Abstract
Background
Prompt diagnosis and treatment of acute mesenteric ischemia (AMI) requires a high index of suspicion for timely management. Poor clinical outcomes and delays in surgical treatment are demonstrated even in modern clinical series. Recognition of exhaled volatile organic compounds (VOC’s) specific to AMI may facilitate early detection and diagnosis and improve patient outcomes.
Study Design
Adult Wistar Rats (n=5) were intubated and anesthetized and control tracheostomy breath samples were collected using Tedlar gas sample bags. Intestinal ischemia was induced by placing an occlusive clip across the superior mesenteric artery and breath samples were collected following one hour of intestinal ischemia and following 15 minutes of intestinal reperfusion. Gas chromatography was used to identify and measure levels the VOC’s obtained and measured retention indices were compared with known values in the Kovats Retention Index.
Results
Multiple retention indices (n=41)) were noted on gas chromatography representing a variety of VOC’s detected. Z,Z,-farnesol (C15H26O), an isoprenoid, was the only compound detected which was undetectable during the control phase (median=0 Cts/sec, range=0) but significantly elevated during the ischemic and reperfusion phases(median=34 Cts/sec, range=25–37) and (median=148 Cts/sec. range=42–246), respectively. Three other isoprenoid compounds: E,E-alpha-farnesene, germacrene-A, and Z,Z-4,6,8-megastigmatriene were also detected in all five animals, but their levels did not differ significantly between control, ischemic and reperfusion phases.
Conclusions
This pilot study demonstrates the feasibility of analyzing exhaled VOC’s using a novel rat model for acute mesenteric ischemia. These findings may be useful for the development and identification of similar assays for the rapid diagnosis of acute mesenteric ischemia.
Introduction
Acute mesenteric ischemia (AMI) is associated with significant morbidity and mortality especially when time to diagnosis and surgical revascularization is indicated and prolonged.1, 2 Nonspecific symptoms and the lack of a single diagnostic modality which is pathognomonic for this syndrome may lead to bowel infarction, delays in diagnosis and poor patient outcomes. A high index of suspicion by the initial evaluating provider is usually required for prompt diagnosis and treatment to occur.3
The detection of distinct volatile organic compounds (VOC’s) in exhaled breath by gas chromatography (GC) has been described for other pathologic disease processes.4, 5, 6 Recognition of volatile markers specific to intestinal ischemia by breath analysis may provide additional information to expedite the decision for surgical intervention. In this study, gas chromatography was used to measure levels of VOC’s in alveolar breath before and after abrupt occlusion of the superior mesenteric artery using a rat model. Our aim was to determine the feasibility of detecting changes in VOC levels during a pilot animal model of acute mesenteric ischemia and reperfusion.
Materials and Methods
All studies and care of laboratory animals were performed in agreement with the guidelines of the University of California as established by US National Institutes of Health.
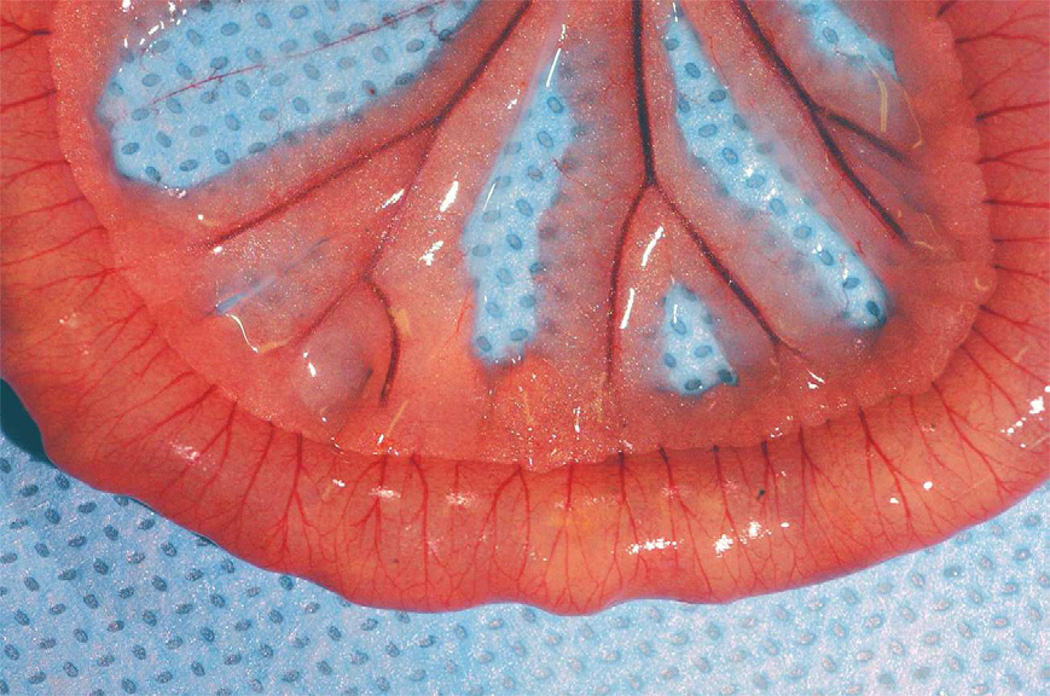
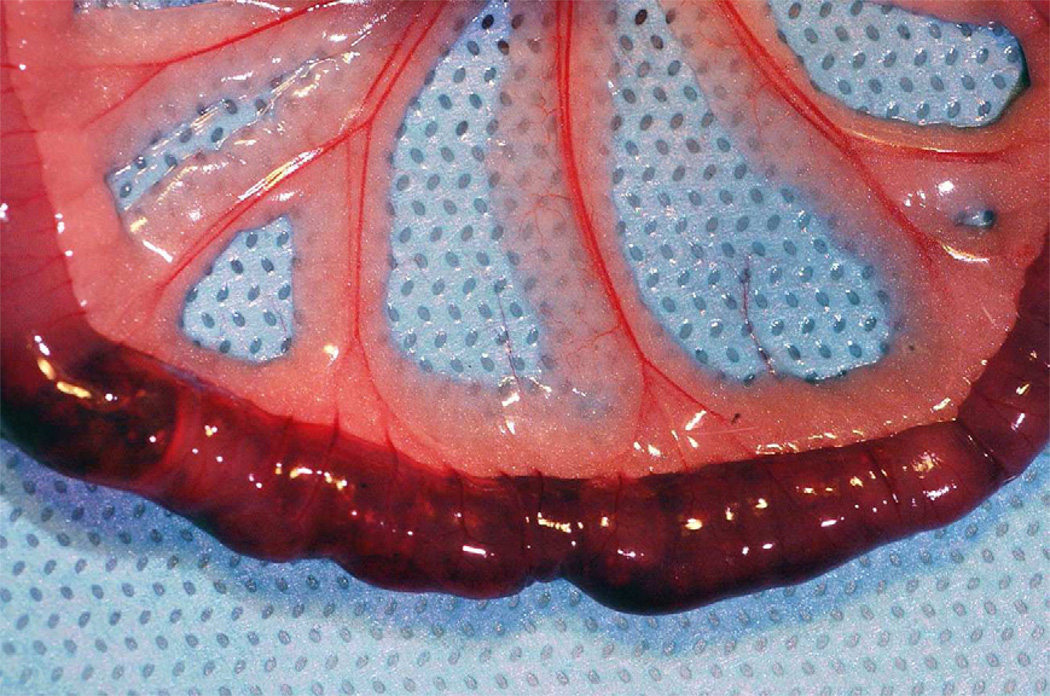
Adult Wistar rats (n=5) each weighing 350 mg were intubated and sedated using general anesthesia (Nembutal). Prior to laparotomy, control exhaled breath samples were collected using Tedlar gas sample bags (1L) at the end of the tracheotomy tube attached to a one-way valve. Surgical exposure of the superior mesenteric artery was performed via a midline incision. Intestinal ischemic injury was achieved by superior mesenteric artery occlusion (SMAO) with a micro surgical clip placed across the vascular pedicle for one hour in the anesthetized rat. Exhaled tracheostomy samples were collected in similar fashion at one hour of ischemia, and after release of the occlusive clamp following 15 minutes of intestinal reperfusion. Visual evaluation of the small intestine was performed at these same time intervals to confirm the presence of bowel ischemia. (Figure 1A and 1B).
Figure 1.
1A and 1B: Small intestine in the animals prior to (1A) superior mesenteric occlusion and following one hour of ischemia (1B). Visual confirmation of intestinal ischemia was achieved prior to collection of exhaled breath samples
Each sample was run through a gas chromatograph (ZNOSE Model 4500, Newbury Park, Ca, USA), and results were matched to the Kovats retention index database to identify VOC’s. The Kovats retention index is a standardized parameter used in gas chromatography to compare and identify these compounds.7 Measured retention indices were compared with known values in the Kovats Retention Index database.
Results
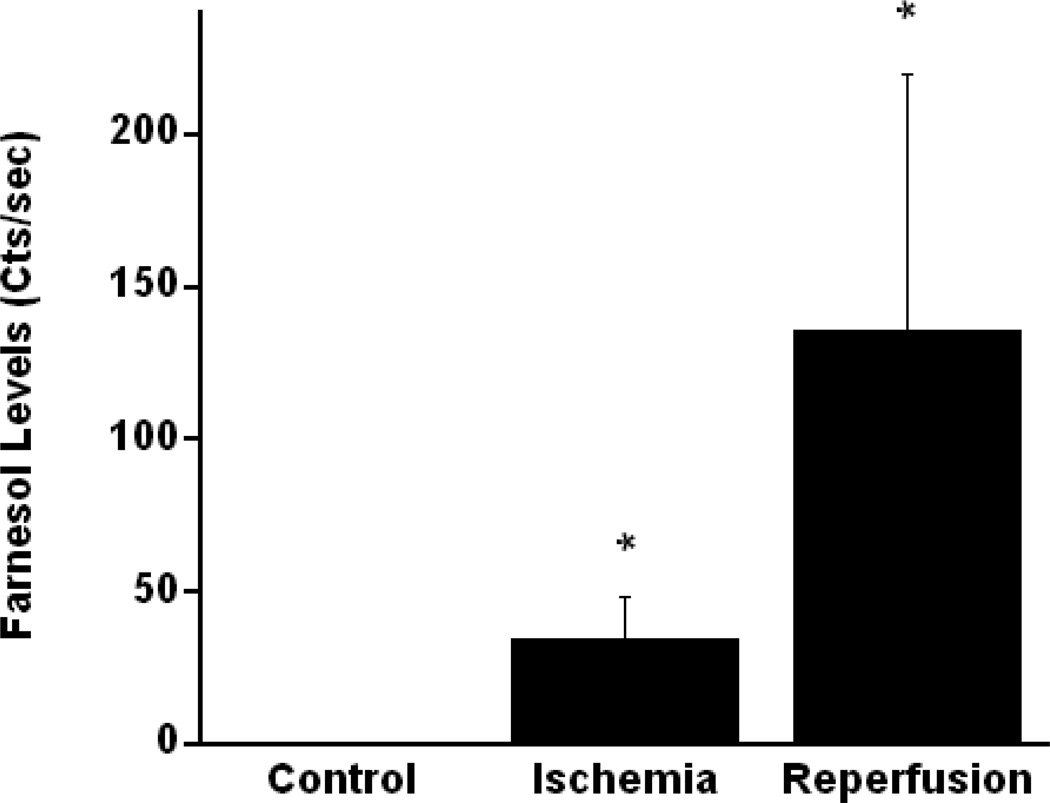
Multiple retention indices (n=41)) were noted on gas chromatography representing a variety of VOC’s detected. The most commonly detected compounds are listed in Table 1. Of the multiple gases identified, trimethyldodecatrienol (Z,Z-farnesol-C15H260, molar wt-222.37 g/mol), an isoprenoid, was the only compound detected in significantly elevated amounts in all five animals following one hour of ischemia (median=34 Cts/sec, range=25–37) and at 15 minutes reperfusion (median=148 Cts/sec, range=42–246) compared with pre-ischemic measurements (median=0 Cts/sec, range=0; p<0.01). Figure 2 demonstrates Z,Z-farnesol levels at each time point for all five individual animals.
Table 1.
Compounds Detected on Gas Chromatography
| Number of Animals Compound Detected | |||||
|---|---|---|---|---|---|
| Control | Ischemic | Reperfusion | |||
| Compounds Detected | Common Name | Molecular Formula | Phase | Phase | Phase |
| (ZZ)-3,7,11-Trimethyl-2,6,10-dodecatrien-1-ol | Z,Z-farnesol | C15H26 | 0 | 5 | 5 |
| (Z,E)-1,5-Dimethyl-8-(prop-1-en-2-yl)-1,5-cyclodecadiene | germacrene A (Z,Z)-4,6,8- | C15H24 | 3 | 4 | 5 |
| 4-Methoxybenzylformate | Megastigmatriene | C13H20 | 3 | 3 | 5 |
| (E,E)-3,7,11-Trimethyl-1,3,6,10-dodecatetraene | E,E-alpha-farnesene | C15H24 | 3 | 4 | 5 |
| 1-Octen-3-ol | delta-1-octene-3-ol | C8H16O | 0 | 1 | 2 |
| (Z,E)-3,7,11-Trimethyl-2,6,10-dodecatrienyl acetate | Z,E-farnesyl acetate | C17H28O2 | 2 | 2 | 4 |
| (Z,Z)-4,7-Tridecadien-(2S)-2-yl acetate | 2S-Z4Z7-13Ac | C15H26O2 | 1 | 0 | 4 |
| (Z)-3-Dodecen-1-ol | Z3-12OH | C12H24O | 2 | 2 | 3 |
| (Z)-2,6-Dimethyl-5,7-octadien-4-one | Z-tagetone | C10H160 | 3 | 3 | 3 |
| (Z)-8-Dodecenyl acetate | Z8-12Ac | C14H26O2 | 1 | 1 | 3 |
| Decanal | 10Ald | C10H20O | 1 | 3 | 3 |
| (E)-3,7-Dimethyl-2,6-octadienal | geranial | C10H16O | 0 | 0 | 2 |
Figure 2.
Figure 2 demonstrates Z,Z-farnesol levels at each time point for all five individual animals.
E,E-alpha-farnesene (C15H24, molar wt 204.35 g/mol), an isoprenoid analogue of Z,Z-farnesol was another compound detected in all five animals. The median detected value during the control phase was 227 Cts/sec (range=189–289). Levels of this compound following 15 minutes of ischemia and following reperfusion were 173 Cts/sec (range=28–179) and 164 Cts/sec (range=29–288), respectively. In contrast to Z,Z,-farnesol, these levels were not statistically significant (p> 0.05).
Germacrene A (C15H24, molar wt-204.35 g/mol), another isoprenoid similar in structure to Z,Z-farnesol, was also noted in all five rats but levels did not vary significantly between the control, ischemia, and reperfusion phases. (p> 0.05) Levels of this compound were 189 Cts/sec (0–298) during the control phase, 166 Cts/sec (range=0–279) following one hour of ischemia, and 164 Cts/sec (range=29–288) during the reperfusion phase.
Z,Z-4,6,8-Megastimatriene (C13H20, molar wt-176.3 g/mol), another isoprenoid, was also present in all five rats during reperfusion. The median value of this compound during the reperfusion phase was 306 Cts/sec (range=25–422). The median values prior to ischemia and 15 minutes following SMA occlusion, respectively, were 227 Cts/sec (range=0–346) and 138 Cts/sec (range=0–414). These levels were also not significantly different.(p> 0.05)
Discussion
Poor clinical outcomes in patients with AMI and delays in surgical treatment are demonstrated even in modern clinical series’. 1,2 Delayed diagnosis and treatment may occur when radiologic findings are equivocal, or misinterpreted since there are no specific, pathognomonic findings on computed tomography or magnetic resonance imaging specific to the early stages of AMI. Furthermore, an increase in the severity of systemic manifestations and increased patient mortality has been associated with longer times to revascularization.8 In a study by Kougias, et al, the mean duration between the onset of patient symptoms and surgical intervention was 16 hours. 1 The overall 30-day mortality was 26% and intestinal resection was required in 31% of patients due to infarction and/or necrosis at the time of initial exploration.1 Another similar review by Park, et al reported a 32% 30-day mortality in their series of 58 patients treated for AMI.2 Five percent of patients presented with extensive bowel necrosis and unsalvageable disease and 57% of patients required intestinal resection at the time of their initial operation.
To our knowledge, this is the first model to detect exhaled VOC’s during and following acute mesenteric ischemia. Our findings from this pilot study demonstrate that elevated levels of Z,Z-farnesol were measured following acute mesenteric ischemia using a rat model. Three other isoprenoids were detected in all five rats, however no significant difference was seen during control and experimental measurements.
Breath analysis can be used in the clinical diagnosis of other pathologic conditions include the detection of acetone in ketotic patients, ammonia in patients with uremia, and a bitter almond aroma suggesting cyanide poisoning9. More formal laboratory breath testing has been developed to aid in the diagnosis of other gastrointestinal disorders including: peptic ulcer disease10, delayed gastric emptying11, irritable bowel syndrome12, and lactose intolerance13.
Although the mechanism and significance of this increased release in exhaled VOC’s is unclear in the setting of AMI, decreased intestinal mucosal permeability following ischemia-reperfusion has been well described.14,15,16 Studies have demonstrated that the initial pathologic alterations of inflammatory or ischemic bowel mucosa occur in the digestive fermentation process, resulting in a change in the type and concentration of gases that are produced by a normal healthy bowel.17 Systemic absorption of these gases may, in theory, be detected using breath analysis although no prior studies have demonstrated increased levels of these specific compounds during and following ischemic bowel injury.
The only compounds detected in all five animals, Z,Z-farnesol, EE,-alpha-farnesene, Germacrene A and 4,6,8-Megastimatriene, are known to be part of a class of compounds known as isoprenoids, also referred to as terpenoids. Z,Z-farnesol, was the only compound undetectable during the control phase which was significantly elevated during the ischemia and reperfusion phases. This compound originates as a byproduct of the mevalonate pathway via the conversion of the intermediate farnesol pyrophosphate (FPP) which is known to be a key pathway in the formation of cholesterol, along with key intermediates essential for cellular processes. In addition, farnesol has been implicated in the reduction of serum triglyceride levels in rats.18
The oxidation of Z,Z-farnesol into its aldehyde intermediate in the rat has been found to occur mainly19 in the liver, colon, stomach, and lung and mediated by alcohol dehydrogenases. Although its precise role in the gastrointestinal tract is presently unclear, recent human studies have identified farnesol metabolism in the liver, kidney, and intestines.20 Farnesene, a close derivative of Z,Z-farnesol, has been found to play a significant role during oxidative injury of eukaryotic cells.21,22,23 When the autoxidation of this compound occurs in-vivo, cell injury and death have been observed. The inhibition of farnesene production has been shown to significantly decrease oxidative cell injury. 21,22 This effect, however has not been previously demonstrated in mammalian studies
Limitations of our study include the inability to determine whether the compounds detected originate from the gut lumen or are actual by-products of cell injury during bowel ischemia. Measurement of control samples following laparotomy but prior to ischemia would more accurately delineate whether increased levels of VOC’s occurred from intestinal ischemia or surgical trauma. . Additionally, the significance of the detectable levels of other isoprenoids in our study is unclear and it is difficult to draw conclusions from this measurement. Additional studies are required to further delineate the importance of these findings. Although the aim of the study was to identify measurable compounds during the early onset of acute mesenteric ischemia, we hypothesized that elevated levels during reperfusion would support the theory that these compounds were more likely to originate in the rat intestine. It is probable that during clamping of the vascular pedicle, the superior mesenteric vein was occluded. This may explain elevated levels of VOC’s originating from the intestine due to the presence of accumulated compounds in the absence of splanchnic venous outflow.
Blood samples were not obtained concurrently from the animals for analysis of these VOC’s. Theoretically, if systemic absorption of these compounds is the source of these exhaled gases, serologic detection should also be possible. Serologic sampling of both the peripheral and portal veins may also help confirm the origin of these compounds in this model. The addition of mass spectrometry to gas chromatography increases the accuracy of substance determination and reduces the possibility of error inherent to GC alone. We are currently conducting further analysis of these compounds in a similar model using both modalities simultaneously.
This pilot study demonstrates the feasibility of analyzing exhaled VOC’s using a rat model for acute mesenteric ischemia. Four distinct isoprenoids were detected by GC in all animals following ischemic bowel injury, and Z,Z-farnesol was the only compound detected in significantly increased levels during ischemia and after reperfusion compared with control. These findings may be useful for the development and identification of similar assays for the rapid diagnosis of acute mesenteric ischemia and facilitate management of this complex disease process.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the Pacific Coast Surgical Association, 82nd Annual Meeting, Scottsdale, AZ, February 19th, 2011.
References
- 1.Kouglas P, Lau D, El Sayed HF, Zhou W, Huynh TT, Lin P. Determinants of mortality and treatment outcome following surfical interventions for acute mesenteric ischemia. J Vasc Surg. 2007;46:467–474. doi: 10.1016/j.jvs.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 2.Park WM, Gloviczki P, Cherry KJ, Jr, Hallet JW, Bower TC, Panneton JM, et al. Contemporary management of acute mesenteric ischemia: Factors associated with survival. J Vasc Surg. 2002;35:445–452. doi: 10.1067/mva.2002.120373. [DOI] [PubMed] [Google Scholar]
- 3.Eltawary IG, Etman YM, Zenati M, Simmons RL, Rosengart MR. Acute mesenteric ischemia: the importance of early surgical consultation. Am Surg. 2009;75:212–219. [PubMed] [Google Scholar]
- 4.Mortensen PB, Clausen MR. Short-chain fatty acids in the human colon:Relation to gastrointestinal health and disease. Scand J Gastroenterol suppl. 1996:132–148. doi: 10.3109/00365529609094568. [DOI] [PubMed] [Google Scholar]
- 5.Bajtarevic A, Ager C, Pienz M, Klieber M, Schwarz K, Ligor T, et al. Noninvasive detection of lung cancer by analysis of exhaled breath. BMC Cancer. 2009;9:348. doi: 10.1186/1471-2407-9-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carpenter CT, Price PV, Christman BW. Exhaled breath condensate isoprostanes are elevated in patients with acute lung injury or ARDS. Chest. 1998;114:1653–1659. doi: 10.1378/chest.114.6.1653. [DOI] [PubMed] [Google Scholar]
- 7.Kováts E. Gas-chromatographische Charakterisierung organischer Verbindungen. Teil 1: Retentionsindices aliphatischer Halogenide, Alkohole, Aldehyde und Ketone. Helvetica Chimica Acta. 2004;41:1915–1932. 1958. [Google Scholar]
- 8.Pontell L, Sharma P, Rivera LR, Thacker M, Tan YH, Brock YA, Furness JB. Damaging effects of ischemia/reperfusion on intestinal muscle. Cell Tissue Res. 2011;34:411–419. doi: 10.1007/s00441-010-1096-z. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez-Verlaan P, Geeraerts T, Buys S, Riu-Poulenc B, Cabot C, Fourcade O, et al. An unusual cause of severe lactic acidosis: cyanide poisoning after bitter almond ingestion. Intensive Care Med. 2011;37:168–169. doi: 10.1007/s00134-010-2029-8. [DOI] [PubMed] [Google Scholar]
- 10.Calvet X, Sanchez-Delgado J, Montserrat A, Lario S, Ramirez-Lazaro MJ, Quesada M, et al. Accuracy of diagnostic tests for Helicobacter pylori: a reappraisal. Clin Intect Dis. 2009;15:1385–1391. doi: 10.1086/598198. [DOI] [PubMed] [Google Scholar]
- 11.Ron Y, Sperber AD, Levine A, Shevah O, Dickman R, Avni Y, et al. Early satiety is the only patient-reported symptom associated with delayed gastric emptying, as assessed by breath-test. J Neurogastroenterol Motil. 2011;17:61–66. doi: 10.5056/jnm.2011.17.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Youn YH, Park JS, Jahng JH, Lim HC, Kim JH, Pimentel M, et al. Relationships among the lactulose breath test, intestinal gas volume, and gastrointestinal symptoms in patients with irritable bowel syndrome. Dig Dis Sci. 2011 doi: 10.1007/s10620-011-1569-2. In press. [DOI] [PubMed] [Google Scholar]
- 13.Law D, Conklin J, Pimentel M. Lactose intolerance and the role of the lactose breath test. Am J Gastroenterol. 2010;105:1276–1278. doi: 10.1038/ajg.2010.146. [DOI] [PubMed] [Google Scholar]
- 14.Qin X, Sheth SU, Sharpe SM, Dong W, Lu Q, Xu D, Deitch EA. The mucus layer is critical in protecting against ischemia-reperfusion-mediated gut injury and in the restitution of gut barrier function. Shock. 2011;35:275–281. doi: 10.1097/SHK.0b013e3181f6aaf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang JX, Chen S, Ma LP, Jiang LY, Chen JW, Chang RM, et al. Functional and morphological changes of the gut barrier during the restitution process after hemorrhagic shock. World J Gastroenterol. 2005;21:5485–5491. doi: 10.3748/wjg.v11.i35.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grotz MR, Deitch EA, Ding J, Xu D, Huang Q, Regel G. Intestinal cytokine response after gut ischemia: role of gut barrier failure. Ann Surg. 1999;229:478–486. doi: 10.1097/00000658-199904000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen C, Lindholt JS, Erlandsen EJ, Mortensen FV. D-Lactate as a marker of venous-induced intestinal ischemia: An experimental study in pigs. Int J Surg. 2011 doi: 10.1016/j.ijsu.2011.04.004. In Press. [DOI] [PubMed] [Google Scholar]
- 18.Duncan RE, Archer MC. Farnesol decreases serum triglycerides in rats:Identification of mechanisms including up-regulation of PPARalpha and down-regulation of fatty acid synthase in hepatocytes. Lipids. 2008;43:619–627. doi: 10.1007/s11745-008-3192-3. [DOI] [PubMed] [Google Scholar]
- 19.Endo S, Matsunaga T, Ohta C, Soda M, Kanamori A, Kitade Y, et al. Roles of rat and human aldo-keto reductases in metabolism of farnesol and geranylgeraniol. Chem Biol Interact. 2011 doi: 10.1016/j.cbi.2010.12.017. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Staines AG, Sindelar P, Coughtrie MWH, Burchell B. Farnesol is glucuronidated in human liver, kidney and intestine in vitro, and is a novel substrate for UGT2B7 and UGT1A1. Biochem J. 2004;384:637–645. doi: 10.1042/BJ20040997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wills RBH, Bailey WM, Scott KJ. Possible involvement of alpha-Farnesene in the development of chilling injury in bananas. Plant Physiol. 1975;56:550–551. doi: 10.1104/pp.56.4.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pechous SW, Watkins CB, Whitaker BD. Expression of alpha-farnesene synthase gene AFS1 in relation to levels of alpha-farnesene and conjugated trienols in peel tissue of scald-susceptible ‘Law Rome’ and scald-resistant ‘Idared’apple fruit. Postharvest Biology and Technology. 2005;35:125–132. [Google Scholar]
- 23.Rudell DR, Mattheis JP, Fellman JK. Relationship of superficial scald development and alpha-Farnesene oxidation to reactions of diphenylamine and diphenylamine derivatives in Cv. Granny Smith apple peel. J Agric Food Chem. 2005;53:8382–8389. doi: 10.1021/jf0512407. [DOI] [PubMed] [Google Scholar]