Abstract
We present a developmental perspective on the concept of phylotypic and phenotypic stages of craniofacial development. Within Orders of avians and mammals, a phylotypic period exists when the morphology of the facial prominences is minimally divergent. We postulate that species-specific facial variations arise as a result of subtle shifts in the timing and the duration of molecular pathway activity (e.g., heterochrony), and present evidence demonstrating a critical role for Wnt and FGF signaling in this process. The same molecular pathways that shape the vertebrate face are also implicated in craniofacial deformities, indicating that comparisons between and among animal species may represent a novel method for the identification of human craniofacial disease genes.
Keywords: phylotype, phenotype, Wnt, Fgf, neural crest
1. Introduction
It is the common wonder of all men, how among so many millions of faces, there should be none alike.
Sir Thomas Browne
The face is an animal’s calling card; distinctive, unique, and with a form that is embedded during the fetal period. Yet there is a prior stage in embryonic development when vertebrates share a common craniofacial morphology: Their facial prominences are arranged in a similar fashion, the sensory organs are positioned in comparable sites; even the cellular origins of the facial tissues are conserved among vertebrates [1, 2]. Karl Ernst von Baer was one of the first scientists to suggest that there was an embryonic stage when many vertebrate species resemble one another [3]. Over the intervening decades the definition of a phylotype (from the Greek phūlon, meaning class) has changed but it is now generally considered to be a developmental period characterized by reduced phenotypic divergence [4].
Since its initial description, the actual existence of a phylotypic stage has been the subject of intense debate and occasionally, experimentation. Opponents argue that the existence of a phylotypic stage has not been supported by comparative quantitative analyses [5], while proponents of the theory contend that morphological [6] and genome-wide [4, 7] data demonstrate a highly constrained period during which the body plan for vertebrates is established [8].
Even while the debate continues there are still useful ideas that arise from this concept of a phylotypic stage of development. For us, one particularly compelling feature of this theory is that among some vertebrates there is an embryonic period where craniofacial phenotypic divergence appears to be quite minimal. If the phylotype theory is true, then such a model provides a foundation on which to consider first, when and how the vertebrate craniofacial “bauplan” is established and second, to experimentally determine the molecular mechanisms that are responsible for generating facial variation between and among animal species. The identification of a phylotypic stage of craniofacial development (if one exists) would also be a powerful tool towards identifying genes that are responsible for craniofacial malformations because candidate disease genes are also most likely responsible for generating normal phenotypic variation in vertebrate facial features [9-11].
2. Defining a phylotypic period of craniofacial development
The most vigorous criticism of the phylotypic theory is that it represents a nebulous concept with poorly defined key characteristics. For example, one specific (and valid) criticism is that comparative approaches are rarely quantitative [5,12]. Therefore, in this review we will restrict ourselves to presenting data that provide a quantitative measure of phenotypic divergence in the face.
We begin with a comparison of birds. In our own studies the skulls of ducks (Anseriformes), quails and chickens (both Galliformes), and pigeons (Columbiformes), were skeletonized then subjected to scanning, and the resulting micro-CT images were used to generate three-dimensional models. The models were land-marked [13], and information regarding scale, position and orientation of the landmarks was removed from the data [14]. This new dataset contained information about face shape separate from the variable of size and these mean shape differences were then compared among the various bird species [14].
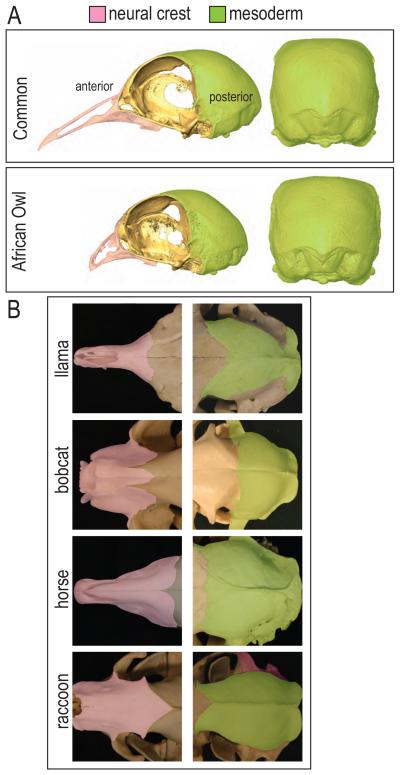
These quantitative analyses revealed that not all regions of the skull show equivalent phenotypic divergence. For example, the posterior head skeletons had very similar morphologies. In sharp contrast, the various anterior (facial) skeletons exhibited dramatically different morphologies. Even within a single species, Columbia livia (the domesticated pigeon), the posterior skull regions were consistent in shape while the anterior, facial skeletons exhibited remarkable variation (Fig. 1A). We examined a wide variety of mammalian skulls and made the same observation: there were minor shape differences in the posterior mammalian head skeleton yet enormous variability in the anterior skeleton (Fig. 1B).
Figure 1.
Phenotypic divergence of skull morphology. (A) Within a single species, Columbia livia, phenotypic divergence can be seen more dramatically among the neural crest derived anterior skull. (B) Within the mammals, the mesoderm derived posterior skull shows similar morphology while the anterior skull exhibits greater phenotypic divergence.
3. Defining the onset of phenotypic divergence in the face
Our quantitative approach demonstrated that the facial skeleton exhibits extreme phenotypic divergence in adulthood, but even embryos exhibit species-specific facial characteristics. Therefore, our next goal was to determine when this species-specific phenotypic divergence was first obvious and, by extension, identify when bird embryos show minimal phenotypic divergence.
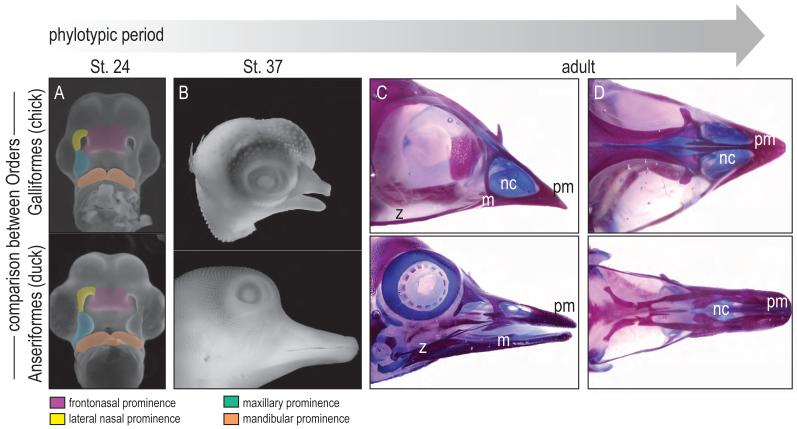
Using geometric morphometrics to analyze the facial prominences, we have found that prior to Hamilton Hamburger St. 24 the facial features of bird embryos show little phenotypic divergence (Fig. 2A, and see [15, 16]). After this embryonic time-point, species-specific differences in mean face shape are readily detectable (Fig. 2B and see [14]). Even if the variable of size is removed, these shape differences become exacerbated with age (Fig. 2C). Therefore, we can reasonably conclude that in avians, the beginning of phenotypic divergence occurs around St. 22-24 (Fig. 2). Prior to this stage, Anseriforms, Galliformes, and Columbiformes show no species-specific differences. We can also reasonably conclude that the genetic program(s) controlling facial growth (and hence species-specific differences in facial form) must be enacted around this time.
Figure 2.
Phylotypic and phenotypic stages of facial prominence development in Aves. (A) At St.24, the facial prominences of avian embryos show little divergence. (B) The species-specific differences of facial morphology are evident at St. 37. (C, D) The shape differences become exacerbated with age. pm, premaxilla; nc, nasal capsule; m, mandible, z, zygoma.
4. Is there a phylotypic period of mammalian craniofacial development?
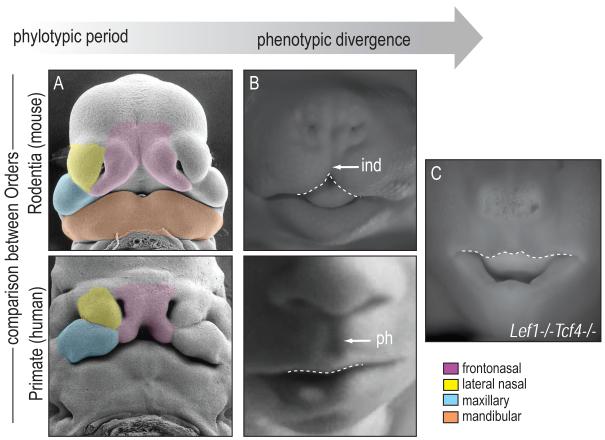
Avians clearly share a phylotypic period of development. What about other Orders in the Animal Kingdom? We compared two mammalian faces, the mouse (Order Rodentia) with the human (Order Primate) and during their early development were struck by similarities in the general shape, position, and the size of the facial prominences (Fig. 3A). For example, the sizes of the murine and human frontonasal prominences relative to the lateral nasal, maxillary, and mandibular prominences are comparable (Fig. 3A). With time, a distinct change in frontonasal shape becomes evident: the mouse frontonasal prominence exhibits a “split” in the upper lip called the infranasal depression, whereas the human develops a continuous pro-labium (the central portion of the upper lip) with a philtrum (the groove between the upper lip and lower nose; Fig. 3B). These phenotypic differences later manifest as an elongated rostrum in the case of the mouse, and a foreshortened rostrum in the case of the human (and other Haplorhines; see [17]). Therefore, mammals also appear to share a phylotypic period of craniofacial development, and phenotypic divergence is most obviously detectable in the growth of the mammalian frontonasal prominence relative to the other facial prominences.
Figure 3.
Phylotypic and phenotypic period in Mammals. (A) Around Carnegie 15, the facial prominences of mouse and human embryos are nearly indistinguishable. (B) Further in development, the frontonasal prominence begins to exhibit phenotypic divergence. The mouse frontonasal prominence exhibits a “split” in the upper lid called the infranasal depression. The human frontonasal prominence develops a continuous pro-labium with a philtrum. (C) Deletion of Lef1 and Tcf4 transforms the face of the embryonic mouse to resemble that of a human. ind, infranasal depression; ph, philtrum.
5. Variations in Wnt activity alter species-specific facial morphogenesis
Although there is a clear difference in their post-natal appearance, during embryonic development mouse and human faces are remarkably similar. What signaling pathways are responsible for these species-specific variations in facial morphology? Some insights have come from our analyses of mouse mutants, in particular Lef1-/-/Tcf4-/- embryos [18]. In these mouse mutants the characteristic infranasal depression is replaced by a philtrum-like structure, which appears to transform the embryonic mouse face into something more closely resembling a human (Fig. 3C). How can such a dramatic transformation occur? Our data suggest that at an earlier stage of development (the phylotypic period, perhaps?) null mutations in Lef1 and Tcf4 produce subtle shifts in Wnt pathway activity within the murine frontonasal and maxillary prominences [18].
The cell biological and morphological consequences of these changes in the timing (and perhaps duration) of Wnt signaling support the theory of heterochrony. Heterochrony is a theory which proposes that alterations in the activity of a molecular signal lead to changes in size and shape of a body part or an entire animal [19]. Two related species can have heterochronic processes that alter the shape of a tissue or organ [20]; here, we speculate that heterochronic events may also explain why the faces of mice and humans start out looking so much alike and then exhibit such radical variation. The resemblance between a murine Lef1-/-/Tcf4-/- face and that of a normal human fetus suggests that Wnt signaling plays an important role in regulating the growth of the maxillary and frontonasal prominences and that subtle shifts in the timing or duration of that Wnt signal may lie at the heart of species-specific facial growth.
6. Is there a shared phylotypic period between vertebrate Classes?
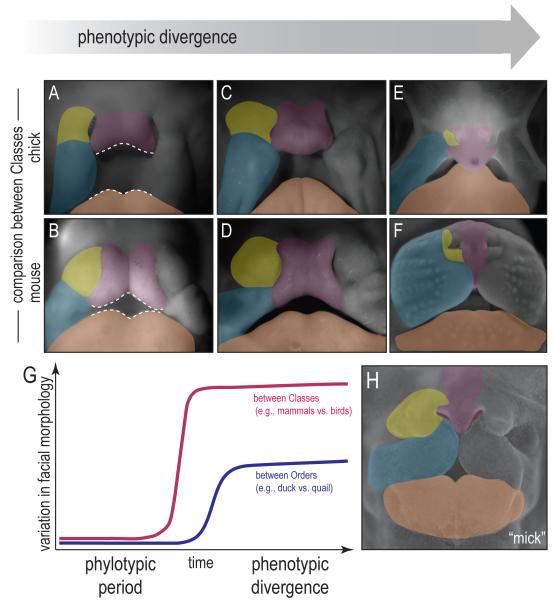
Within an Order there appears to be a phylotypic period of craniofacial development. Does this theory of a phylotypic period of craniofacial development apply more broadly to include different Classes of animals? We compared the facial prominences of Aves and Mammals, using the chick and the mouse as representatives (Fig. 3A). Around Carnegie St. 15 (a universal system for staging the embryonic development of most vertebrates) we found that the general shape, position, and the size of the chick and mouse facial prominences are similar (Fig. 4A). Perhaps most obvious, the size of the chick and mouse frontonasal prominences (pink) relative to the lateral nasal (yellow), maxillary (blue) and mandibular (orange) prominences, are comparable (Fig. 4A,B).
Figure 4.
Morphologic progression of facial prominences between Classes embryos during development. (A, B) In the early stage of development, the mouse and chick embryos share similar facial morphology. (C, D) With time, phenotypic divergence becomes evident within the frontonasal prominence. The chick frontonasal prominence shows a bulbous appearance whereas the mouse frontonasal prominence has an infranasal depression. (E, F) Later in development, the frontonasal prominence forms an upper beak in chick, and a muzzle in the mouse. (G) The variation in facial morphology increases over time during development. Between Classes (e.g. Mammals vs. Aves) the variation is greater and the phylotypic period is reduced. Between Orders (e.g. duck vs. quail), the phylotypic period is longer and there is less variation in facial morphology. (H) Disruption of Fgf signaling transforms a chick face to resemble that of a mouse.
With time, a distinct change in shape is evident within the frontonasal prominence: in the chick, the frontonasal prominence adopts a bulbous appearance whereas in the mouse, it develops an infranasal depression (Fig. 4C,D). Later, this manifests as a difference in the relative contribution of the frontonasal prominence to the face as a whole: in chicks, the frontonasal prominence contributes to the majority of the upper beak (Fig. 4E). In mice, the frontonasal prominence is reduced to a thin sliver of tissue interposed between the well-developed maxillary prominences (Fig. 4F). Thus, the Ave develops a beak and the mammal, a muzzle.
7. The transition from a phylotypic period to species-specific phenotypic divergence
We graphically represented the theoretical progression of a vertebrate embryo from a phylotypic period of development to a stage of phenotypic divergence (Fig. 4G). The first point to appreciate from this graph is an obvious one: more distantly related animal embryos (e.g., from different Classes, such as Mammals versus Aves) show greater phenotypic divergence than do more closely related embryos (e.g., from different Orders). The second point is still theoretical: namely, that there exists a phylotypic period during which vertebrates have a similar organization to their facial prominences. If such a phylotypic period of craniofacial development exists, then it would be reasonable to expect that within a given Class of animals (i.e., Aves), the phylotypic period lasts longer than the phylotypic period between two Classes of animals (i.e., Mammals versus Aves). The third point is also theoretical and presents a testable hypothesis: if there is phylotypic period among Classes of animals then presumably one could manipulate the molecular machinery responsible for establishing species-specific facial phenotypes and in effect, transform the facial appearance of one animal into that of another.
In previous experiments done in my (JAH) lab, we achieved such a facial “transformation”, by grafting cranial neural crest cells from a quail donor into a duck host (the “quck”; [21]). We now speculate that an analogous transformation can be achieved simply be “recapitulating” the molecular code for the embryonic mouse face onto a bird embryo at the phylotypic stage of development. But what is the “molecular code” controlling facial morphology? Rather than being a cadre of ‘species-specific” genes, we favor the theory that changes in the spatiotemporal domains of gene expression are ultimately responsible for generating different facial morphologies. In our own experiments we manipulated the activity of one molecular pathway, controlled by Fibroblast Growth Factors (FGFs) and found that such a transformation could be achieved. We found that by blocking FGF signaling with a soluble form of the FGF receptor [22], facial development is perturbed, but in a particularly interesting way. Chick embryos treated with FGFR1-IgG exhibited a frontonasal prominence that instead of elongating, is reduced in size relative to grossly enlarged maxillary prominences (Fig. 4H). The result is a chick embryo with a mouse face (in our parlance, a ”mick”). This experimental result serves as a clear illustration of the remarkable plasticity of the vertebrate face.
8. Conclusions
Quantitative analyses support the concept of a phylotypic period of craniofacial development, when vertebrate faces share a similar morphology. The development of species-specific facial characteristics is most obvious in the neural crest-derived facial skeleton, and one can pinpoint a stage when these species-specific characteristics first arise. The ability to interrogate facial tissues at the phylotypic period will undoubtedly lead to the identification of gene pathways that control species-specific facial growth. Our own data clearly implicate Wnt and FGF signaling in the development of species-specific facial characteristics, and the ability to “transform” the face of one animal into that resembling another reveals a previously unappreciated plasticity in the vertebrate face. Last, it has become increasingly obvious that subtle changes in the temporal or spatial pattern of genes that shape the face also underlie a myriad of human deformities. Perhaps all that stands between the human face and that of our closest ancestors is some minor tweaking of these molecular pathways.
Abbreviations
- FGF
fibroblast growth factors
- Lef1
lymphoid enhancer-binding factor 1
- Tcf4
transcription factor 4
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Trainor PA, Melton KR, Manzanares M. Origins and plasticity of neural crest cells and their roles in jaw and craniofacial evolution. Int J Dev Biol. 2003;47(7-8):541–53. [PubMed] [Google Scholar]
- 2.Cerny R, et al. Developmental origins and evolution of jaws: new interpretation of “maxillary” and “mandibular”. Dev Biol. 2004;276(1):225–36. doi: 10.1016/j.ydbio.2004.08.046. [DOI] [PubMed] [Google Scholar]
- 3.Baer K.E.v. Über Entwicklungsgeschichte der Thiere: Beobachtung und Reflexion. Bornträger; Königsberg: 1828. p. 264. [Google Scholar]
- 4.Irie N, Sehara-Fujisawa A. The vertebrate phylotypic stage and an early bilaterian-related stage in mouse embryogenesis defined by genomic information. BMC Biol. 2007;5:1. doi: 10.1186/1741-7007-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bininda-Emonds OR, Jeffery JE, Richardson MK. Inverting the hourglass: quantitative evidence against the phylotypic stage in vertebrate development. Proc Biol Sci. 2003;270(1513):341–6. doi: 10.1098/rspb.2002.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Virta VC, Cooper MS. Ontogeny and phylogeny of the yolk extension in embryonic cypriniform fishes. J Exp Zoolog B Mol Dev Evol. 2009;312B(3):196–223. doi: 10.1002/jez.b.21284. [DOI] [PubMed] [Google Scholar]
- 7.Hazkani-Covo E, Wool D, Graur D. In search of the vertebrate phylotypic stage: a molecular examination of the developmental hourglass model and von Baer’s third law. J Exp Zoolog B Mol Dev Evol. 2005;304(2):150–8. doi: 10.1002/jez.b.21033. [DOI] [PubMed] [Google Scholar]
- 8.Slack JMW, Holland PWH, Graham CF. The zootype and the phylotypic stage. Science. 1993 Feb 11;361:490–492. doi: 10.1038/361490a0. [DOI] [PubMed] [Google Scholar]
- 9.Juriloff DM, Harris MJ. Mouse genetic models of cleft lip with or without cleft palate. Birth Defects Res A Clin Mol Teratol. 2008;82(2):63–77. doi: 10.1002/bdra.20430. [DOI] [PubMed] [Google Scholar]
- 10.Vaglia JL, Smith KK. Early differentiation and migration of cranial neural crest in the opossum, Monodelphis domestica. Evol Dev. 2003;5(2):121–35. doi: 10.1046/j.1525-142x.2003.03019.x. [DOI] [PubMed] [Google Scholar]
- 11.Hallgrimsson B, et al. Epigenetic interactions and the structure of phenotypic variation in the cranium. Evol Dev. 2007;9(1):76–91. doi: 10.1111/j.1525-142X.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 12.Richardson MK, et al. There is no highly conserved embryonic stage in the vertebrates: implications for current theories of evolution and development. Anat Embryol (Berl) 1997;196(2):91–106. doi: 10.1007/s004290050082. [DOI] [PubMed] [Google Scholar]
- 13.Wiley DA, Alcantra N, Ghosh DA, Kil D, Delson YJ, Harcourt-Smith E, Rohlf W, St. John FJ, Hamann K, B. Evolutionary Morphing. Proceedings of IEEE Visualization 2005. 2005 [Google Scholar]
- 14.Brugmann S, et al. Molecular basis for species-specific variation in facial morphology. Development. 2009 In review. [Google Scholar]
- 15.Abzhanov A, et al. Bmp4 and morphological variation of beaks in Darwin’s finches. Science. 2004;305(5689):1462–1465. doi: 10.1126/science.1098095. [DOI] [PubMed] [Google Scholar]
- 16.Wu P, et al. Molecular shaping of the beak. Science. 2004;305(5689):1465–1466. doi: 10.1126/science.1098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hershkovitz P. Introduction to Primates. Vol. 1. The University of Chicago Press; Chicago: 1977. Living New World Monkeys (Platyrrhini) p. 1132. [Google Scholar]
- 18.Brugmann SA, et al. Wnt signaling mediates regional specification in the vertebrate face. Development. 2007;134(18):3283–95. doi: 10.1242/dev.005132. [DOI] [PubMed] [Google Scholar]
- 19.Raff RA, Kaufman TC. Embryos, Genes, and Evolution. Macmillan Publishing Co., Inc.; New York: 1983. [Google Scholar]
- 20.Nunn CL, Smith KK. Statistical analyses of developmental sequences: the craniofacial region in marsupial and placental mammals. Am Nat. 1998;152(1):82–101. doi: 10.1086/286151. [DOI] [PubMed] [Google Scholar]
- 21.Schneider RA, Helms JA. The cellular and molecular origins of beak morphology. Science. 2003;299(5606):565–8. doi: 10.1126/science.1077827. [DOI] [PubMed] [Google Scholar]
- 22.Wang JF, et al. A soluble fibroblast growth factor receptor is released from HL-60 promyelocytic leukemia cells: implications for paracrine growth control. Growth Factors. 2000;17(3):203–14. doi: 10.3109/08977190009001069. [DOI] [PubMed] [Google Scholar]