Abstract
Background
It is controversial whether starting highly active antiretroviral therapy (HAART) during primary HIV infection (PHI) is beneficial.
Methods
Subjects in this observational cohort began HAART <30 days (group 1: acute treatment, n=40), 31–180 days (group 2: early treatment, n=82), or >180 days (group 3: delayed treatment, n=35) after HIV infection and were compared to 27 historical and 60 contemporary controls.
Results
Time to HIV-related diagnoses did not differ for group 1 (aHR 1.44, p=.3) or group 2 (aHR 1.17, p=.5) compared to contemporary controls, but it was delayed for both treated groups (aHR 0.38 for group 1, p=.01; and aHR 0.28 for group 2, p<.0001) compared to historical controls.
Conclusions
Although rates of HIV-related diagnoses were similar in acutely-treated subjects and contemporary controls, results were confounded by associations between higher CD4 counts, lower HIV RNA levels and delayed disease progression as reasons for deferring treatment. Randomized trials are needed to address benefits of HAART during PHI.
Background
Initiation of highly active antiretroviral treatment (HAART) during primary human immunodeficiency virus type 1 (HIV) infection has many theoretical benefits, including the possibility of preserving immune system functions1, limiting viral diversity2–4, and reducing viral “set point”5–8. However, these benefits have been difficult to demonstrate. In two randomized, placebo-controlled trials, zidovudine increased the mean CD4+ T-cell count9,10 and decreased the frequency of Centers for Disease Control and Prevention (CDC) Class B conditions compared to placebo when initiated during primary HIV infection (PHI)9. However, after longer follow-up, there was no difference in rates of progression to acquired immunodeficiency syndrome (AIDS) between treated and untreated subjects11.
In some studies from observational cohorts, analyses have found associations between HAART initiated during PHI and improvements in virologic, immunologic, and clinical outcomes, but other studies have not identified these same associations 6,7,12–21. However, results from observational studies must be interpreted cautiously due to potential for measured and unmeasurable confounding. To date, three randomized studies that evaluated the effects of HAART during PHI have been attempted (ACTG A521722, Primo-SHM8, and SPARTAC23,24), but none has yet been published. The first of these was unable to evaluate the impact of HAART because of rapid progression among subjects in the untreated arm. In SPARTAC, preliminary results suggest that a short course of HAART could delay re-initiation of treatment by about four months.
Since 1992, individuals with PHI have enrolled into an observational cohort at the University of Washington Primary Infection Clinic1,14,25–27. Over time, some subjects initiated treatment during PHI based on the availability of HAART, strength of consensus treatment recommendations, and personal preferences. We undertook this analysis to evaluate the rate of HIV disease progression among treated subjects. We were particularly interested in whether there was an advantage to initiating HAART immediately following HIV acquisition, as it has been suggested that benefits of HAART would be most pronounced among these subjects6,28. We planned analyses to compare separate control groups comprised of historical and contemporary subjects because of differences between these groups at baseline.
Methods
Patient population
This analysis updates a prior evaluation of the cohort14 that compared treated subjects to historical control subjects. At the time of cohort entry, all subjects were either HIV antibody-negative with detectable HIV RNA (acute infection) or HIV antibody-positive with a negative or indeterminate Western Blot, negative “detuned” antibody test, or negative HIV test within one year of screening (early infection). All subjects were enrolled within 240 days after infection, estimated to be the date of onset of seroconversion symptoms25 or, for asymptomatic subjects, the midpoint between the last negative and first positive HIV tests. The UW Institutional Review Board approved this study, and all subjects provided written consent.
HAART became readily available after February 1996. We considered antiretroviral regimens to be HAART if they included three or more agents representing at least two classes of antiretroviral medications; the triple nucleoside regimen of zidovudine, lamivudine, and abacavir was also considered HAART. We excluded subjects who received hydroxyurea because of the associated toxicity without immunologic or virologic benefits29,30.
HIV RNA quantification in blood plasma
Beginning in 1996, plasma HIV quantitation was performed using a variety of branched DNA assays with lower limits of detection of 10000, 500, or 50 copies/mL (Chiron Corporation, Emeryville, CA). When specimens were available, we retested stored specimens that had not been previously evaluated and those results which were below the lower limits of detection of less-sensitive assays using an ultra-sensitive reverse transcription polymerase chain reaction (RT-PCR) assay (Roche; Branchburg, NJ) or an independently-validated real-time RT-PCR amplification assay with lower limits of detection equal to 50 copies/mL [42]. Since 2002, all specimens were evaluated by an RT-PCR assay.
Data collection
AIDS-defining opportunistic infections (OIs) included CDC Category C conditions from the 1993 revised classification system for HIV infection31. The list of “HIV-related diagnoses”14 included CDC Category B and C conditions plus bacterial infections (pneumonia, bronchitis, and sinusitis) and mucocutaneous conditions (folliculitis, acute herpesvirus outbreaks, genital and rectal warts, molluscum contagiosum, psoriasis, and seborrheic dermatitis) that occur with increased frequency in HIV-infected persons32. All diagnoses were documented prospectively by mid-level providers and physicians using standardized data collection forms.
Statistical analysis
Our primary objective was to evaluate whether the clinical impact of HAART varied in a time-dependent manner following HIV infection. Treated subjects were divided a priori into subjects who initiated therapy within 30 days after HIV infection (group 1: acute treatment), 31 to 180 days after HIV infection (group 2: early treatment), and more than 181 days after HIV infection (group 3: delayed treatment). Untreated subjects were divided into historical and contemporary control groups based on enrollment before or after availability of HAART. Subjects in the treatment groups contributed data to one of these control groups until they initiated HAART. However, untreated subjects who were HIV-infected before 1996 contributed data only to the historical control group, even if follow-up continued beyond 1996. Survival analyses evaluated the time to OI or death and, because this analysis was clearly underpowered, time to the first HIV-related diagnosis or death. These analyses excluded clinical conditions that occurred within four weeks of HIV infection because of overlap with the acute retroviral syndrome. Cox proportional hazard models, with treatment group as a time-varying covariate, were adjusted for age, race/ethnicity, severity of acute retroviral syndrome symptoms, and baseline (i.e. initial) CD4+ T-cell count and HIV RNA level.
Evaluations of pre-treatment CD4+ T-cell counts and HIV RNA levels used the closest measurement obtained within 30 days before starting HAART. Changes in CD4+ T-cell counts over time were evaluated by linear mixed modeling with an unstructured covariance structure. All analyses were conducted as intent-to-treat analyses and performed using Stata 10.1 (StataCorp LP, College Station, TX), SAS (SAS Institute Inc., Cary, NC) or R statistical software.
Results
Between August 1992 and January 2009, 328 individuals enrolled into the cohort. Subjects without follow-up beyond study enrollment (n=5) and those who received hydroxyurea (n=9) or other non-HAART regimens (n=35) were excluded from analysis. The remaining 279 subjects included 274 men and 5 women contributing 1191 person-years for analysis. At study referral, 96 (34%) subjects were HIV antibody-negative/RNA-positive, and 28 (10%) were HIV antibody-positive with a negative or indeterminate Western Blot assay. Subjects were first evaluated at the Primary Infection Clinic a median of 43 [interquartile range (IQR) 21-83] days following HIV infection (Table 1); at study screening, 26 (9%) were HIV-antibody negative, and 19 (7%) were HIV antibody-positive with a negative or indeterminate Western Blot assay.
Table 1.
Demographic and other characteristics of subjects, by study group
| Group 1: Acute Treatment (≤30 days) (n= 47) | Group 2: Early Treatment (31–180 days) (n=89) | Group 3: Delayed Treatment (≥181 days) (n=47) | Never Treated Historical Controls (n=27) | Never Treated Contemporary Controls (n=69) | Total (n=279) | |||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Age (mean years, s.d.) | 36 (8) | 34 (7) | 35 (8) | 29 (9) | 34 (8) | 34 (8) | p=.006 | |
|
| ||||||||
| Male | 100% | 99% | 98% | 93% | 98% | 98% | NS | |
|
| ||||||||
| Caucasian, non-Hispanic | 83% | 89% | 89% | 81% | 80% | 85% | NS | |
|
| ||||||||
| HIV risk factor | MSM | 44 (94%) | 83 (93%) | 43 (91%) | 20 (74%) | 64 (93%) | 254 (91%) | NS |
| MSM-IVDU | 1 (2%) | 2 (2%) | 2 (4%) | 3 (11%) | 4 (6%) | 12 (4%) | ||
| IVDU | - | 1 (1%) | - | 1 (4%) | - | 2 (1%) | ||
| Heterosexual | 1 (2%) | 3 (3%) | 1 (2%) | 2 (7%) | 1 (1%) | 8 (3%) | ||
| Other | 1 (2%) | - | 1 (2%) | 1 (4%) | - | 3 (1%) | ||
|
| ||||||||
| Severity of symptoms of acute retroviral syndrome | none | - | 12 (13%) | 9 (19%) | 4 (15%) | 20 (29%) | 45 (16%) | p<.001 |
| mild | 3 (6%) | 17 (19%) | 8 (17%) | 4 (15%) | 17 (25%) | 49 (18%) | ||
| moderate | 12 (26%) | 24 (27%) | 13 (28%) | 6 (22%) | 12 (17%) | 67 (24%) | ||
| severe | 27 (57%) | 33 (37%) | 17 (36%) | 8 (30%) | 18 (26%) | 103 (37%) | ||
| hospitalized | 5 (11%) | 3 (3%) | - | 5 (19%) | 2 (3%) | 15 (5%) | ||
|
| ||||||||
| Number of days from HIV infection to study screening (median, IQR) | 13 (10–18) | 50 (29–79) | 83 (28–125) | 78 (50–105) | 45 (24–81) | 43 (21–83) | p=.0001 | |
|
| ||||||||
| Date of study screening (median month/year, IQR) | 12/02 (6/00–2/05) | 12/00 (6/98–4/03) | 8/99 (3/96–10/03) | 1/94 (6/93–11/94) | 9/04 (1/01–2/07) | 6/01 (1/98–7/04) | ||
|
| ||||||||
| Baseline CD4+ T-cell count (mean cells/mm3, s.d.) | 437 (229)1 | 559 (211) 1 | 650 (277) 1 | 617 (250) 2 | 652 (244) 2 | 582 (248) | ||
|
| ||||||||
| Baseline HIV RNA level, (mean log10 copies/mL, s.d.) | 6.0 (1.0) 1 | 4.9 (0.9) 1 | 4.4 (1.1) 1 | 4.8 (0.6) 2 | 4.1 (1.3) 2 | 4.8 (1.2) | ||
|
| ||||||||
| Number of days from infection to HAART (median, IQR) | 21 (15–24) | 80 (45–108) | 822 (410–1424) | NA | NA | |||
|
| ||||||||
| Initial HAART regimen | PI | 34 (72%) | 40 (45%) | 21 (46%) | NA | NA | 95 (52%) | p<.001 |
| NNRTI | 8 (17%) | 34 (38%) | 24 (52%) | 66 (36%) | ||||
| PI/NNRTI | 4 (9%) | 14 (16%) | 0 (0%) | 18 (10%) | ||||
| II or II/NNRTI | 1 (2%) | 0 (0%) | 1 (2%) | 2 (1%) | ||||
| NRTI only | 0 (0%) | 1 (1%) | 1 (2%) | 2 (1%) | ||||
|
| ||||||||
| Duration of study follow-up (median years, IQR) | 3.6 (1.2–6.4) | 4.9 (2.7–7.8) | 6.0 (3.4–9.6) | 0.8 (0.3–1.8) | 1.8 (0.5–3.3) | 3.5 (1.2–6.6) | p=.0001 | |
s.d.: standard deviation; NS: not significant; MSM: men who have sex with men; IVDU: intravenous drug use; IQR: interquartile range; HAART: highly active antiretroviral therapy; PI: protease inhibitor therapy; NNRTI: non-nucleoside reverse transcriptase inhibitor therapy; II: integrase inhibitor; NRTI: nucleoside reverse transcriptase inhibitor; NA: not applicable
Tests for linear trend across treatment groups, by ordinary least squares regression with robust standard errors, adjusted for time since HIV infection: p=.003 (CD4) and p=.001 (HIV RNA).
Tests for differences between control groups, by ordinary least squares regression with robust standard errors, adjusted for time since infection, p=0.29 (CD4) and p<.0001 (RNA)
Forty-seven subjects initiated HAART within 30 days of infection (group 1: acute treatment); dates of HIV infection of all of the acutely treated subjects were estimated as the date of symptom onset. Eight of these subjects were HIV antibody-negative when starting HAART. Eighty-nine subjects initiated HAART between 31 and 180 days following infection (group 2: early treatment), and 47 subjects initiated HAART more than 181 days after HIV infection (group 3: delayed treatment). There were 27 historical and 69 contemporary control subjects who never initiated HAART.
Most subjects in the cohort were young, Caucasian men who have sex with men (Table 1). Most (84%) subjects experienced symptoms consistent with the acute retroviral syndrome. There were significant baseline differences between groups in age, seroconversion symptom severity, time from HIV infection to study screening, baseline CD4+ T-cell counts and HIV RNA levels, initial HAART regimens, and duration of study follow-up.
In the first year following infection, after adjusting for baseline CD4+ T-cell count, age, and severity of seroconversion symptoms, the mean CD4+ T-cell count declined 177 (95% CI 88-266) cells/mm3 for historical controls in the year following HIV infection and 128 (95% CI 82-174) cells/mm3 for contemporary controls. At treatment initiation, the mean CD4+ T-cell count was lower among subjects in the delayed treatment group (group 3, 319 cells/mm3, n=22) compared to subjects who received acute treatment (group 1, 469 cells/mm3, n=43) or early treatment (group 2, 531 cells/mm3, n=87). At HAART start, the mean HIV RNA level was higher among acutely treated subjects (5.6 log10 copies/mL, n=43) compared to subjects who received early treatment in group 2 (4.7 log10 copies/mL, n=88) and those who delayed treatment in group 3 (4.7 log10 copies/mL, n=23).
Only 23 subjects were observed to have CD4+ T-cell counts below 200 cells/mm3 at any time during the study. Of the fourteen subjects who had CD4+ T-cell counts below 200 cells/mm3 at least 90 days after infection, three did not receive OI prophylaxis, five received OI prophylaxis at least part of the time, and the remaining six had only single measurements below 200 and did not receive prophylaxis. Five subjects were diagnosed with candidal esophagitis immediately after HIV infection. There were an additional ten OIs in two treated and eight untreated subjects that occurred at least four weeks after infection, and five subjects died (including one who had experienced an OI). Only one death, in a subject who had discontinued HAART, was thought to be related to HIV infection. Among the 14 subjects, the first OI or death occurred a median of 404 (IQR 154-1207) days after infection. Further analysis of these endpoints was not performed due to small numbers.
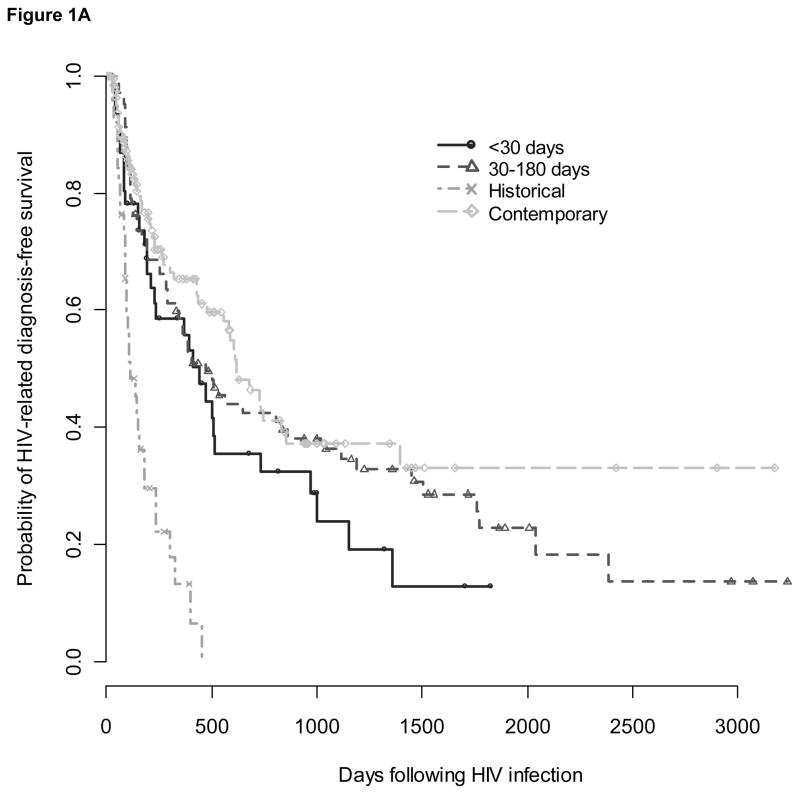
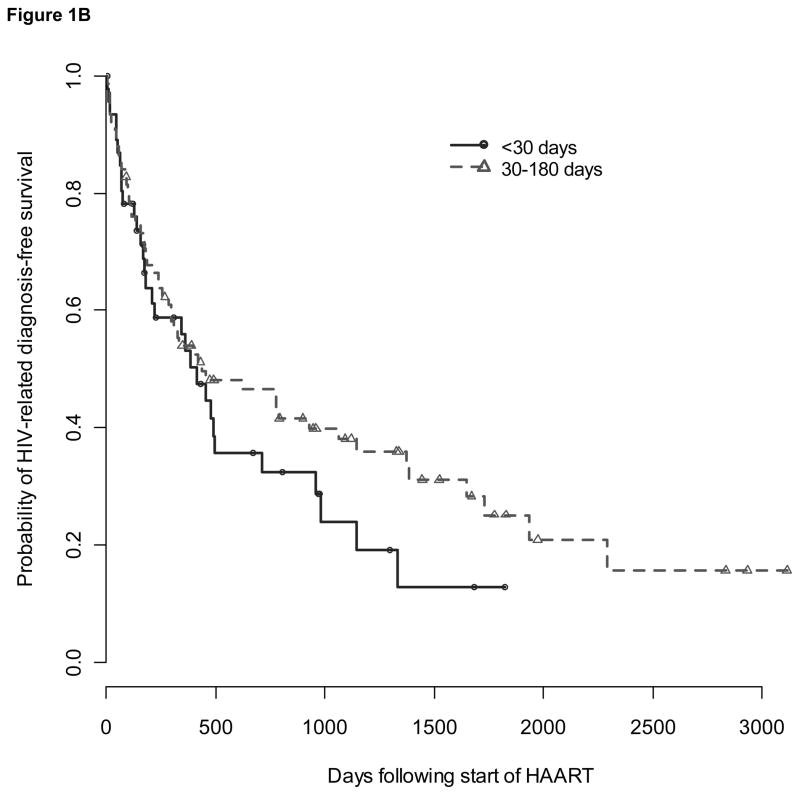
There were 178 subjects who experienced at least one HIV-related diagnosis greater than four weeks after infection (Table 2). Among 47 subjects who delayed the start of HAART greater than 180 days (group 3), 36 (77%) had an HIV-related diagnosis before initiating therapy and contributed to time-to-event analyses only as part of control groups. Due to small sample size, the remaining group of eleven subjects was not analyzed further. Median time to diagnosis of an HIV-related condition based on Kaplan-Meier time-to-event analysis was 443 (IQR 154-1003) days for group 1, 474 (IQR 142-1774) days for group 2, 616 (IQR 206- not available) days for contemporary controls, and 113 (IQR 83-237) days for historical controls (Figure 1A). Compared to contemporary controls, time to diagnosis of an HIV-related condition did not differ for group 1 [adjusted hazard ratio (aHR) 1.60, 95% CI 0.95-2.69, p=.08] or group 2 (aHR 1.18, 95% CI 0.78-1.79, p=.4) but was shorter for historical controls (aHR 3.74, 95% CI 2.35-5.95, p<0.0001). However, compared to historical controls, time to diagnosis of an HIV-related condition was significantly delayed for both the acute and early treatment groups (aHR 0.43, 95% CI 0.24-0.76, p=.004 for group 1; aHR 0.32, 95% CI 0.19-0.53, p<.0001 for group 2). There was no difference in time from HAART start to the diagnosis of an HIV-related condition between group 1 subjects who started HAART within 30 days and group 2 subjects who started HAART between 31 and 180 days after infection (aHR comparing group 2 to group 1: 0.66, 95% CI 0.38-1.14, p=.14) (Figure 1B).
Table 2.
List of the initial1 HIV-related diagnoses in 178 subjects
| HIV-related condition | # | Median # of days (IQR, range) following HIV infection | |
|---|---|---|---|
| Opportunistic infections (CDC Category C conditions) | 3 | 58 (48,432) | |
| Candidal esophagitis | 1 | ||
| Herpes simplex virus > 30 days | 2 | ||
| CDC Category B conditions | 27 | 116 (53–196, 30–853) | |
| Candidiasis - oropharyngeal | 18 | ||
| Candidiasis - vulvovaginal | 2 | ||
| Cryptosporidiosis < 30 days | 1 | ||
| Diarrhea > 30 days | 2 | ||
| Oral hairy leukoplakia | 2 | ||
| Peripheral neuropathy (not drug-related) | 2 | ||
| Bacterial infections | 43 | 263 (131–678, 34–1774) | |
| Pneumonia | 1 | ||
| Bronchitis | 18 | ||
| Sinusitis | 24 | ||
| Mucocutaneous conditions | 107 | 198 (89–481, 30–2387) | |
| Folliculitis | 7 | ||
| Herpes simplex virus - genital2 | 7 | ||
| Herpes simplex virus - oral2 | 20 | ||
| Herpes simplex virus - rectal2 | 12 | ||
| Herpes zoster virus (shingles) | 5 | ||
| Molluscum contagiosum | 3 | ||
| Psoriasis | 3 | ||
| Seborrheic dermatitis | 20 | ||
| Warts - genital/rectal | 18 | ||
| Warts - non-genital | 12 | ||
| Death3 | 3 | 154 (60,376) | |
| Total | 1834 | 192 (91–474, 30–2387) | |
Additional HIV-related diagnoses, OIs, or deaths subsequent to the initial HIV-related diagnosis are not included in this table.
Herpesvirus outcomes do not include asymptomatic seropositivity.
Deaths were due to a subarachnoid hemorrhage, a motor vehicle accident, and a suicide.
Five individuals reported two HIV related diagnoses with concurrent start dates.
Figure 1. Survival from HIV-related diagnoses over time since infection (Fig 1A) and since start of HAART (Fig 1B).
Survival plots showing the probability of remaining without an HIV-related diagnosis over time since infection (1A) and time since start of HAART (1B) for groups that received acute (circles), or early treatment (triangles) and historical (x’s) and contemporary (diamonds) controls. Pluses indicate censoring of subjects.
Conclusions
Although this analysis does not answer the question whether HAART should be initiated during PHI, it does provide insight into whether this question can ever be definitively answered by observational cohort studies. Our results suggest that there should be caution in interpreting other studies that aim to evaluate measures of HIV disease progression in observational cohorts when HAART is available and used in a non-randomized manner.
When treated subjects were compared to untreated contemporary control subjects, our data do not support a benefit of initiating HAART during PHI compared to delaying treatment. However, when treated subjects were compared to historical control subjects, we found a significant association between HAART and longer interval to the first HIV-related diagnosis. These differing conclusions underscore the importance of understanding the limitations of control groups in observational cohorts. Comparisons using the contemporary control group conservatively biased our analysis because reasons for initiating HAART (e.g. low CD4+ T-cell counts) are associated with worse clinical outcomes33. Based on baseline characteristics, we would have predicted that subjects in the acutely treated group would have poorer prognosis compared to subjects in the contemporary control group, whose higher CD4+ T-cell counts and lower HIV RNA levels allowed them to defer therapy. In contrast, historical control subjects may be a more representative population for comparison at baseline because HAART was not available when they enrolled. However, the decreased risk of disease progression among treated subjects compared to historical controls could be attributed to factors other than HAART, including the potential for changing HIV virulence or secular trends in OI prophylaxis or vaccination following the 1995 publication of guidelines for OI prevention34,35. We adjusted analyses to account for baseline differences between groups, but there are likely to be confounding variables that could not be measured or estimated without relying on untested assumptions.
Randomized studies of subjects with PHI have previously been impeded by the perceived lack of equipoise, small numbers of individuals identified during PHI, the length of follow-up time required to evaluate mortality, and the difficulty of obtaining informed consent when individuals may be experiencing physical and psychological stresses associated with recent HIV infection. Our results are consistent with much of the published literature and suggest that there should be equipoise about the benefit of initiating HAART during acute HIV infection. There are a growing number of public health-based pooled HIV RNA testing programs that screen for PHI36–39. These programs should not only focus on the public health benefits of safer sex counseling and partner services, but they should also continue to develop strong collaborations with research programs and specialists who understand the nuances of this field. Unless a “test and treat” approach is adopted in which HAART is universally recommended for all HIV-infected persons to benefit their individual health or to reduce transmission40 or until data from definitive randomized trials are published, individuals diagnosed with PHI and their care providers will continue to face a quandary about whether to initiate or defer HAART.
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health [grant numbers K23 AI-65243 (to JS), R01 AI-55343 (to SH), U01 AI-41535, and P01 AI-57005] and the University of Washington Center for AIDS and Research (AI-27757). Pharmaceutical support was provided by: Bristol Myers Squibb (formerly DuPont Pharmaceuticals Co.), GlaxoSmithKline, and Merck.
We would like to thank the subjects who participated in this cohort, the astute clinicians who recognized PHI and referred subjects, Terri Smith and Krista Yuhas for data support and analysis, and the many past and current collaborators in the Seattle Primary Infection Program. This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health [grant numbers K23 AI-65243 (to JS), R01 AI-55343 (to SH), U01 AI-41535, and P01 AI-57005] and the University of Washington Center for AIDS and Research (AI-27757). Pharmaceutical support was provided by: Bristol Myers Squibb (formerly DuPont Pharmaceuticals Co.), GlaxoSmithKline, and Merck.
Dr. Collier has current or past research support from Merck & Company, Schering-Plough (which is now part of Merck & Company), Boehringer-Ingelheim, Gilead Sciences, and Tibotec-Virco (Johnson and Johnson). She was a member of a Data, Safety, and Monitoring Board for a Merck-sponsored study, and has participated in Advisory Boards for GlaxoSmithKline, Merck & Company, and Pfizer. She and an immediate family member own stock in Abbott Laboratories, Bristol Myers Squibb, Johnson and Johnson and Pfizer, Inc.
Footnotes
Presented in part at the 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA; February 25-28, 2007.
Other authors report no conflicts of interest.
References
- 1.Malhotra U, Berrey MM, Huang Y, et al. Effect of combination antiretroviral therapy on T-cell immunity in acute human immunodeficiency virus type 1 infection. The Journal of infectious diseases. 2000 Jan;181(1):121–131. doi: 10.1086/315202. [DOI] [PubMed] [Google Scholar]
- 2.Pilcher CD, Shugars DC, Fiscus SA, et al. HIV in body fluids during primary HIV infection: implications for pathogenesis, treatment and public health. AIDS. 2001 May 4;15(7):837–845. doi: 10.1097/00002030-200105040-00004. [DOI] [PubMed] [Google Scholar]
- 3.Karlsson AC, Birk M, Lindback S, Gaines H, Mittler JE, Sonnerborg A. Initiation of therapy during primary HIV type 1 infection results in a continuous decay of proviral DNA and a highly restricted viral evolution. AIDS research and human retroviruses. 2001 Mar 20;17(5):409–416. doi: 10.1089/088922201750102463. [DOI] [PubMed] [Google Scholar]
- 4.Strain MC, Little SJ, Daar ES, et al. Effect of treatment, during primary infection, on establishment and clearance of cellular reservoirs of HIV-1. The Journal of infectious diseases. 2005 May 1;191(9):1410–1418. doi: 10.1086/428777. [DOI] [PubMed] [Google Scholar]
- 5.Desquilbet L, Goujard C, Rouzioux C, et al. Does transient HAART during primary HIV-1 infection lower the virological set-point? AIDS. 2004 Dec 3;18(18):2361–2369. [PubMed] [Google Scholar]
- 6.Hecht FM, Wang L, Collier A, et al. A Multicenter Observational Study of the Potential Benefits of Initiating Combination Antiretroviral Therapy during Acute HIV Infection. The Journal of infectious diseases. 2006 Sep 15;194(6):725–733. doi: 10.1086/506616. [DOI] [PubMed] [Google Scholar]
- 7.Streeck H, Jessen H, Alter G, et al. Immunological and Virological Impact of Highly Active Antiretroviral Therapy Initiated during Acute HIV-1 Infection. The Journal of infectious diseases. 2006 Sep 15;194(6):734–739. doi: 10.1086/503811. [DOI] [PubMed] [Google Scholar]
- 8.Steingrover R, Schellens I, Verbon A, et al. Temporary ART during primary HIV-1 infection lowers the viral set-point: the prospective, randomized Primo-SHM Study. Paper presented at: 15th Conference on Retroviruses and Opportunistic Infections; Boston, MA. February 3–6, 2008; [abstract #698b] [Google Scholar]
- 9.Kinloch-De Loes S, Hirschel BJ, Hoen B, et al. A controlled trial of zidovudine in primary human immunodeficiency virus infection. The New England journal of medicine. 1995 Aug 17;333(7):408–413. doi: 10.1056/NEJM199508173330702. [DOI] [PubMed] [Google Scholar]
- 10.Niu MT, Bethel J, Holodniy M, Standiford HC, Schnittman SM. Zidovudine treatment in patients with primary (acute) human immunodeficiency virus type 1 infection: a randomized, double-blind, placebo-controlled trial. DATRI 002 Study Group. Division of AIDS Treatment Research Initiative. The Journal of infectious diseases. 1998 Jul;178(1):80–91. doi: 10.1086/515612. [DOI] [PubMed] [Google Scholar]
- 11.Kinloch-de Loes S, Perneger TV. Primary HIV infection: follow-up of patients initially randomized to zidovudine or placebo. J Infect. 1997 Sep;35(2):111–116. doi: 10.1016/s0163-4453(97)91413-4. [DOI] [PubMed] [Google Scholar]
- 12.Hoen B, Dumon B, Harzic M, et al. Highly active antiretroviral treatment initiated early in the course of symptomatic primary HIV-1 infection: results of the ANRS 053 trial. The Journal of infectious diseases. 1999 Oct;180(4):1342–1346. doi: 10.1086/315002. [DOI] [PubMed] [Google Scholar]
- 13.Smith D, Berrey MM, Robertson M, et al. Virological and immunological effects of combination antiretroviral therapy with zidovudine, lamivudine, and indinavir during primary human immunodeficiency virus type 1 infection. The Journal of infectious diseases. 2000 Sep;182(3):950–954. doi: 10.1086/315753. [DOI] [PubMed] [Google Scholar]
- 14.Berrey MM, Schacker T, Collier AC, et al. Treatment of primary human immunodeficiency virus type 1 infection with potent antiretroviral therapy reduces frequency of rapid progression to AIDS. The Journal of infectious diseases. 2001 May 15;183(10):1466–1475. doi: 10.1086/320189. [DOI] [PubMed] [Google Scholar]
- 15.Fidler S, Oxenius A, Brady M, et al. Virological and immunological effects of short-course antiretroviral therapy in primary HIV infection. AIDS. 2002 Oct 18;16(15):2049–2054. doi: 10.1097/00002030-200210180-00010. [DOI] [PubMed] [Google Scholar]
- 16.Lillo FB, Ciuffreda D, Veglia F, et al. Viral load and burden modification following early antiretroviral therapy of primary HIV-1 infection. AIDS. 1999 May 7;13(7):791–796. doi: 10.1097/00002030-199905070-00007. [DOI] [PubMed] [Google Scholar]
- 17.Kassutto S, Maghsoudi K, Johnston MN, et al. Longitudinal analysis of clinical markers following antiretroviral therapy initiated during acute or early HIV type 1 infection. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2006 Apr 1;42(7):1024–1031. doi: 10.1086/500410. [DOI] [PubMed] [Google Scholar]
- 18.Pantazis N, Touloumi G, Vanhems P, Gill J, Bucher HC, Porter K. The effect of antiretroviral treatment of different durations in primary HIV infection. Aids. 2008 Nov 30;22(18):2441–2450. doi: 10.1097/QAD.0b013e328319ea4e. [DOI] [PubMed] [Google Scholar]
- 19.Steingrover R, Pogany K, Fernandez Garcia E, et al. HIV-1 viral rebound dynamics after a single treatment interruption depends on time of initiation of highly active antiretroviral therapy. Aids. 2008 Aug 20;22(13):1583–1588. doi: 10.1097/QAD.0b013e328305bd77. [DOI] [PubMed] [Google Scholar]
- 20.Koegl C, Wolf E, Hanhoff N, et al. Treatment during primary HIV infection does not lower viral set point but improves CD4 lymphocytes in an observational cohort. Eur J Med Res. 2009 Jul 22;14(7):277–283. doi: 10.1186/2047-783X-14-7-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steingrover R, Garcia EF, van Valkengoed IG, et al. Transient lowering of the viral set point after temporary antiretroviral therapy of primary HIV type 1 infection. AIDS research and human retroviruses. 2010 Apr;26(4):379–387. doi: 10.1089/aid.2009.0041. [DOI] [PubMed] [Google Scholar]
- 22.Hogan C, DeGruttola V, Daar E, et al. A Finite Course of ART during Early HIV-1 Infection Modestly Delays Need for Subsequent ART Initiation: ACTG A5217. The SETPOINT Study Paper presented at: 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA. February 16–19, 2010; [#134] [Google Scholar]
- 23.Fidler S, Ewings F, Porter K, et al. A Comparison of HIV Viral Rebound following ART Cessation in Primary and Chronic HIV Infection. Paper presented at: 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA. February 16–19, 2010; [#474] [Google Scholar]
- 24.Fidler S. The effect of short-course antiretroviral therapy in primary HIV infection: final results from an international randomised controlled trial; SPARTAC. 6th IAS Conference on HIV Pathogenesis, Treatment and Prevention; Rome, Italy. July 17–20, 2011; 2011. [abstract #WELBX06] [Google Scholar]
- 25.Schacker T, Collier AC, Hughes J, Shea T, Corey L. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996 Aug 15;125(4):257–264. doi: 10.7326/0003-4819-125-4-199608150-00001. [DOI] [PubMed] [Google Scholar]
- 26.Schacker TW, Hughes JP, Shea T, Coombs RW, Corey L. Biological and virologic characteristics of primary HIV infection. Ann Intern Med. 1998 Apr 15;128(8):613–620. doi: 10.7326/0003-4819-128-8-199804150-00001. [DOI] [PubMed] [Google Scholar]
- 27.Stekler J, Sycks BJ, Holte S, et al. HIV Dynamics in Seminal Plasma during Primary HIV Infection. AIDS research and human retroviruses. 2008 Oct;24(10):1269–1274. doi: 10.1089/aid.2008.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenberg ES, Altfeld M, Poon SH, et al. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000 Sep 28;407(6803):523–526. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- 29.Zala C, Salomon H, Ochoa C, et al. Higher rate of toxicity with no increased efficacy when hydroxyurea is added to a regimen of stavudine plus didanosine and nevirapine in primary HIV infection. J Acquir Immune Defic Syndr. 2002 Apr 1;29(4):368–373. doi: 10.1097/00126334-200204010-00007. [DOI] [PubMed] [Google Scholar]
- 30.Blanckenberg DH, Wood R, Horban A, et al. Evaluation of nevirapine and/or hydroxyurea with nucleoside reverse transcriptase inhibitors in treatment-naive HIV-1-infected subjects. AIDS. 2004 Mar 5;18(4):631–640. doi: 10.1097/00002030-200403050-00007. [DOI] [PubMed] [Google Scholar]
- 31.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992 Dec 18;41(RR-17):1–19. [PubMed] [Google Scholar]
- 32.Mandell, Bennett, & Dolin: Principles and Practice of Infectious Diseases. 6. Philadelphia: Elsevier; 2005. [Google Scholar]
- 33.Clements M, Law M, Pedersen C, Kaldor J. Estimating the effect of antiretroviral treatment during HIV seroconversion: impact of confounding in observational data. HIV medicine. 2003 Oct;4(4):332–337. doi: 10.1046/j.1468-1293.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- 34.Kaplan JE, Hanson D, Dworkin MS, et al. Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2000 Apr;30 (Suppl 1):S5–14. doi: 10.1086/313843. [DOI] [PubMed] [Google Scholar]
- 35.Kaplan JE, Masur H, Holmes KK, et al. USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus: introduction. USPHS/IDSA Prevention of Opportunistic Infections Working Group. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 1995 Aug;21 (Suppl 1):S1–11. [PubMed] [Google Scholar]
- 36.Pilcher CD, Fiscus SA, Nguyen TQ, et al. Detection of acute infections during HIV testing in North Carolina. The New England journal of medicine. 2005 May 5;352(18):1873–1883. doi: 10.1056/NEJMoa042291. [DOI] [PubMed] [Google Scholar]
- 37.Stekler J, Swenson PD, Wood RW, Handsfield HH, Golden MR. Targeted screening for primary HIV infection through pooled HIV-RNA testing in men who have sex with men. AIDS. 2005 Aug 12;19(12):1323–1325. doi: 10.1097/01.aids.0000180105.73264.81. [DOI] [PubMed] [Google Scholar]
- 38.Patel P, Klausner JD, Bacon OM, et al. Detection of acute HIV infections in high-risk patients in California. J Acquir Immune Defic Syndr. 2006 May;42(1):75–79. doi: 10.1097/01.qai.0000218363.21088.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Priddy FH, Pilcher CD, Moore RH, et al. Detection of Acute HIV Infections in an Urban HIV Counseling and Testing Population in the United States. J Acquir Immune Defic Syndr. 2006 Nov 2; doi: 10.1097/01.qai.0000254323.86897.36. [DOI] [PubMed] [Google Scholar]
- 40.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. The New England journal of medicine. 2011 Aug 11;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]