Abstract
Background
Previous national and international studies of quality of life (QoL) in patients with skin diseases have revealed different levels of QoL impairment. The aims of this study were to assess QoL in patients with skin diseases in central Saudi Arabia using the newly validated Skindex-16 instrument and to determine the association between QoL in patients with skin disease, sociodemographic data, and disease characteristics.
Methods
A cross-sectional study was conducted in 283 adult patients who visited the outpatient dermatology clinics of King Abdulaziz Medical City, Riyadh, Saudi Arabia, over 3 months. The patients were interviewed using a pretested Arabic version of the Skindex-16 to measure the effect of skin disorders on their QoL during the previous 7 days. Patient characteristics, medical history, and clinical findings were collected. Multiple linear regression analyses were used to relate the demographic and clinical characteristics to the percentage mean QoL score, and P ≤ 0.05 was considered to be statistically significant.
Results
QoL was good in 69% of the respondents, with a total percent mean score of 31.80 ± 20.16. The emotional domain was the most affected (mean percentage score 44.27 ± 27.06), followed by symptoms (31.45 ± 28.40) and functioning (14.61 ± 22.75). After adjustment for potential confounders, poorer QoL was significantly associated with female gender (P = 0.03), older age (P = 0.003), rural origin (P = 0.03), positive family history of the same lesion(s) (P = 0.01), shorter duration of ≤6 months (P = 0.02), generalized spread (P ≤ 0.02), and lack of isotretinoin treatment (P = 0.02).
Conclusion
. The QoL results in this study were generally more optimistic than those of many previous studies. This discrepancy may be due to biases in questionnaire responses or to cultural differences in experience of skin disease and perception of disability. Significant predictors of QoL were not the same for the three domains of the Skindex scale. Further studies of specific diseases and educational programs targeting patients at higher risk for QoL impairments are recommended.
Keywords: quality of life, skin disease, Saudi Arabia
Introduction
Skin diseases are a common health condition responsible for considerable disability.1 Patients with skin disease may experience severe symptoms, such as itching, pain, and discomfort, that can have a profound psychological impact. Furthermore, patients’ social and physical activities, including sports and work, may be adversely affected because of reluctance to allow others to see their skin disease. Some treatment modalities can situationally decrease quality of life (QoL) due to the difficulties involved in using topical creams and ointments.2
Although mortality rates are generally low, skin diseases have significant effects on QoL.3 The disability-adjusted life years associated with skin diseases have been reported to be similar to that of other medical conditions. The evidence has also shown deficits in objective QoL measurements associated with common skin diseases (eg, acne) that are comparable with those of chronic disorders, such as asthma, diabetes, and arthritis.4
QoL measures can potentially allow dermatologists to record and monitor a patient’s progress with valid, reliable methods that allow intrapersonal and interpersonal comparisons.5 Measuring dermatological health-related QoL warrants more methodological studies to test and possibly refine existing instruments or to create new ones. Cultural differences should be considered when creating a tool that scientifically measures dermatological health-related QoL.6
Previous worldwide studies of QoL in patients with different skin diseases, such as acne,7 psoriasis,8 vitiligo,9–11 eczema,8 and cutaneous melanoma12 have revealed different levels of QoL impairment. Different levels of QoL impairment in patients with skin diseases have also been noted in developing countries, such as Saudi Arabia.13–16 However, many methodological issues remain, including the problem of cultural differences when the same measure is used in different countries.6
The Skindex is an instrument that measures the effects of a skin disease on a patient’s QoL, and has been refined and studied extensively.17 It is a self-administered questionnaire that was originally created in English. It initially included 61 questions but was then modified to 29 questions. Later, a 16-question short questionnaire, known as the Skindex-16, was introduced. The Skindex-16 assesses the domains considered to be essential in any QoL assessment instrument, such as burden of symptoms, social function, and emotional state.17 The Skindex-16 has advantages over the Skindex-29, in that it is a brief, single-page version with fewer items, and measures the inconvenience rather than frequency of a patient’s experiences.17 An Arabic version of the Skindex-16 has been validated and adapted to Saudi culture.18 The present study assessed QoL in patients with skin disease using the newly validated Skindex-16 for Saudis at the King Abdulaziz Medical City (KAMC), Riyadh, Saudi Arabia, and determined the association between QoL in patients with skin disease, sociodemographic data, and disease characteristics.
Materials and methods
This study was conducted at King Abdulaziz Medical City (KAMC), which is located in Riyadh, Kingdom of Saudi Arabia. KAMC is a tertiary hospital with a 690-bed capacity, including 25 beds for expected surgical procedures and 132 beds for emergency admissions. Eight consultants run the KAMC dermatology department, which includes eight outpatient clinics that see approximately 650 patients each month. The study subjects were all adult patients (ie, age 15 years or older) who were not suffering from psychological conditions and who had visited the KAMC dermatology clinics between July 16, 2011 and October 15, 2011.
The main goal of this study was to assess QoL in Saudi Arabian patients with skin disease. Based on previous studies, the expected prevalence of psychiatric morbidity in these patients was 24.4%.19 Assuming a statistical significance level of 5% and a margin of error of 5%, the estimated sample size needed was 307 subjects. All of the patients attending the KAMC dermatology clinics during the 3-month study period who fulfilled the inclusion criteria and were willing to participate in the study were enrolled in the study until the required sample size was obtained.
Data collection
Following approval by our institutional review board and informed verbal consent from the patients, a pretested Arabic version of the Skindex-16 scale18 was administered to measure the effect of skin disorders on QoL over the previous 7 days. The Skindex-16 questionnaire is simple and brief (16 questions) and focuses on three domains, ie, a symptoms domain (questions 1–4), an emotional domain (questions 5–11), and a functional domain (questions 12–16). The patients responded to each question with “never bothered”, “rarely bothered”, “sometimes bothered”, “bothered most of time”, or “always bothered”. A five-point Likert scale (from 0 to 4 points) was used to score the questions. The scores for each domain were summed over the patients, and the percent score was calculated. A higher score reflects a greater impact of skin disease. The QoL range for each domain and the overall QoL were categorized as follows: poor QoL (<50%), moderate QoL (50%–75%), and good QoL (>75%).
The questionnaire consisted of the following types of questions:
Sociodemographic characteristics, which included age, gender, educational level, marital status, and monthly income.
Disease information, which included the skin disease diagnosis, course, and duration, and site of the lesion. The skin diagnoses were classified into eight categories, ie, papulosquamous disorders, connective tissue and immunological disorders, eczematous dermatitis, sebaceous and apocrine gland disorders, disorders of hair follicles and related disorders, cutaneous infections, pigmentary disorders, and miscellaneous (eg, sexually transmitted diseases and tumors).
Information about treatment, which included route of administration, cortisone and retinoid use, and duration, if applicable. Patient charts were examined to collect this information.
Ethical considerations
Participation in the study was completely voluntary. The investigators explained the purpose of the research and how the survey would be conducted. Each patient was able to withdraw from the study at any time. Confidentiality was maintained throughout the study, and the subjects were assured that the results would be used only for the stated scientific research purposes. The patients knew that their responses would not be available to the dermatologist and would not influence the treatment they received. Approval by the institutional review board of the King Abdullah International Medical Research Center (KAIMRC) was obtained before conducting the study (RR011/103).
Data analysis
SPSS software (version 17.0, SPSS Inc, Chicago, IL) was used for data analysis. The χ2 test was used to compare the categorical data. The Student’s t-test, analysis of variance, Mann-Whitney U test, and Kruskal-Wallis test were used to compare the numerical data. Multiple regression analyses were used to determine the significant predictors of the QoL scores for each domain and for the overall score. For all of the statistical analyses, a P ≤ 0.05 was considered to be statistically significant.
Results
Patient characteristics
A total of 283 patients with skin diseases were interviewed during the study period, representing a response rate of 92.2%. The patients were mostly of urban origin (91.2%), with approximately two thirds being females (63.3%), and one half being married (53.4%). One third were younger than 25 years (33.9%), and 20.1% were aged 40 years or more. The majority had completed their secondary education (79.5%), and the monthly family income was more than 5000 Saudi riyals for approximately two-thirds (68.6%) of the participants (Table 1). More males than females had completed their secondary education (P = 0.025) and were married (P < 0.001).
Table 1.
Patient, disease, and treatment characteristics for 283 patients with skin diseases
| Female | Male | Total | P value | |
|---|---|---|---|---|
|
|
||||
| n (%) | n (%) | n (%) | ||
| Patient characteristics | ||||
| Age, years# | ||||
| 15–24 | 69 (40.4) | 27 (29.7) | 96 (36.6) | P = 0.14 |
| 25–39 | 64 (37.4) | 45 (49.5) | 109 (41.6) | |
| 40–59 | 38 (22.2) | 19 (20.9) | 57 (21.8) | |
| Origin | ||||
| Urban | 165 (92.2) | 93 (89.4) | 258 (91.2) | P = 0.43 |
| Rural | 14 (7.8) | 11 (10.6) | 25 (8.8) | |
| Marital status | ||||
| Single | 78 (43.6) | 36 (34.6) | 114 (40.3) | P < 0.001 |
| Married | 85 (47.5) | 66 (63.5) | 151 (53.4) | |
| Divorced/widow | 16 (8.9) | 2 (1.9) | 18 (6.4) | |
| Education level | ||||
| Less than secondary | 44 (24.6) | 14 (13.5) | 58 (20.5) | P = 0.025 |
| Secondary or more | 135 (75.4) | 90 (86.5) | 225 (79.5) | |
| Monthly income | ||||
| <3000 SR | 15 (8.4) | 6 (5.8) | 21 (7.4) | P = 0.31 |
| 3000–5000 SR | 47 (26.3) | 21 (20.2) | 68 (24.0) | |
| >5000 SR | 117 (65.4) | 77 (74.0) | 194 (68.6) | |
| Disease characteristics | ||||
| Duration of disease | ||||
| <1 week | 3 (1.7) | 2 (1.9) | 5 (1.8) | P = 0.35 |
| 1–4 weeks | 13 (7.3) | 6 (5.8) | 19 (6.7) | |
| 1–6 months | 27 (15.1) | 12 (11.5) | 39 (13.8) | |
| >6 months | 136 (76.0) | 84 (80.8) | 220 (77.7) | |
| Family history of same lesion | ||||
| No | 130 (72.6) | 73 (70.2) | 203 (71.7) | P = 0.66 |
| Yes | 49 (27.4) | 31 (29.8) | 80 (28.3) | |
| Spread of skin lesion | ||||
| Generalized | 77 (43.0) | 47 (45.2) | 124 (43.8) | P = 0.72 |
| Localized | 102 (57.0) | 57 (54.8) | 159 (56.2) | |
| Presence of comorbidity | ||||
| Yes | 58 (32.4) | 31 (29.8) | 89 (31.4) | P = 0.65 |
| No | 121 (67.6) | 73 (70.2) | 194 (68.6) | |
| Treatment characteristics | ||||
| Using steroids | 33 (25.8) | 22 (27.2) | 55 (19.5) | P = 0.58 |
| Using isotretinoin | 25 (19.5) | 10 (12.3) | 35 (12.5) | P = 0.29 |
| Using other medications | 70 (54.7) | 49 (60.5) | 119 (41.9) | |
| Topical* | 74 (46.8) | 54 (50.0) | 128 (64.3) | |
| Tablets* | 54 (34.2) | 30 (27.8) | 84 (42.2) | |
| Psoralen + ultraviolet A* | 14 (8.9) | 11 (10.2) | 25 (12.6) | |
| Injection* | 2 (1.3) | 4 (3.7) | 6 (3.0) | |
| Others (eg, laser, surgery)* | 14 (8.9) | 9 (8.3) | 23 (11.6) | |
Notes: Duration was categorized into “>6 months” and “other” during the statistical analysis.
Exact age was unknown for 21 patients.
not mutually exclusive.
Abbreviation: SR, Saudi riyals.
Disease and treatment characteristics
Sebaceous and apocrine gland disorders were the most common diagnoses (24.4%), followed by pigmentary disorders (19.4%), eczematous dermatitis (14.5%), cutaneous infections (13.8%), and papulosquamous disorders (eg, psoriasis, 11.0%). Disorders of the hair follicles and connective tissue and immunological disorders were the least common disorders (6.4% and 5.3%, respectively). The head and face was most common lesion site (66.1%), followed by the upper and lower limbs (34.7% and 31.8%), back (32.9%), lower limbs (31.8%), chest or abdomen (31.4%), hands and feet (28.5% and 28.7%), and genitalia (13.4%). In the majority of patients, the condition was chronic (81.6%) and had lasted longer than 6 months (77.7%). Generalized lesions were seen in 43.8% of the patients, and a positive family history of skin disease was present in 28.3% of them.
The majority of patients were taking medication (73.9%). Topical treatments were the most common modality (64.3%), followed by tablets (42.2%), psoralen + ultraviolet A therapy (12.6%), and injections (3.0%). Other therapies, such as laser therapy and surgery, were used by 11.6% of the patients. Corticosteroid and isotretinoin medication were used by 19.4% and 12.4% of the patients, respectively.
Comorbidities were present in 31.4% of patients. Diabetes and hypertension were the most common disorders (43.8% and 47.2%, respectively), followed by heart disease (22.5%), thyroid disease (21.3%), psychiatric disorders (20.2%), and others (25.8%). No significant gender differences were observed for any of the disease or treatment characteristics.
Table 2 shows the responses of the study subjects on the Skindex-16 QoL scale. The overall QoL of the patients with skin disease was good, with a total mean percent score of 31.80 ± 20.16. The emotional domain was affected most (mean percentage score 44.27 ± 27.06), followed by the symptom domain (mean percentage score 31.45 ± 28.40). The functional domain was least affected (mean percentage score 14.61 ± 22.75).
Table 2.
Responses for 283 patients on three Skindex-16 domains*
| Domains | Never bothered (%) | Rarely bothered (%) | Sometimes (%) | Bothered most of the time (%) | Always bothered (%) |
|---|---|---|---|---|---|
| Symptom domain questions | |||||
| Itching | 37.8 | 18.4 | 14.1 | 12 | 17.7 |
| Burning or stinging | 56.5 | 12.7 | 12 | 9.2 | 9.5 |
| Pain | 64.3 | 13.1 | 8.1 | 7.8 | 6.7 |
| Skin irritation | 32.2 | 19.4 | 15.2 | 14.8 | 18.4 |
| Total mean percent score (±SD) | 31.45 ± 28.40 | ||||
| Emotional domain questions | |||||
| Persistence/recurrence | 19.1 | 12 | 23.3 | 21.6 | 24 |
| Worry (eg, spreading, getting worse, scarring, unpredictability) | 17.3 | 11.3 | 14.1 | 22.6 | 34.6 |
| Appearance | 18 | 14.1 | 24.4 | 18.7 | 24.7 |
| Frustration | 42.4 | 14.5 | 16.6 | 13.4 | 13.1 |
| Embarrassment | 37.8 | 10.6 | 18.4 | 14.5 | 18.7 |
| Being annoyed | 36.7 | 18.4 | 17.3 | 13.1 | 14.5 |
| Feeling depressed | 56.2 | 14.8 | 10.2 | 10.2 | 8.5 |
| Total mean % score (±SD) | 44.27 (±27.06) | ||||
| Functional domain questions | |||||
| Effects on interactions with others | 66.1 | 11.3 | 7.1 | 8.5 | 7.1 |
| Effects on the desire to be with people | 71.7 | 8.8 | 5.7 | 5.7 | 8.1 |
| Difficult to show affection | 78.8 | 6.4 | 6 | 3.2 | 5.7 |
| Effects on daily activities | 85.5 | 3.5 | 6 | 2.8 | 2.1 |
| Hard to work or do enjoyable activities | 74.2 | 8.5 | 6.4 | 4.6 | 6.4 |
| Total mean % score (±SD) | 14.61 (±22.75) | ||||
| Total score percentage (±SD) | 31.80 (±20.16) | ||||
Note:
Higher score reflects a greater impact on quality of life.
Abbreviation: SD, standard deviation.
The symptom that most commonly bothered the patients always or most of the time was irritation from the skin condition (33.2%), followed by itching (29.7%). Worry was the emotional problem that most commonly bothered the patients always or most of the time (57.2%), followed by recurrence (45%) and embarrassment (33%). The effect on interactions with others was the functional problem that most commonly bothered the patients always or most of the time (15.6%).
Table 3 shows the QoL scores on the Skindex-16 and its three domains. The majority of the patients reported good QoL (69.2%), with no significant gender differences (χ2 0.153, P = 0.926). The highest mean score occurred in the functional domain (87.3%), followed by the symptom domain (72.4%) and the emotional domain (57.6%), with no statistically significant gender differences in any of these domains (P > 0.05).
Table 3.
Quality of life levels and scores in different Skindex-16 domains for 283 patients by gender*
| Quality of life domains | Poor (<50%) | Moderate (50%–75%) | Good (>75%) | % mean score | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| No | % | No | % | No | % | ||
| Symptoms domain | |||||||
| Male | 10 | 9.6 | 19 | 18.3 | 75 | 72.1 | 32.21 (±28.14) |
| Female | 19 | 10.6 | 30 | 16.8 | 130 | 72.6 | 31.1 (±28.63) |
| Total | 29 | 10.2 | 49 | 17.3 | 205 | 72.4 | 31.45 (±28.40) |
| χ2 = 0.153, df 2, P = 0.926 | |||||||
| Emotion domain | |||||||
| Male | 15 | 14.4 | 49 | 27.4 | 61 | 58.7 | 40.73 (±28.79) |
| Female | 28 | 15.6 | 28 | 26.9 | 102 | 57.0 | 46.33 (±25.87) |
| Total | 43 | 15.2 | 77 | 27.2 | 163 | 57.6 | 44.27 (±27.06) |
| χ2 = 0.101, df 2, P = 0.951 | |||||||
| Functioning domain | |||||||
| Male | 3 | 2.9 | 12 | 11.5 | 89 | 85.6 | 17.07 (±25.09) |
| Female | 2 | 1.1 | 19 | 10.6 | 158 | 88.3 | 13.18 (±21.22) |
| Total | 5 | 1.8 | 31 | 11.0 | 247 | 87.3 | 14.61 (±22.75) |
| χ2 = 1.269, df 2, P = 0.530 | |||||||
| Total Skindex scale | |||||||
| Male | 3 | 2.9 | 19 | 18.3 | 82 | 78.8 | 31.20 (±21.60) |
| Female | 7 | 3.9 | 30 | 16.8 | 142 | 79.3 | 32.14 (±19.32) |
| Total | 10 | 3.5 | 49 | 17.3 | 224 | 79.2 | 31.80 (±20.16) |
| χ2 = 0.284, df 2, P = 0.867 | |||||||
Notes:
Higher score reflects a greater impact on quality of life; χ2, Pearson Chi-squared test.
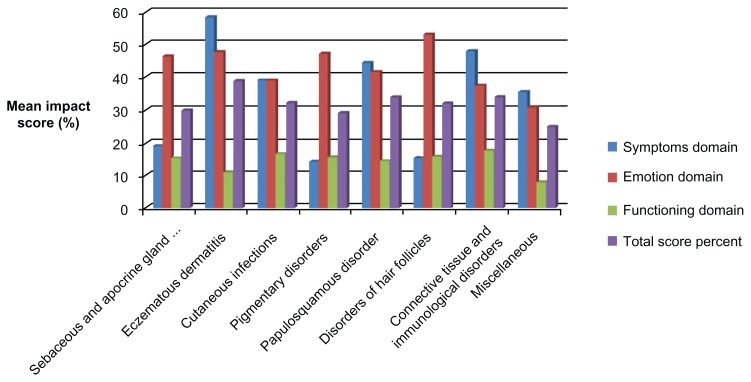
Figure 1 shows the distribution of the study sample by diagnosis and mean percent QoL score in the three domains of the Skindex-16 scale. Patients with eczematous dermatitis and those with connective tissue and immunological disorders had significantly higher symptom domain scores, which reflects a significantly greater physical burden (P < 0.001). There were no significant differences in the mean percentage scores for the emotional (P = 0.186) or functional domains (P = 0.877) by diagnosis.
Figure 1.
Mean percentage quality of life impact scores by diagnosis on three domains of the Skindex-16 scale.
Table 4 shows the statistical associations of the total mean scores on the Skindex scale and its domains with patient disease and treatment characteristics. The symptom domain scores were significantly higher for patients who were older (t = 9.35, P < 0.001), of rural origin (t = 2.05, P = 0.041), not single (t = 11.53, P < 0.001), less educated (t = 2.03, P = 0.044), had acute disease onset (t = 2.36, P = 0.019), had their disease for 6 months or less (t = 2.87, P = 0.004), had comorbidities (t = 2.41, P = 0.017), or were taking isotretinoin (t = 5.61, P < 0.001). However, after adjusting for all of these variables, the following factors were significant predictors of poor QoL scores in this domain: old age (t = 2.95, P = 0.003), generalized spreading (t = 3.08), disease for less than 6 months in duration (t = 2.40, P = 0.017), and not taking isotretinoin therapy (t = 2.34, P = 0.02).
Table 4.
Association of mean (standard deviation) percent mean scores on the Skindex-16 scale and its domains, with patient and disease characteristics
| Symptom domain score % | Emotion domain score % | Functioning domain score % | Total Skindex score % | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| T value (P value) | T value (adjusted P value) | T value (P value) | T value (adjusted P value) | T value (P value) | T value (adjusted P value) | T value (P value) | T value (adjusted P value) | |
| Gender (male = 1) | 0.35 (NS) | −0.59 (NS) | 1.64 (NS) | −2.25 (0.03)* | 1.33 (NS) | 0.95 (NS) | 0.38 (NS) | −1.16 (NS) |
| Age | 9.35 (0.001)* | 2.95 (0.003)* | −2.99 (0.03)* | −0.58 (NS) | 2.23 (NS) | 0.05 (NS) | 0.99 (NS) | 0.64 (NS) |
| Origin (urban = 1) | −2.05 (0.04)* | −1.84 (NS) | 0.06 (NS) | −0.42 (NS) | −2.36 (0.02)* | −2.22 (0.03)* | 1.58 (NS) | −1.64 (NS) |
| Marital status (married = 1) | 11.53 (0.001)* | 1.14 (NS) | 1.65 (NS) | −0.27 (NS) | 0.41 (NS) | −0.22 (NS) | 0.45 (NS) | 0.13 (NS) |
| Education (less than secondary = 1) | 2.03 (0.04)* | −0.74 (NS) | −2.03 (0.04)* | −1.60 (NS) | 1.31 (NS) | −0.64 (NS) | 0.94 (NS) | −1.39 (NS) |
| Duration (≤6 months = 1) | 2.87 (0.004)* | 2.40 (0.02)* | 1.40 (NS) | −0.49 (NS) | 0.38 (NS) | −0.7 (NS) | 0.04 (NS) | 0.25 (NS) |
| Spread (generalized = 1) | 2.78 (0.006)* | 3.08 (0.002)* | 2.73 (0.007)* | 2.40 (0.02)* | 1.31 (NS) | 1.27 (NS) | 3.09 (0.002)* | 2.85 (0.005)* |
| Family history (positive = 1) | 1.00 (NS) | 0.79 (NS) | 3.40 (0.001)* | 2.88 (0.01)* | 0.70 (NS) | 0.74 (NS) | 1.86 (NS) | 2.19 (NS) |
| Comorbidity (yes = 1) | 2.41 (0.017)* | 0.06 (NS) | 1.34 (NS) | −0.76 (NS) | −2.07 (0.04)* | −1.58 (NS) | 0.59 (NS) | −0.99 (NS) |
| Use of medications (yes = 1) | 0.31 (NS) | 1.42 (NS) | 1.35 (NS) | 1.26 (NS) | 1.08 (NS) | 1.02 (NS) | 1.28 (NS) | 1.56 (NS) |
| Psoralen + ultraviolet A therapy (yes = 1) | 1.61 (NS) | −1.95 (0.05)* | 0.69 (NS) | 0.13 (NS) | 1.15 (NS) | 0.86 (NS) | 0.25 (NS) | −0.25 (NS) |
| Cortisone (yes = 1) | 2.75 (0.006)* | 1.61 (NS) | 0.04 (NS) | 0.17 (NS) | 0.52 (NS) | −0.53 (NS) | 0.75 (NS) | 0.43 (NS) |
| Isotretinoin (yes = 1) | 5.61 (0.001)* | −2.34 (0.02)* | 0.76 (NS) | −2.31 (0.02)* | 0.53 (NS) | −0.94 (NS) | 1.80 (NS) | −2.44 (0.02)* |
Note:
Statistically significant. negative sign denotes inverse association of the impact score with the independent variable.
Abbreviations: NS, not statistically significant; T, student’s t-test was applied.
The following factors were significant predictors of poor QoL scores in the emotional domain: being female (t = 2.25, P = 0.025), having generalized spreading (t = 2.40, P = 0.017), having a positive family history of the same lesions (t = 2.88, P = 0.004), and not taking isotretinoin (2.31, P = 0.022). The only significant predictor of poor QoL on the functional domain was being from a rural area (t = 2.22, P = 0.027). The following factors were significant predictors of poor overall QoL on the Skindex scale: having generalized spread (t = 2.85, P = 0.005) and not taking isotretinoin therapy (t = 2.44, P = 0.016).
Discussion
Patients with skin diseases experience a wide variety of symptoms which affect their lives, ranging from trivial problems to major handicaps.20 Although studies aimed at estimating the extent of their symptoms have been conducted in advanced societies, the QoL for patients with skin disease in developing countries has continued to be a major problem because related issues have not been adequately addressed.21 In the present study, a reaction to health changes was clearly seen in all of the QoL domains. Some patients had good QoL, but others showed a poor QoL. This finding seems to suggest that people react differently to stressful experiences. However, the majority of patients reported good QoL (69.2%), with no significant gender differences. This result was generally more favorable than those seen in several previous studies that reported a significantly greater impact from skin disease.22 This discrepancy may be explained by biases in questionnaire responses or cultural differences in the skin disease experience and perception of disability. Further studies are needed to investigate this issue.
The skin is undoubtedly the most visible organ determining appearance, and plays a major role in social and sexual communication. Appearance is important in social situations, and it influences social perceptions.23 In the present study, being irritated by the skin condition was the most frequent symptom that bothered the patients always or most of time (33.2%), followed by itching (29.7%). In addition to causing physical discomfort and inconvenience, these symptoms have been found to influence the personal and social lives of patients, as well as their daily functioning and psychological status.5,20,24 In the present study, worry was the most common emotional problem that bothered the patients always or most of the time (57.2%), followed by recurrence (45%), and embarrassment (33%). The effect on interaction with others was the most common functional problem that bothered patients always or most of the time (15.6%). Therefore, it is important that treatment, nursing, and care for these patients consider the patients’ own QoL experiences.8
QoL measures in patients with skin lesions can supplement measures of clinical severity for comprehensively assessing disease and treatment outcomes, and they are an important area for future research. In the present study, patients with eczematous dermatitis, connective tissue diseases and immunological disorders scored significantly higher in the symptom domain, which reflects significantly greater burden. In previous studies, papulosquamous disorders, connective tissue disease, and immunological disorders were the more disabling conditions.13 However, poor adherence to therapy is correlated with poor QoL and is a barrier to successful treatment of skin diseases, such as acne24 and psoriasis.25,26
The disability suffered by female patients was significantly greater than that suffered by males. This finding is similar to the results of international studies, which have reported higher scores for females.27,28 This gender difference has also been reported in patients with atopic eczema,29–31 acne,32 and various other skin diseases.33 The increased QoL impact on females in this and other studies may be explained by the fact that women are often more emotional, sensitive, and conscious of their appearance in public; therefore, even slight dermatological manifestations may cause distress. This phenomenon may explain the significantly higher scores on the emotional domain seen for females in the present study. However, gender differences have not been consistent across all studies. A study conducted in the Qassim province of central Saudi Arabia15 found no significant gender difference in QoL for patients with vitiligo. This finding is similar to some international studies that did not find a major gender effect on QoL.34 These differences may be explained by differences in populations, sampling methods, study settings, and the spectrum of diseases studied.13
The relationship between age and impairment caused by skin disease was also investigated. In many previous studies, no significant association was found.13,20 Other studies have reported significantly higher QoL impairment in younger patients. However, the present study found significantly higher scores in the symptom domain with increasing age and significantly higher scores in the emotional domain with decreasing age. However, after adjusting for other confounding variables, significantly higher scores with a significantly higher burden were evident in the older patients. This finding agrees with the findings of a previous study35 in which older adults with acne vulgaris reported significantly greater overall QoL effects than did younger patients. This finding is interesting because of the prevailing perception of younger patients being more susceptible to the psychological effects of skin lesions.
The patients in the present study with lower education levels scored better in the emotional domain, and this finding agrees with other previous studies.14,16 This finding may be due to greater awareness of the problem and having more knowledge about the disease, leading to greater psychological impact from the disease.16 The patients in our study with lower educational levels had worst scores in the symptom domain; however, after adjusting for potential confounders, no significant association was found between educational level and QoL scores. A previous study of Saudi subjects showed that patients with higher educational levels had the lowest scores on the social relationship dimension, which is probably due to this dimension representing the greatest level of interaction with others outside the nuclear family.14
A lack of social support is an important predictor of poor physical and psychological functioning.33,36–39 This fact explains the better QoL findings in patients of rural origin due to better family and social support.16 In the present study, patients of rural origin had significantly higher scores on the symptom and functional QoL domains. However, after adjusting for potential confounders, this significant association between rural origin and poor QoL was evident only in the functional domain, which may reflect the uncertain access to medical care experienced by patients with rural origins.
Married subjects experience less severe impact of QoL than do the single and divorced subjects.14 This finding may be explained by the more secure and stable life enjoyed by married subjects.21 In the present study, married and divorced patients showed significantly greater effects in the symptom domain than did the single patients, reflecting greater suffering. The male patients were more likely to be married, and the female patients were more likely to be divorced. A married woman who develops vitiligo, for example, may experience marital problems that could lead to a divorce.9 However, after adjusting for potential confounders, the association between marital status and QoL was not significant.
Studies of disease duration and QoL have yielded inconsistent results in adult patients,40,41 with some studies reporting that a longer disease duration was a predictor of impaired QoL41 and others concluding the opposite.40 It has been reported that a shorter duration of disease improves the bodily pain dimension and that the role of emotional and mental health dimensions is greater in patients with a disease duration of less than one year.16 In the present study, patients who had skin lesions for 6 months or less scored significantly higher on the symptom domain than did patients who suffered for more than 6 months, even after adjusting for all potential confounders. A prolonged disease duration may enhance compliance and coping skills.42
It has been suggested that the location of skin lesions is a significant factor that leads to QoL impairment, possibly because of its effects on identity development.10 In the present study, 56% of the patients experienced localized skin lesions, most of which were on the hands and face. Adult studies have usually reported that dermatological diseases on visible areas of the body caused more impairment in QoL, especially in women.43–45 However, women may effectively hide the lesions with cosmetics so that facial involvement may have no particular effect on their QoL.10,46 In the present study, the patients with generalized spreading to the extremities, face, and neck had significantly lower QoL in the symptom, emotional, and overall domains than did the patients with localized lesions, both before and after adjusting for all of the potential confounders; this finding is consistent with other studies.14
QoL measures are increasingly used as a tool to assess treatment effectiveness.47 Isotretinoin therapy is used to treat severe or recalcitrant acne. In the present study, patients treated with isotretinoin had significantly lower scores in all of the Skindex scale domains, denoting a significantly favorable impact on QoL. This finding agreed with many other studies that have reported significantly improved QoL in such patients48 and a poorer QoL in those patients receiving topical treatments rather than isotretinoin therapy. 48 However, the treated patient population may influence the reported effects of systemic treatment on QoL. The patients who receive therapy may be more motivated and optimistic, and thus more likely to report benefits and endure more drug-induced toxicities than those not receiving treatment.12
The presence of comorbidities with acne had the greatest impact on all of the SF-36 subscales. Its effects were statistically significant in areas all except for the role emotional subscale.16 In the present study, comorbidities were noted in 31.4% of all patients, and their presence was significantly associated with higher scores on the symptom domain (reflecting a higher physical burden on patients) and a significantly lower functional domain score (probably due to managing these comorbidities in ways that might have diminished the functional impact of the skin lesion). However, these associations were not statistically significant after adjusting for potential confounders,.
One of the main limitations of our study was the small sample size for each subgroup of skin lesions, which limited the generalizability of our conclusions and the power to detect differences in important subgroups. Another limitation was that the samples were selected from patients attending the dermatology clinics of a tertiary care hospital, implying that they were probably evaluated during the chronic stage of their conditions; therefore, the results cannot be generalized. In addition, the study was a cross-sectional design for which the causal association between QoL and different characteristics could not be guaranteed. Future prospective trials may more clearly identify the impact of skin diseases on QoL. Finally, the Skindex scale is a generic instrument that may not be sensitive for detecting effects that are associated with specific diseases, and the combination of a generic and specific health-related QoL instrument would be most informative.49
Aside from these limitations, the results of our study suggest that the QoL of patients with skin diseases, as measured using the culturally adapted Skindex-16 to suit the needs of the Saudi population, is generally good. However, marked differences in QoL scores from other studies may be due to cultural differences in the experience of skin diseases and the perception of disability. Significant predictors of QoL are not the same for the three domains of Skindex scale. However, generally speaking, a poorer QoL was significantly associated with female gender, older age, rural origin, positive family history of the same lesion(s), a shorter duration of lesion (less than 6 months), generalized spread, and absence of retinoid treatment. Educational programs are recommended for patients who are more vulnerable to having poorer QoL to prevent further impairment, as well as further studies of QoL in Saudi patients for specific diseases using disease-specific instruments.
Acknowledgment
This study was initiated and funded by the King Abdullah International Medical Research Center (KAIMRC). We thank all of the members who contributed. We are particularly grateful to Ms Aisha Mahfouz and Mr Mahmoud Salam, the research coordinators at the KAIMRC, whose collaboration made the study possible. We also thank the editorial office at KAIMRC for English language editing of the manuscript via a specialist English language copy editor. This work was presented at the third research summer school program of the KAIMRC in July 2011.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Saw SM, Koh D, Adjani MR, et al. A population based prevalence survey of skin diseases in adolescents and adults in rural Sumatra, Indonesia, 1999. Trans R Soc Trop Med Hyg. 2001;95:384–388. doi: 10.1016/s0035-9203(01)90189-0. [DOI] [PubMed] [Google Scholar]
- 2.Finlay AY. Quality of life indices. Indian J Dermatol Venerol Leprol. 2004;70:143–148. [PubMed] [Google Scholar]
- 3.Rani Z, Khan MS, Aman S, Nadeem M, Hameed A, Kazmi AH. Quality of life issues and new benchmarks in the assessment of skin diseases. Journal of Pakistan Association of Dermatologists. 2005;15:339–344. [Google Scholar]
- 4.Beattie PE, Lewis-Jones MS. A comparative study of impairment of quality of life in children with skin diseases and children with other chronic diseases. Br J Dermatol. 2006;155:145–151. doi: 10.1111/j.1365-2133.2006.07185.x. [DOI] [PubMed] [Google Scholar]
- 5.Halioua B, Beumont MG, Lunel F. Quality of life in dermatology. Int J Dermatol. 2000;39:801–806. doi: 10.1046/j.1365-4362.2000.00793.x. [DOI] [PubMed] [Google Scholar]
- 6.Nijsten T, Meads DM, De Korte J, et al. Cross-cultural inequivalence of dermatology-specific health-related quality of life instruments in psoriasis patients. J Invest Dermatol. 2007;127:2315–2322. doi: 10.1038/sj.jid.5700875. [DOI] [PubMed] [Google Scholar]
- 7.Dunn LK, O’Neill JL, Feldman SR. Acne in adolescents: quality of life, self-esteem, mood, and psychological disorders. Dermatol Online J. 2011;17:1. [PubMed] [Google Scholar]
- 8.Wahl AK, Mørk C, Lillehol BM, et al. Changes in quality of life in persons with eczema and psoriasis after treatment in departments of dermatology. Acta Derm Venereol. 2006;86:198– 201. doi: 10.2340/00015555-0062. [DOI] [PubMed] [Google Scholar]
- 9.Parsad D, Dogra S, Kanwar A. Quality of life in patients with vitiligo. Health Qual Life Outcomes. 2003;1:58. doi: 10.1186/1477-7525-1-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bilgiç O, Bilgiç A, Akis H, Eskioglut F, Kilict EZ. Depression, anxiety and health-related quality of life in children and adolescents with vitiligo. Clin Exp Dermatol. 2011;36:360–365. doi: 10.1111/j.1365-2230.2010.03965.x. [DOI] [PubMed] [Google Scholar]
- 11.Ongenae K, Beelaert L, Van Geel N, Naeyaert JM. Psychosocial effects of vitiligo. J Eur Acad Dermatol Venereol. 2006;20:1–8. doi: 10.1111/j.1468-3083.2005.01369.x. [DOI] [PubMed] [Google Scholar]
- 12.Cornish D, Holterhues C, Van de Poll-Franse LV, Coebergh JW, Nijsten T. A systematic review of health-related quality of life in cutaneous melanoma. Ann Oncol. 2009;20(Suppl 6):vi51–vi58. doi: 10.1093/annonc/mdp255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Hoqail I. Impairment of quality of life among adults with skin disease in King Fahad Medical City, Saudi Arabia. J Family Community Med. 2009;16:105–109. [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Mubarak L, Al-Mohanna H, Al-Issa A, Jabak M, Mulekar SV. Quality of life in Saudi vitiligo patients. J Cutan Aesthet Surg. 2011;4:33–37. doi: 10.4103/0974-2077.79188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al Robaee AA. Assessment of quality of life in Saudi patients with vitiligo in a medical school in Qassim province, Saudi Arabia. Saudi Med J. 2007;28:1414–1417. [PubMed] [Google Scholar]
- 16.Al Robaee AA. Assessment of general health and quality of life in patients with acne using a validated generic questionnaire. Acta Dermatovenerol Alp Panonica Adriat. 2009;18:157–164. [PubMed] [Google Scholar]
- 17.Chren MM, Lasek RJ, Sahay AP, Sands L. Measurement properties of Skindex-16: a brief quality of life measure for patients with skin diseases. J Cutan Med Surg. 2001;5:105–110. doi: 10.1007/BF02737863. [DOI] [PubMed] [Google Scholar]
- 18.Alghamdi KM, Alshammari SA. Arabic version of Skindex-16: translation and cultural adaptation, with assessment of reliability and validity. Int J Dermatol. 2007;46:247–252. doi: 10.1111/j.1365-4632.2007.03013.x. [DOI] [PubMed] [Google Scholar]
- 19.Prinsen CA, Lindeboom R, Sprangers M, Legierse CM, De Korte J. Health-related quality of life assessment in dermatology: interpretation of Skindex-29 scores using patient-based anchors. J Invest Dermatol. 2010;130:1318–1322. doi: 10.1038/jid.2009.404. [DOI] [PubMed] [Google Scholar]
- 20.Harlow D, Poyner T, Finlay AY, Dykes PJ. Impaired quality of life of adults with skin disease in primary care. Br J Dermatol. 2000;143:979–982. doi: 10.1046/j.1365-2133.2000.03830.x. [DOI] [PubMed] [Google Scholar]
- 21.Abasiubong F, Akpan N, Ukpong DI, Umanah I, Udoh SB. Quality of life in patients with skin diseases in UYO, a community in south-south Nigeria. Adv Trop Med Pub Health Int. 2011;1:55–65. [Google Scholar]
- 22.Hahm BJ, Min SU, Yoon MY, et al. Changes of psychiatric parameters and their relationships by oral isotretinoin in acne patients. J Dermatol. 2009;36:255–261. doi: 10.1111/j.1346-8138.2009.00635.x. [DOI] [PubMed] [Google Scholar]
- 23.Porter J. The psychological effects of vitiligo: response to impaired appearance. In: Hann SK, Nordlund JJ, editors. Vitiligo: a Monograph on the Basic and Clinical Science. Oxford, UK: Blackwell Science; 2000. [Google Scholar]
- 24.Tan JK, Vasey K, Fung KY. Beliefs and perceptions of patients with acne. J Am Acad Dermatol. 2001;44:439–445. doi: 10.1067/mjd.2001.111340. [DOI] [PubMed] [Google Scholar]
- 25.Zaghloul SS, Goodfield MJ. Objective assessment of compliance with psoriasis treatment. Arch Dermatol. 2004;140:408–414. doi: 10.1001/archderm.140.4.408. [DOI] [PubMed] [Google Scholar]
- 26.Renzi C, Picardi A, Abeni D, et al. Association of dissatisfaction with care and psychiatric morbidity with poor treatment compliance. Arch Dermatol. 2002;138:337–342. doi: 10.1001/archderm.138.3.337. [DOI] [PubMed] [Google Scholar]
- 27.Zachariae R, Zachariae C, Ibsen HH, Mortensen JT, Wulf HC. Psychological symptoms and quality of life of dermatology outpatients and hospitalized dermatology patients. Acta Derm Venereol. 2004;84:205–212. doi: 10.1080/00015550410023284. [DOI] [PubMed] [Google Scholar]
- 28.Gelford J, Feldman S, Stern R, Thomas J, Rolsted T, Margolis D. Determinants of quality of life in patients with psoriasis: a study from US population. J Am Acad Dermatol. 2004;51:704–708. doi: 10.1016/j.jaad.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Holm EA, Wulf HC, Stegman H, Jemec GBE. Life quality assessment among patients with atopic eczema. Br J Dermatol. 2005;154:719–725. doi: 10.1111/j.1365-2133.2005.07050.x. [DOI] [PubMed] [Google Scholar]
- 30.Whalley D, McKenna SP, Dewar AL, et al. A new instrument for assessing quality of life in atopic dermatitis: international development of the Quality of Life Index for Atopic Dermatitis (QoLIAD) Br J Dermatol. 2004;150:274–283. doi: 10.1111/j.1365-2133.2004.05783.x. [DOI] [PubMed] [Google Scholar]
- 31.Holm EA, Esmann S, Jemec GB. Does visible atopic dermatitis affect quality of life more in women than in men? Gend Med. 2005;1:125–230. doi: 10.1016/s1550-8579(04)80017-2. [DOI] [PubMed] [Google Scholar]
- 32.Mulder MM, Sigurdsson V, van Zuuren EJ, et al. Psychosocial impact of acne vulgaris: evaluation of the relation between a change in clinical acne severity and psychosocial state. Dermatology. 2001;203:124–130. doi: 10.1159/000051726. [DOI] [PubMed] [Google Scholar]
- 33.Stangier U, Ehlers A, Gieler U. Measuring adjustment to chronic skin disorders: validation of a self-report measure. Psychol Assess. 2003;15:532–549. doi: 10.1037/1040-3590.15.4.532. [DOI] [PubMed] [Google Scholar]
- 34.Skoet R, Zachariae R, Agner T. Contact dermatitis and quality of life: a structured review of the literature. Br J Dermatol. 2003;149:452–456. doi: 10.1046/j.1365-2133.2003.05601.x. [DOI] [PubMed] [Google Scholar]
- 35.Farage M, Miller K, Sherman S, Tsevat J. Assessing quality of life in older adult patients with skin disorders. Glob J Health Sci. 2012;4:119–131. doi: 10.5539/gjhs.v4n2p119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trask PC, Paterson AG, Hayasaka S, Dunn RL, Riba M, Johnson T. Psychosocial characteristics of individuals with non-stage IV melanoma. J Clin Oncol. 2001;19:2844–2850. doi: 10.1200/JCO.2001.19.11.2844. [DOI] [PubMed] [Google Scholar]
- 37.Koo JYM, Lee CS, editors. Psychocutaneous Medicine. New York, NY: Marcel Dekker; 2003. [Google Scholar]
- 38.Lu Y, Duller P, van der Valk PGM, Evers AWM. Helplessness as predictor of stigmatization in patients with psoriasis and atopic dermatitis. Dermatol Psychosom. 2003;4:146–150. [Google Scholar]
- 39.Evers AW, Kraaimaat FW, van Lankveld W, Jongen PJ, Jacobs JW, Bijlsma JW. Beyond unfavorable thinking: the Illness Cognition Questionnaire for chronic diseases. J Consult Clin Psychol. 2001;69:1026–1036. [PubMed] [Google Scholar]
- 40.Kostopoulou P, Jouary T, Quintard B, et al. Objective vs subjective factors in the psychological impact of vitiligo: the experience from a French referral centre. Br J Dermatol. 2009;161:128–133. doi: 10.1111/j.1365-2133.2009.09077.x. [DOI] [PubMed] [Google Scholar]
- 41.Borimnejad L, Parsa Yekta Z, Nikbakht-Nasrabadi A, Firooz A. Quality of life with vitiligo: comparison of male and female Muslim patients in Iran. Gend Med. 2006;3:124–130. doi: 10.1016/s1550-8579(06)80201-9. [DOI] [PubMed] [Google Scholar]
- 42.Bilgic A, Bilgic Ö, Akış HK, Eskioğlu F, Kılıç EZ. Psychiatric symptoms and health related quality of life in children and adolescents with psoriasis. Pediatr Dermatol. 2010;27:614–617. doi: 10.1111/j.1525-1470.2010.01195.x. [DOI] [PubMed] [Google Scholar]
- 43.Ongenae K, Dierckxsens L, Brochez L, van Geel N, Naeyaert JM. Quality of life and stigmatization profile in a cohort of vitiligo patients and effect of the use of camouflage. Dermatology. 2005;210:279–285. doi: 10.1159/000084751. [DOI] [PubMed] [Google Scholar]
- 44.Radtke MA, Schäfer I, Gajur A, Langenbruch A, Augustin M. Willingness-to-pay and quality of life in patients with vitiligo. Br J Dermatol. 2009;161:134–139. doi: 10.1111/j.1365-2133.2009.09091.x. [DOI] [PubMed] [Google Scholar]
- 45.Picardi A, Abeni D, Renzi C, et al. Increased psychiatric morbidity in female outpatients with skin lesions on visible parts of the body. Acta Derm Venereol. 2001;81:410–414. doi: 10.1080/000155501317208345. [DOI] [PubMed] [Google Scholar]
- 46.Sampogna F, Raskovic D, Guerra L, et al. Identification of categories at risk for high quality of life impairment in patients with vitiligo. Br J Dermatol. 2008;159:351–359. doi: 10.1111/j.1365-2133.2008.08678.x. [DOI] [PubMed] [Google Scholar]
- 47.Tan SR, Solish N. Long-term efficacy and quality of life in the treatment of focal hyperhidrosis with botulinum toxinum A. Dermatol Surg. 2002;28:495–499. doi: 10.1046/j.1524-4725.2002.01159.x. [DOI] [PubMed] [Google Scholar]
- 48.Kaymak Y, Taner E, Taner Y. Comparison of depression, anxiety and life quality in acne vulgaris patients who were treated with either isotretinoin or topical agents. Int J Dermatol. 2009;48:41–46. doi: 10.1111/j.1365-4632.2009.03806.x. [DOI] [PubMed] [Google Scholar]
- 49.Both H, Essink-Bot ML, Busschbach J, Nijsten T. Critical review of generic and dermatology-specific health-related quality of life instruments. J Invest Dermatol. 2007;127:2726–2739. doi: 10.1038/sj.jid.5701142. [DOI] [PubMed] [Google Scholar]