Summary
Background
Trousseau’s syndrome is a pro-thrombotic state associated with malignancy that is poorly understood pathophysiologically.
Methods and Results
Here we report studies on the blood of a 55-year-old man with giant-cell lung carcinoma who developed a severe form of Trousseau’s syndrome. His clinical course was dominated by an extremely hypercoagulable state. Despite receiving potent antithrombotic therapy, he suffered eleven major arterial and venous thrombotic events over a 5 month period. We examined the patient’s blood for tissue factor (TF), the major initiator of coagulation, and found its concentration in his plasma to be forty-one-fold higher than the mean concentration derived from testing of 16 normal individuals.
Conclusion
Almost all of the TF in the patient’s plasma was associated with cell-derived microvesicles, likely shed by the cancer cells.
Keywords: cancer, microvesicles, thrombosis, tissue factor, Trousseau’s syndrome
Introduction
Thrombosis imposes a tremendous burden on patients with cancer in terms of morbidity, mortality, and cost. It is estimated that up to 20% of patients with cancer will experience thrombosis at some time during their illness [1]. Occasionally, cancer-associated thrombosis takes on a particularly malignant form termed Trousseau’s syndrome, an entity first described by Armand Trousseau in 1865 [2]. Ironically, this syndrome heralded Trousseau’s own malignancy, to which he eventually succumbed. Although the term Trousseau’s syndrome has historically referred to migratory superficial thrombophlebitis associated with malignancy, it now also encompasses a florid, cancer-associated prothrombotic state. In this syndrome, thrombosis becomes the dominant feature of the illness, frequently leading to the patient’s demise. The individual patient may experience a myriad of thrombotic complications, including arterial and venous thrombosis, non-bacterial thrombotic endocarditis, disseminated intravascular coagulation (DIC), and microangiopathic hemolytic anemia independent of DIC. Trousseau’s syndrome is a relatively common condition associated with a variety of cancers, particularly pancreatic, gastric, ovarian, and lung adenocarcinomas; yet, its mechanisms are poorly understood. Recent work by Wahrenbrock et al. [3] provided insight into the frequent association of Trousseau’s syndrome with mucin-secreting adenocarcinomas. These investigators showed that mucins secreted by adenocarcinoma cells can bind and cross-link P-selectin on platelets and L-selectin on leukocytes, causing these cells to aggregate and form thrombi. This may explain why heparins (which bind and block selectins) are much more effective than warfarin in preventing thrombosis in these patients [4,5]. However, other mechanisms may operate in the pathogenesis of Trousseau’s syndrome. For example, certain types of cancer cells appear to secrete a cysteine protease known as ‘cancer procoagulant’, capable of directly activating factor X (FX) to FXa [6]. Also, some cancers have been found to express tissue factor (TF) [7], the main initiator of coagulation in vivo. Here, we report the case of a patient with giant-cell lung carcinoma who developed a severe form of Trousseau’s syndrome, characterized by extremely high levels of micro-vesicle-associated TF in his blood. This case may illustrate a more general principle applicable not only to other cases of cancer-associated thrombosis, but to diseases characterized by elevated levels of TF in blood.
Materials and methods
The patient
This report is based on studies on a 55-year-old patient with metastatic giant cell-lung carcinoma who sustained several myocardial infarctions, deep vein thromboses of the lower extremities, occipital infarcts, peripheral arterial infarcts, kidney and spleen infarcts and a fatal ischemic stroke despite receiving warfarin, clopidogrel, low-molecular weight heparin and lepirudin at various times during his clinical course.
Plasma preparation
Blood was drawn from the patient (1 week before his death) and from 16 healthy male volunteers into ethylenediamine-tetraacetic acid-anticoagulated vacutainers. Cell-free plasma was obtained within 30 min of the blood draw by sequential centrifugation, as described previously [8]. Plasma aliquots were stored at −80 °C until analyzed. Informed consent from both the patient and the healthy volunteers was obtained under a protocol approved by the Institutional Review Board of Baylor College of Medicine.
TF antigen
Plasma TF antigen was measured by enzyme-linked immunoassay (ELISA), according to the manufacturer’s instructions (American Diagnostica, Stamford, CT, USA).
Flow cytometry analysis of microvesicles
Plasma microvesicles were counted by flow cytometry using a method based on that of Sims et al. [9], labeling the microvesicles from 100 µL of plasma with 5 µg mL−1 flourescein isothiocyanate (FITC)-conjugated annexin-V for 30 min at room temperature. Four hundred microliters of Tris-buffered saline with calcium were added to the plasma, and the sample was then analyzed by flow cytometry, counting the number of fluorescent events in 150 s. To determine whether the patient’s TF-bearing microvesicles originated from monocytes or macrophages, we used dual-label flow cytometry with a phycoerythrin (PE)-conjugated anti-CD14 antibody (Becton Dickinson, Franklin Lakes, NJ, USA) and a FITC-conjugated anti-TF monoclonal antibody (mAb) (American Diagnostica).
TF activity
TF-VIIa activity was measured using a chromogenic assay based on the one described by Bogdanov et al. [10]. Briefly, 50 µL of cell-free plasma were incubated for 10 min at 37 °C with 10 nm FVIIa, 100 nm FX, and 1 mm FXa-specific chromogenic substrate (DiaPharma, West Chester, OH, USA), in the presence of 5 mm CaCl2. The background signal was determined in the absence of chromogenic substrate, and was subtracted from all measurements. After 30 min, absorbance at 405 nm was measured using a spectrophotometer.
Tissue factor pathway inhibitor
Plasma TF pathway inhibitor (TFPI) was measured by ELISA, as described previously [11].
Immunohistochemistry
Paraffin-embedded tissue specimens were stained with hematoxylin and eosin (H&E), and were processed for immunohistochemistry for TF using 2 µg mL−1 rabbit anti-TF polyclonal antibody. As a control, 2 µg mL−1 non-immune rabbit IgG was used instead of the anti-TF antibody.
Results
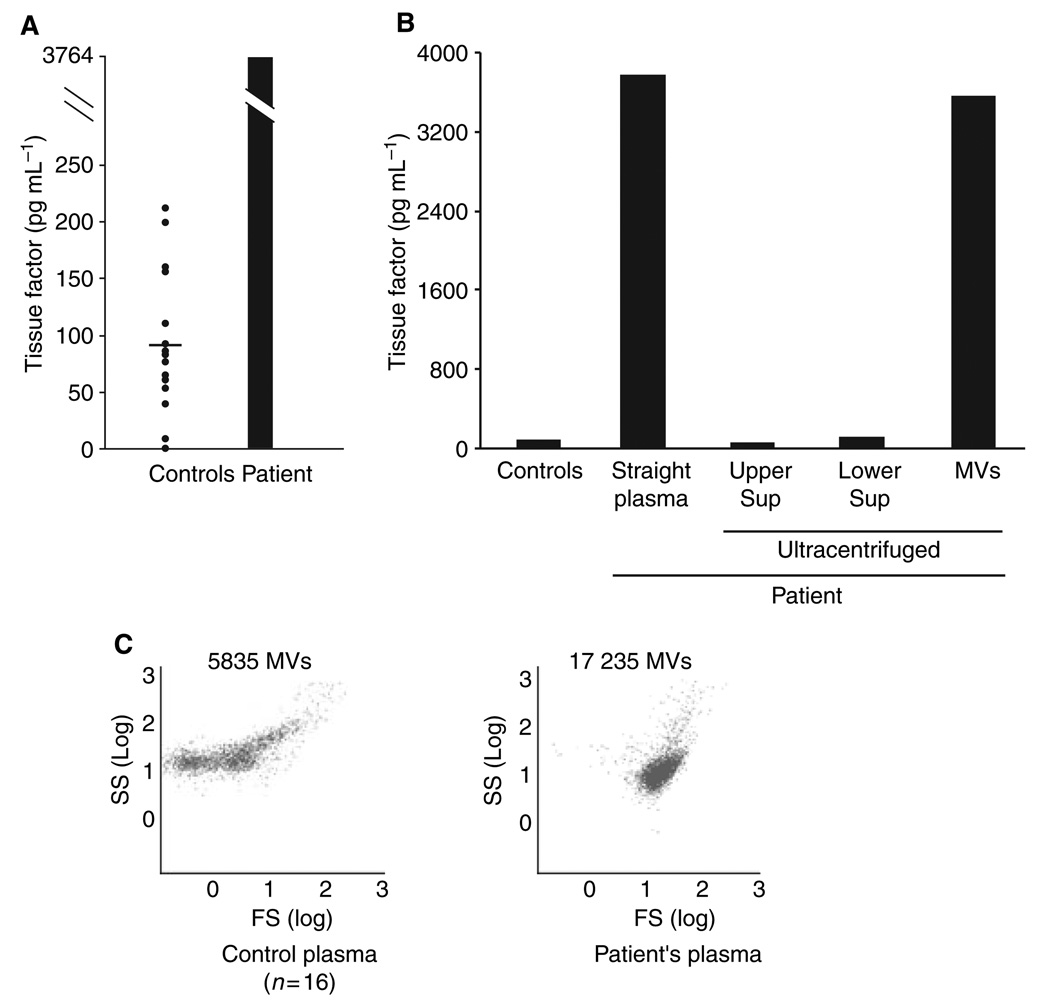
To gain insight into this patient’s relentless thrombosis, we analyzed his blood for the presence of TF. We measured TF in the patient’s cell-free plasma and in the plasma from 16 healthy males (mean age, 45.8 ± 11 years). The TF plasma concentration in the controls was 90.8 ± 62.2 pg mL−1 (±SD). The TF level in the patient’s plasma was 3764 pg mL−1, forty-one-fold higher than the mean control value (Fig. 1A). Ninety-four percent of the patient’s TF was associated with microvesicles (Fig. 1B). The microvesicle concentration in the patient’s plasma was also markedly elevated (17 235 events in 150 s vs. 5835 in control plasma) (Fig. 1C).
Fig. 1.
Tissue factor (TF) levels in plasma. Using enzyme-linked immunoassay, we measured TF antigen in the cell-free plasma of 16 healthy men and in that of the patient. Panel A shows the plasma level of TF in healthy subjects and in the patient. The horizontal bar indicates the mean value for the normal group. Panel B shows the distribution of TF in the patient’s plasma. The plasma was centrifuged (200 000 g × 5 h), and the TF levels were measured in the upper and lower halves of the supernatant (Sup), and in the microvesicle (MV) pellet at the bottom of the tube. Almost all of the TF localized to the MV fraction. The flow cytometry dot plot in Panel C shows the MV counts in the control and patient’s plasma, each dot representing a single annexin-V-positive microvesicle. The plasmas were diluted 1:5 in buffer, and the number of microvesicles was counted over 150 s.
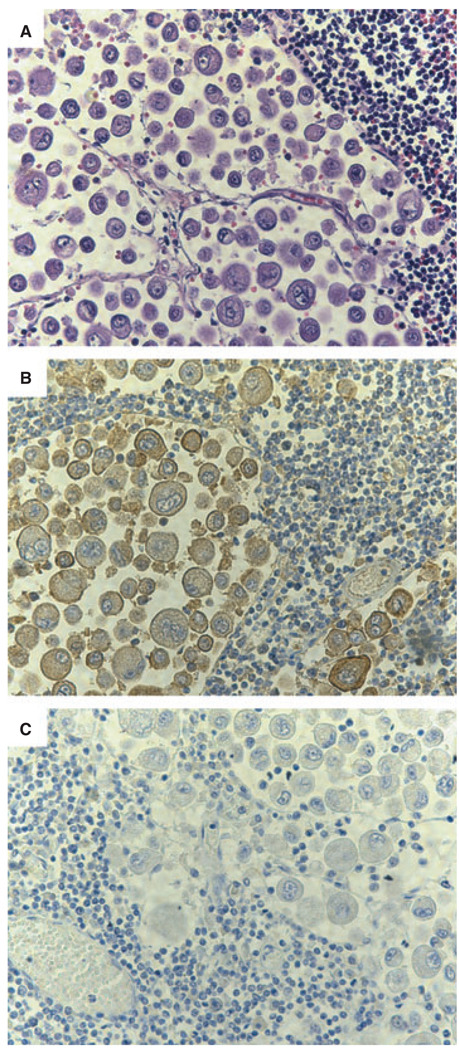
Because TF may be functionally cryptic, TF antigen may not always correlate with TF-VIIa activity [12]. We therefore measured the TF-VIIa activity in the patient’s plasma, and found it to be markedly greater than the average value for controls (Fig. 2A). The TF-VIIa activity in the patient’s plasma was greatly reduced by an anti-TF mAb (Fig. 2A). In contrast to the TF results, the concentration of TFPI, the major inhibitor of the TF pathway, in the patient’s plasma was well within the normal range: 16 ± 4.1 ng mL−1 in the plasma of healthy controls, and 13 ng mL−1 in the patient’s plasma (Fig. 2B). Lastly, we investigated the cellular origin of the TF-bearing microvesicles in the patient’s plasma. The two likeliest sources of the TF-bearing microvesicles were the cancer cells and monocytes/macrophages, the latter being the only cells within the vascular space conclusively shown to express TF in vivo [13].Analyzed by flow cytometry, only a small proportion of TF-positive microvesicles stained for CD14 (data not shown), while the patient’s monocytes did stain with the same anti-CD14 antibody, rendering it unlikely that the TF-bearing microvesicles arose from monocytes or macrophages. We therefore examined the patient’s cancer cells for the presence of TF. Tumor cells from a lymph node stained intensely for TF (Fig. 3). Further, the blood vessels infiltrating the tumor also contained cell-free material that stained intensely for TF. These studies strongly suggest that the microvesicle-associated TF in the patient’s blood originated primarily from the cancer.
Fig. 2.
Tissue factor (TF)-VIIa activity and tissue factor pathway inhibitor (TFPI) levels in plasma. Panel A shows the TF-VIIa activity in the plasma of controls and of the patient, measured using a chromogenic assay. Whereas treatment of the patient’s plasma with a control IgG had no effect on the TF-VII activity, an anti-TF monoclonal antibody markedly decreased it. The addition of anti-TF antibody to normal plasma had no significant effect on its TF-VIIa activity. Panel B shows the plasma levels of TF pathway inhibitor antigen in controls and in the patient. The horizontal bar indicates the mean value.
Fig. 3.
Tissue factor (TF) in a mediastinal lymph node with metastasis. During the autopsy of the patient, a mediastinal lymph node with a metastasic tumor was obtained. Panel A shows metastatic giant-cell lung carcinoma cells stained with hematoxylin and eosin. Panel B shows that the cancerous cells immunostained intensely (seen as a brownish color) with an anti-TF rabbit polyclonal antibody. As shown in panel C, the TF signal observed was specific because non-immune rabbit IgG gave very little background staining.
Discussion
TF has been reported in association with many types of cancers, including adenocarcinomas of the pancreas, stomach, colon, ovary, lung, and breast [7]. With the exception of leukemias, the mechanism by which TF from cancer cells gains access to the blood has not been clearly elucidated. In one patient with Trousseau’s syndrome, Callander and Rapaport observed tumor cells that stained intensely for TF invading a blood vessel and in direct contact with the blood [14]. This observation supported the long-standing conjecture that cancer cells within blood were responsible for the hypercoagulable state of cancer [15,16]. Our studies indicate that an additional mechanism for exposure of blood to TF is through membrane microvesicles. This mechanism was suggested a quarter of a century ago by Dvorak et al., [17] who demonstrated in 1981 that several mouse carcinoma cell lines shed procoagulant membrane microvesicles both in culture and into ascites when implanted in vivo. Nevertheless, only a few studies have reported the presence of microvesicles in the blood of cancer patients, and none, to our knowledge, have specifically investigated the role of TF-bearing microvesicles in Trousseau’s syndrome. We describe a patient who developed a severe form of Trousseau’s syndrome with an underlying giant-cell lung carcinoma and who had extraordinarily high concentrations of TF in his blood, mostly associated with microvesicles. As in previously reported cases [14], the thrombotic diathesis in this patient began long before there was any evidence of malignancy. However, certain aspects of this patient’s course suggest that the high TF level may not fully explain his clinical syndrome. For example, both animals that receive TF [18] and patients with sepsis [19] – a condition characterized by a high level of TF in blood – develop DIC rather than overt macrovascular thrombosis. It is thus somewhat surprising that the patient we report did not have full-blown DIC. A possible explanation for this is that most of the TF in the patient’s blood was cryptic [12], incapable of initiating coagulation. The TF-VIIa activity in his plasma was only 5-fold greater than in controls, despite the TF antigen level being forty-onefold higher. However, the activity measurement was complicated by the fact that the patient’s plasma contained heparin; the assay therefore likely grossly underestimated the TF activity in his plasma during those times when he was not receiving heparins.
A clue as to how TF-VIIa activity may be regulated comes from the study of monocyte-derived TF-bearing microvesicles. The TF-VIIa activity in these microvesicles increases when they interact with platelets [8,20]. Could a similar mechanism operate in the case we describe? Monocyte-derived TF-bearing microvesicles bind and fuse with activated platelets through a mechanism dependent on P-selectin on platelets and P-selectin glycoprotein ligand-1 (PSGL-1) on the monocyte microvesicles [8]. PSGL-1 is a membrane mucin [21] and, as with carcinoma mucins, its interaction with P-selectin is blocked by heparin [22]. Could mucins on the surface of cancer-derived TF-bearing microvesicles bind P-selectin on activated platelets and initiate thrombosis? Another intriguing possibility is that TF may play a role in Trousseau’s syndrome independent of its coagulant function. TF has been shown to also possess adhesive functions [23,24]. Could TF-bearing microvesicles cross-link other cells by virtue of their dense coating of TF, producing cell aggregates capable of occluding a vessel? Such a scenario would explain the relative inefficacy of oral anticoagulants in the treatment of Trousseau’s syndrome. Therapies targeted to the mechanisms of Trousseau’s syndrome will probably lead to more effective treatments of this frequently devastating condition. Anti-TF agents, including recombinant TFPI [25] and anti-TF monoclonal antibodies [26], are currently being tested in various clinical settings (e.g. sepsis and coronary heart disease). We believe that anti-TF therapies may prove to be particularly useful in cases of Trousseau’s syndrome with elevated blood TF levels.
Acknowledgments
This work was supported in part by grants 0325389Y from the American Heart Association (to I. del Conde) and NHLBI R01HL64796 (to J. A. López).
Footnotes
Disclosure of Conflict of Interests
References
- 1.Donati MB. Cancer and thrombosis. Haemostasis. 1994;24:128–131. doi: 10.1159/000217092. [DOI] [PubMed] [Google Scholar]
- 2.Trousseau A. Phlegmasia alba dolens. Clinique medicinale de lHotel-Dieu de Paris. London: The New Sydenham Society; 1865. [Google Scholar]
- 3.Wahrenbrock M, Borsig L, Le D, Varki N, Varki A. Selectin-mucin interactions as a probable molecular explanation for the association of Trousseau syndrome with mucinous adenocarcinomas. J Clin Invest. 2003;112:853–862. doi: 10.1172/JCI18882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell WR, Starksen NF, Tong S, Porterfield JK. Trousseau’s syndrome. Devastating coagulopathy in the absence of heparin. Am J Med. 1985;79:423–430. doi: 10.1016/0002-9343(85)90028-2. [DOI] [PubMed] [Google Scholar]
- 5.Sack GH, Jr, Levin J, Bell WR. Trousseau’s syndrome and other manifestations of chronic disseminated coagulopathy in patients with neoplasms: clinical, pathophysiologic, and therapeutic features. Medicine (Baltimore) 1977;56:1–37. [PubMed] [Google Scholar]
- 6.Falanga A, Gordon SG. Isolation and characterization of cancer procoagulant: a cysteine proteinase from malignant tissue. Biochemistry. 1985;24:5558–5567. doi: 10.1021/bi00341a041. [DOI] [PubMed] [Google Scholar]
- 7.Rao LV. Tissue factor as a tumor procoagulant. Cancer Metastasis Rev. 1992;11:249–266. doi: 10.1007/BF01307181. [DOI] [PubMed] [Google Scholar]
- 8.del Conde I, Shrimpton CN, Thiagarajan P, López JA. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106:1604–1611. doi: 10.1182/blood-2004-03-1095. [DOI] [PubMed] [Google Scholar]
- 9.Sims PJ, Faioni EM, Wiedmer T, Shattil SJ. Complement proteins C5b-9 cause release of membrane vesicles from the platelet surface that are enriched in the membrane receptor for coagulation factor Va and express prothrombinase activity. J Biol Chem. 1988;263:18205–18212. [PubMed] [Google Scholar]
- 10.Bogdanov VY, Balasubramanian V, Hathcock J, Vele O, Lieb M, Nemerson Y. Alternatively spliced human tissue factor: a circulating, soluble, thrombogenic protein. Nat Med. 2003;9:458–462. doi: 10.1038/nm841. [DOI] [PubMed] [Google Scholar]
- 11.Dietzen DJ, Jack GG, Page KL, Tetzlo TA, Hall CL, Mast AE. Localization of tissue factor pathway inhibitor to lipid rafts is not required for inhibition of factor VIIa/tissue factor activity. Thromb Haemost. 2003;89:65–73. [PubMed] [Google Scholar]
- 12.Bach RR. Initiation of coagulation by tissue factor. CRC Crit Rev Biochem. 1988;23:339–368. doi: 10.3109/10409238809082548. [DOI] [PubMed] [Google Scholar]
- 13.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134:1087–1097. [PMC free article] [PubMed] [Google Scholar]
- 14.Callander N, Rapaport SI. Trousseau’s syndrome. West J Med. 1993;158:364–371. [PMC free article] [PubMed] [Google Scholar]
- 15.Kenney WE. The association of carcinoma in the body and tail of the pancreas with multiple venous thrombi. Surgery. 1943;14:600–609. [Google Scholar]
- 16.McKay DG, Mansell H, Hertig AT. Carcinoma of the body of the pancreas with fibrin thrombosis and fibrinogenopenia. Cancer. 1953;6:862–869. doi: 10.1002/1097-0142(195309)6:5<862::aid-cncr2820060503>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 17.Dvorak HF, Quay SC, Orenstein NS, Dvorak AM, Hahn P, Bitzer AM, Carvalho AC. Tumor shedding and coagulation. Science. 1981;212:923–924. doi: 10.1126/science.7195067. [DOI] [PubMed] [Google Scholar]
- 18.Warr TA, Rao LV, Rapaport SI. Disseminated intravascular coagulation in rabbits induced by administration of endotoxin or tissue factor: effect of anti-tissue factor antibodies and measurement of plasma extrinsic pathway inhibitor activity. Blood. 1990;75:1481–1489. [PubMed] [Google Scholar]
- 19.McKay DG. Clinical significance of intravascular coagulation. Bibl Haematol. 1983;49:63–78. doi: 10.1159/000408448. [DOI] [PubMed] [Google Scholar]
- 20.Falati S, Liu Q, Gross P, Merrill-Skolo G, Chou J, Vandendries E, Celi A, Croce K, Furie BC, Furie B. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J Exp Med. 2003;197:1585–1598. doi: 10.1084/jem.20021868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore KL, Stults NL, Diaz S, Smith DF, Cummings RD, Varki A, McEver RP. Identification of a specific glycoprotein ligand for P-selectin (CD62) on myeloid cells. J Cell Biol. 1992;118:445–456. doi: 10.1083/jcb.118.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koenig A, Norgard-Sumnicht K, Linhardt R, Varki A. Differential interactions of heparin and heparan sulfate glycosaminoglycans with the selectins. Implications for the use of unfractionated and low molecular weight heparins as therapeutic agents. J Clin Invest. 1998;101:877–889. doi: 10.1172/JCI1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller M, Albrecht S, Golfert F, Hofer A, Funk RH, Magdolen V, Flossel C, Luther T. Localization of tissue factor in actin-filament-rich membrane areas of epithelial cells. Exp Cell Res. 1999;248:136–147. doi: 10.1006/excr.1999.4395. [DOI] [PubMed] [Google Scholar]
- 24.Randolph GJ, Luther T, Albrecht S, Magdolen V, Muller WA. Role of tissue factor in adhesion of mononuclear phagocytes to and trafficking through endothelium in vitro. Blood. 1998;92:4167–4177. [PubMed] [Google Scholar]
- 25.Abraham E, Reinhart K, Opal S, Demeyer I, Doig C, Rodriguez AL, Beale R, Svoboda P, Laterre PF, Simon S, Light B, Spapen H, Stone J, Seibert A, Peckelsen C, De Deyne C, Postier R, Pettila V, Artigas A, Percell SR, et al. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. JAMA. 2003;290:238–247. doi: 10.1001/jama.290.2.238. [DOI] [PubMed] [Google Scholar]
- 26.Morrow DA, Murphy SA, McCabe CH, Mackman N, Wong HC, Antman EM. Potent inhibition of thrombin with a monoclonal antibody against tissue factor (Sunol-cH36): results of the PROXIMATE-TIMI 27 trial. Eur Heart J. 2005;26:682–688. doi: 10.1093/eurheartj/ehi094. [DOI] [PubMed] [Google Scholar]