Abstract
Introduction
Large randomized trials assessing the benefit of adjuvant trastuzumab in early-stage breast cancer positive for the human epidermal growth factor receptor 2 (her2) have demonstrated a significant improvement in survival. The objective of the present study was to describe the outcomes of women who received adjuvant trastuzumab for her2-positive breast cancer in British Columbia since publicly funded population-based use was initiated in July 2005.
Methods
Women from British Columbia, newly diagnosed with stage i–iii breast cancer between July 2004 and December 2006, who were positive for her2 overexpression by immunohistochemistry (3+) or amplification by fluorescence in situ hybridization (ratio ≥ 2.0) were included in the study. Data were collected from the prospectively assembled BC Cancer Agency Outcomes Unit, with cases linked to the provincial pharmacy data repository to determine the proportion of women who received adjuvant trastuzumab.
Results
Our retrospective study identified 703 her2-positive patients, of whom 480 (68%) received trastuzumab. In patients receiving trastuzumab, the 2-year relapse-free survival was 96.1% [95% confidence interval (CI): 93.6% to 97.7%] and the overall survival was 99.3% (95% CI: 97.9% to 99.8%). Among node-negative and -positive patients, the 2-year relapse-free survival was 97.8% and 94.8% respectively (p = 0.09) for the trastuzumab-treated group and 90.9% and 77.3% (p = 0.01) for the group not receiving trastuzumab (n = 223). Site of first distant metastasis was the central nervous system in 19.5% of the entire cohort and in 37.5% of patients treated with trastuzumab.
Discussion
This population-based analysis of adjuvant trastuzumab use among Canadian women demonstrates highly favorable outcomes at the 2-year follow-up.
Keywords: Adjuvant, trastuzumab, breast cancer, her2
1. INTRODUCTION
The gene for the human epidermal growth factor receptor 2 (HER2) encodes for a transmembrane tyrosine kinase receptor protein (her2) that has both prognostic and predictive significance for invasive breast cancer. In approximately 15%–25% of breast cancers, the HER2 gene is amplified, which is associated with poor prognosis1,2.
Trastuzumab is a humanized monoclonal antibody against the extracellular domain of her2. A landmark study by Slamon et al. demonstrated a significant reduction in mortality associated with trastuzumab use in the metastatic setting2. Subsequently, several major trials of adjuvant trastuzumab demonstrated significantly reduced disease recurrence and improved survival in more than 13,000 participants3–6. In the province of British Columbia, trastuzumab for newly diagnosed her2-positive early breast cancer became publicly funded during 2005. Furthermore, women with that diagnosis who had completed adjuvant chemotherapy from July 2004 to introduction of the new policy and who had remained clinically disease-free were offered 1 year of trastuzumab treatment.
The objectives of the present study were to describe the proportion of women receiving adjuvant trastuzumab for her2-positive early-stage breast cancer during the period between July 1, 2004, and December 31, 2006, and to determine their outcomes.
2. METHODS
Data were abstracted from the prospectively assembled BC Cancer Agency (bcca) Breast Cancer Outcomes Unit database, which includes demographic, pathologic, treatment, and outcomes information on referred B.C. residents diagnosed with breast cancer since 1989. Women in the province who were referred to the bcca with newly diagnosed stage i–iii breast cancer and who were positive for her2 overexpression by immunohistochemistry (ihc 3+) or amplification by fluorescence in situ hybridization (fish ratio ≥ 2.0) were included in the study. Women with previous or synchronous contralateral breast cancers were excluded. Each case was linked to the provincial bcca pharmacy data repository to determine the proportion of women who received adjuvant trastuzumab. Trastuzumab dosing in British Columbia during the study period consisted of a loading dose of 8 mg per kilogram of body weight given intravenously, followed by a 6-mg/kg intravenous dose every 3 weeks thereafter for a total of 17 trastuzumab treatments (duration of 1 year).
In this study of her2-positive women, 2 cohorts were analyzed: those who had received adjuvant trastuzumab and those who had not. Baseline characteristics between the groups were compared using the chi-square test for categorical variables and the Mann–Whitney test for continuous variables. Kaplan–Meier curves were used to illustrate survival outcomes. All p values were two-sided and statistically significant if less than 0.05. Relapse-free survival (rfs) was defined as time from diagnosis to local, regional, or distant relapse, or death from breast cancer with distant rfs. Breast cancer–specific survival (bccs) was defined as time from diagnosis to death from breast cancer, and overall survival (os) was from diagnosis to death from any cause. Approval was obtained from the University of British Columbia Research Ethics Board before the study commenced.
3. RESULTS
This retrospective study identified 703 her2-positive patients. In 479 patients (68% of the total study population), her2 overexpression was found by ihc 3+, and therefore no fish analysis was performed. In 3 patients (0.4% of the total study population), the ihc score was deemed positive, but without quantification, and no fish analysis was performed. In the remaining patients, fish analysis was performed (Table i). Of the total study population, 480 (68%) received adjuvant trastuzumab, and almost all patients in that cohort received adjuvant chemotherapy. Of the patients receiving adjuvant trastuzumab, 72% received anthracycline- and taxane-based chemotherapy; 14% received anthracycline-based chemotherapy alone. The remainder received non-anthracycline-based chemotherapy or were switched from one chemotherapy regimen to another during treatment. In the cohort that did not receive trastuzumab (n = 223), only 28% received adjuvant chemotherapy. Median follow-up was 2.1 years.
TABLE I.
Expression of the human epidermal growth factor receptor 2 (her2) by immunohistochemistry (ihc) in women with her2-amplified breast cancers by fluorescence in situ hybridization (fish)
| ihc result | Patients [n (%)] with her2 amplification by fish |
|---|---|
| Negative | 8 (3.6) |
| Positive | |
| 1+ | 28 (12.7) |
| 2+ | 117 (52.9) |
| 3+ | 62 (28.1) |
| Not quantified | 2 (0.9) |
| Unknown | 4 (1.8) |
Overall, patients not receiving trastuzumab had better prognostic features at baseline (Table ii). Compared with the untreated group, patients receiving adjuvant trastuzumab were significantly younger (p < 0.001) and had larger tumours (p < 0.001) and an increased presence of lymphovascular invasion (p = 0.004); they were also more likely to be node-positive (p = 0.001). Site of first distant metastasis was the central nervous system (cns) for 19.5% of the entire cohort and for 37.5% of the patients treated with trastuzumab.
TABLE II.
Baseline characteristics of the study patients by trastuzumab treatment status.
| Characteristic |
Adjuvant trastuzumab?
|
p Valuea | |
|---|---|---|---|
| Yes | No | ||
| Patients [n (%)] | 480 (68.3) | 224 (31.7) | |
| Median age at Dx (years) | 52 | 64 | <0.001b |
| Median tumour size (cm) | 2.2 | 1.9 | <0.001b |
| Positive nodes (%) | |||
| 0 | 44.0 | 53.8 | 0.001 |
| 1–3 | 32.9 | 20.2 | |
| 4+ | 20.8 | 15.7 | |
| Unknown | 2.3 | 10.3 | |
| Lymphovascular invasion (%) | |||
| Negative | 60.2 | 70.0 | 0.004 |
| Positive | 33.1 | 22.4 | |
| Unknown | 6.7 | 7.6 | |
| Grade (%) | |||
| 1 | 1.9 | 5.8 | <0.001 |
| 2 | 21.9 | 35.0 | |
| 3 | 74.8 | 58.3 | |
| Unknown | 1.5 | 0.9 | |
| Estrogen receptor status (%) | |||
| Negative | 44.2 | 37.7 | 0.13 |
| Positive | 55.8 | 61.4 | |
| Unknown | 0 | 0.9 | |
| Initial local therapy (%) | |||
| No surgery | 2.3 | 1.3 | <0.001 |
| Mastectomy ± radiotherapy | 51.0 | 48.0 | |
| bcs + radiotherapy | 43.5 | 35.9 | |
| bcs alone | 3.1 | 14.8 | |
| Systemic therapy at Dx (%) | |||
| None | 0.2 | 33.2 | <0.001 |
| Hormone therapy | 0.2 | 39.0 | |
| Chemo | 50.2 | 16.6 | |
| Chemo + hormonal therapy | 49.4 | 11.2 | |
By chi-square test unless otherwise indicated.
By Mann–Whitney test.
Dx = diagnosis; bcs = breast-conserving surgery; Chemo = chemotherapy.
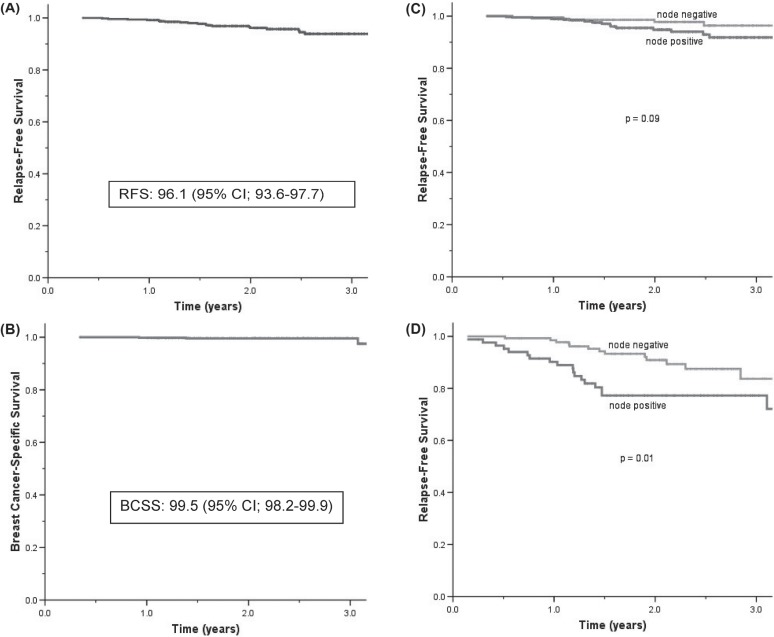
The 2-year rfs for patients receiving adjuvant trastuzumab was 96.1% [95% confidence interval (ci): 93.6% to 97.7%]. Kaplan–Meier curves for bccs and os were similar for the trastuzumab-treated patients, with 2-year rates of 99.5% (95% ci: 98.2% to 99.9%) and 99.3% (95% ci: 97.9% to 99.8%) respectively. Comparing the node-negative and -positive patients, both in the trastuzumab-treated group (2-year rfs: 97.8% vs. 94.8%, p = 0.09) and in the untreated cohort [2-year rfs: 90.9% vs. 77.3%, p = 0.01 (Figure 1)], the rfs approached statistical significance or was significant. The corresponding 2-year distant rfs rates in the non-trastuzumab cohort were 94.5% and 81.0% respectively (p = 0.01).
FIGURE 1.
Survival outcomes in women with breast cancer positive for the human epidermal growth factor receptor 2 (her2). For patients receiving adjuvant trastuzumab (n = 480), (A) 2-year relapse-free survival (rfs) and (B) breast cancer–specific survival (bcss). The 2-year rfs (C) in patients receiving and (D) not receiving adjuvant trastuzumab (n = 223), by nodal status.
4. DISCUSSION
The results from this study demonstrate excellent outcomes for women with her2-positive breast cancer treated with adjuvant trastuzumab. Although the data are retrospective and cannot be directly compared with those from the large adjuvant clinical trials, our population had better survival outcomes. These findings may be a result of a different proportion of subjects with node-negative disease. In addition, our study included a subset of women who had completed chemotherapy up to 1 year before they started adjuvant trastuzumab and who had remained disease-free, which may have improved outcomes for that group.
In the patients who did not receive trastuzumab, 28% received adjuvant chemotherapy and would have been eligible for anti-her2 treatment, thus raising the question of why this group of patients did not receive trastuzumab. Several reasons can be hypothesized, including the possibility that some patients were medically unfit to receive adjuvant trastuzumab; some patients may have decided against 1 year of systemic therapy, especially in the group that was offered retroactive trastuzumab months after chemotherapy completion; and some physicians may have been biased against offering treatment to this group of patients.
Very few women with small node-negative tumours (T1a–b N0) were enrolled in the large adjuvant trastuzumab studies, and so outcomes in those patients are of considerable interest. Retrospective studies in this population demonstrate worse outcomes for patients having small her2-positive tumours than for their counterparts having her2-negative disease, suggesting that adjuvant trastuzumab therapy may be warranted7–9. These studies included women who had not received anti-her2 therapy. In our study, 72 patients had her2-positive T1a–b N0 tumours; 30 received adjuvant trastuzumab, and 42 did not. A meaningful comparison of survival at this time point could not be performed, but it represents a direction for future research.
In terms of recurrent her2-positive breast cancer, cns disease is a concern. One study demonstrated that the observed increase in cns metastases as first events among patients treated with adjuvant trastuzumab could be attributed to earlier failures at other distant sites in the untreated group10, but a meta-analysis showed an increase in risk among treated patients in the randomized trials11. In our study, 19.5% of the overall population had cns recurrence as the first site of distant metastasis, but such recurrences reached 37.5% in the trastuzumab-treated group. That observation raises the question of whether adjuvant therapy with trastuzumab might alter the initial site of relapse, possibly reflecting the ability of trastuzumab to control systemic disease more effectively outside than within the cns.
The limitations of our study include a short follow-up (median of 2 years), but the 2-year point was when most of the adjuvant trastuzumab trials were first reported. Information on the decision not to use adjuvant trastuzumab in eligible patients was not captured, nor were cardiac or comorbidity statuses.
5. CONCLUSIONS
This population-based analysis of adjuvant trastuzumab use demonstrates excellent survival outcomes among women with her2-positive early breast cancer. That finding is especially profound in an aggressive disease that is known to have a significant relapse rate within the first few years after diagnosis. Future clinical trials studying targeted anti-her2 agents in comparison or in combination with adjuvant trastuzumab [altto (search for NCT00490139 and NCT00553358 at http://clinicaltrials.gov/ct2/search) and beth (NCT00625898)] may find event rates lower than anticipated—all of which bode well for the population of women diagnosed with this biologic subtype of breast cancer in the future.
6. ACKNOWLEDGMENTS
The authors acknowledge an AACR Translational Research Scholar-in-Training Award (Susan G. Komen for the Cure).
7. CONFLICT OF INTEREST DISCLOSURES
MDS, CHS, SO, and SLE have no financial conflicts to declare. KAG has held a consultant/advisory role with Roche. SKC has received remuneration and funding and has held a consultant/advisory role with Roche.
8. REFERENCES
- 1.Mackey J, McLeod D, Ragaz J, et al. Adjuvant targeted therapy in early breast cancer. Cancer. 2009;115:1154–68. doi: 10.1002/cncr.24114. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Leyland–Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against her2 for metastatic breast cancer that overexpresses her2. N Engl J Med. 2001;344:783–892. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 3.Gianni L, Dafni U, Gelber RD, et al. Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with her2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial. Lancet Oncol. 2011;12:236–44. doi: 10.1016/S1470-2045(11)70033-X. [DOI] [PubMed] [Google Scholar]
- 4.Perez EA, Romond EH, Suman VJ, et al. Four-year followup of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2–positive breast cancer: joint analysis of data from ncctg N9831 and NSABP B-31. J Clin Oncol. 2011;29:3366–73. doi: 10.1200/JCO.2011.35.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slamon D, Eiermann W, Robert N, et al. Phase iii randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (ac→t) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (ac→th) with docetaxel, carboplatin and trastuzumab (tch) in her2 positive early breast cancer patients: bcirg 006 study [abstract 62] Breast Cancer Res Treat. 2005;94(suppl 1):S5. [Google Scholar]
- 6.Joensuu H, Bono P, Kataja V, et al. Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer Trial. J Clin Oncol. 2009;27:5685–92. doi: 10.1200/JCO.2008.21.4577. [DOI] [PubMed] [Google Scholar]
- 7.Chia S, Norris B, Speers C, et al. Human epidermal growth factor receptor 2 overexpression as a prognostic factor in a large tissue microarray series of node-negative breast cancers. J Clin Oncol. 2008;26:5697–704. doi: 10.1200/JCO.2007.15.8659. [DOI] [PubMed] [Google Scholar]
- 8.Curigliano G, Viale G, Bagnardi V, et al. Clinical relevance of her2 overexpression/amplification in patients with small tumor size and node-negative breast cancer. J Clin Oncol. 2009;27:5693–9. doi: 10.1200/JCO.2009.22.0962. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez–Angulo AM, Litton JK, Broglio KR, et al. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2–positive, node-negative tumors 1 cm or smaller. J Clin Oncol. 2009;27:5700–6. doi: 10.1200/JCO.2009.23.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable her2-positive breast cancer. N Engl J Med. 2005;353:1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 11.Bria E, Cuppone F, Fornier M, et al. Cardiotoxicity and incidence of brain metastases after adjuvant trastuzumab for early breast cancer: the dark side of the moon? A metaanalysis of the randomized trials. Breast Cancer Res Treat. 2008;109:231–9. doi: 10.1007/s10549-007-9663-z. [DOI] [PubMed] [Google Scholar]