Abstract
Background
The benefits of breastfeeding for improved health and developmental outcomes in mothers and their infants have been widely recognized. The purpose of the present study was to assess whether feeding modes influence maternal blood pressure at one month postpartum.
Methods
The pregnancy charts of 407 women who delivered at a birthing center in rural Japan between August 1998 and September 2007 were analyzed. The criteria for inclusion were low-risk, full-term pregnancy (duration, 37–42 weeks) resulting in spontaneous vaginal deliveries, intrapartum hemorrhage < 500 mL, and a healthy infant (Apgar score ≥ 8 at one minute).
Results
The subjects were classified into three groups based on feeding modes. The proportion of each mode was 28.3% in the breastfeeding group, 56.5% in the mixed-feeding group, and 15.2% in the formula-feeding group. The systolic blood pressure (SBP) in mothers at one month postpartum for each feeding mode was 118.4 ± 8.7 mmHg in the breastfeeding group, 120.6 ± 9.3 mmHg in the mixed-feeding group, and 122.0 ± 9.9 mmHg in the formula-feeding group. SBP at one month postpartum in the breastfeeding group was significantly lower than that in the other groups. No significant differences were observed in diastolic blood pressure in the three groups at one month postpartum.
Conclusion
Breastfeeding resulted in lower SBP in mothers at one month postpartum compared with those using other feeding modes, thus indicating an effect of breastfeeding on maternal blood pressure.
Keywords: breastfeeding, blood pressure, feeding mode, postpartum
Introduction
Human milk is species-specific and contains optimal nutrients, growth factors, and immunological components beneficial for infants.1 Breastfeeding in infants has short-term benefits, such as prevention of infectious diseases, including diarrhea,2–5 respiratory tract infection,6–9 and otitis media.10,11 Recent studies have reported that the long-term health benefits of breastfeeding in infants include reduction in the risk of adult diseases, such as obesity,12,13 hypertension,14,15 and type 1 diabetes mellitus.16,17 In addition, other studies have suggested that the long-term health benefits of breastfeeding for mothers include prevention of the development of ovarian18–20 and breast cancer21,22 and type 2 diabetes mellitus.20 Furthermore, from an economic viewpoint, breastfeeding is less expensive than formula-feeding.23
Milk production initially occurs as a result of endocrine (prolactin, cortisol, thyroid-stimulating hormone, prolactin-inhibiting factor, oxytocin) activity.24 Among these physiological factors, oxytocin is released in a pulsatile fashion from the anterior pituitary gland as a result of nipple/areola stimulation. The main role of oxytocin is maintenance of lactogenesis, as well as decreased postpartum bleeding and more rapid uterine involution. In addition, it also functions as a neurotransmitter, sedative, and promoter of mother-child attachment.25–27 Johnston and Amico reported that plasma oxytocin levels in mothers who were exclusively breastfeeding were higher than those who were formula-feeding, and basal plasma oxytocin levels were maintained at a high level.28 Light et al suggested that oxytocin decreases blood pressure; mothers with high oxytocin levels had lower blood pressure than those with low oxytocin levels.29 In addition, Jonas et al reported that both systolic blood pressure (SBP) and diastolic blood pressure (DBP) fall during breastfeeding.30 However, although the relationship between breastfeeding and blood pressure has been studied to a reasonable extent, not enough evidence in terms of a link between breastfeeding and lower blood pressure has yet been obtained. In particular, little information is available on whether blood pressure differs in women among various feeding modes, including breastfeeding, mixed-feeding, and formula-feeding. An understanding of the relationship between breastfeeding and maternal blood pressure is very important for long-term maternal health benefits and disease prevention. The purpose of the present study was to assess whether feeding modes influence maternal blood pressure at one month postpartum.
Materials and methods
Study population
The present retrospective chart review was conducted on the pregnancy charts of 407 women who delivered at a birthing center located in Aomori Prefecture, Japan. This study was conducted in accordance with the principles of the Declaration of Helsinki (Seoul 2008) and the ethical guidelines for epidemiological research provided by the Ministry of Education, Culture, Sports, Science, and Technology as well as the Ministry of Health, Labour, and Welfare in Japan (2008). All data used in the present study were coded and obtained from the medical records of subjects without disclosing their identity from the medical records. During the period from August 1998 until the end of September 2007, 579 pregnancy records of women who had a singleton pregnancy and delivered a live infant were studied. The inclusion criteria were low-risk, full-term pregnancy (duration 37–42 weeks) resulting in a spontaneous vaginal delivery, intrapartum hemorrhage < 500 mL, and a healthy infant (Apgar score ≥ 8 at one minute). Mothers with chronic diseases (eg, diabetes, hypertension, hyperthyroidism), gestational diabetes, and pregnancy-induced hypertension were excluded from the study. Records with unknown or missing data regarding obstetric factors were not included. A total of 407 cases were finally available for analysis.
Perinatal factors
Perinatal factors extracted from the pregnancy charts were maternal age, parity, self-reported prepregnancy weight, prepregnancy body mass index, gestational weight gain, blood pressure, chronic diseases, delivery mode, duration of pregnancy, duration of labor, neonatal gender, neonatal weight and height, Apgar score at one minute, admission to hospital, and first month checkup (maternal weight, blood pressure, feeding modes, infant weight).
Feeding modes
Subjects were divided into three groups according to feeding modes at one month postpartum. The first group was exclusively breastfeeding and the mothers gave breast milk only; the second group was mixed-feeding with breast milk and infant formula; and the third group was formula-feeding with infant formula only. Each feeding mode at one month postpartum was determined by self-assessment from mothers.
Statistical analysis
Statistical analysis was performed using SPSS software, version 16.0 (SPSS Japan Inc, Tokyo, Japan) for Windows. Descriptive statistics are shown as the arithmetic mean ± standard deviation. All data showed a normal distribution. A two-sample t-test, one-way analysis of variance, and Tukey’s honestly significant difference test were performed to determine differences across the three groups. The χ2 statistic was used to analyze categorical variables. Univariate analysis was performed using Pearson’s correlation coefficient. Multiple linear regression analysis was performed to determine any association between SBP (object functions) and maternal factors (explanatory variables). A value of P < 0.05 was considered to be statistically significant.
Results
Characteristics of study population
Characteristics of the study population are summarized in Table 1. The study population was classified into three groups depending upon the feeding mode, ie, breastfeeding 28.3%, mixed-feeding 56.5%, and formula-feeding 15.2%. The overall smoking rate was 18.2%, and the values in each group were 7.8%, 20.9%, and 27.4%, respectively, indicating that the smoking rate in the breastfeeding group was significantly lower than that in the other groups. No significant difference was observed between feeding modes and maternal/infant factors, except for maternal smoking status.
Table 1.
Summary and comparison of characteristics in the feeding modes
| Maternal/infant factors | Feeding modes group | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| All population n = 407 (100%) |
Breastfeeding n = 115 (28.3%) |
Mixed-feeding n = 230 (56.5%) |
Formula-feeding n = 62 (15.2%) |
|||||
|
|
|
|
|
|||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Maternal age yearsb | 26.7 | 4.5 | 26.7 | 4.1 | 26.6 | 4.6 | 26.7 | 4.7 |
| Nulliparousc | 154 | 37.8 | 43 | 37.4 | 91 | 39.6 | 20 | 32.3 |
| Smokersc | 74 | 18.2 | 9 | 7.8 | 48 | 20.9 | 17 | 27.4** |
| Prepregnancy weight kgb | 53.4 | 8.5 | 52.6 | 6.8 | 53.6 | 9.3 | 54.2 | 8.3 |
| Prepregnancy body mass indexa,b | 21.2 | 3.2 | 20.9 | 2.4 | 21.4 | 3.6 | 21.4 | 3.4 |
| Delivery weight | 65.2 | 8.7 | 64.3 | 7.4 | 65.6 | 9.1 | 65.4 | 9.4 |
| Gestational weight gain kgb | 11.8 | 4.0 | 11.7 | 3.5 | 12.0 | 4.4 | 11.2 | 3.7 |
| Duration of pregnancy weeksb | 39.5 | 1.2 | 39.5 | 1.1 | 39.6 | 1.2 | 39.3 | 1.1 |
| Birth weight gb | 3213.5 | 373.1 | 3200.2 | 321.9 | 3226.1 | 403.6 | 3188.2 | 345.5 |
| One-minute Apgar scoreb | 9.8 | 0.4 | 9.9 | 0.4 | 9.8 | 0.4 | 9.7 | 0.5 |
| Maternal weight at one month postpartum kgb | 57.9 | 8.3 | 57.1 | 7.1 | 58.3 | 8.8 | 57.8 | 8.8 |
| Postpartum weight loss kgb | 7.3 | 2.3 | 7.2 | 2.1 | 7.3 | 2.3 | 7.6 | 2.5 |
| Infant weight at one month gb | 4510.1 | 455.7 | 4496.2 | 448.5 | 4523.5 | 476.0 | 4486.0 | 392.5 |
Notes:
Body mass index, weight in kilograms divided by the square of the height in meters (kg/m2);
one-way analysis of variance, not significant;
presented as number and percent and analyzed with the χ2 test:
P < 0.01.
Abbreviation: SD, standard deviation.
Assessment of feeding modes and blood pressure
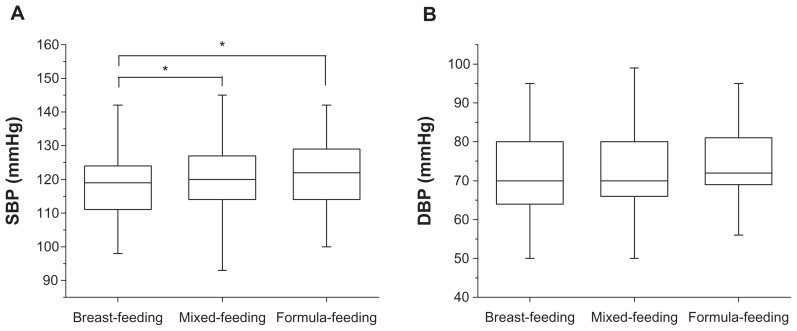
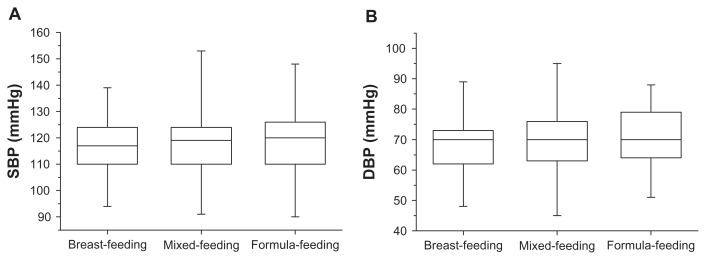
Results for maternal blood pressure at one month postpartum obtained for each feeding mode are shown in Figure 1. The mean SBP value 118.4 ± 8.7 mmHg in the breastfeeding group, 120.6 ± 9.3 mmHg in the mixed-feeding group, and 122.0 ± 9.9 mmHg in the formula-feeding group, demonstrating that blood pressure increased in a stepwise manner across the groups. The breastfeeding group showed the lowest value among the three groups, whereas no significant differences in one-month postpartum DBP were observed among the three groups. However, SBP and DBP values of these groups, as shown in Figure 2, showed no statistically significant differences in the third trimester. In addition, multiple linear regression analysis identified maternal weight at one month postpartum, feeding modes, and parity as independent predictive factors for SBP elevation at one month postpartum; however, the value of R2 was low (R2 = 0.09, one-way analysis of variance; P < 0.001).
Figure 1.
Distribution of SBP and DBP at one month postpartum in three feeding modes, breastfeeding, mixed-feeding, and formula-feeding. SBP observed in the breastfeeding group was significantly lower than that in the other groups (P < 0.05) (A) No significant difference was observed in DBP. (B) One-way analysis of variance: *P < 0.05.
Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure.
Figure 2.
Distribution of SBP/DBP in the third trimester observed according to three feeding modes, namely breastfeeding, mixed-feeding, and formula-feeding. No statistically significant differences were observed in SBP and DBP between mothers who used any of the three feeding modes (A and B).
Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure.
Relationship between maternal factors and blood pressure
A positive weak correlation was observed between SBP at one month postpartum and prepregnancy weight (r = 0.231, P < 0.001), prepregnancy body mass index (r = 0.214, P < 0.001), delivery weight (r = 0.249, P < 0.001), and maternal weight at one month postpartum (r = 0.250, P < 0.001, Table 2). However, no significant correlation was observed between SBP at one month postpartum and maternal age, gestational weight gain, duration of pregnancy, or postpartum weight loss (Table 2). Furthermore, multiple linear regression analysis was used to identify independent variables associated with elevated blood pressure. The mother’s weight at one month postpartum, feeding mode, parity, and SBP in the third trimester of pregnancy were identified as variables that can independently predict increased SBP at one month postpartum (R2 = 0.14, one-way analysis of variance, P < 0.001, data not shown), although R2 was a low value. Because it may be considered that smoking affected blood pressure as a confounding factor, the data were adjusted by smoking, and a regression analysis was conducted. As a result, delivery weight, parity, and feeding mode were identified as factors (R2 = 0.076, one-way analysis of variance, P < 0.001, data not shown).
Table 2.
Correlations between maternal factors and blood pressure
| Third trimester of pregnancy | One month postpartum | |||
|---|---|---|---|---|
|
|
|
|||
| SBP | DBP | SBP | DBP | |
| Maternal age | 0.036 | −0.014 | 0.133** | 0.049 |
| Duration of pregnancy | −0.015 | 0.063 | −0.002 | 0.031 |
| Prepregnancy weight | 0.203** | 0.071 | 0.231** | 0.096 |
| Prepregnancy BMI | 0.177** | 0.067 | 0.214** | 0.059 |
| Delivery weight | 0.202** | 0.073 | 0.249** | 0.146** |
| Gestational weight gain | 0.009 | 0.011 | 0.043 | 0.043 |
| Maternal weight one month postpartum | 0.184** | 0.060 | 0.250** | 0.143** |
Notes: Pearson’s correlation coefficient:
P < 0.05;
P < 0.01.
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Next, the effects of parity and maternal smoking on blood pressure were analyzed (Table 3). SBP in multiparous mothers was higher than that in primiparous mothers (121.1 ± 9.0 mmHg versus 118.7 ± 9.5 mmHg, P < 0.05). At this time, the mean maternal age of nulliparous and multiparous women was 24.2 ± 3.9 years and 28.1 ± 4.2 years, respectively, showing that maternal age in multiparous women was significantly higher than that in nulliparous women (P < 0.001). In contrast, smoking did not affect blood pressure, although the mean maternal age of nonsmokers was significantly higher than that of smokers (26.9 ± 4.5 years versus 25.4 ± 4.4 years, P < 0.05). However, parity did not affect blood pressure in either case (data not shown).
Table 3.
Influence of parity and maternal smoking status on blood pressure
| Mean age | Third trimester of pregnancy | One month postpartum | |||
|---|---|---|---|---|---|
|
|
|
||||
| SBP | DBP | SBP | DBP | ||
| Nulliparous (n = 154) | 24.2 (3.9)* | 117.2 (9.9) | 69.6 (8.7) | 118.7 (9.5) | 71.8 (9.8) |
| Multiparous (n = 253) | 28.1 (4.2)* | 117.3 (10.0) | 69.7 (9.2) | 121.1 (9.0)* | 73.0 (10.0) |
| Nonsmokers (n = 333) | 26.9 (4.4)* | 117.4 (10.0) | 69.3 (9.1) | 120.3 (9.3) | 72.7 (9.7) |
| Smokers (n = 74) | 25.4 (4.5)* | 117.5 (9.9) | 71.5 (8.1) | 119.7 (9.3) | 71.7 (10.6) |
Notes: Values in parentheses are the standard deviation. A statistically significant difference was found between nulliparous and multiparous and nonsmokers and smokers by the two-sample t-test
P < 0.05.
Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure.
Discussion
In this retrospective study, the pregnancy charts of 407 women who delivered at a birthing center in rural Japan between August 1998 and September 2007 were analyzed. When blood pressure in each group was compared, one-month postpartum SBP in the breastfeeding group was significantly lower than that in the other groups (Figure 1). At that time, various factors such as maternal age, prepregnancy weight, prepregnancy body mass index, delivery weight, and maternal weight at one month postpartum correlated with SBP (Table 2). Generally, aging and smoking cause high blood pressure. Actually, maternal age in the multiparous group was significantly higher than that in the nulliparous group, which showed a lower SBP than that in the multiparous group (Table 3). The present results showed that the smoking rate in the breastfeeding group was significantly lower than that in the other groups (Table 1), indicating that mothers in the mixed-feeding and formula-feeding groups were more likely to be smokers. Therefore, it may be considered that smoking affected blood pressure as a confounding factor. However, no significant relationships were observed with any factors, suggesting that a direct effect of smoking does not affect the lower SBP caused by breastfeeding in mothers at one month postpartum. Lee et al reported that women who breastfeed for 1–6 months have a lower risk of hypertension than those who do not breastfeed.31 Jonas et al and Altemus et al reported that both SBP and DBP reduce during a breastfeeding session, and that blood pressure before breastfeeding decreases during at least the first 6 months of breastfeeding in a homelike environment.30,32 Under these circumstances, there is a possibility that breastfeeding reduces blood pressure, particularly SBP, and that aging acted as a factor that upregulated blood pressure in the present population.
Oxytocin is released in a pulsatile manner from the anterior pituitary gland because of nipple/areolar stimulation. Previous studies have reported that oxytocin levels in breastfeeding mothers are higher than those in mixed-feeding mothers.28 Additionally, mothers with high oxytocin levels have lower blood pressure than those with low oxytocin levels.29 In animal studies, frequent oxytocin administration induces calm and long-term antistress effects, such as lowering of blood pressure in rats.33,34 Because mothers in the breastfeeding group fed their infants several times per day, oxytocin was released whenever the infant suckled the breast; thus, oxytocin was repeatedly released during breastfeeding. Therefore, these findings suggest that blood pressure in breastfeeding mothers decreased in response to oxytocin release, and that these mothers were likely to benefit from oxytocin at least while breastfeeding their infant. In the present study, plasma levels of oxytocin in mothers were unknown, because this study was a retrospective chart review. Additional approaches will be needed to clarify these issues.
The present results showed that the exclusive breastfeeding rate at one month was 28.3% (Table 1). In 1989, the World Health Organization/United Nations Children’s Fund released a joint document called “The Ten Steps to Successful Breastfeeding”.35 The American Academy of Pediatrics23 and World Health Organization36 both recommend exclusive breastfeeding for infants in the first 6 months of life. In a US national survey conducted in 2001, 33% of infants were breastfed (exclusive breastfeeding 17%) at the age of 6 months.37 In Japan, breastfeeding has been promoted since 1989, according to the abovementioned guidelines. According to a national Japanese study in 2005, 42.4% of infants at the age of one month, 38.0% at 3 months, and 34.7% at 6 months were exclusively breastfed.38 The breastfeeding rate obtained herein was much lower than the national average of Japan reported in 2005. These results may be because of regional characteristics and lack of knowledge or motivation for breastfeeding.
It is well known that breast milk is the ideal food for infants. Numerous worldwide studies have demonstrated improved health and developmental outcomes in breastfed infants and their mothers. The present study showed that breastfeeding resulted in lower SBP in mothers at one month postpartum compared with those using other feeding modes. Although the present study was performed in one specific district in Japan, the results support previous findings that breast feeding may induce long-term positive effects on maternal health. One important benefit of breast feeding is the prevention of adult diseases, and since chronic diseases, including metabolic disorders, are one of the largest causes of death in adults, promoting breastfeeding is essential. Thus, promoting breastfeeding is recommended, and further studies on the benefits of breast feeding for both the mother and child are needed to achieve this aim.
Acknowledgments
We are indebted to the midwives of the Fukushi Birth Center for collecting the pregnancy charts. This study was supported by a grant for Hirosaki University Institutional Research in 2011.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Prentice A. Constituents of human milk. [Accessed June 14, 2012]. Available at: http://archive.unu.edu/unupress/food/8F174e/8F174E04.htm#Constituents of human milk.
- 2.Howie PW, Forsyth JS, Ogston SA, Clark A, Florey CD. Protective effect of breast feeding against infection. BMJ. 1990;300:11–16. doi: 10.1136/bmj.300.6716.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clemens J, Rao M, Ahmed F, et al. Breast-feeding and the risk of life-threatening rotavirus diarrhea: prevention or postponement? Pediatrics. 1993;92:680–685. [PubMed] [Google Scholar]
- 4.Lopez-Alarcon M, Villalpando S, Fajardo A. Breast-feeding lowers the frequency and duration of acute respiratory infection and diarrhea in infants under six months of age. J Nutr. 1997;127:436–443. doi: 10.1093/jn/127.3.436. [DOI] [PubMed] [Google Scholar]
- 5.Bhandari N, Bahl R, Mazumdar S, Martines J, Black RE, Bhan MK. Effect of community-based promotion of exclusive breastfeeding on diarrhoeal illness and growth: a cluster randomized controlled trial. Infant Feeding Study Group. Lancet. 2003;361:1418–1423. doi: 10.1016/S0140-6736(03)13134-0. [DOI] [PubMed] [Google Scholar]
- 6.Blaymore Bier J, Oliver T, Ferguson A, Vohr BR. Human milk reduces outpatient upper respiratory symptoms in premature infants during their first year of life. J Perinatol. 2002;22:354–359. doi: 10.1038/sj.jp.7210742. [DOI] [PubMed] [Google Scholar]
- 7.Bachrach VR, Schwarz E, Bachrach LR. Breastfeeding and the risk of hospitalization for respiratory disease in infancy: a meta-analysis. Arch Pediatr Adolesc Med. 2003;157:237–243. doi: 10.1001/archpedi.157.3.237. [DOI] [PubMed] [Google Scholar]
- 8.Chantry CJ, Howard CR, Auinger P. Full breastfeeding duration and associated decrease in respiratory tract infection in US children. Pediatrics. 2006;117:425–432. doi: 10.1542/peds.2004-2283. [DOI] [PubMed] [Google Scholar]
- 9.Wright AL, Holberg CJ, Martinez FD, Morgan WJ, Taussig LM. Breast feeding and lower respiratory tract illness in the first year of life. Group Health Medical Associates. BMJ. 1989;299:946–949. doi: 10.1136/bmj.299.6705.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Owen MJ, Baldwin CD, Swank PR, Pannu AK, Johnson DL, Howie VM. Relation of infant feeding practices, cigarette smoke exposure, and group child care to the onset and duration of otitis media with effusion in the first two years of life. J Pediatr. 1993;123:702–711. doi: 10.1016/s0022-3476(05)80843-1. [DOI] [PubMed] [Google Scholar]
- 11.Aniansson G, Alm B, Andersson B, et al. A prospective cohort study on breast-feeding and otitis media in Swedish infants. Pediatr Infect Dis J. 1994;13:183–188. doi: 10.1097/00006454-199403000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Von Kries R, Koletzko B, Sauerwald T, et al. Breast feeding and obesity: cross sectional study. BMJ. 1999;319:147–150. doi: 10.1136/bmj.319.7203.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics. 2005;115:1367–1377. doi: 10.1542/peds.2004-1176. [DOI] [PubMed] [Google Scholar]
- 14.Singhal A, Cole TJ, Lucas A. Early nutrition in preterm infants and later blood pressure: two cohorts after randomised trials. Lancet. 2001;357:413–419. doi: 10.1016/S0140-6736(00)04004-6. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Evidence on the long-term effects of breastfeeding. Systematic reviews and meta-analysis. [Accessed June 14, 2012]. Available at: http://www.who.int/maternal_child_adolescent/documents/9241595230/en/
- 16.Virtanen SM, Räsänen L, Aro A, et al. Infant feeding in Finnish children less than 7 yr of age with newly diagnosed IDDM. Childhood Diabetes in Finland Study Group. Diabetes Care. 1991;14:415–417. doi: 10.2337/diacare.14.5.415. [DOI] [PubMed] [Google Scholar]
- 17.Gerstein HC. Cow’s milk exposure and type I diabetes mellitus. A critical overview of the clinical literature. Diabetes Care. 1994;17:13–19. doi: 10.2337/diacare.17.1.13. [DOI] [PubMed] [Google Scholar]
- 18.Gwinn ML, Lee NC, Rhodes PH, Layde PM, Rubin GL. Pregnancy, breast feeding, and oral contraceptives and the risk of epithelial ovarian cancer. J Clin Epidemiol. 1990;43:559–568. doi: 10.1016/0895-4356(90)90160-q. [DOI] [PubMed] [Google Scholar]
- 19.Rosenblatt KA, Thomas DB. Lactation and the risk of epithelial ovarian cancer. WHO Collaborative Study of Neoplasia and Steroid contraceptives. Int J Epidemiol. 1993;22:192–197. doi: 10.1093/ije/22.2.192. [DOI] [PubMed] [Google Scholar]
- 20.Stuebe A. The risks of not breastfeeding for mothers and infants. Rev Obstet Gynecol. 2009;2:222–231. [PMC free article] [PubMed] [Google Scholar]
- 21.Labbok MH. Effects of breastfeeding on the mother. Pediatr Clin North Am. 2001;48:143–158. doi: 10.1016/s0031-3955(05)70290-x. [DOI] [PubMed] [Google Scholar]
- 22.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet. 2002;360:187–195. doi: 10.1016/S0140-6736(02)09454-0. [DOI] [PubMed] [Google Scholar]
- 23.American Academy of Pediatrics. Breastfeeding and the use of human milk. Pediatrics. 2005;115:196–506. [Google Scholar]
- 24.Neville MC, Neifert MR, editors. Lactation: Physiology, Nutrition and Breast Feeding. New York, NY: Plenum Press; 1983. [Google Scholar]
- 25.Uvnäs-Moberg K. Oxytocin linked antistress effects – the relaxation and growth response. Acta Physiol Scand Suppl. 1997;640:38–42. [PubMed] [Google Scholar]
- 26.Uvnäs-Moberg K, Petersson M. Oxytocin, a mediator of anti-stress, well-being, social interaction, growth and healing. Z Psychosom Med Psychother. 2005;51:57–80. doi: 10.13109/zptm.2005.51.1.57. German. [DOI] [PubMed] [Google Scholar]
- 27.Nemsadze K, Silagava M. Neuroendocrine foundation of maternal-child attachment. Georgian Med News. 2010:21–26. [PubMed] [Google Scholar]
- 28.Johnston JM, Amico JA. A prospective longitudinal study of the release of oxytocin and prolactin in response to infant suckling in long term lactation. J Clin Endocrinol Metab. 1986;62:653–657. doi: 10.1210/jcem-62-4-653. [DOI] [PubMed] [Google Scholar]
- 29.Light KC, Smith TE, Johns JM, Brownley KA, Hofheimer JA, Amico JA. Oxytocin responsivity in mothers of infants: a preliminary study of relationships with blood pressure during laboratory stress and normal ambulatory activity. Health Psychol. 2000;19:560–567. doi: 10.1037//0278-6133.19.6.560. [DOI] [PubMed] [Google Scholar]
- 30.Jonas W, Nissen E, Ransjö-Arvidson AB, Wiklund I, Henriksson P, Uvnäs-Moberg K. Short- and long-term decrease of blood pressure in women during breastfeeding. Breastfeed Med. 2008;3:103–109. doi: 10.1089/bfm.2007.0031. [DOI] [PubMed] [Google Scholar]
- 31.Lee SY, Kim MT, Jee SH, Yang HP. Does long-term lactation protect premenopausal women against hypertension risk? A Korean women’s cohort study. Prev Med. 2005;41:433–438. doi: 10.1016/j.ypmed.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 32.Altemus M, Redwine LS, Leong YM, Frye CA, Porges SW, Carter CS. Responses to laboratory psychosocial stress in postpartum women. Psychosom Med. 2001;63:814–821. doi: 10.1097/00006842-200109000-00015. [DOI] [PubMed] [Google Scholar]
- 33.Petersson M, Alster P, Lundeberg T, Uvnäs-Moberg K. Oxytocin causes a long-term decrease of blood pressure in female and male rats. Physiol Behav. 1996;60:1311–1315. doi: 10.1016/s0031-9384(96)00261-2. [DOI] [PubMed] [Google Scholar]
- 34.Petersson M, Lundeberg T, Uvnäs-Moberg K. Oxytocin decreases blood pressure in male but not in female spontaneously hypertensive rats. J Auton Nerv Syst. 1997;66:15–18. doi: 10.1016/s0165-1838(97)00040-4. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization. Protecting, promoting and supporting breastfeeding: The special role of maternity services. [Accessed June 14, 2012]. Available at: http://whqlibdoc.who.int/publications/9241561300.pdf.
- 36.World Health Organization. Global strategy for infant and young child feeding. [Accessed June 14, 2012]. Available at: http://www.who.int/nutrition/publications/gs_infant_feeding_text_eng.pdf.
- 37.Ryan AS, Wenjun Z, Acosta A. Breastfeeding continues to increase into the new millennium. Pediatrics. 2002;110:1103–1109. doi: 10.1542/peds.110.6.1103. [DOI] [PubMed] [Google Scholar]
- 38.Ministry of Health, Labour and Welfare [http://www.mhlw.go.jp/] Summury of the results of nutrition survey in infants (FY2005) Japan: Breast-feeding division; [Accessed December 27, 2011]. [updated 2006 Jun 29]. Available from: [ http://www.mhlw.go.jp/shingi/2007/03/dl/s0314-17b-1.pdf (in Japanese)] [Google Scholar]