1016/s0021-9150(02)00234-4. [DOI] [PubMed] [Google Scholar]
- 11.Lima DS, Sato EI, Lima VC, et al. Brachial endothelial function is impaired in patients with systemic lupus erythematosus. J Rheumatol. 2002;29:292–297. [PubMed] [Google Scholar]
- 12.Filer AD, Gardner-Medwin JM, Thambyrajah J, et al. Diffuse endothelial dysfunction is common to ANCA associated systemic vasculitis and polyarteritis nodosa. Ann Rheum Dis. 2003;62:162–167. doi: 10.1136/ard.62.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hürlimann D, Forster A, Noll G, et al. Anti-tumor necrosis factor-α treatment improves endothelial function in patients with rheumatoid arthritis. Circulation. 2002;106:2184–2187. doi: 10.1161/01.cir.0000037521.71373.44. [DOI] [PubMed] [Google Scholar]
- 14.Raza K, Thambyrajah J, Townend JN, et al. Suppression of inflammation in primary systemic vasculitis restores vascular endothelial function: lessons for atherosclerotic disease? Circulation. 2000;102:1470–1472. doi: 10.1161/01.cir.102.13.1470. [DOI] [PubMed] [Google Scholar]
- 15.Bergholm R, Leirisalo-Repo M, Vehkavaara S, et al. Impaired responsiveness to NO in newly diagnosed patients with rheumatoid arthritis. Arterioscler Thromb Vasc Biol. 2002;22:1637–1641. doi: 10.1161/01.atv.0000033516.73864.4e. [DOI] [PubMed] [Google Scholar]
- 16.Hingorani AD, Cross J, Kharbanda RK, et al. Acute systemic inflammation impairs endothelium-dependent dilatation in humans. Circulation. 2000;102:994–999. doi: 10.1161/01.cir.102.9.994. [DOI] [PubMed] [Google Scholar]
- 17.Pleiner J, Heere-Ress E, Langenberger H, et al. Adrenoceptor hyporeactivity is responsible for Escherichia coli endotoxin-induced acute vascular dysfunction in humans. Arterioscler Thromb Vasc Biol. 2002;22:95–100. doi: 10.1161/hq0102.101818. [DOI] [PubMed] [Google Scholar]
- 18.London GM, Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar M. Arterial wave reflections and survival in end-stage renal failure. Hypertension. 2001;38:434–438. doi: 10.1161/01.hyp.38.3.434. [DOI] [PubMed] [Google Scholar]
- 19.Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 20.Vlachopoulos C, Dima I, Aznaouridis K, et al. Acute systemic inflammation increases arterial stiffness and decreases wave reflections in healthy individuals. Circulation. 2005;112:2193–2200. doi: 10.1161/CIRCULATIONAHA.105.535435. [DOI] [PubMed] [Google Scholar]
- 21.Wilkinson IB, Franklin SS, Cockcroft JR. Nitric oxide and the regulation of large artery stiffness: from physiology to pharmacology. Hypertension. 2004;44:112–116. doi: 10.1161/01.HYP.0000138068.03893.40. [DOI] [PubMed] [Google Scholar]
- 22.Peters TS, Lewis SJ. Lipopolysaccharide inhibits acetylcholine and nitric oxide-mediated vasodilation in vivo. J Pharmacol Exp Ther. 1996;279:918–925. [PubMed] [Google Scholar]
- 23.Pleiner J, Mittermayer F, Schaller G, et al. High doses of vitamin C reverse Escherichia coli endotoxin-induced hyporeactivity to acetylcholine in the human forearm. Circulation. 2002;106:1460–1464. doi: 10.1161/01.cir.0000030184.70207.ff. [DOI] [PubMed] [Google Scholar]
- 24.Kharbanda RK, Walton B, Allen M, et al. Prevention of inflammation-induced endothelial dysfunction. A novel vasculoprotective action of aspirin. Circulation. 2002;105:2600–2604. doi: 10.1161/01.cir.0000017863.52347.6c. [DOI] [PubMed] [Google Scholar]
- 25.Bhagat K, Vallance P. Inflammatory cytokines impair endothelium-dependent dilatation in human veins in vivo. Circulation. 1997;96:3042–3047. doi: 10.1161/01.cir.96.9.3042. [DOI] [PubMed] [Google Scholar]
- 26.Pleiner J, Schaller G, Mittermayer F, et al. Simvastatin prevents vascular hyporeactivity during inflammation. Circulation. 2004;110:3349–3354. doi: 10.1161/01.CIR.0000147774.90396.ED. [DOI] [PubMed] [Google Scholar]