Abstract
Rationale: The current hypothesis that human pulmonary alveolarization is complete by 3 years is contradicted by new evidence of alveolarization throughout adolescence in mammals.
Objectives: We reexamined the current hypothesis using helium-3 (3He) magnetic resonance (MR) to assess alveolar size noninvasively between 7 and 21 years, during which lung volume nearly quadruples. If new alveolarization does not occur, alveolar size should increase to the same extent.
Methods: Lung volumes were measured by spirometry and plethysmography in 109 healthy subjects aged 7–21 years. Using 3HeMR we determined two independent measures of peripheral airspace dimensions: apparent diffusion coefficient (ADC) of 3He at FRC (n = 109), and average diffusion distance of helium by q-space analysis (n = 46). We compared the change in these parameters with lung growth against a model of lung expansion with no new alveolarization.
Measurements and Main Results: ADC increased by 0.19% for every 1% increment in FRC (95% confidence interval [CI], 0.13–0.25), whereas the expected change in the absence of neoalveolarization is 0.41% (95% CI, 0.31–0.52). Similarly, increase of with FRC was significantly less than the predicted increase in the absence of neoalveolarization. The number of alveoli is estimated to increase 1.94-fold (95% CI, 1.64–2.30) across the age range studied.
Conclusions: Our observations are best explained by postulating that the lungs grow partly by neoalveolarization throughout childhood and adolescence. This has important implications: developing lungs have the potential to recover from early life insults and respond to emerging alveolar therapies. Conversely, drugs, diseases, or environmental exposures could adversely affect alveolarization throughout childhood.
Keywords: growth and development, lung development, alveolarization
At a Glance Commentary
Scientific Knowledge on the Subject
It is believed that human pulmonary alveolarization ceases in early childhood and that new alveoli do not form after about 2–3 years of life.
What This Study Adds to the Field
We provide direct evidence that supports ongoing alveolarization throughout childhood and adolescence in humans.
The process and timing of postnatal development of alveoli (alveolarization) in the human lung is poorly understood (1, 2) despite a number of studies in recent decades (3, 4). Apart from clarifying the prognosis of diseases of early childhood, accurate knowledge of alveolarization can contribute to understanding the factors affecting lung growth and development and lung structure–function relationships. Further, it can provide the basis for the development and evaluation of new treatment modalities designed to restore damaged alveolar function.
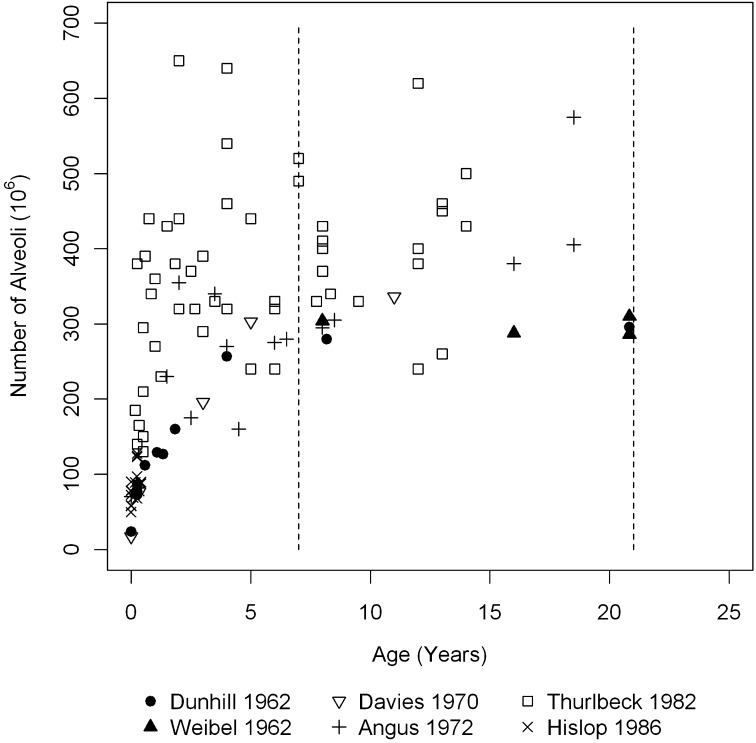
Currently, pulmonary alveoli are thought to stop multiplying by 2–3 years of age in humans (5, 6), although some have suggested 7–8 years as the limit (7, 8). This hypothesis is based on studies using older morphometric histologic techniques to analyze whole lungs from autopsies of children. However, there is a disparity between these studies both in the measured alveolar number and in the estimated age at which alveolarization is complete (Figure 1) (3, 4, 7–10). Repeating these studies with modern techniques of morphometry is impractical because of severe ethical constraints in access to adequate specimens from children (11). However, new evidence based on modern techniques has shown that alveolarization continues beyond early life and through maturity in some mammals (12–14). Post-pneumonectomy alveolarization can occur in mature animals (13). Although there is increasing acceptance of the possibility of late alveolarization in humans (1), there is no direct evidence for this.
Figure 1.
Number of alveoli in the developing human lung estimated by morphometry from previously published studies. Dotted lines (7 yr and 21 yr) represent the age range reported in the present study.
In vivo measurement methods are currently the only realistic options to study human alveolarization. One such novel method depends on the measurement of self-diffusion of hyperpolarized helium-3 (3He) in the lung periphery during a brief breath-hold using magnetic resonance (MR). A parameter known as apparent diffusion coefficient (ADC) can be determined, which is related to the dimensions of the enclosing structure, in this case the lung peripheral airspaces including alveolar ducts and alveoli (15, 16). Recently, it has also become possible to determine the average linear dimensions of the peripheral airspaces and alveoli by 3HeMR (17).
Using 3HeMR, we tested the hypothesis that human alveolarization stops by 3 years of age. Because human lung volume increases three- to fourfold between 7 years of age and adulthood (18), the volume of individual alveoli should increase to the same extent over this period of growth if no more alveoli form. 3HeMR can reliably detect such an increase in alveolar size. Some of the results of these studies have been previously reported in the form of an abstract (19).
Methods
Subjects
Children and young adults (7–21 yr of age) were recruited from the Leicester respiratory cohorts (20) and a community database. Of the 173 who attended, we excluded 53 born before 36 weeks of gestation, admitted to a neonatal unit or with significant respiratory illnesses, and 11 because data did not meet validity criteria, leaving 109 subjects. The study had Research Ethics Committee approval. Written informed consent was obtained from all subjects and, for minors less than 18 years, from their parents or legal guardians.
Physiologic Measurements
We measured height, weight, and at least three technically acceptable recordings of spirometry and plethysmography (Jaeger Masterscreen Body, Wuerzburg, Germany) and recorded the highest values of FEV1 and FVC and the mean value of FRC (21, 22). Z-scores for height and weight (23) and spirometry and plethysmography (18, 24) were based on United Kingdom reference values.
Hyperpolarized 3HeMR
3HeMR was undertaken in a 0.15-T permanent magnet system (Intermagnetics General Corp., New York, NY), where the subjects lie supine and breath-hold after inhalation of 600 ml of a mixture of 3He in 4He from FRC. We used two techniques of MR.
First, we obtained the global ADC using a modified rapid acquisition with refocused echoes MR sequence (25). At least three technically satisfactory ADC values were obtained in all subjects and the mean was taken as the raw ADC. Separate measurements done with constant bolus volume but different concentrations of helium were used to adjust the raw ADC for differences in 3He concentration: the concentration-corrected value is ADC0. We also conducted measurements with different bolus sizes in volunteers (bolus effect study) to determine the effect of lung inflation on ADC.
The second technique (q-space technique) (17) was used in 46 subjects. The mean displacement of 3He atoms is derived from the displacement probability profile of the 3He atoms obtained from this technique. This gives a direct measure of mean linear dimensions of peripheral airspaces. We also applied the Yablonskiy acinar model (26) to the q-space data and obtained values for mean alveolar duct diameter, R (including alveolar sleeve), and mean alveolar sleeve depth, h. The effect of helium concentration and bolus size on these parameters was estimated.
Statistical Analysis
We fitted a multilevel linear regression model of log-transformed ADC0 against log-transformed FRC and relative inflation volume combining the data from single measurements from the main study with measurements at varying bolus volumes from the bolus effect study. The first level of the model represents subjects and the second level different measurements on a given subject. The null hypothesis of no neoalveolarization is satisfied if the regression slopes of ADC against FRC and inflation volume are equal. We tested this using the Wald test.
The model was also used to estimate the fully concentration- and volume-corrected ADC: ADCcorr. We assessed associations of log-transformed ADCcorr with age, height, weight, and log FRC separately using linear regression models with and without adjusting for confounding factors. All tests were two-sided with a significance threshold of 0.05. Similarly, we tested for trends in mean peripheral airspace dimension with height, weight, age, and FRC. For this outcome, we did not adjust for potential confounding because of limited sample size. Data were analyzed using Stata Version 11 (StataCorp, College Station, TX). Details of methods are provided in the online supplement.
Results
Demographics and Potential Confounders
The study population consisted of 109 subjects (50 males) aged 7–21 years (median, 12.8 yr): 79 (72.4%) whites; 22 (20.2%) of south Asian origin; and 8 (7.3%) of other or mixed ethnicity. Forty six participants (42.2%) had current or previous passive exposure to tobacco smoke (before birth or during childhood). Seven (6.4%) had tried cigarette smoking but none were current smokers. Previous inhaled corticosteroid treatment was recorded in 18 (16.5%).
Physiologic and Lung Function Measurements
All subjects were appropriately grown (Table 1) and measurements of FEV1, FVC, and FRC (Table 1) were approximately normally distributed. The range of the measured parameters was as expected given the wide age range. Linear regression of FRC on age gave a 3.4-fold increase in mean estimated FRC from 7 (1.02 L) to 21 years (3.50 L).
TABLE 1.
MEAN AND DISTRIBUTION OF MEASUREMENTS INCLUDING LUNG FUNCTION TESTS AND HELIUM MAGNETIC RESONANCE DATA
| Measurement | N* | Mean (SD) | Range |
| Anthropometry | |||
| Height, cm | 109 | 154.7 (14.9) | 122.6 to 184.5 |
| Weight, kg | 109 | 49.7 (14.8) | 22.5 to 99.7 |
| Lung function | |||
| FEV1, L | 108 | 2.8 (0.89) | 1.34 to 5.16 |
| FVC, L | 108 | 3.2 (1.02) | 1.51 to 5.85 |
| FRC, L | 109 | 1.98 (0.7) | 1.02 to 3.95 |
| FEV1 z-score | 108 | 0.2 (0.94) | −2.5 to 2.9 |
| FVC z-score | 108 | 0.09 (1.00) | −2.4 to 2.6 |
| FRC z-score | 109 | −0.55 (0.95) | −3.2 to 1.9 |
| 3HeMR data | |||
| ADCcorr, cm2sec−1 | 109 | 0.094 (0.012) | 0.070 to 0.139 |
| , mm | 45† | 0.418 (0.039) | 0.354 to 0.515 |
| Alveolar duct radius including alveolar sleeve (R)‡, mm | 46† | 0.426 (0.040) | 0.318 to 0.503 |
| Alveolar sleeve height (h)‡, mm | 46† | 0.244 (0.043) | 0.164 to 0.345 |
Definition of abbreviations: ADC = apparent diffusion coefficient of 3He; = mean peripheral airspace diameter.
Number of technically acceptable observations.
Measurements were done only in a subset of the volunteers.
Alveolar duct radius (R) and alveolar sleeve height (h) derived from application of the Yablonskiy model of alveolar duct (26).
Hyperpolarized 3HeMR
Within-subject values of ADC were internally consistent (mean coefficient of variation, 3.1%). The concentration-corrected ADC (ADC0) increased by 0.19% (95% confidence interval [CI], 0.13–0.25) for every 1% increment in FRC in the study population (slope α) but by 0.41% (95% CI, 0.31–0.52) for every 1% inflation in the bolus-effect study (slope β) (P value for difference between α and β < 0.001). The change of ADC0 with FRC remained less than in the predicted scenario of “no alveolarization” after adjusting for confounders (P = 0.02). From α and β, the rate of neoalveolarization was estimated as 0.54 (95% CI, 0.41–0.68). Based on these results, we estimated that the observed 3.4-fold increase in FRC between 7 and 21 years of age was accompanied by a 1.94-fold (95% CI, 1.64–2.30) increase in alveolar number and a 1.75-fold (95% CI, 1.48–2.07) increase in alveolar volume (see online supplement for derivation).
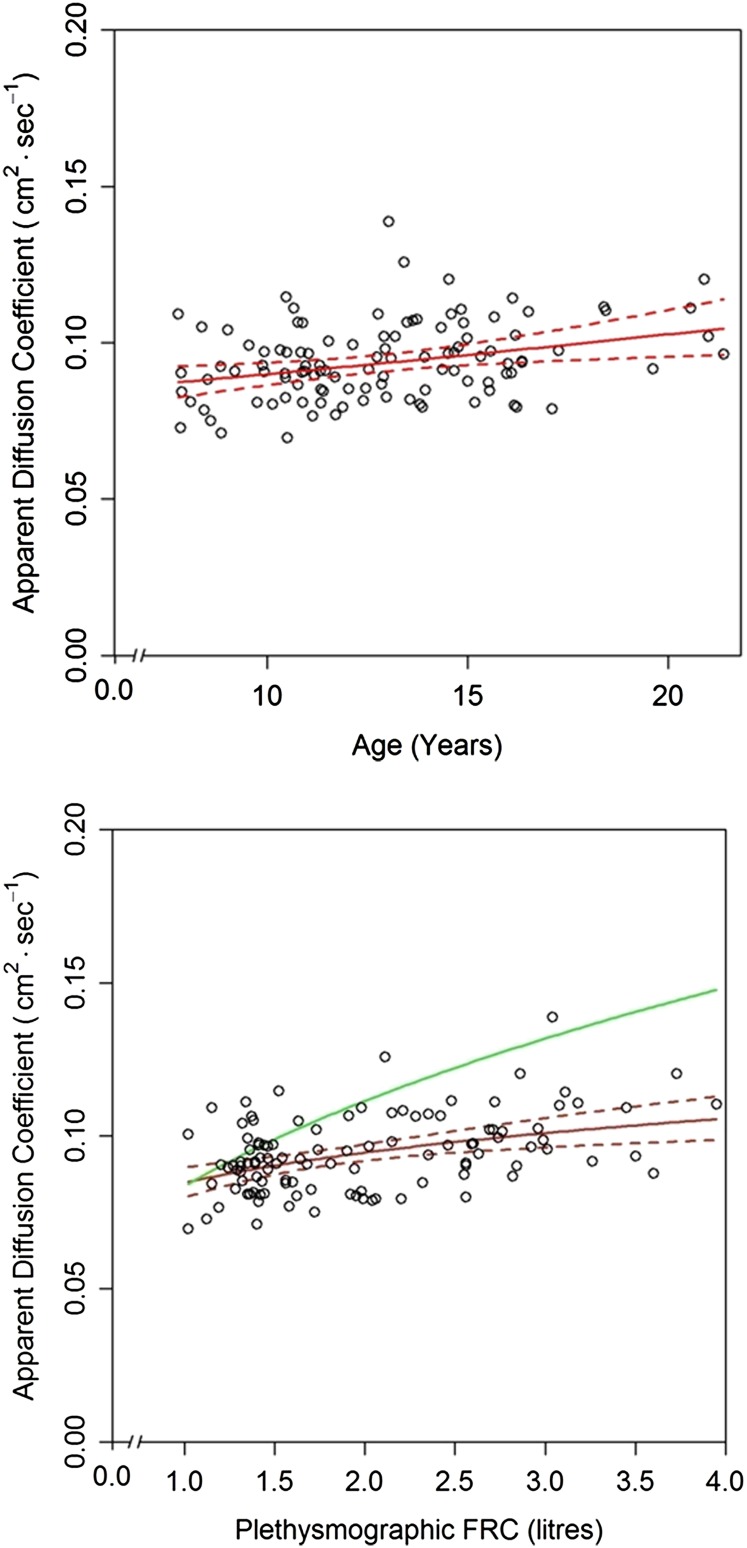
The slope β was used to calculate ADCcorr, the fully concentration- and volume-corrected ADC. Mean (SD) ADCcorr by 3HeMR was 0.094 (0.012) cm2s−1, and was similar in males and females (P = 0.90). ADCcorr increased significantly with most measures of growth including age (slope of log[ADCcorr] vs. age = 0.013; 95% CI, 0.006–0.021; r2 = 0.10; P < 0.001) (Figure 2, top panel); FRC (slope of log[ADCcorr] vs. log[FRC] = 0.16; 95% CI, 0.09–0.23; r2 = 0.18; P < 0.001) (Figure 2, bottom panel); and height (slope of log[ADCcorr] vs. height = 0.0031; 95% CI, 0.0016–0.0047; r2 = 0.13; P < 0.001). Adjustment for potential confounders had negligible effects on the measured relationships between ADC with age or FRC.
Figure 2.
Scatterplot of apparent diffusion coefficient (ADC)0 against (top panel) age and (bottom panel) resting lung volume (FRC). Values are weighted mean ADC from three measurements in each individual child and corrected to zero helium (He) concentration (ADC0) and FRC. The solid red lines indicate back-transformed linear fits of log(ADC) on (top panel) age and on (bottom panel) log(FRC) and the areas between the dotted red lines are 95% simultaneous confidence bands (Scheffe method). The green curve in the bottom panel shows the estimated change in ADC for a child with an initial FRC of 1 L assuming that the growth of the lung occurs only by proportional enlargement of preexisting alveoli without new alveolarization. This curve was estimated from the multilevel model (see Methods).
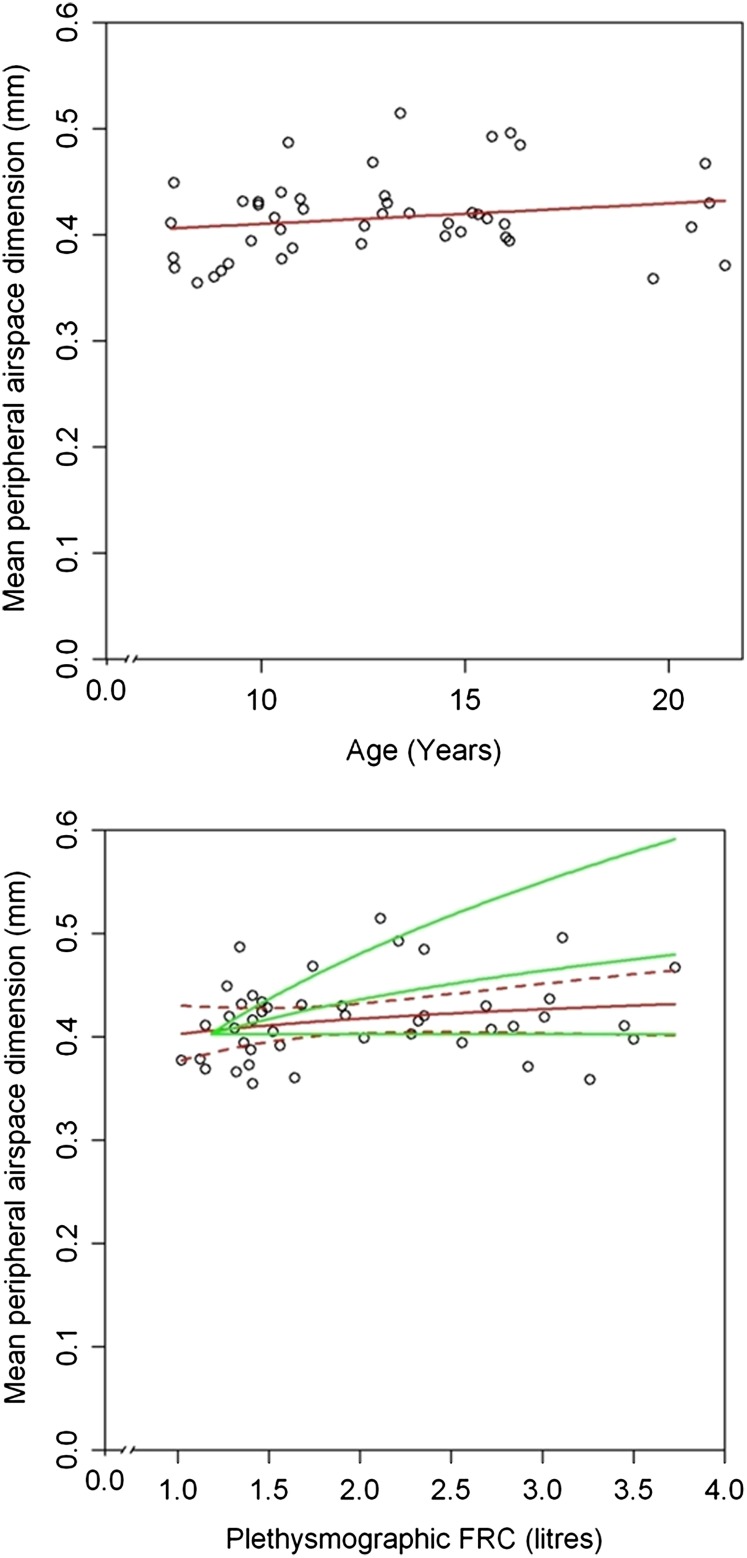
The mean peripheral airspace dimension ; range and mean reported in Table 1) did not change with helium concentration. There was no significant increase with age (slope of log vs. age = 0.005; 95% CI, −0.003 to 0.012; r2 = 0.04; P = 0.2) (Figure 3, top panel) and with FRC (slope of log vs. log[FRC] = 0.053; 95% CI, −0.021 to 0.128; r2 = 0.05; P = 0.16) (Figure 3, bottom panel). Because measurements of with different bolus sizes changed as the cubic root of volume, in the absence of neoalveolarization the slope of log against log(FRC) should be 0.33. data are compatible with an even higher rate of alveolarization than estimated from ADC data (see online supplement and Figure 3, bottom panel). Similarly, both parameters derived from the Yablonskiy acinar model, R and h, changed significantly less with increasing FRC (slope of log[R] vs. log[FRC] = 0.105; 95% CI, −0.03 to 0.18; r2 = 0.159; P = 0.006) (slope of log[h] vs. log[FRC] = 0.16; 95% CI, −0.013 to 0.30; r2 = 0.09; P = 0.03) than expected from the scenario of no new alveolarization. These results did not alter after adjusting for potential confounders.
Figure 3.
Scatterplot of mean peripheral airspace dimension against (top panel) age and (bottom panel) FRC. The red lines indicate back transformed log–linear fits in the top panel and log–log fits in the bottom panel. Dotted red lines in the bottom panel indicate 95% confidence intervals. Green lines in the bottom panel indicate the following in a child with an initial FRC of 1 L: top line, predicted change in with FRC if lung growth was accomplished only by expansion of preexisting alveoli (scenario of no alveolarization); middle line, predicted change if rate of neoalveolarization was 0.54 (predicted from apparent diffusion coefficient vs. FRC multilevel model); lower line, predicted change if all growth of lung was by neoalveolarization. The data suggest that the rate of neoalveolarization may be even higher than predicted from the apparent diffusion coefficient versus FRC model (Figure 2, bottom panel).
Discussion
We have shown that dimensions of alveoli determined by 3HeMR increase with age and lung size during childhood and adolescence at a rate much less than would be expected if lung growth occurred only by expansion of the preexisting airspaces. This is best explained by postulating that lung grows largely by neoalveolarization through childhood and adolescence. This contradicts the prevailing hypothesis that alveolarization is restricted to fetal life and early childhood. If confirmed by future studies, our findings have important clinical implications for the prognosis of lung disease and for the impact of drugs and environmental exposures during childhood.
The reliability of the 3HeMR technique is critical to this interpretation. Our measurements were highly repeatable, with a within-subject coefficient of variation of ADC of 3.1%. The validity of ADC as a measure of pulmonary alveolar dimensions has been demonstrated by morphometry in animal models (27, 28) and in human lungs (29, 30). These studies demonstrate the use of ADC in noninvasively assessing peripheral airspace dimensions. Parameters derived from the second technique of MR (R and h) have also been validated against human lung morphometry (30).
We considered the possibility that our results could be explained by changes in geometry of lung acinus with growth rather than neoalveolarization. However, our q-space data, analyzed using the acinar model of Yablonskiy and coworkers (26), show that both alveolar sleeve diameter and alveolar duct diameter increased less than expected with lung growth. In addition, because ADC and the parameters derived from q-space MR are measured with diffusion times of 14 and 5 milliseconds, respectively, they measure different geometric aspects of the peripheral airspaces and changes in relative geometry should be reflected in different relationships of these parameters with growth. However, both increase with FRC at a rate considerably slower than expected in the absence of neoalveolarization. Therefore, it is unlikely that changes in geometry occur with growth.
In the only other report of ADC in childhood, Altes and coworkers (31) measured ADC in 29 healthy subjects ranging from 4–30 years. They also report an increase of ADC with age. However, they did not measure lung size by independent means and there was no attempt to determine an expected line for increase of ADC with age. Shanbhag and coworkers (17) measured in five children aged 6 years and in adults and found that was lower in children than adults. There was no assessment of lung volume in this study. The mean age of adult subjects was 49 years, when effects of alveolar enlargement caused by senescence (25, 32) may have begun. In support of this, reported for the five children was very close to the values in the two youngest adults (both 28 yr) in their study.
The belief that alveolarization in humans was complete by 3 years was based on studies using older methods of morphometry, the drawbacks of which have been discussed previously (33). New methods of measuring alveolar number and size have since been developed (34, 35). However, human alveolarization has not yet been studied using these new techniques, largely because of ethical constraints that preclude the acquisition of suitable postmortem lung tissue.
Recent studies in mammals using new morphometric techniques support continued alveolarization to adulthood in rabbits (14), rhesus monkeys (12), and after pneumonectomy in mature dogs (13). Massaro and coworkers (36) showed evidence of calorie intake-related alveolar gain in adult mice refed after starvation. Schittny and coworkers (37) observed an increase in alveolar number after completion of microvascular maturation in rats using design-based stereology. Using three-dimensional synchrotron radiation X-ray tomography, they showed local duplication of single capillary layers in areas of postmaturity septal growth, which indicated a potential mechanism for postmature alveolarization. This dispelled the notion that the immature double capillary layer in alveolar walls is a prerequisite for new septation. Thus, there is emerging evidence of alveolarization in maturing lungs in other mammals. There is indirect evidence that this also happens in humans. Brown and coworkers (38) used electrical impedance tomography to determine an average alveolar number of 90 million at age 2–3 years compared with 300 million in adults, implying that alveolarization continues to take place after 3 years of age. Using a new unbiased morphometric technique, Ochs and coworkers (35) showed that alveolar number was closely related to adult lung volume and that mean alveolar size was almost constant between subjects. If alveolarization were completed by 2–3 years, the final number of alveoli, and by extrapolation the final size of the lung, would have to be set by then, which is implausible. This provides more indirect evidence in favor of human alveolarization beyond early childhood.
The strengths of the study include the large number of volunteers from a wide age range spanning most of the period of lung growth. Prospectively collected data on early life exposures and preexisting lung disease were available (20), which allowed selection of volunteers known to be healthy. Repeated MR measurements at varying inflation volumes were performed in some subjects, which facilitated the statistical test of the hypothesis of no new alveolarization. Independent measurement of lung size by plethysmography enabled the calculation of expected slope in the models. Finally, the application of two different techniques of MR permitted evaluation of lung geometry.
Studies of alveolar size and number in subjects at different ages, including our own, share the common assumption that the cross-sectional data are representative of longitudinal changes. We found wide interindividual variability in ADC, but our large number of volunteers and the repeatability of ADC measurements (within subjects) minimize the effect of this problem. Also, preliminary longitudinal data (not shown) corroborate the evidence from this study. Further longitudinal measurements of ADC will help refine the results of this study and begin to explain the wide population scatter of ADC values. The assumptions underpinning the multilevel regression model are transparent and the model fits the data well, suggesting that the assumptions are realistic (see online supplement).
There are important implications to our observation that alveolarization is not confined to early life. Children who die after extremely preterm birth have been shown to have larger, simpler, and fewer alveoli (39), based on histologic studies of fatal cases. There have been no studies of alveolarization in survivors of neonatal chronic lung disease and it is possible that there is catch-up alveolarization. There may be hope of recovery from diseases that result in diminished alveolar number at birth (e.g., pulmonary hypoplasia caused by severe oligohydramnios or congenital diaphragmatic hernia) or later in life (e.g., surgical lung resection). Adverse environmental exposures can affect alveolar structure. Systemic corticosteroids in early postnatal life can inhibit alveolarization (40, 41). If alveolarization is not confined to early life, it is a possible that inhaled corticosteroids may be deleterious throughout childhood. Passive tobacco smoke exposure in childhood is linked to adult chronic obstructive pulmonary disease (42). Impaired alveolarization may be a potential mechanism linking passive smoking during childhood to increased lung senescence and emphysema, and therefore chronic obstructive pulmonary disease. Finally, the advent of potential “alveolar therapy” to restore damaged alveolar structure (43) requires safe, noninvasive repeatable measurements to study the outcome of future therapeutic trials. Hyperpolarized 3HeMR provides such a method.
We conclude that there is evidence for continuing alveolarization through childhood and adolescence in humans. The exciting possibility of late alveolarization implies that the lung may be able to recover from damage occurring in early life. Emerging therapies have the potential to enhance this process. Conversely, environmental exposures during childhood could have a longer window of opportunity to impair the process of alveolarization, with potential adverse consequences throughout later life.
Supplementary Material
Acknowledgments
Cris Dogaru helped with data cleaning, compilation, and analysis. Jenny Phillips helped with performing the physiologic measurements and management of laboratory visits. Tony Davis performed the stratified random selection of subjects from the Leicester Specialist Community Child Health Services Database.
Footnotes
Supported by the Wellcome Trust, London, United Kingdom (grant number 081367/B/06/Z). M.M. was funded by Marie Curie Actions 7th Framework Program. B.D.S. was funded by the Swiss National Science Foundation (3200B0-122341) and Asthma UK (07/048).
Author Contributions: M.N. was responsible for the practical conduct of the research including planning and coordination, preliminary analyses, and interpretation of the data, and wrote the first draft of the manuscript. J.O.-B. had overall responsibility for the 3He MR measurements. C.S.B. had overall responsibility for the physiologic measurements. M.M. set up the protocols for hyperpolarized gas production and collection and for the 3He MR measurements using the rapid acquisition with refocused echoes sequence. I.B. and R.G. performed the 3He MR measurements and analyzed the 3He data. K.S.P. programmed data collection using the MR spectroscopy sequence and contributed to many of the measurements. C.E.K. and B.D.S. were responsible for the selection of subjects, the statistical analysis, and model-fitting. S.E.W. performed many of the physiologic measurements and had responsibility for much of the study administration. M.S., J.O.-B., C.S.B., and C.E.K. planned the study. All authors contributed to the different drafts of the manuscript and approved the final version. M.S. had the overall responsibility for the project and a major input into study design, analysis, and manuscript. M.S. acts as the guarantor.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201107-1348OC on October 27, 2011
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Burri PH. Structural aspects of postnatal lung development: alveolar formation and growth. Biol Neonate 2006;89:313–322 [DOI] [PubMed] [Google Scholar]
- 2.Merkus PJ, ten Have-Opbroek AA, Quanjer PH. Human lung growth: a review. Pediatr Pulmonol 1996;21:383–397 [DOI] [PubMed] [Google Scholar]
- 3.Thurlbeck WM. Postnatal human lung growth. Thorax 1982;37:564–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angus GE, Thurlbeck WM. Number of alveoli in the human lung. J Appl Physiol 1972;32:483–485 [DOI] [PubMed] [Google Scholar]
- 5.Zeltner TB, Burri PH. The postnatal development and growth of the human lung. II. Morphology. Respir Physiol 1987;67:269–282 [DOI] [PubMed] [Google Scholar]
- 6.Hislop AA. Airway and blood vessel interaction during lung development. J Anat 2002;201:325–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunnill MS. Postnatal growth of the lung. Thorax 1962;17:329–333 [Google Scholar]
- 8.Weibel ER, Gomez DM. A principle for counting tissue structures on random sections. J Appl Physiol 1962;17:343–348 [DOI] [PubMed] [Google Scholar]
- 9.Davies G, Reid L. Growth of the alveoli and pulmonary arteries in childhood. Thorax 1970;25:669–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hislop AA, Wigglesworth JS, Desai R. Alveolar development in the human fetus and infant. Early Hum Dev 1986;13:1–11 [DOI] [PubMed] [Google Scholar]
- 11.Hislop AA. Lung growth and computed tomography. Eur Respir J 2003;22:195–196 [DOI] [PubMed] [Google Scholar]
- 12.Hyde DM, Blozis SA, Avdalovic MV, Putney LF, Dettorre R, Quesenberry NJ, Singh P, Tyler NK. Alveoli increase in number but not size from birth to adulthood in rhesus monkeys. Am J Physiol Lung Cell Mol Physiol 2007;293:L570–L579 [DOI] [PubMed] [Google Scholar]
- 13.Hsia CC, Herazo LF, Fryder-Doffey F, Weibel ER. Compensatory lung growth occurs in adult dogs after right pneumonectomy. J Clin Invest 1994;94:405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovar J, Sly PD, Willet KE. Postnatal alveolar development of the rabbit. J Appl Physiol 2002;93:629–635 [DOI] [PubMed] [Google Scholar]
- 15.Mayo JR, Hayden ME. Hyperpolarized helium 3 diffusion imaging of the lung. Radiology 2002;222:8–11 [DOI] [PubMed] [Google Scholar]
- 16.Salerno M, de Lange EE, Altes TA, Truwit JD, Brookeman JR, Mugler JP., III Emphysema: hyperpolarized helium 3 diffusion MR imaging of the lungs compared with spirometric indexes–initial experience. Radiology 2002;222:252–260 [DOI] [PubMed] [Google Scholar]
- 17.Shanbhag DD, Altes TA, Miller GW, Mata JF, Knight-Scott J. q-Space analysis of lung morphometry in vivo with hyperpolarized 3He spectroscopy. J Magn Reson Imaging 2006;24:84–94 [DOI] [PubMed] [Google Scholar]
- 18.Rosenthal M, Cramer D, Bain SH, Denison D, Bush A, Warner JO. Lung function in white children aged 4 to 19 years. II. Single breath analysis and plethysmography. Thorax 1993;48:803–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narayanan M, Owers-Bradley J, Ball I, Mada M, Kuehni C, Spycher B, Garipov R, Silverman M, Beardsmore CS. Evidence for continuous alveolisation during childhood, using 3He magnetic resonance. Eur Respir J 2009;34(Suppl. 53):267s [Google Scholar]
- 20.Kuehni CE, Brooke AM, Strippoli MP, Spycher BD, Davis A, Silverman M. Cohort profile: the Leicester respiratory cohorts. Int J Epidemiol 2007;36:977–985 [DOI] [PubMed] [Google Scholar]
- 21.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. Standardisation of spirometry. Eur Respir J 2005;26:319–338 [DOI] [PubMed] [Google Scholar]
- 22.Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, van der Grinten CP, et al. Standardisation of the measurement of lung volumes. Eur Respir J 2005;26:511–522 [DOI] [PubMed] [Google Scholar]
- 23.Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med 1998;17:407–429 [PubMed] [Google Scholar]
- 24.Rosenthal M, Bain SH, Cramer D, Helms P, Denison D, Bush A, Warner JO. Lung function in white children aged 4 to 19 years. I. Spirometry. Thorax 1993;48:794–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waters B, Owers-Bradley J, Silverman M. Acinar structure in symptom-free adults by helium-3 magnetic resonance. Am J Respir Crit Care Med 2006;173:847–851 [DOI] [PubMed] [Google Scholar]
- 26.Yablonskiy DA, Sukstanskii AL, Leawoods JC, Gierada DS, Bretthorst GL, Lefrak SS, Cooper JD, Conradi MS. Quantitative in vivo assessment of lung microstructure at the alveolar level with hyperpolarized 3He diffusion MRI. Proc Natl Acad Sci USA 2002;99:3111–3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peces-Barba G, Ruiz-Cabello J, Cremillieux Y, Rodriguez I, Dupuich D, Callot V, Ortega M, Rubio Arbo ML, Cortijo M, Gonzalez-Mangado N. Helium-3 MRI diffusion coefficient: correlation to morphometry in a model of mild emphysema. Eur Respir J 2003;22:14–19 [DOI] [PubMed] [Google Scholar]
- 28.Chen XJ, Hedlund LW, Moller HE, Chawla MS, Maronpot RR, Johnson GA. Detection of emphysema in rat lungs by using magnetic resonance measurements of 3He diffusion. Proc Natl Acad Sci USA 2000;97:11478–11481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woods JC, Choong CK, Yablonskiy DA, Bentley J, Wong J, Pierce JA, Cooper JD, Macklem PT, Conradi MS, Hogg JC. Hyperpolarized 3He diffusion MRI and histology in pulmonary emphysema. Magn Reson Med 2006;56:1293–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yablonskiy DA, Sukstanskii AL, Woods JC, Gierada DS, Quirk JD, Hogg JC, Cooper JD, Conradi MS. Quantification of lung microstructure with hyperpolarized 3He diffusion MRI. J Appl Physiol 2009;107:1258–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Altes TA, Mata J, de Lange EE, Brookeman JR, Mugler JP., III Assessment of lung development using hyperpolarized helium-3 diffusion MR imaging. J Magn Reson Imaging 2006;24:1277–1283 [DOI] [PubMed] [Google Scholar]
- 32.Gillooly M, Lamb D. Airspace size in lungs of lifelong non-smokers: effect of age and sex. Thorax 1993;48:39–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weibel ER, Hsia CC, Ochs M. How much is there really? Why stereology is essential in lung morphometry. J Appl Physiol 2007;102:459–467 [DOI] [PubMed] [Google Scholar]
- 34.Hyde DM, Tyler NK, Putney LF, Singh P, Gundersen HJ. Total number and mean size of alveoli in mammalian lung estimated using fractionator sampling and unbiased estimates of the Euler characteristic of alveolar openings. Anat Rec A Discov Mol Cell Evol Biol 2004;277:216–226 [DOI] [PubMed] [Google Scholar]
- 35.Ochs M, Nyengaard JR, Jung A, Knudsen L, Voigt M, Wahlers T, Richter J, Gundersen HJ. The number of alveoli in the human lung. Am J Respir Crit Care Med 2004;169:120–124 [DOI] [PubMed] [Google Scholar]
- 36.Massaro D, Massaro GD, Baras A, Hoffman EP, Clerch LB. Calorie-related rapid onset of alveolar loss, regeneration, and changes in mouse lung gene expression. Am J Physiol Lung Cell Mol Physiol 2004;286:L896–L906 [DOI] [PubMed] [Google Scholar]
- 37.Schittny JC, Mund SI, Stampanoni M. Evidence and structural mechanism for late lung alveolarization. Am J Physiol Lung Cell Mol Physiol 2008;294:L246–L254 [DOI] [PubMed] [Google Scholar]
- 38.Brown BH, Primhak RA, Smallwood RH, Milnes P, Narracott AJ, Jackson MJ. Neonatal lungs: maturational changes in lung resistivity spectra. Med Biol Eng Comput 2002;40:506–511 [DOI] [PubMed] [Google Scholar]
- 39.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med 2007;357:1946–1955 [DOI] [PubMed] [Google Scholar]
- 40.Massaro D, Teich N, Maxwell S, Massaro GD, Whitney P. Postnatal development of alveoli. Regulation and evidence for a critical period in rats. J Clin Invest 1985;76:1297–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Massaro D, Massaro GD. Dexamethasone accelerates postnatal alveolar wall thinning and alters wall composition. Am J Physiol 1986;251:R218–R224 [DOI] [PubMed] [Google Scholar]
- 42.Svanes C, Omenaas E, Jarvis D, Chinn S, Gulsvik A, Burney P. Parental smoking in childhood and adult obstructive lung disease: results from the European Community Respiratory Health Survey. Thorax 2004;59:295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ravikumar P, Dane DM, McDonough P, Yilmaz C, Estrera AS, Hsia CCW. Long- term post-pneumonectomy pulmonary adaptation following all-trans-retinoic acid supplementation. J Appl Physiol 2011;110:764–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.