Abstract
Background
Mansonella perstans infection is common in areas of Africa where Wuchereria bancrofti, a causative agent of lymphatic filariasis, is endemic. M. perstans is refractory to standard antifilarial therapies. The recent discovery of bacterial endosymbionts (e.g., wolbachia) in most filarial species, including M. perstans, provides new therapeutic options for reducing microfilaremia.
Methods
In an open-label, randomized trial, we recruited subjects with M. perstans microfilaremia, with or without concomitant W. bancrofti infection, from four villages in Mali and randomly assigned them to receive doxycycline, at a dose of 200 mg daily for 6 weeks (106 subjects), or no treatment (110). At 6 months, subjects who were co-infected with W. bancrofti underwent a second random assignment, to treatment with a single dose of albendazole (400 mg) and ivermectin (150 μg per kilogram of body weight) or no treatment. Subjects were monitored daily during the first 6-week study period for adverse events. M. perstans and W. bancrofti microfilarial levels were assessed at 6, 12, and 36 months.
Results
At 12 months, 67 of 69 subjects who had received treatment with doxycycline only (97%) had no detectable M. perstans microfilariae per 60 μl of blood, as compared with 10 of 63 subjects who had received no treatment (16%) (relative risk, 6.18; 95% confidence interval, 3.63 to 11.89; P<0.001). At 36 months, M. perstans microfilaremia remained suppressed in 48 of 64 subjects who had received treatment with doxycycline only (75%), a finding that was consistent with a macrofilaricidal effect of doxycycline. Vomiting was more frequent in the doxycycline-treated group than in the untreated group (17% vs. 4%).
Conclusions
These results are consistent with previous findings that M. perstans harbors the intracellular endosymbiont, wolbachia, and suggest that doxycycline is an effective therapy for M. perstans infection.
The filarial parasite Mansonella perstans is endemic in central and western Africa, with a distribution that overlaps that of Wuchereria bancrofti, Loa loa, and Onchocerca volvulus. Transmitted through the bite of an infected midge (culicoides species), infective M. perstans larvae develop over the course of months into adult worms that reside in the serous cavities and mesentery and retroperitoneal tissues. Microfilariae are carried through the bloodstream, and those of M. perstans can be distinguished from those of L. loa and W. bancrofti by their small size, lack of periodicity, and the absence of a sheath. As is true of other bloodborne filarial infections, most M. perstans infections are subclinical, although a wide range of symptoms has been attributed to M. perstans infection, including angioedema, pruritus, fever, headaches, arthralgias, serositis, and neurologic manifestations.1 The contribution of M. perstans to overall disability among populations in regions where filariae are endemic has been difficult to determine, however, because of the high prevalence of coinfection with other filariae and the lack of specificity of the symptoms. Furthermore, conventional antifilarial agents, including diethylcarbamazine, ivermectin, and albendazole, have limited efficacy in the treatment of M. perstans.2,3
Doxycycline, given at a dose of 200 mg daily for 4 to 8 weeks, has been shown to decrease the development, embryogenesis, fertility, and viability of filarial worms in species that harbor the intracellular endosymbiont wolbachia.4–6 This effect results in a dramatic and sustained decrease in microfilarial levels. Thus, the results of a recent study showing that M. perstans harbors wolbachia7 suggest a unique opportunity to treat this infection effectively and to examine the clinical and immunologic manifestations attributable to M. perstans. The current study was designed to evaluate the efficacy of doxycycline for the treatment of M. perstans infection in subjects with M. perstans microfilaremia with or without concomitant infection with W. bancrofti.
METHODS
STUDY POPULATION
We conducted the study in the villages of Sabougou, Fougan, Yerba, and Ben, which are located approximately 180 km northwest of Bamako, Mali. Previous studies had shown a prevalence of M. perstans microfilaremia of 67%, a prevalence of W. bancrofti infection of 48.3%, and a high frequency of coinfection.8 Onchocerciasis is not endemic in this region. The study was approved by the ethics review committees at the University of Bamako (Bamako, Mali) and the National Institute of Allergy and Infectious Diseases (Bethesda, MD). Community permission was obtained from village elders, and individual oral or written informed consent was obtained from all participants (and their parents, in the case of subjects who were younger than 18 years of age) in French or Bambara.
A total of 1035 volunteers of both sexes, 14 to 65 years of age, were screened with the use of a calibrated thick blood smear (60 μl), obtained during the daytime, for the detection of M. perstans microfilariae and with the use of an immunochromatographic card test (ICT) for the assessment of W. bancrofti antigenemia (circulating antigen). On the basis of the screening results, 694 subjects who were infected with M. perstans were identified for further evaluation, which included a detailed history-taking and physical examination, serum pregnancy test, calibrated thick blood smear (60 μl) obtained between 10 p.m. and 2 a.m., assessment of circulating antigen levels of W. bancrofti by means of an enzyme-linked immunosorbent assay (ELISA) (TropBio), complete blood count, and measurement of serum alanine aminotransferase, bilirubin, and creatinine levels by means of a dipstick test (Reflotron Plus, Roche Diagnostics). Subjects were excluded from the study if they were pregnant or breast-feeding; had a hemoglobin level of 10 g per deciliter or less, a creatinine level greater than 1.4 mg per deciliter (123.8 μmol per liter), an alanine aminotransferase level greater than 45 U per liter, or a bilirubin level greater than 1.5 mg per deciliter (25.6 μmol per liter); weighed less than 40 kg; were classified as a heavy user of alcohol (i.e., drank more than one alcohol-containing drink per day); had a temperature higher than 37.5°C; had a serious medical illness; had a history of allergy to doxycycline; or had a suspected immunodeficiency.
STUDY DESIGN
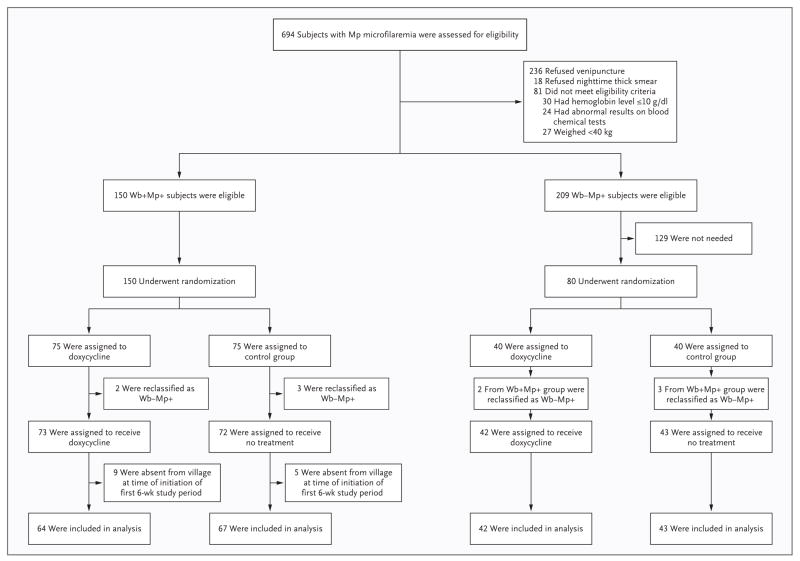
On the basis of the screening results, eligible subjects were separated into two groups: subjects who were positive for both M. perstans and W. bancrofti and subjects who were positive for M. perstans and negative for W. bancrofti. The subjects in each group were randomly assigned to receive doxycycline (200 mg daily for 6 weeks) or no treatment (Fig. 1). Doxycycline was administered under the direct observation of a study physician. All 216 subjects who were included in the two groups at the start of the first 6-week study period were assessed for adverse events daily throughout the 6-week period and monthly thereafter for the duration of the study. Adverse events were graded on the basis of the National Cancer Institute’s Common Toxicity Criteria, version 2. Local residents (guides) were trained in each village to perform interim assessments as needed and to serve as liaisons between the study participants and study personnel.
Figure 1. Screening and Random Assignment.
Wb+Mp+ refers to the group that was positive for both Mansonella perstans (Mp) and Wuchereria bancrofti (Wb); Wb−Mp+ refers to the group that was negative for W. bancrofti and positive for M. perstans. Although it was anticipated that 40 subjects would be assigned to each of the subgroups of persons who were negative for W. bancrofti and positive for M. perstans, the initial assignment was made on the basis of the results of an immunochromatographic card test (ICT). Five subjects who had been classified as positive for W. bancrofti on the basis of the ICT were found to be negative for W. bancrofti on the basis of an enzyme-linked immunosorbent assay before the initiation of doxycycline therapy and were reassigned to the corresponding subgroup within the group that was negative for W. bancrofti and positive for M. perstans.
Venipuncture was performed at baseline, 3 weeks, and 6 weeks to obtain blood for serum pregnancy testing and for measurement of serum creatinine, alanine aminotransferase, and bilirubin levels. At 6 months, subjects who were positive for both M. perstans and W. bancrofti underwent a second random assignment, to treatment with a single dose of albendazole (400 mg) and ivermectin (150 μg per kilogram of body weight) or no therapy (Fig. 1B in the Supplementary Appendix, available with the full text of this article at NEJM.org). Daytime venipuncture and nighttime fingerstick were performed at 6, 12, and 36 months for the assessment of M. perstans and W. bancrofti microfilarial levels and the determination of W. bancrofti circulating antigen levels by means of ELISA. Whole blood was stored at baseline and at 12 months for the quantitation of wolbachia DNA as described previously.7
All laboratory assessments were performed by trained personnel who were unaware of the group assignments. In addition, 10% of the slides were reread in a blinded fashion by one of the senior investigators to confirm the accuracy of microfilarial counts. Ultrasound examinations, as described by Mand et al.,9 were performed at baseline and 12 months in men who were positive for W. bancrofti, for assessment of hydrocele, lymphatic dilatation, and the presence of worm nests in scrotal lymphatic vessels. Ultrasonography was performed by an experienced radiologist, who was unaware of the group assignments.
STATISTICAL ANALYSIS
A reduction of 50% or more in M. perstans microfilaremia at 1 year in response to doxycycline treatment was chosen as the primary study end point because it was considered, on the basis of previous experience with measurements of M. perstans microfilariae over time, to be unlikely to represent random variation in the counts. We estimated that with a sample of 40 subjects in each group, the study would have 90% power to show a 50% mean reduction in the percent of baseline levels of M. perstans microfilariae, with a standard deviation of 2 on a square-root transformation scale and a dropout rate of 20%. Secondary end points included clearance of microfilaremia, further reduction of M. perstans microfilaremia after albendazole–ivermectin therapy, and reduction of pretreatment symptoms that were possibly related to filarial infection.
Owing to unforeseen problems with the specificity of the ICT, five subjects who were negative for W. bancrofti microfilariae were initially classified as positive for both M. perstans and W. bancrofti (two in the doxycycline group and three in the no-treatment group); these subjects were reclassified as positive for M. perstans and negative for W. bancrofti, on the basis of the results of the ELISA for circulating antigens (Fig. 1). This reclassification occurred before the administration of doxycycline. For simplicity of reporting and analysis, the data for the therapy groups are reported according to their definitive classification. With the data handled this way, only the blocking on the ICT is affected, and all randomization and analyses are valid.
Fourteen randomly assigned subjects who were positive for both M. perstans and W. bancrofti were absent from their villages at the beginning of the first 6-week study period (Fig. 1B in the Supplementary Appendix). These subjects were unaware of their study-group assignment and have been excluded from the analyses. For subjects who completed the first 6-week study period but missed either the 6-month or 12-month assessment, or both, we made the assumption that the missing data were missing at random. Nevertheless, an “extreme analysis” has been used to show that, regardless of the reason for the missing M. perstans clearance data, there would still be a highly significant effect of doxycycline.
The baseline levels of M. perstans microfilariae and the changes in circulating antigen levels within groups were compared with the use of the exact Wilcoxon–Mann–Whitney test with the associated Hodges–Lehmann confidence intervals. Exact tests and the associated confidence intervals for relative risk were used to test the primary end point of a reduction of 50% or more in M. perstans microfilaremia at 12 months. A logistic-regression analysis was performed to check for the significance of other factors and included terms for the baseline presence of W. bancrofti and for doxycycline and albendazole–ivermectin treatments. Similar analyses were performed for total clearance of microfilaremia and for microfilarial levels. Differences in proportions (represented as percentages) were used to compare the frequencies of adverse events. Calculations were performed with the use of the coin package for conditional inference, version 0.6–7, of R statistical software, version 2.7.0 (logistic regression) for the Wilcoxon–Mann–Whitney test10,11 and with the use of StatXact 8 PROCs for the tests of exact relative risk and differences in proportions. The Wilcoxon signed-rank test was used for paired comparisons of results of polymerase-chain-reaction (PCR) assays.
RESULTS
BASELINE CHARACTERISTICS
Subjects were recruited and screened between December 16, 2004, and March 3, 2005. The prevalence of M. perstans microfilaremia, as determined by the results of the screening, was 67.1% (95% confidence interval [CI], 64.1 to 69.9). Of the 359 eligible subjects, 216 (131 who were positive for both M. perstans and W. bancrofti and 85 who were positive for M. perstans and negative for W. bancrofti) were assigned to receive either doxycycline over the course of a 6-week period in May and June 2005 or no treatment (Fig. 1). Baseline demographic and parasitologic characteristics are shown in Table 1. Two subjects in the doxycycline group and three subjects in the no-treatment group withdrew before the completion of the initial 6-week study period because they had missed more than two visits (four subjects) or because of pregnancy (one subject in the no-treatment group) (Fig. 1A and 1B in the Supplementary Appendix). These five subjects were included in the analysis of adverse events and in the extreme analysis.
Table 1.
Baseline Characteristics of the Study Population.*
| Variable | Positive for Both M. perstans and W. bancrofti | Positive for M. perstans and Negative for W. bancrofti | Total Population | |||
|---|---|---|---|---|---|---|
| No Treatment (N=67) | Doxycycline (N=64) | No Treatment (N=43) | Doxycycline (N=42) | No Treatment (N=110) | Doxycycline (N=106) | |
| Sex (no.) | ||||||
|
| ||||||
| Male | 37 | 33 | 27 | 26 | 64 | 59 |
|
| ||||||
| Female | 30 | 31 | 16 | 16 | 46 | 47 |
|
| ||||||
| Age (yr) | ||||||
|
| ||||||
| Median | 46 | 46 | 45 | 45 | 46 | 45 |
|
| ||||||
| Range | 14–65 | 14–65 | 15–64 | 15–65 | 14–65 | 14–65 |
|
| ||||||
| Mansonella perstans (microfilariae/ml) | ||||||
|
| ||||||
| Geometric mean | 307 | 335 | 275 | 301 | 321 | 294 |
|
| ||||||
| Range | 17–19733 | 17–8313 | 17–3519 | 17–5712 | 17–19733 | 17–8313 |
|
| ||||||
| Wuchereria bancrofti (microfilariae/ml) | ||||||
|
| ||||||
| Geometric mean | 5.8 | 5.1 | NA | NA | NA | NA |
|
| ||||||
| Range | 0–6100 | 0–8684 | NA | NA | NA | NA |
|
| ||||||
| Circulating antigen of W. bancrofti (U/ml)† | ||||||
|
| ||||||
| Geometric mean | 1311 | 1986 | NA | NA | NA | NA |
|
| ||||||
| Range | 133–61,299 | 155–146,305 | NA | NA | NA | NA |
|
| ||||||
| Positive for worm nests (no./total no.)‡ | 11/33 | 13/30 | NA | NA | NA | NA |
NA denotes not applicable.
Subjects with undetectable or indeterminate levels of circulating antigen as assessed by enzyme-linked immunosorbent assay have been excluded.
Ultrasonography to detect the presence of worm nests in scrotal lymphatic vessels was performed only in men who were infected with W. bancrofti. Four men in the no-treatment group and three in the doxycycline group did not undergo ultrasonography at baseline.
ADVERSE EVENTS
Mild adverse events, reported in both the doxycycline and no-treatment groups, included headache, diarrhea, and respiratory symptoms (Tables 1 and 2 in the Supplementary Appendix). Only vomiting was more frequent in the doxycycline group than in the no-treatment group (17% vs. 4%, P = 0.001). Adverse events resolved in all subjects, without discontinuation of the study treatment in the subjects receiving doxycycline, and no serious adverse events were reported in either group.
EFFICACY
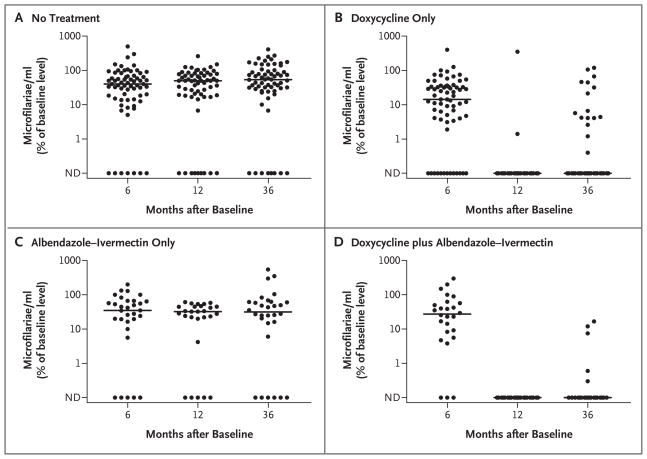
Doxycycline treatment alone led to a dramatic decrease in levels of M. perstans microfilariae, with median levels decreasing to 23% of pretreatment levels at 6 months after treatment and to 0% of pretreatment levels at 12 months after treatment, as compared with median levels in the no-treatment group that were 38% and 50% of baseline levels at 6 months and 12 months, respectively (P<0.001 for both comparisons, as assessed by the Wilcoxon–Mann–Whitney test) (Fig. 2A and 2B and Table 2). At 12 months, the primary end point of a 50% decrease in M. perstans microfilarial levels had been achieved in 92 of 93 subjects who had received doxycycline, as compared with 58 of 90 subjects who had not received doxycycline (relative risk, 1.54; 95% CI, 1.33 to 1.83; P<0.001). In fact, complete clearance of detectable M. perstans microfilariae at 12 months was achieved in 67 of 69 subjects (97%) who received doxycycline only, as compared with 10 of 63 subjects (16%) who had received no treatment (relative risk, 6.18; 95% CI, 3.63 to 11.89; P<0.001) (Fig. 2) and 5 of 27 subjects (19%) who had received albendazole–ivermectin but not doxycycline.
Figure 2. Efficacy of Doxycycline in Reducing Mansonella perstans Microfilarial Levels at 6, 12, and 36 Months after Treatment.
Levels of M. perstans are shown as percents of baseline levels for the groups that received no treatment, doxycycline only, a single dose of albendazole–ivermectin at 6 months, and doxycycline plus a single dose of albendazole–ivermectin at 6 months. Each circle represents the value for an individual patient. The horizontal lines represent the median values for each group at each time point. All groups received a single dose of albendazole–ivermectin between 12 and 36 months. ND denotes not detectable.
Table 2.
Efficacy of Doxycycline with or without Albendazole–Ivermectin in Reducing Mansonella perstans Microfilarial Levels.
| Group | Baseline | 6 Months | 12 Months | 36 Months* |
|---|---|---|---|---|
| No treatment | ||||
|
| ||||
| No. in group | 75 | 67 | 63 | 64 |
|
| ||||
| M. perstans (microfilariae/ml) | ||||
|
| ||||
| Median | 323 | 136 | 153 | 264 |
|
| ||||
| Range | 17–20,128 | 0–5916 | 0–11,441 | 0–8305 |
|
| ||||
| Doxycycline only | ||||
|
| ||||
| No. in group | 76 | 67 | 69 | 64 |
|
| ||||
| M. perstans (microfilariae/ml) | ||||
|
| ||||
| Median | 357 | 51 | 0 | 0 |
|
| ||||
| Range | 17–5712 | 0–1955 | 0–17 | 0–383 |
|
| ||||
| Doxycycline with albendazole–ivermectin | ||||
|
| ||||
| No. in group | 30 | 26 | 25 | 23 |
|
| ||||
| M. perstans (microfilariae/ml) | ||||
|
| ||||
| Median | 332 | 51 | 0 | 0 |
|
| ||||
| Range | 17–8313 | 0–3315 | 0 | 0–102 |
|
| ||||
| Albendazole–ivermectin only | ||||
|
| ||||
| No. in group | 35 | 31 | 27 | 29 |
|
| ||||
| M. perstans (microfilariae/ml) | ||||
|
| ||||
| Median | 340 | 102 | 153 | 102 |
|
| ||||
| Range | 17–6120 | 0–2108 | 0–799 | 0–3783 |
All subjects received a single dose of albendazole–ivermectin as part of the mass distribution program before the 36-month visit.
A single dose of albendazole–ivermectin was distributed in all the study villages between 12 months and 36 months as part of the national mass treatment program for the elimination of lymphatic filariasis. Although 146 of the 180 subjects who were included in the 36-month analysis (81%) reported receiving at least one dose of albendazole–ivermectin, M. perstans levels remained stable at 36 months in the group that had received no study treatment and in the group that had received the 6-month study dose of albendazole–ivermectin but not doxycycline. Levels of M. perstans microfilariae remained suppressed at 36 months in subjects who had received doxycycline; there was a complete absence of detectable M. perstans microfilariae in 48 of 64 subjects (75%) in the group that had received doxycycline only, as compared with 8 of 64 subjects (12%) in the group that had received no study treatment at all (relative risk, 6.0; 95% CI, 3.27 to 12.81; P<0.001) (Fig. 2). Even if the missing data on M. perstans clearance were included, such that all missing values for subjects who had received doxycycline were treated as if clearance of M. perstans had not been achieved and all missing values for subjects who had received no treatment were treated as if clearance of M. perstans had been achieved, the effect of doxycycline would be highly significant at both 12 months and 36 months (P<0.001). After adjustment for doxycycline treatment, neither treatment with albendazole–ivermectin nor the presence of W. bancrofti microfilariae had a significant effect on the complete clearance of M. perstans microfilariae at 12 months or 36 months (P>0.10 for all comparisons, by logistic regression), although because of the large effect of doxycycline there was little power to detect a treatment effect of albendazole–ivermectin.
Depletion of wolbachia in M. perstans by doxycycline was shown with the use of real-time PCR analysis that was specific for the 16S ribosomal DNA of M. perstans wolbachia. Among 182 paired whole-blood samples that were available at baseline and at 12 months (Table 3), 45 of 88 samples from the groups that received doxycycline and 39 of 94 from the groups that received no doxycycline had detectable M. perstans wolbachia at baseline. The ratios of copy numbers of M. perstans wolbachia to microfilariae were similar between the two groups (P = 0.11). The ratios of M. perstans wolbachia to microfilariae remained unchanged from baseline to 12 months in subjects who did not receive doxycycline (P = 0.69) (Table 3). Among subjects in the doxycycline groups, M. perstans wolbachia DNA could be evaluated only in the two subjects who were positive for M. perstans microfilariae at 12 months; in those two subjects, M. perstans wolbachia DNA was undetectable after doxycycline therapy.
Table 3.
Effect of Doxycycline on Mansonella perstans wolbachia.
| Group | Detectable M. perstans wolbachia 16S rDNA | |
|---|---|---|
| no. of subjects/no. tested | geometric mean copies/microfilaria (95% CI) | |
| Doxycycline | ||
|
| ||
| Baseline | 45/88 | 2.6 (1.3–5.0) |
|
| ||
| 12 mo | 0/45* | 0 |
|
| ||
| No treatment | ||
|
| ||
| Baseline | 39/94 | 5.97 (3.1–11.4) |
|
| ||
| 12 mo | 39/94 | 7.33 (3.0–18.0) |
Only two subjects had detectable M. perstans microfilariae as assessed by a thick blood smear.
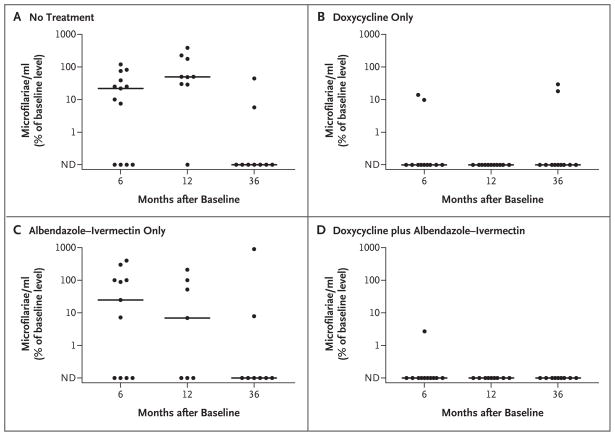
As expected, doxycycline was also effective in reducing levels of W. bancrofti microfilariae (Fig. 3, and Table 3 in the Supplementary Appendix). A total of 17 of 21 subjects in the doxycycline group, as compared with 4 of 20 in the group that received no treatment, had clearance of detectable W. bancrofti microfilariae at 6 months (relative risk, 4.25; 95% CI, 1.84 to 13.46; P<0.001). After the 12-month results for the subjects who were treated with albendazole–ivermectin were excluded, 11 of 11 subjects in the doxycycline group, as compared with 2 of 10 in the no-treatment group, had clearance of detectable W. bancrofti microfilariae at 12 months (relative risk with exclusion of subjects treated with albendazole–ivermectin, 5.00; 95% CI, 1.80 to 45.60; P<0.001). Fewer than half the subjects who received only albendazole–ivermectin (3 of 7, or 43%) had clearance of detectable W. bancrofti microfilariae by 6 months after administration of the drugs, an effect that was similar to that in the no-treatment group (relative risk, 2.14; 95% CI, 0.41 to 19.98; P = 0.40). Median levels of W. bancrofti microfilariae did decrease from 68 to 17 microfilariae per milliliter in the albendazole–ivermectin group; however, this effect was not significant, probably owing to the small number of subjects in this group (Table 3 in the Supplementary Appendix). The effect of doxycycline on levels of W. bancrofti microfilariae could not be assessed at 36 months owing to the dramatic effect of the additional albendazole–ivermectin treatment in the control groups (Fig. 3).
Figure 3. Efficacy of Doxycycline in Reducing Wuchereria bancrofti Microfilarial Levels at 6, 12, and 36 Months after Treatment.
Levels of W. bancrofti are shown as percents of baseline levels for groups that received no treatment, doxycycline only, a single dose of albendazole–ivermectin at 6 months, and doxycycline plus a single dose of albendazole– ivermectin at 6 months. Each circle represents the value for an individual patient. The horizontal lines represent the median values for each group at each time point. All groups received a single dose of albendazole–ivermectin between 12 and 36 months. ND denotes not detectable.
Circulating antigen levels of W. bancrofti had declined significantly at 12 months in the group that received doxycycline only and in the group that received doxycycline plus albendazole–ivermectin but not in the groups that received no treatment or only albendazole–ivermectin treatment (median level in the doxycycline-only group, 60% of baseline level; 95% CI, 39 to 90; P = 0.01; median level in the group that received doxycycline plus albendazole–ivermectin, 57% of baseline; 95% CI, 26 to 81; P = 0.02; median level in the no-treatment group, 79% of baseline; 95% CI, 44 to 126; P = 0.36; and median level in the group that received only albendazole–ivermectin, 58% of baseline; 95% CI, 30 to 127; P = 0.19). Worm nests were detected by ultrasonography in the scrotal lymphatic vessels of 24 of 63 of the men infected with W. bancrofti (38%) who were tested at baseline. There was no change in the number of worm nests detected by ultrasonography in any of the groups at 12 months.
DISCUSSION
M. perstans infection is widely distributed across central Africa and northern South America and the Caribbean islands, with prevalences as high as 80 to 100%.12,13 Nevertheless, little is known about the clinical manifestations of M. perstans infection. In contrast to the other filarial pathogens in humans, M. perstans is relatively refractory to therapy with conventional antifilarial agents.7,14,15 The lack of efficacy of a single dose of albendazole–ivermectin in reducing M. perstans microfilaremia was confirmed in the present study. Although some studies have described a reduction in levels of M. perstans microfilariae after antifilarial treatment,16,17 complete clearance of microfilariae appears to be uncommon, even with prolonged drug treatment. In an open-label, nonrandomized study that compared the effects of seven different drug regimens, the most effective treatment (diethylcarbamazine at a dose of 200 mg twice daily for 21 days plus mebendazole at a dose of 100 to 200 mg daily for 21 days) effected clearance of M. perstans microfilaremia in only 10 of 27 patients (37%) by 30 days after treatment.2
Wolbachia has been shown to be present in all the major human filarial pathogens,18,19 with the exception of L. loa.20–22 Consistent with these data, doxycycline treatment has shown efficacy in decreasing microfilarial levels in lymphatic filariasis3 and onchocerciasis,4 but not in loiasis.23 In addition, doxycycline has been shown in several studies to have macrofilaricidal activity.4–6 Although we recently showed, with the use of molecular and Western blot analyses, that M. perstans microfilariae from Mali contain wolbachia,7 previous studies did not detect wolbachia in M. perstans microfilariae from Gabon or Uganda.20,21 The data from the current study, showing a clear effect of doxycycline on M. perstans microfilariae, provide further support for the presence of wolbachia in this species in Mali, raising the possibility that some geographic isolates of M. perstans may have lost (or gained) the endosymbiont.7,21
The substantial decreases at 6 and 12 months in microfilarial levels of both W. bancrofti and M. perstans in the groups that were not treated with doxycycline were unexpected. Such natural variability in microfilarial levels has been reported previously6,24 and underscores the need for appropriate control groups in filarial chemotherapy trials. The factors that determine microfilarial levels are poorly understood but are likely to include host factors as well as the burden of infestation with adult female worms. Although natural attrition of adult worms in the setting of decreasing transmission (and reinfection) could lead to a reduction in microfilarial levels in a given population over time, the lack of a decrease in circulating antigen levels of W. bancrofti (which are thought to reflect the burden of infestation with adult worms25) in the absence of treatment provides evidence against this hypothesis. Variations in the timing of the collection of blood samples could systematically affect levels of W. bancrofti microfilariae because of their nocturnal periodicity; however, this would not account for the decrease in levels of M. perstans microfilariae, since this species is not periodic. Moreover, the strict adherence to the timing of blood collection in the present study should minimize such concerns.
In summary, doxycycline, given at a dose of 200 mg daily for 6 weeks, was well tolerated and effective in reducing levels of M. perstans microfilariae in Malian adults with or without concomitant infection with W. bancrofti, providing further evidence that M. perstans in Mali contains the endosymbiont wolbachia. Furthermore, the sustained suppression of M. perstans microfilariae 36 months after treatment suggests that doxycycline has an effect on M. perstans adult worms. Most important, this study shows that doxycycline is an effective therapy for M. perstans infection.
Supplementary Material
Acknowledgments
Supported by the Division of Intramural Research of the National Institutes of Health, National Institute of Allergy and Infectious Diseases, Bethesda, MD.
We thank the Kolokani District health workers, Richard Sakai, Housseini Dolo, Dramane Sanogo, Fode Keita, and Cheickna Diakite for their assistance in the implementation of the study at the village level, and Mady Sissoko for his help with the laboratory assays.
Footnotes
Dr. Nutman reports owning equity in Johnson & Johnson. No other potential conflict of interest relevant to this article was reported.
References
- 1.Klion AD, Nutman TB. Loiasis, M. ozzardi, M. perstans, M. streptocerca. In: Guerrant DL, Walker DH, Weller PF, editors. Tropical infectious diseases: principles, pathogens, and practice. 2. New York: Churchill Livingstone; 2006. pp. 1163–75. [Google Scholar]
- 2.Bregani ER, Rovellini A, Mbaidoum N, Magnini MG. Comparison of different anthelminthic drug regimens against Mansonella perstans filariasis. Trans R Soc Trop Med Hyg. 2006;100:458–63. doi: 10.1016/j.trstmh.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Asio SM, Simonsen PE, Onapa AW. Mansonella perstans: safety and efficacy of ivermectin alone, albendazole alone and the two drugs in combination. Ann Trop Med Parasitol. 2008;102:1–7. doi: 10.1179/136485909X384929. [DOI] [PubMed] [Google Scholar]
- 4.Debrah AY, Mand S, Specht S, et al. Doxycycline reduces plasma VEGF-C/sVEGFR-3 and improves pathology in lymphatic filariasis. PLoS Pathog. 2006;2(9):e92. doi: 10.1371/journal.ppat.0020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoerauf A, Specht S, Buttner M, et al. Wolbachia endobacteria depletion by doxycycline as antifilarial therapy has macrofilaricidal activity in onchocerciasis: a randomized placebo-controlled study. Med Microbiol Immunol. 2008;197:295–311. doi: 10.1007/s00430-007-0062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor MJ, Makunde WH, McGarry HF, Turner JD, Mand S, Hoerauf A. Macrofilaricidal activity after doxycycline treatment of Wuchereria bancrofti: a double-blind, randomised placebo-controlled trial. Lancet. 2005;365:2116–21. doi: 10.1016/S0140-6736(05)66591-9. [DOI] [PubMed] [Google Scholar]
- 7.Keiser PB, Coulibaly YI, Kubofcik J, et al. Molecular identification of Wolbachia from the filarial nematode Mansonella perstans. Mol Biochem Parasitol. 2008;160:123–8. doi: 10.1016/j.molbiopara.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keiser PB, Coulibaly YI, Keita F, et al. Clinical characteristics of post-treatment reactions to ivermectin/albendazole for Wuchereria bancrofti in a region co-endemic for Mansonella perstans. Am J Trop Med Hyg. 2003;69:331–5. [PubMed] [Google Scholar]
- 9.Mand S, Marfo-Debrekyei Y, Dittrich M, Fischer K, Adjei O, Hoerauf A. Animated documentation of the filaria dance sign (FDS) in bancroftian filariasis. Filaria J. 2003;2:3. doi: 10.1186/1475-2883-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hothorn T, Hornik K, van de Wiel MA, Zeileis A. A Lego system for conditional inference. Am Stat. 2006;60:257–63. [Google Scholar]
- 11.Team RDCR. A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2007. [Google Scholar]
- 12.Wanji S, Tendongfor N, Esum M, Ndindeng S, Enyong P. Epidemiology of concomitant infections due to Loa loa, Mansonella perstans, and Onchocerca volvulus in rain forest villages of Cameroon. Med Microbiol Immunol. 2003;192:15–21. doi: 10.1007/s00430-002-0154-x. [DOI] [PubMed] [Google Scholar]
- 13.Akue JP, Hommel M, Devaney E. Markers of Loa loa infection in permanent residents of a loiasis endemic area of Gabon. Trans R Soc Trop Med Hyg. 1996;90:115–8. doi: 10.1016/s0035-9203(96)90105-4. [DOI] [PubMed] [Google Scholar]
- 14.Gardon J, Kamgno J, Gardon-Wendel N, Demanaga N, Duke BO, Boussinesq M. Efficacy of repeated doses of ivermectin against Mansonella perstans. Trans R Soc Trop Med Hyg. 2002;96:325–6. doi: 10.1016/s0035-9203(02)90112-4. [DOI] [PubMed] [Google Scholar]
- 15.Schulz-Key H, Albrecht W, Heuschkel C, Soboslay PT, Banla M, Görgen H. Efficacy of ivermectin in the treatment of concomitant Mansonella perstans infections in onchocerciasis patients. Trans R Soc Trop Med Hyg. 1993;87:227–9. doi: 10.1016/0035-9203(93)90504-j. [DOI] [PubMed] [Google Scholar]
- 16.Kyelem D, Sanou S, Boatin B, Medlock J, Coulibaly S, Molyneux DH. Impact of long-term ivermectin (Mectizan) on Wuchereria bancrofti and Mansonella perstans infections in Burkina Faso: strategic and policy implications. Ann Trop Med Parasitol. 2003;97:827–38. doi: 10.1179/000349803225002462. [DOI] [PubMed] [Google Scholar]
- 17.Duong TH, Kombila M, Ferrer A, Nguiri C, Richard-Lenoble D. Decrease in Mansonella perstans microfilaraemia after albendazole treatment. Trans R Soc Trop Med Hyg. 1998;92:459. doi: 10.1016/s0035-9203(98)91093-8. [DOI] [PubMed] [Google Scholar]
- 18.Taylor MJ, Bilo K, Cross HF, Archer JP, Underwood AP. 16S rDNA phylogeny and ultrstructural characterization of Wolbachia intracellular bacteria of the filarial nematodes, Brugia malayi, B. pahangi, and Wuchereria bancrofti. Exp Parasitol. 1999;91:356–61. doi: 10.1006/expr.1998.4383. [DOI] [PubMed] [Google Scholar]
- 19.Casiraghi M, Favia G, Cancrini G, Bartoloni A, Bandi C. Molecular identification of Wolbachia from the filarial nematode Mansonella ozzardi. Parasitol Res. 2001;87:417–20. doi: 10.1007/s004360000368. [DOI] [PubMed] [Google Scholar]
- 20.Grobusch MP, Kombila M, Autenrieth I, Mehlhorn H, Kremsner PG. No evidence of Wolbachia endosymbiosis with Loa loa and Mansonella perstans. Parasitol Res. 2003;90:405–8. doi: 10.1007/s00436-003-0872-z. [DOI] [PubMed] [Google Scholar]
- 21.Büttner DW, Wanji S, Bazzocchi C, Bain O, Fischer P. Obligatory symbiotic Wolbachia endobacteria are absent from Loa loa. Filaria J. 2003;2:10. doi: 10.1186/1475-2883-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGarry HF, Pfarr K, Egerton G, et al. Evidence against Wolbachia symbiosis in Loa loa. Filaria J. 2003;2:9. doi: 10.1186/1475-2883-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brouqui P, Fournier PE, Raoult D. Doxycycline and eradication of microfilaremia in patients with loaisis. Emerg Infect Dis. 2001;7(Suppl):604–5. doi: 10.3201/eid0707.010747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Debrah AY, Mand S, Marfo-Debrekyei Y, Larbi J, Adjei O, Hoerauf A. Assessment of microfilarial loads in the skin of onchocerciasis patients after treatment with different regimens of doxycycline plus ivermectin. Filaria J. 2006;5:5. doi: 10.1186/1475-2883-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chanteau S, Moulia-Pelat JP, Glaziou P, et al. Og4C3 circulating antigen: a marker of infection and adult worm burden in Wuchereria bancrofti filariasis. J Infect Dis. 1994;170:247–50. doi: 10.1093/infdis/170.1.247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.