Abstract
Objective
To assess retrospectively 30-day, 1-year and 3-year patency of chronically occluded iliofemoral venous thrombotic lesions treated with stent placement in a case series from our institution.
Patients and methods
Records of 189 consecutive patients treated by interventional radiology for iliofemoral venous occlusions between March 01, 2003 and December 01, 2008 were retrospectively reviewed. 89 patients (27 males, median age 46.2 years) with chronic iliac or iliofemoral deep vein thrombosis without involvement of the inferior vena cava met criteria for analysis.
Results
89 patients (91 limbs) successfully underwent placement of venous self-expanding stents. Discharge patency was 100%. Following the index procedure, the mean pressure gradient across the lesion decreased from 5.63 mmHg (95% CI: 3.51 – 7.75) to 0.71 mmHg (95% CI: 0.08 – 1.34) mmHg (p<0.0001). There were no bleeding complications. Median follow-up was 11.3 months (range 0.8 – 72.4). Follow-up at 30 days demonstrated 90 of 91 limbs patent. Primary patency of treated limbs at 1 and 3 years was 81 and 71% respectively. Primary patency was lost in 17 (19.1%) cases; interventions to maintain or restore stent patency were performed in 13 (14.6%) cases. Primary assisted limb patency at 1 and 3 years was 94% and 90% respectively; secondary patency was 95%.
Conclusion
Angioplasty with stent placement for treatment of chronically thrombosed iliofemoral veins is a low risk procedure with acceptable patency rates for up to 3 years. The outcomes in patients treated in a quaternary referral center are similar to those reported by other centers.
Keywords: iliac vein occlusion, femoral vein occlusion, deep venous thrombosis, venous thrombosis, DVT, postthrombotic syndrome, chronic venous insufficiency, May-Thurner syndrome, venous disease, vascular disease
Post-thrombotic syndrome (PTS) is a common complication of deep venous thrombosis (DVT) observed in up to 40% of patients following an episode of DVT. Even when anticoagulant therapy and elastic compression stockings are regularly used, 25% of proximal DVT patients will develop PTS.(1, 2) Extensive thrombosis of iliofemoral veins portends a greater than twofold risk of recurrent DVT compared to thrombosis without iliac segment involvement.(3) Iliac vein thrombosis will result in disabling venous claudication in a substantial number of patients and those with thrombosis involving the iliac venous segments are even more prone to developing PTS compared to patients with thrombus in the more distal locations.(4-6) Moreover, recurrent, ipsilateral DVT has been shown to be an important predictor of the PTS(7), which results in significant disability and worsened quality of life.(8) Optimal treatment of iliac vein thrombosis to date remains a subject of discussion as it has not been adequately studied in randomized clinical trials.(9) Some DVT cases never result in recanalization with conservative treatment and end-up as chronic occlusions, contributing to the prevalence of PTS.
To re-establish venous patency, placement of stents is now performed to recanalize chronically occluded iliac and common femoral veins.(5, 10, 11) Among possible clinical benefits may be decreased incidence and severity of PTS.(5, 12) While the cumulative experience of iliofemoral venous stent placement continues to increase, our quaternary referral center routinely sees many complex patients. It was assumed that even in these difficult patients with chronic venous thrombosis, endovascular stents can offer meaningful benefits. The purpose of this study was to retrospectively assess 30-day, 1-year and 3-year patency of chronically occluded iliac and femoral venous thrombotic lesions treated with stent placement in a case series from our institution. Considering that many our patients are referred after it is felt that treatment options are exhausted, we were interested to compare patency rates in our institution to those reported by other authors.
Materials and methods
Patient selection
The study was approved by the Mayo Clinic Institutional Review Board. Consecutive cases of technically successful recanalization of chronically thrombosed iliac and femoral veins from March 01, 2003 through December 01, 2008 were identified through review of the Vascular and Interventional Radiology Database (Hi-iQ, ConexSys, Lincoln, RI). Key inclusion criteria were age greater than 18 years, confirmed uni- or bi-lateral iliac or iliofemoral thrombotic occlusions of over 30-day duration, treated with percutaneous iliac or iliofemoral venous stents. Only patients who underwent successful recanalization procedure were included. Using the current method, we had no ability to capture the scale and number of unsuccessful cases. Index procedure was angioplasty and stent placement of the chronically thrombosed iliac veins and/or common femoral veins performed at our institution regardless of the duration of symptoms, time from initial diagnosis, or previous attempts at recanalization. Excluded were the cases of acute thrombosis (except for acute-on-chronic cases of previously diagnosed iliac vein thrombosis); cases involving IVC occlusions; extrinsic compression by a tumor or aneurysm; retroperitoneal fibrosis; polycystic liver disease since many of them have additional etiological factors, different from the more common lower extremity DVT cases, and likely a different natural history. In addition, excluded were the cases that required combined surgical and endovascular approach or cases encompassing prior stent placement at outside institutions since the focus of this study was to evaluate the primary endovascular approach. Finally, cases with absence of at least one follow-up with lumen patency assessment by either a venogram or ultrasound were also excluded.
All patients were routinely scheduled for follow-up at 3 months, 6 months, 12 months, and annually thereafter (emergently if acute loss of patency was suspected). Follow-up consisted of patency assessment of the treated veins by ultrasound or venography, clinical evaluation, and, in select cases, strain-gauge venous plethysmography. Patients were followed for the maximum time length possible up to 2011. Patency data were based on the results of imaging studies (ultrasound or venography).
Data abstraction
Data were abstracted from the medical records by 3 independent abstractors (AK, SM, HB) who reviewed procedural, imaging and laboratory reports, clinical notes from vascular physicians and other specialists. Records were analyzed for clinical descriptors, such as pain, edema, and stasis ulcers. Noninvasive vascular laboratory data, including exercise and outflow venous plethysmography, passive drainage and refill, and continuous wave Doppler readings were abstracted from vascular laboratory reports. Procedural characteristics, such as stent details, approaches, specific techniques, and anatomical descriptors were analyzed. Records were reviewed for comorbidities, such as cancers or hypercoagulable status, and peri-procedural placement of inferior vena cava (IVC) filters. When only the month of the initial diagnosis or symptom onset was known, the date was entered as the first day of that month; for remote cases when only the year was recalled by the patient, the date was recorded as January 1st of that year. When transduced pressures were documented as a fluctuation between two adjacent numbers, the lower numbers for both pre- and post-procedural gradients was used. All questionable cases were reviewed collectively.
Procedure
The procedure was performed with local anesthetics and intravenous sedation. Right internal jugular vein or ipsilateral common femoral vein access was used. Pressure gradient between the IVC and the distal open vein was measured when possible and as soon as the occlusion had been traversed. The tract was pre-dilated using a 12 or 14 mm balloon. An effort was made to cover the entire diseased iliac and common femoral venous segment with stents and in most cases 14 mm diameter stents were used, but a few patients had smaller and/or larger stents placed at the operator’s discretion. In the central common iliac vein (CIV), the stents were extended approximately 1 cm into the IVC bifurcation, but avoiding coverage of the contralateral iliac vein ostium. The stents were then dilated using the same diameter balloon and a venogram was repeated. Finally, pressure measurements were obtained between the IVC bifurcation and the distal end of the stented segment.
During the procedure the patients were fully anticoagulated. Typically, unfractionated heparin was given as a bolus of 5000 units followed by additional doses with a target activated clotting time (ACT) of 300 seconds. Immediately following the procedure, the patients were started on therapeutic doses of low molecular weight heparin and warfarin. Therapeutic doses of warfarin were continued with a target INR range of 2.0 to 3.0 for no less than 2 months. Patients with known hypercoagulability, poor inflow, or history of extensive or recurrent thrombotic episodes were kept on anticoagulation longer according to current guidelines.(13)
Clinical and imaging follow-up was obtained at 3, 6, and 12 months, and annually thereafter. Routine imaging follow-up was with ultrasound which was considered jointly with clinical assessment. Venography was performed in cases where patency was compromised (defined as luminal diameter reduction of ≥50%) based on the combination of ultrasound assessment and clinical picture. Intervention was performed if venography revealed a 50% diameter narrowing of the stent.
Statistical analysis
Cox proportional hazards analysis was performed to test the year of intervention, age at the procedure, and gender as covariates in the model of primary patency survival. The patency was evaluated using Kaplan-Meier method. Matched pairs t-test was used to analyze changes in the pressure gradients across the lesion before and after the index procedure. Chi-square analysis was used for categorical variables. Last-Observation-Carried-Forward (LOCF) method was used for patients who eventually stopped following-up. The database was set up to capture only patients with successful recanalization thus precluding intention-to-treat analysis.
Results
An initial group of 189 consecutive patients who underwent successful recanalization with angioplasty and stent placement of occluded iliac and common femoral veins during the specified time frame was identified. Most patients were referred for chronic symptoms clinically consistent with post-thrombotic syndrome (venous claudication, stasis ulcers, and hemosiderine depositions, etc. after DVT); some patients had venous outflow obstruction confirmed by non-invasive vascular studies. After applying exclusion criteria (see Figure 1), 69 cases with IVC involvement, 5 cases of non-IVC abdominal tumor compression, 2 cases of prior recanalization elsewhere, 10 combined surgical-endovascular cases, and 14 cases lacking imaging follow-up were excluded. A total of 89 cases were selected for analysis. Patient demographics are summarized in Table 1. Median age at symptom onset was 42.2 (Interquartile range, IQR 26.6 – 51.2) years (men 40.4; women 42.7). Median age at index procedure was 46.2 years (IQR 38.0 – 54.0) (men 47.2; women 46.0). Two patients had procedures performed in the setting of cancers: 1 with actively treated lung and colon cancers; 1 with renal cell carcinoma in remission. Two patients received a diagnosis of cancer within a year following the index procedure (papillary thyroid; urothelial cell carcinoma). Eleven patients (12.4%) had history of pulmonary embolism with IVC filter placement before the index procedure; 1 patient with a repeat acute DVT had a filter placed prophylactically after the index procedure. Twenty seven patients (30.3%) had a confirmed diagnosis of thrombophilia (see Table 1).
Figure 1.
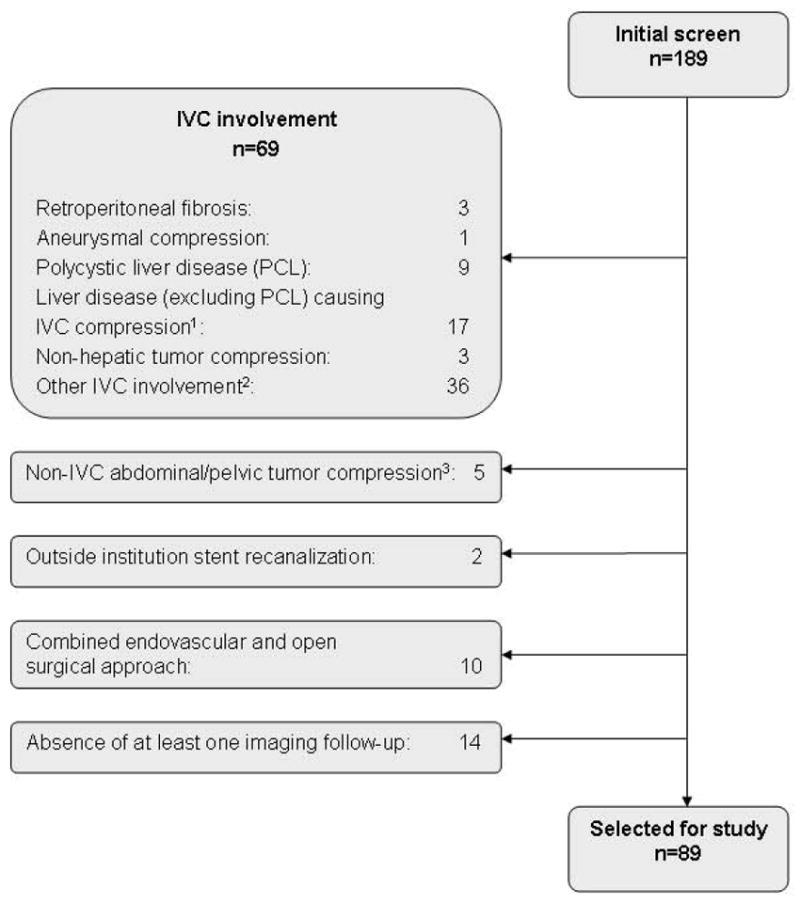
Patient Identification
Table 1.
Patient demographics
| Total: | Men: N (%) | Women: N (%) | |
|---|---|---|---|
| 89 | 27 (30) | 62 (70) | |
| Total | Men | Women | |
| Age at symptom onset (yrs)1 | 42.15 (26.54; 51.15) | 40.36 (29.31; 51.38) | 42.66 (25.92; 51.15) |
| Age at diagnosis (yrs) | 44.06 (30.09; 52.38) | 44.83 (32.10; 54.17) | 43.58 (26.55; 51.82) |
| Age at index procedure (yrs) | 46.16 (37.98; 83.97) | 47.17 (37.62; 55.86) | 45.94 (37.40; 52.78) |
| Malignancy within 1 year of index procedure, N (%) | 4 (4.5) | ||
| History of pulmonary embolism, N (%) | 11 (12.4) | ||
| IVC filter placement | 12 (13.5) | ||
| Confirmed coagulopathies, total (N,%) | 27 (30) | ||
| Prothrombin G20210 | 1 (1) | ||
| Prothrombin G20210 and Factor V Leiden heterozygosity | 1 (1) | ||
| Factor V Leiden heterozygosity | 12 (13) | ||
| Factor V Leiden homozygosity | 2 (2) | ||
| Hyperhomocysteinemia | 1 (1) | ||
| Hyperhomocysteinemia with sickle cell trait | 1 (1) | ||
| Positive lupus anticoagulant / Antiphospholipid syndrome | 5 (6) | ||
| Protein C deficiency | 2 (2) | ||
| Protein S deficiency | 1 (1) | ||
| Unspecified thrombophilia | 1 (1) |
Median and interquartile range.
Most cases were unilateral (87; 97.8%) and involved the veins of the left leg (81; 91%); 2 cases (2%) involved both sides (Table 2). Iliac segments only were involved in 35 (39.3%) cases; iliofemoral segments were involved in 54 (60.7%) cases.
Table 2.
Primary patency.
| Number of cases with stenting (index procedure) n (%) |
Primary patency length (mo.)1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Combined across all years | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | |||
|
| |||||||||
| Total | 89 | (n=89) | (n=12) | (n=8) | (n=18) | (n=16) | (n=18) | (n=17) | |
| 11.31 (4.09; 28.47) | 43.26 (16.88; 61.13) | 10.71 (5.22; 38.72) | 11.03 (2.21; 42.58) | 8.14 (3.84; 29.13) | 12.40 (5.53; 22.33) | 7.76 (3.26; 15.83) | |||
|
| |||||||||
| Right lower extremity | 6 (7) | (n=6) | (n=1) | - | - | (n=2) | - | (n=3) | |
| 6.69 (3.96; 14.12) | 5.62 | 19.30 (8.94; 29.66) | 4.93 (1.05; 7.76) | ||||||
|
| |||||||||
| Left lower extremity | 81 (91) | (n=81) | (n=11) | (n=8) | (n=17) | (n=14) | (n=18) | (n=13) | |
| 13.32 (4.1; 29.89) | 54.84 (23.24; 62.20) | 10.72 (5.22; 38.72) | 11.31 (2.55; 43.56) | 6.86 (3.03; 29.63) | 12.40 (5.53; 22.33) | 8.71 (3.26; 15.83) | |||
|
| |||||||||
| Bilateral | 2 (2) | (n=2) | |||||||
| 9.86 (1.64; 18.08) | |||||||||
Median (interquartile range)
Follow-up at 30 days demonstrated 90 of 91 limbs patent. Primary patency of treated limbs at 1 and 3 years was 81% and 71% respectively. In the statistical model, the year of the intervention, age at the time of the procedure, and gender were not significant predictors of patency (p=0.89; p=0.12; p=0.42 respectively). Median primary patency of treated limbs appeared longest in the patients operated in the first year of the study (43.3 months) because of the follow-up lead time. Combined across all years, median primary patency was 11.3 months (range 0.78 – 72.5), limited mostly by the duration of follow-up, and was greater in cases involving only the left lower extremity (13.3 months) compared to the right lower extremity-only or bilateral cases (6.7 and 9.9 months respectively). However, the study did not have sufficient power to perform a meaningful comparison of outcomes between interventions on the right and left sides.
Table 3 summarizes data on the number and length of stents and the anatomical segments intervened on. During the index procedure, 225 stents were placed in 91 limbs. Additional 7 stents were placed in assist procedures. In the early months of the study period, primarily Wallstents (Boston Scientific Scimed, Maple Grove, MN) (n=36) were used, but later on most stents were nitinol self-expandable ones: Protégé (ev3 Inc., Plymouth, MN, USA) (140 stents), Smart (Cordis Endovascular, Warren, NJ) (n=48), and Luminexx (Bard Peripheral Vascular, Tempe, AZ) (1 stent). In most limbs, the stents were 14 mm in diameter or larger (n=65, 71.42%). In 4 limbs (4.40%), stents were 10 mm in combination with larger stents. In 18 limbs (19.78%), a combination of 12 mm and larger stents was used. Only 4 limbs (4.40%) were stented with 16 mm diameter stents.
Table 3.
Stenting per limb and segment.
| Extremity | Number of cases | Number of stents | Average number of stents per case | Segments stented | |
|---|---|---|---|---|---|
|
| |||||
| RLE only | 6 | 16 (first procedure only) | 2.66 | RCIV | 6 |
|
| |||||
| REIV | 6 | ||||
|
| |||||
| RCFV | 6 | ||||
|
| |||||
| LLE only | 81 (first procedure) | 199 (first procedure) | 2.46 | LCIV | 77 |
|
| |||||
| LEIV | 66 | ||||
|
| |||||
| 4 (second procedure) | 7 (second procedure) | 1.75 | LCFV | 46 | |
|
| |||||
| Bilateral | 2 | 10 (first procedure only) |
|
RCIV | 2 |
|
| |||||
| REIV | 2 | ||||
|
| |||||
| RCFV | 1 | ||||
|
| |||||
| LCIV | 1 | ||||
|
| |||||
| LEIV | 2 | ||||
|
| |||||
| LCFV | 2 | ||||
Changes of the pressure gradients across the lesions could be reliably analyzied only in 27 (30.3%) patients since pressures could not be transduced through chronically occluded segments. The mean recorded pressure gradient was 5.63 mmHg (95% CI: 3.51 – 7.75) before the index procedure and 0.71 mmHg (95% CI: 0.08 – 1.34) following recanalization and stent placement, indicating a highly significant change of -4.9 mmHg (CI: -2.84 to -7.0; p<0.0001).
Of the 89 patients, 17 (19.1%) developed image-confirmed stenosis or occlusion of the recanalized segment. Of these, 11 (12.4%) were found to have stenosis of the treated segments and primary assisted patency was esablished. Eight (9.0%) of them received repeat angioplasty only; 3 (3.4%) had additional stents placed. Subsequently, 3 (3.4%) of these 11 patients were lost to follow-up but the remaining 8 (9.0%) had primary assisted patency preserved throughout the last follow-up. Primary assisted patency was 94% at 1 year, and 90% at 3 years. (see Figure 3)
Figure 3.
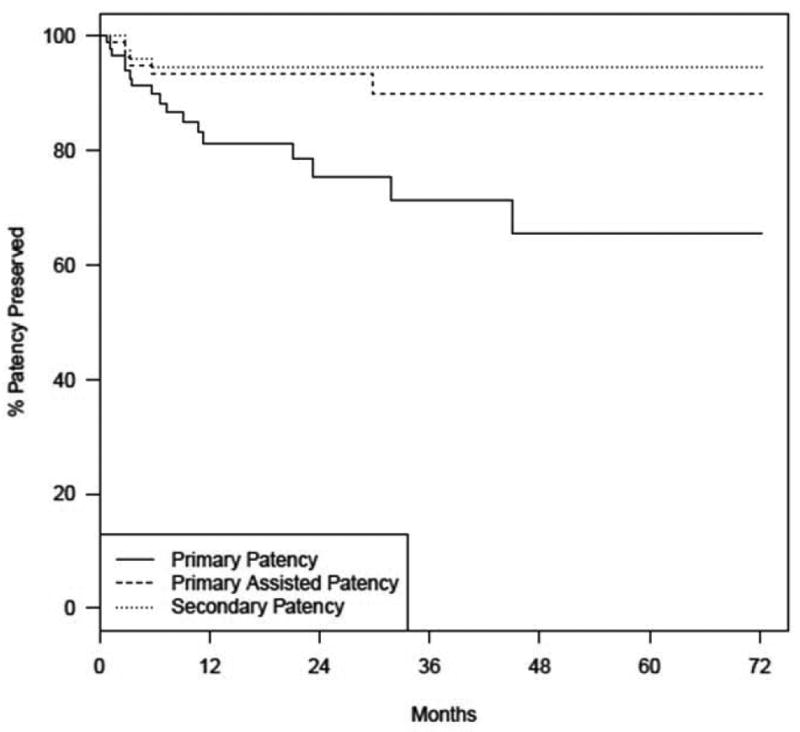
Patency survival curves
Six of the 17 patients developed occlusions of the treated segments. Two of these patients underwent successful recanalization of the veins but were all lost to follow-up. One patient had an unsuccessful attempt at recanalization. The remaining 3 patients either declined, were not available for, or were not deemed to benefit further from a repeat intervention. (see Figure 2) Secondary patency was 95% at years 1 and 3. Figure 3 shows the Kaplan-Meier survival curves of the primary patency after the index procedure compared with primary assisted and secondary patency survival.
Figure 2.
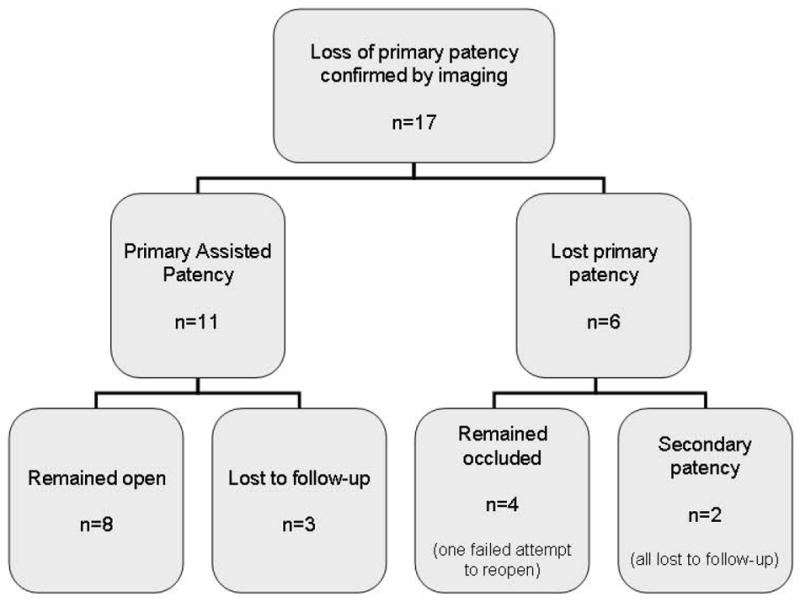
Confirmed Loss of Patency
Early complications were limited to acute recurrent thrombosis on post-operative day 30 in 1 patient. An attempt to restore flow with thrombolysis and balloon thrombectomy was unsuccessful with persistent flow via collaterals. No patients had postoperative hematomas or bleeding complications.
Given the limitations of time for many of the referred patients with well established diagnoses, often only limited assessment had been performed prior to the intervention. Subsequently, precise venous severity score changes could not be calculated in those cases. However, clinical descriptors suggested overall improvement after the procedure up to one year. As a result, documented reports of pain decreased from 43 (48%) cases before the intervention to 25 (28%) cases after the intervention, i.e. symptom report reduction of 58% (95% CI: 43 – 72). Ulcer reports decreased from 17 (19%) cases to 8 (9%) cases after the intervention; symptom frequency reduction of 47% (95% CI: 26 – 69). Edema reports decreased from 59 (66%) cases to 36 (40%) cases after the intervention; symptom frequency reduction of 61% (95% CI: 48 – 72). There was only 1 patient with a report of edema after the intervention with no documentation of this complaint before the intervention. Edema was better controlled in patients who consistently used compression garments. While many patients had repeat follow-ups that included clinical assessment and vascular laboratory tests, only 34 (38%) patients had vascular laboratory assessment completed both before and after the index procedure. Based on these data, venous incompetence based on venous plethysmography data remained unchanged in 20, improved in 7 and worsened in 7 patients, suggesting no overall change. Venous obstruction remained unchanged in 18 patients, definitely improved in 8, possibly improved in 4; it possibly worsened in 1, and definitely worsened in 3 patients, suggesting a modest improvement.
Discussion
Recanalization of chronically thrombosed iliofemoral veins through angioplasty and stent placement is a viable procedure. The limited time of follow-up and patient attrition in this study likely lead to underestimation of the primary patency. Patency rates in our series for the duration of the study (over 72 months) are similar to those reported earlier. In a slightly older group (median age 54 years), Neglen et al. reported post-stent patency in thrombotic venous disease at 72 months to be 57%, 80%, and 86% for primary, primary assisted and secondary patency respectively. Patency rates in non-thrombotic disease were markedly (14-22%) higher.(5) Titus et al. reported similar patency rates and thrombophilia in a group of mixed acute and chronic thrombotic iliofemoral venous occlusive disease patients.(14) Overall, stent placement for treatment of thrombosed venous lesions appears durable for at least 3 years. Most cases of primary patency loss occur within the first year of follow-up. Therefore, we feel that the chosen periodic interval schedule of imaging follow-up at 1, 3, 6 and 12 months, and afterwards annually or sooner, based on changes in clinical symptoms, was reasonable.
With the use of catheter directed thrombolysis, the risk of major hemorrhagic complications is relatively low. Whether all patients with a history of proximal DVT irrespective of symptoms should undergo imaging of iliac veins for potential endovascular treatment remains unclear. Recently published early results of randomized controlled CaVenT trial(15) showed significantly better short-term patency of iliofemoral segment in patients after catheter-directed thrombolysis compared to those treated with anticoagulation alone. ATTRACT, another randomized controlled trial designed to evaluate catheter-directed thrombolysis to prevent (PTS), is currently recruiting patients with iliac and femoral venous thrombosis.(16) However, both trials aim to evaluate clinical outcomes of endovascular treatment for acute venous thrombosis (<21 days of symptoms). Chronically thrombosed cases may present a greater challenge.
In this study, while in general post-procedural outcomes indicated clinical improvement, retrospective review of existing records limited the precision of evaluation of relevant clinical parameters that define venous clinical severity score (VCSS) and CEAP class.(17, 18) It is plausible that frequency and severity of pain and stasis ulcerations decreased thanks to reduced venous pressure through minimized outflow obstruction. These changes were more notable than reduction of edema as the latter would persist in the absence of competent valves and would not be corrected by stents. To arrive at reliable conclusions about clinical outcomes, a concerted effort should be applied to assess functional and clinical parameters in prospective studies.
This retrospective study has multiple limitations. Many of our patients travel from afar in anticipation of an expeditious treatment procedure and with a previously well established diagnosis. Therefore, pre-procedural evaluations were limited and ollow-ups were more problematic in many cases fraught with assessment and follow-up biases. Recall bias was hard to eliminate. Exclusion of patients without at least one imaging follow-up after the index procedure (typically occurred in patients who traveled far back home) certainly affected our estimates. Due to travel distance and cost involved, some of the patients after initial improvement and follow-up, eventually stop coming. This made us rely on the Last-Observation-Carried-Forward (LOCF) method with its own limitations. Changes of pressure gradients across lesions before and after the procedure were hard to document in many cases. Nonetheless, they were highly statistically significant. In most cases, only the start date of medical therapy was documented, but not the discontinuation date, which made it hard to assess ongoing anticoagulation compliance. Thrombophilia status is prone to diagnostic suspicion bias as these expensive tests are not performed routinely. Important risk factors, such as malignancies, pregnancy, and hormonal use, and other potential confounders could not be reliably ascertained without access to outside records in patients who did not receive regular care in our clinic.
In summary, angioplasty with stent placement for treatment of chronically thrombosed iliofemoral veins is a low risk procedure with acceptable patency rates for up to 3 years. It may provide symptomatic improvement in a significant number of patients for up to one year. The outcomes in patients treated in a quaternary referral center are similar to those reported by other centers.
Acknowledgments
Financial Support:
This project was supported by NIH/NCRR CTSA Grant Number UL1 RR024150. Andrew K. Kurklinsky, M.D. is a vascular medicine fellow at Mayo Clinic, Rochester, MN, supported by NIH Grant Number K12HL083797.
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Conflicts of interest:
Dr. Kurklinsky and Dr. Bjarnason received an unrestricted educational grant from ev3.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prandoni P, Lensing AWA, Prins MH, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med. 2004;141:249–256. doi: 10.7326/0003-4819-141-4-200408170-00004. [DOI] [PubMed] [Google Scholar]
- 2.Vedantham S. Valvular dysfunction and venous obstruction in the post-thrombotic syndrome. Thromb Res. 2009;123(Suppl 4):S62–65. doi: 10.1016/S0049-3848(09)70146-X. [DOI] [PubMed] [Google Scholar]
- 3.Douketis JD, Crowther MA, Foster GA, et al. Does the location of thrombosis determine the risk of disease recurrence in patients with proximal deep vein thrombosis? Am J Med. 2001;110:515–519. doi: 10.1016/s0002-9343(01)00661-1. [DOI] [PubMed] [Google Scholar]
- 4.Delis KT, Bountouroglou D, Mansfield AO. Venous claudication in iliofemoral thrombosis: long-term effects on venous hemodynamics, clinical status, and quality of life. Ann Surg. 2004;239:118–126. doi: 10.1097/01.sla.0000103067.10695.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neglen P, Hollis KC, Olivier J, et al. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg. 2007;46:979–990. doi: 10.1016/j.jvs.2007.06.046. [DOI] [PubMed] [Google Scholar]
- 6.Comerota AJ, Throm RC, Mathias SD, et al. Catheter-directed thrombolysis for iliofemoral deep venous thrombosis improves health-related quality of life. J Vasc Surg. 2000;32:130–137. doi: 10.1067/mva.2000.105664. [DOI] [PubMed] [Google Scholar]
- 7.Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1–7. doi: 10.7326/0003-4819-125-1-199607010-00001. [DOI] [PubMed] [Google Scholar]
- 8.Kahn SR, Shbaklo H, Lamping DL, et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost. 2008;6:1105–1112. doi: 10.1111/j.1538-7836.2008.03002.x. [DOI] [PubMed] [Google Scholar]
- 9.Wysokinska EM, Sobande F, Wysokinski WE, et al. Iliac vein thrombosis: feasibility assessment of randomized controlled trials of endovascular pharmacomechanical thrombolysis. J Thromb Haemost. 8:1943–1949. doi: 10.1111/j.1538-7836.2010.03968.x. [DOI] [PubMed] [Google Scholar]
- 10.Neglen P, Raju S. Balloon dilation and stenting of chronic iliac vein obstruction: technical aspects and early clinical outcome. J Endovasc Ther. 2000;7:79–91. doi: 10.1177/152660280000700201. [DOI] [PubMed] [Google Scholar]
- 11.Hartung O, Otero A, Boufi M, et al. Mid-term results of endovascular treatment for symptomatic chronic nonmalignant iliocaval venous occlusive disease. J Vasc Surg. 2005;42:1138–1144. doi: 10.1016/j.jvs.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Neglen P. Chronic venous obstruction: diagnostic considerations and therapeutic role of percutaneous iliac stenting. Vascular. 2007;15:273–280. doi: 10.2310/6670.2007.00071. [DOI] [PubMed] [Google Scholar]
- 13.Kearon C, Kahn SR, Agnelli G, et al. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(6 Suppl):454S–545S. doi: 10.1378/chest.08-0658. [DOI] [PubMed] [Google Scholar]
- 14.Titus JM, Moise MA, Bena J, et al. Iliofemoral stenting for venous occlusive disease. J Vasc Surg. 2011;53:706–712. doi: 10.1016/j.jvs.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Enden T, Klow NE, Sandvik L, et al. Catheter-directed thrombolysis vs. anticoagulant therapy alone in deep vein thrombosis: results of an open randomized, controlled trial reporting on short-term patency. J Thromb Haemost. 2009;7:1268–1275. doi: 10.1111/j.1538-7836.2009.03464.x. [DOI] [PubMed] [Google Scholar]
- 16.Acute Venous Thrombosis: Thrombus Removal With Adjunctive Catheter-Directed Thrombolysis (ATTRACT) National Institute of Health. Clinical Trial Identifier: NCT00790335 2010 [Google Scholar]
- 17.Eklof B, Rutherford RB, Bergan JJ, et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004;40:1248–1252. doi: 10.1016/j.jvs.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 18.Vasquez MA, Rabe E, McLafferty RB, et al. Revision of the venous clinical severity score: venous outcomes consensus statement: special communication of the American Venous Forum Ad Hoc Outcomes Working Group. J Vasc Surg. 52:1387–1396. doi: 10.1016/j.jvs.2010.06.161. [DOI] [PubMed] [Google Scholar]


