Abstract
Bidirectional transport of intracellular cargo along microtubule tracks is the subject of intense debate in the motility field. In the present review, we provide an overview of the models describing the possible mechanisms driving intracellular saltatory transport, taking into account current experimental results that may at first seem contradictory. We examine the phenomenon of saltatory motion, in an attempt to interpret the mechanistic debate in terms of the utility of saltatory motion.
Keywords: bidirectional intracellular transport, cargo transport, dynein, kinesin, microtubule cytoskeleton, myosin
Introduction
Molecular motors belonging to the kinesin, dynein and myosin families are responsible for transporting various intracellular cargoes along cytoskeletal filaments. These motors are exquisitely fine-tuned machines, utilizing the chemical energy from ATP hydrolysis to power conformational changes that drive motor stepping in a unidirectional manner. The thermodynamic efficiency (a measure of energy input to work output) is at least 40–60% for kinesin-1 [1], as compared with ~25% for a typical petrol (gasoline) automobile. However, an entirely different story is told when examining the motions of intracellular cargo by molecular motors. Rather than moving directly and with a constant velocity from point A to point B, organelles, proteins and nucleic acid complexes are typically transported by motors in a saltatory manner. This saltatory motion is characterized by a series of back-and-forth runs with numerous stops and starts. Given the efficiency of the motor proteins themselves, it seems counterintuitive that cellular cargo is transported in what appears to be a very inefficient way. In the present review, we address the possible functions and mechanisms of bidirectional intracellular cargo transport in the light of recent experimental and theoretical findings.
Does bidirectional intracellular transport serve a function?
What might be the utility of intracellular bidirectional motility (assuming that utility, if not logic, applies to biology)? There is some evidence to suggest at least four possible, not mutually exclusive, functions, which can be described as (i) search and capture, (ii) proofreading, (iii) manoeuvring around roadblocks, and (iv) facilitating interactions between cargoes.
Saltatory motility might be similar to the search-and-capture mechanism used by microtubules to find chromosome kinetochores. During cell division, spindle microtubules bind to sister chromatids at the kinetochores through a process of continual polymerization and depolymerization until the microtubule correctly navigates (perhaps in a facilitated manner) to a kinetochore (for reviews, see [2–5]). Perhaps bidirectional motility is similar to the search-and-capture method, and cargo is not directed towards its destination and randomly wanders the cell until it ‘hits’ the destination and is captured by its target. One of the most well-studied systems in which bidirectional motility has been documented is the melanocyte cell [6], a pigment-producing dendritic cell whose function is to deliver pigment to keratinocytes in hair and skin [7,8]. Melanosomes, the organelle responsible for the manufacture of pigment, were observed to move bidirectionally along microtubules in dendritic processes [6]. However, bidirectional microtubule-based motility in mouse melanocytes could not explain the peripheral distribution of melanosomes. It was shown that myosin Va is required for the melanosomes to accumulate in the tips of dendrites by capturing melanosomes when they encounter peripheral actin at the tips of dendrites [6]. Further evidence to suggest that target capture may play a role in regulating motility comes from Guillaud et al. [9],with the demonstration that CaMKII (Ca2+ /calmodulin-dependent protein kinase II) located near the synapse ‘catches’ the NMDA (N-methyl-d-aspartate) receptor by phosphorylating the kinesin KIF17 motor and causing NMDA to be released at the synapse [9].
The idea for proofreading as a function of bidirectional motility comes not from the motility field, but from other examples of saltatory motion in Nature. Polymerases might be expected to replicate or transcribe nucleic acids in a unidirectional manner to speed up the processes and maintain strand specificity. However, polymerases sometimes need to backtrack slightly as a part of the proofreading process, to remove the incorrect nucleotide and replace it with the correct one. RNA polymerases do just this, backstepping one position if an error occurs (reviewed in [10]). DNA polymerases, which have 3→5′ exonuclease activity, do not require backstepping to excise the incorrect nucleotide. Like RNA polymerases, the bidirectional motility observed in cargo transport may be necessary for proofreading (i.e. reaching the correct destination because the cargo is going astray).
Saltatory motion may simply be necessary to get through road blocks along crowded microtubule and actin tracks. Evidence for this model is supported by finding that cargoes navigate around actin–microtubule junctions [11,12] and use a combination of motor types to do so. Microtubule-associated proteins can act as road blocks by blocking all or some molecular motors from walking along microtubules [13,14]; for example, tau can selectively block kinesin transport without affecting dynein transport along microtubules [15]. In the context of a highly crowded cytoplasm, the ability to move around multiple barricades may be facilitated by opposite-polarity motors to navigate bidirectionally along microtubules, and side-stepping momentarily on to actin filaments with the use of myosin.
Cytoskeletal filaments themselves may partition cargo into discrete locations in order to increase the effective concentration and the likelihood of cargo interactions. There is some evidence that particular motor proteins may preferentially walk along certain subpopulations of microtubules defined by their post-translational modifications [16–22]. These cytoskeletal subdomains may cause a particular motor to bypass the majority of available filaments until it reaches its preferred track type. In this case, the cytoskeleton is the source of cellular compartmentalization allowing particular cargoes to interact. For example, recent data suggests that endoplasmic reticulum and mitochondria interact as a result of their association with a subpopulation of acetylated microtubules [22].
Mechanistic basis for bidirectional motility
If the motors themselves are largely unidirectional, how does the bidirectional motility phenomenon arise? Because of the counterintuitive nature of stochastic transport, the mechanism behind bidirectional motility has become arguably the hot topic in the transport field in the last 5–10 years. A handful of models have been offered as hypotheses in the literature, and each of these models can be supported by select experimental results. In our opinion, the greatest source of confusion in the field is that people compare different systems under the assumption that because the motor proteins themselves are highly conserved, the mechanics of bidirectional motility must also be universally conserved. This is not to say that there is evidence for every model. Recent experimental and theoretical work has clarified and allowed the maturation of each of these models for the underlying mechanisms driving stochastic bidirectional transport.
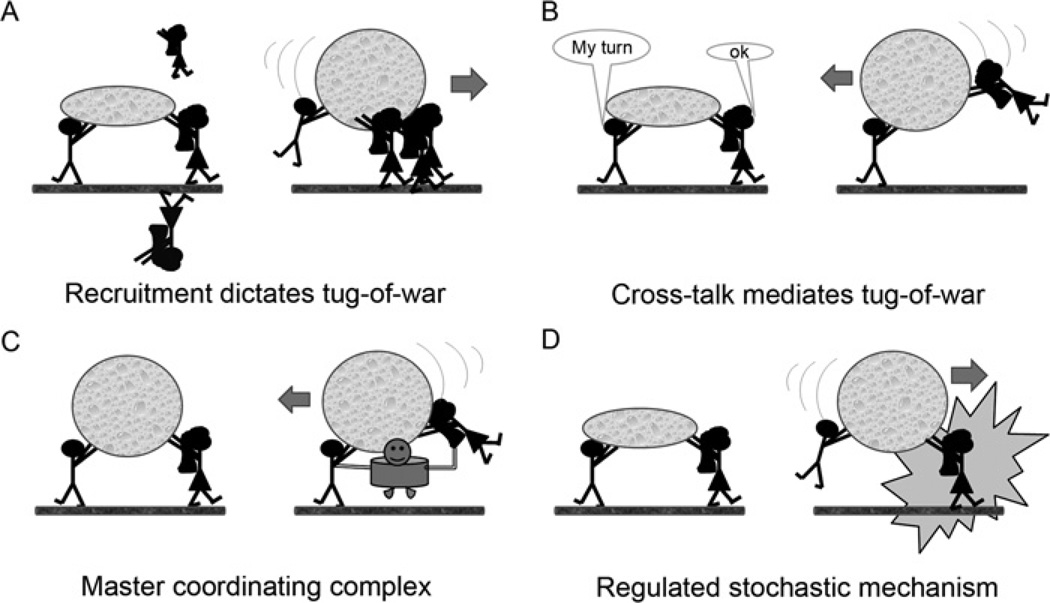
The models presented to date in the literature can be classified as follows. The first model is a tug-of-war between opposite-polarity motors. This model was initially understood as resulting in a stationary cargo caught in the middle of a battle between opposite-polarity motors in cases of equal pulling forces. This model could be further dissected into two distinct mechanisms: a model in which recruitment of new motors of a given polarity dictates a change in directionality of motion (Figure 1A), and a model in which the number of opposite-polarity motors remains constant, but some sort of cross-talk between opposite-polarity motors prevents cargo from being caught in an immobile state (Figure 1B). As a result of this framework, a great deal of effort was put into technically challenging experiments aimed at quantifying the number of each motor type bound to a single organelle at any given time, and to correlate the number of motors bound to the directionality of organelle movement.
Figure 1. Cartoons illustrating different models of bidirectional organelle motility.
These models are not necessarily presumed to be mutually exclusive. (A) Opposite-polarity motors undergo tug-of-war and the direction of motion is decided by the recruitment of additional motors (for example in response to signalling). (B) Tug-of-war dictates the direction of movement of the organelle, and the motors engage in a form of cross-talk such that neither motor can walk independently of the other motor. This model attempts to explain the observation that, in many systems, disruption of one motor also abolishes the activity of the opposite-polarity motor. (C) A co-ordinating complex dictates the directionality of motion. Tug-of-war does not occur in this model, and the activity of each motor is dependent upon the signal from the co-ordinating complex. (D) A stochastic mechanism determines the direction of motion, and opposite-polarity motors compete for cargo motility. The net direction of movement is a result of regulated motor activation and/or inactivation by binding partners or post-translational modifications of the motors themselves.
Recent theoretical work by Müller et al. [23] challenged some of the assumptions regarding a tug-of-war model. Specifically, the authors demonstrated that a tug-of-war mechanism inherently leads to saltatory motion [23]. Rather than resulting in cargo moving in only one direction or not at all, tug-of-war actually causes cargo to transition between plus- and minus-end-directed runs, with pauses in between. The basis for this lies in the stall/detachment ratio for each type of motor involved. This theoretical study is consistent with the motility of peroxisomes in vivo, which were found to have constant velocity no matter the direction of movement [24]. If opposite-polarity motors are pulling simultaneously, it was hypothesized that the velocity would diminish before a change in direction. Our findings suggest that this is not the case. It therefore appears that tug-of-war between molecular motors may not resemble a favourite childhood game.
There are a few systems that are likely to use the mechanism of motor recruitment for bidirectional motility regulation. Unique to this model is the requirement that opposite-polarity motors are not always simultaneously attached. Furthermore, motor attachment must lead to a change in transport direction. Given this mechanism, disruption of one polarity of motor [for example, by silencing kinesin-1 using RNAi (RNA interference)] must result in extreme phenotypes (dispersed or aggregated cargo). To our knowledge, the only example of bidirectional motility that fulfils the above requirements is early endosome movement in the fungus Ustilago maydis [25].
There are numerous examples of cellular cargoes that are transported bidirectionally and retain relatively constant numbers of opposite-polarity motors bound. Endosomes, melanosomes, mitochondria and axonal transport vesicles all co-purify with members of both the dynein and kinesin motor families [9,26–30]. The bidirectional motility of frog pigment granules and mammalian endosomes can be reconstituted in vitro using purified organelles and in vitro polymerized microtubules [26,27]. This shows that all of the components necessary for bidirectional motility (i.e. the motors of both polarities) are already present on the organelle. Moreover, the number of opposite-polarity motors bound to melanosomes and mitochondria remains constant, even when the time the organelle spends making plusend runs compared with minus-end runs changes [28,29]. Detailed investigations into the number of motors bound to individual axonal transport vesicles using photobleaching and quantitative Western blotting revealed that low, but similar, numbers (one to five) of dynein and kinesin proteins are, on average, present on a single vesicle [30]. The above evidence strongly suggests that, in many systems, motors of opposite polarity are present simultaneously and stably on the same organelle.
The second model for bidirectional motility is a coordinated mechanism that does not rely on the motors themselves to battle it out in deciding the fate of the cargo, but instead depends on an external master co-ordinating complex that would be capable of directing transport by turning on kinesin motors at the same time as turning off dynein motors, or vice versa (Figure 1C). This type of co-ordinator was presumed to override the necessity for a tug-of-war battle between opposite-polarity motors, or at least to act as the switch between plus- and minus-end-driven transport. Recent work in our laboratory suggests that such an external master regulator probably does not exist. Replacement of kinesin-1 or cytoplasmic dynein with a variety of other motor domains was able to rescue motility to various degrees depending upon the strength of the replacement motor [31]. If a switch protein exists, it would not be expected to bind all of the replacement motors given the high variability in the structures and sequences, and the replacement motors would not be able to rescue motility.
However, a stochastic model alone cannot account for the remainder of the known cases of bidirectional motility, even if a mechanical form of cross-talk inherent to the motors dictates a change in direction. This is because another attribute of the majority of bidirectionally moving cargo, especially cargo carried by the kinesin-1 and dynein motors, is that the disruption or inactivation of one polarity motor causes the opposite-polarity motor to be rendered inactive, resulting in stationary cargo [32–40]. For example, inhibition of dynein in melanophore cells resulted in the inactivation of both dynein and kinesin, without affecting the binding of either motor to the melanosome [41]. In these cases, opposite-polarity motors require one another for motility and do not compete. An explanation for this may be that motors of different polarity share a common component [dynactin, JIPs (c-Jun N-terminal kinase-interacting proteins), etc.]. In this case, even though the general mechanism is stochastic, affecting this component can inhibit both directions. In fact, in many cases, there is an orderly transition between the plus- and minus-end bias (i.e. melanosomes in Xenopus melanophores and Drosophila lipid droplets). In this case, there might be a regulator that can change the behaviour of one or both motors (Figure 1D). Therefore we might rethink the transport models as we gain a better understanding of the mechanisms by which motors themselves are regulated, including the signalling cascades that trigger cargo dispersion or aggregation in various systems.
The data to date, however, strongly support a kind of stochastic model, in which opposite-polarity motors pull in their respective directions in a form of tug-of-war along the lines of the theoretical study described by Müller et al. [23], but, in addition, the motors are subject to specific regulatory mechanisms. The type of regulation will vary depending upon the particular system and the motors involved.
The field of intracellular transport is progressing rapidly with the use of increasingly sophisticated microscopic approaches and experimental designs. The use of multiple model systems contributes greatly to our understanding of the ways in which cells regulate bidirectional motility. Although a clear understanding of the molecular basis for bidirectional motility is on the horizon, it seems appropriate to appreciate the existence of this strange phenomenon, and to understand the utility of saltatory motion.
Acknowledgments
Funding
This work is supported by the National Institutes of Health [grant numbers GM52111 and HL048129].
Abbreviations used
- NMDA
N-methyl-d-aspartate
References
- 1.Bustamante C, Chemla YR, Forde NR, Izhaky D. Mechanical processes in biochemistry. Annu. Rev. Biochem. 2004;73:705–748. doi: 10.1146/annurev.biochem.72.121801.161542. [DOI] [PubMed] [Google Scholar]
- 2.O’Connell CB, Khodjakov AL. Cooperative mechanisms of mitotic spindle formation. J. Cell Sci. 2007;120:1717–1722. doi: 10.1242/jcs.03442. [DOI] [PubMed] [Google Scholar]
- 3.Bouck D, Bloom K. The role of centromere-binding factor 3 (CBF3) in spindle stability, cytokinesis, and kinetochore attachment. Biochem. Cell Biol. 2005;83:696–702. doi: 10.1139/o05-161. [DOI] [PubMed] [Google Scholar]
- 4.Odde DJ. Mitotic spindle: disturbing a subtle balance. Curr. Biol. 2005;15:R956–R959. doi: 10.1016/j.cub.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Mimori-Kiyosue Y, Tsukita S. “Search-and-capture” of microtubules through plus-end-binding proteins (+TIPs) J. Biochem. (Tokyo) 2003;134:321–326. doi: 10.1093/jb/mvg148. [DOI] [PubMed] [Google Scholar]
- 6.Wu X, Kocher B, Wei Q, Hammer JA., 3rd Myosin Va associates with microtubule-rich domains in both interphase and dividing cells. Cell Motil. Cytoskeleton. 1998;40:286–303. doi: 10.1002/(SICI)1097-0169(1998)40:3<286::AID-CM7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 7.Hearing VJ. Unraveling the melanocyte. Am. J. Hum. Genet. 1993;52:1–7. [PMC free article] [PubMed] [Google Scholar]
- 8.Jimbow K, Iwashina T, Alena F, Yamada K, Pankovich J, Umemura T. Exploitation of pigment biosynthesis pathway as a selective chemotherapeutic approach for malignant melanoma. J. Invest. Dermatol. 1993;100(Suppl.):231S–238S. [PubMed] [Google Scholar]
- 9.Guillaud L, Wong R, Hirokawa N. Disruption of KIF17–Mint1 interaction by CaMKII-dependent phosphorylation: a molecular model of kinesin-cargo release. Nat. Cell Biol. 2008;10:19–29. doi: 10.1038/ncb1665. [DOI] [PubMed] [Google Scholar]
- 10.Sydow JF, Cramer P. RNA polymerase fidelity and transcriptional proofreading. Curr. Opin. Struct. Biol. 2009;19:732–739. doi: 10.1016/j.sbi.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Ali MY, Krementsova EB, Kennedy GG, Mahaffy R, Pollard TD, Trybus KM, Warshaw DM. Myosin Va maneuvers through actin intersections and diffuses along microtubules. Proc. Natl. Acad. Sci. U.S.A. 2007;104:4332–4336. doi: 10.1073/pnas.0611471104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali MY, Lu H, Bookwalter CS, Warshaw DM, Trybus KM. Myosin V and kinesin act as tethers to enhance each others’ processivity. Proc. Natl. Acad. Sci. U.S.A. 2008;105:4691–4696. doi: 10.1073/pnas.0711531105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez LA, Sheetz MP. Steric inhibition of cytoplasmic dynein and kinesin motility by MAP2. Cell Motil. Cytoskeleton. 1993;24:1–16. doi: 10.1002/cm.970240102. [DOI] [PubMed] [Google Scholar]
- 14.Hagiwara H, Yorifuji H, Sato-Yoshitake R, Hirokawa N. Competition between motor molecules (kinesin and cytoplasmic dynein) and fibrous microtubule-associated proteins in binding to microtubules. J. Biol. Chem. 1994;269:3581–3589. [PubMed] [Google Scholar]
- 15.Vershinin M, Xu J, Razafsky DS, King SJ, Gross SP. Tuning microtubule-based transport through filamentous MAPs: the problem of dynein. Traffic. 2008;9:882–892. doi: 10.1111/j.1600-0854.2008.00741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao G, Gundersen GG. Kinesin is a candidate for cross-bridging microtubules and intermediate filaments: selective binding of kinesin to detyrosinated tubulin and vimentin. J. Biol. Chem. 1998;273:9797–9803. doi: 10.1074/jbc.273.16.9797. [DOI] [PubMed] [Google Scholar]
- 17.Reed NA, Cai D, Blasius TL, Jih GT, Meyhofer E, Gaertig J, Verhey KJ. Microtubule acetylation promotes kinesin-1 binding and transport. Curr. Biol. 2006;16:2166–2172. doi: 10.1016/j.cub.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Dompierre JP, Godin JD, Charrin BC, Cordelières FP, King SJ, Humbert S, Saudou F. Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington’s disease by increasing tubulin acetylation. J. Neurosci. 2007;27:3571–3583. doi: 10.1523/JNEUROSCI.0037-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konishi Y, Setou M. Tubulin tyrosination navigates the kinesin-1 motor domain to axons. Nat. Neurosci. 2009;12:559–567. doi: 10.1038/nn.2314. [DOI] [PubMed] [Google Scholar]
- 20.Cai D, McEwen DP, Martens JR, Meyhofer E, Verhey KJ. Single molecule imaging reveals differences in microtubule track selection between kinesin motors. PLoS Biol. 2009;7 doi: 10.1371/journal.pbio.1000216. e1000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammond JW, Huang CF, Kaech S, Jacobson C, Banker G, Verhey KJ. Posttranslational modifications of tubulin and the polarized transport of kinesin-1 in neurons. Mol. Biol. Cell. 2010;21:572–583. doi: 10.1091/mbc.E09-01-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedman JR, Webster BM, Mastronarde DN, Verhey KJ, Voeltz GK. ER sliding dynamics and ER-mitochondrial contacts occur on acetylated microtubules. J. Cell Biol. 2010;190:363–375. doi: 10.1083/jcb.200911024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Müller MJ, Klumpp S, Lipowsky R. Tug-of-war as a cooperative mechanism for bidirectional cargo transport by molecular motors. Proc. Natl. Acad. Sci. U.S.A. 2008;105:4609–4614. doi: 10.1073/pnas.0706825105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kural C, Kim H, Syed S, Goshima G, Gelfand VI, Selvin PR. Kinesin and dynein move a peroxisome in vivo: a tug-of-war or coordinated movement? Science. 2005;308:1469–1472. doi: 10.1126/science.1108408. [DOI] [PubMed] [Google Scholar]
- 25.Schuster M, Lipowsky R, Assmann MA, Lenz P, Steinberg G. Transient binding of dynein controls bidirectional long-range motility of early endosomes. Proc. Natl. Acad. Sci. U.S.A. 2011;108:3618–3623. doi: 10.1073/pnas.1015839108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogers SL, Tint IS, Fanapour PC, Gelfand VI. Regulated bidirectional motility of melanophore pigment granules along microtubules in vitro. Proc. Natl. Acad. Sci. U.S.A. 1997;94:3720–3725. doi: 10.1073/pnas.94.8.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray JW, Bananis E, Wolkoff AW. Reconstitution of ATP-dependent movement of endocytic vesicles along microtubules in vitro: an oscillatory bidirectional process. Mol. Biol. Cell. 2000;11:419–433. doi: 10.1091/mbc.11.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Vos KJ, Sable J, Miller KE, Sheetz MP. Expression of phosphatidylinositol (4,5) bisphosphate-specific pleckstrin homology domains alters direction but not the level of axonal transport of mitochondria. Mol. Biol. Cell. 2003;14:3636–3649. doi: 10.1091/mbc.E02-10-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gross SP, Tuma MC, Deacon SW, Serpinskaya AS, Reilein AR, Gelfand VI. Interactions and regulation of molecular motors in Xenopus melanophores. J. Cell Biol. 2002;156:855–865. doi: 10.1083/jcb.200105055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hendricks AG, Perlson E, Ross JL, Schroeder HW, 3rd, Tokito M, Holzbaur EL. Motor coordination via a tug-of-war mechanism drives bidirectional vesicle transport. Curr. Biol. 2010;20:697–702. doi: 10.1016/j.cub.2010.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ally S, Larson AG, Barlan K, Rice SE, Gelfand VI. Opposite-polarity motors activate one another to trigger cargo transport in live cells. J. Cell Biol. 2009;187:1071–1082. doi: 10.1083/jcb.200908075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waterman-Storer CM, Karki SB, Kuznetsov SA, Tabb JS, Weiss DG, Langford GM, Holzbaur EL. The interaction between cytoplasmic dynein and dynactin is required for fast axonal transport. Proc. Natl. Acad. Sci. U.S.A. 1997;94:12180–12185. doi: 10.1073/pnas.94.22.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin M, Iyadurai SJ, Gassman A, Gindhart JG, Jr, Hays TS, Saxton WM. Cytoplasmic dynein, the dynactin complex, and kinesin are interdependent and essential for fast axonal transport. Mol. Biol. Cell. 1999;10:3717–3728. doi: 10.1091/mbc.10.11.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pilling AD, Horiuchi D, Lively CM, Saxton WM. Kinesin-1 and dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol. Biol. Cell. 2006;17:2057–2068. doi: 10.1091/mbc.E05-06-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gross SP, Guo Y, Martinez JE, Welte MA. A determinant for directionality of organelle transport in Drosophila embryos. Curr. Biol. 2003;13:1660–1668. doi: 10.1016/j.cub.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 36.Barkus RV, Klyachko O, Horiuchi D, Dickson BJ, Saxton WM. Identification of an axonal kinesin-3 motor for fast anterograde vesicle transport that facilitates retrograde transport of neuropeptides. Mol. Biol. Cell. 2008;19:274–283. doi: 10.1091/mbc.E07-03-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uchida A, Alami NH, Brown A. Tight functional coupling of kinesin-1A and dynein motors in the bidirectional transport of neurofilaments. Mol. Biol. Cell. 2009;20:4997–5006. doi: 10.1091/mbc.E09-04-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ling SC, Fahrner PS, Greenough WT, Gelfand VI. Transport of Drosophila fragile X mental retardation protein-containing ribonucleoprotein granules by kinesin-1 and cytoplasmic dynein. Proc. Natl. Acad. Sci. U.S.A. 2004;101:17428–17433. doi: 10.1073/pnas.0408114101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim H, Ling SC, Rogers GC, Kural C, Selvin PR, Rogers SL, Gelfand VI. Microtubule binding by dynactin is required for microtubule organization but not cargo transport. J. Cell Biol. 2007;176:641–651. doi: 10.1083/jcb.200608128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Encalada SE, Szpankowski L, Xia CH, Goldstein LS. Stable kinesin and dynein assemblies drive the axonal transport of mammalian prion protein vesicles. Cell. 2011;144:551–565. doi: 10.1016/j.cell.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deacon SW, Serpinskaya AS, Vaughan PS, Lopez, Fanarraga M, Vernos I, Vaughan KT, Gelfand VI. Dynactin is required for bidirectional organelle transport. J. Cell Biol. 2003;160:297–301. doi: 10.1083/jcb.200210066. [DOI] [PMC free article] [PubMed] [Google Scholar]