Abstract
Objective:
Many adolescents engage in heavy alcohol use. The aim of this study was to disentangle whether brain abnormalities seen in adolescent heavy drinkers are a consequence of heavy drinking, a preexisting risk factor for initiation of alcohol use, or both.
Method:
Study 1 used cross-sectional functional magnetic resonance imaging (fMRI) visual working-memory (VWM) data from 15- to 19-year-olds (20 heavy drinkers, 20 controls) to identify brain regions affected by heavy adolescent alcohol use. Study 2 used longitudinal fMRI VWM data from 12- to 16-year-olds imaged before the onset of drinking and imaged again on the same scanner approximately 3 years later. Those who had transitioned into heavy drinking (n = 20) were matched to continuous nondrinkers (n = 20) on baseline alcohol risk and developmental factors (N = 40; 80 scans).
Results:
Study 1 found that heavy drinkers exhibited more frontal and parietal but less occipital activation than controls, defining the regions of interest for Study 2. In Study 2, adolescents who later transitioned into heavy drinking showed less fMRI response contrast at baseline than continuous nondrinkers, which increased after the onset of heavy drinking, in frontal (1,431 μL, p = .003; η2 = .19) and parietal (810 μL, p = .005; η2 = .23) regions, as in Study 1. Lower baseline activation in the frontal and parietal regions predicted subsequent substance use, more so than commonly observed predictors of youth drinking (p < .05).
Conclusions:
Adolescents who initiated heavy drinking showed different brain activation before the onset of drinking, then less efficient information processing after high-dose alcohol use started. This suggests neural response patterns that could be risk factors for future substance use and also supports prior neuropsychological reports indicating that initiating heavy episodic drinking in adolescence may be followed by subtle alterations in brain functioning.
Adolescence coincides with significant increases in alcohol consumption, with past-year rates increasing from 29% to 65% and past-year drunkenness rising from 12% to 44% between 8th and 12th grade (Johnston et al., 2011). Almost a quarter of all 12th graders report heavy episodic drinking (i.e., five or more drinks on one occasion during the past 2 weeks; Johnston et al., 2011), a pattern related to risky behaviors such as drunk driving, riding with impaired drivers, violence, unsafe sex, and other substance use (Miller et al., 2007).
Heavy alcohol use during this life stage is concerning because the brain undergoes significant maturational changes during adolescence to create a more refined, efficient central nervous system (Giedd et al., 1999; Gogtay et al., 2004; Luna and Sweeney, 2004; Schweinsburg et al., 2005a; Spear and Varlinskaya, 2005). Transformations including cortical thinning, increased fiber track efficiency, and neurochemical and hormonal changes may leave the brain more vulnerable to the deleterious effects of alcohol (Brown et al., 2000; Clark and Tapert, 2008; Spear, 2000; Spear and Varlinskaya, 2005; Squeglia et al., 2009b; Tapert et al., 2002). Given the considerable brain development during adolescence, understanding the effect of neural insults incurred during this period is essential.
Heavy alcohol use during adolescence has been associated with neuropsychological disadvantages (e.g., Brown et al., 2000; Giancola et al., 1998; Sher et al., 1997; Squeglia et al., 2009a, 2009b; Tapert and Brown, 1999), and longitudinal studies suggest adverse effects on visuospatial processing, attention, and working memory (Hanson et al., 2011; Squeglia et al., 2009b; Tapert et al., 2002). Research using functional magnetic resonance imaging (fMRI) has begun to shed light on neural functioning correlates of adolescent heavy drinking. Young heavy drinkers have shown greater parietal and decreased occipital and cerebellar activation during a spatial working-memory task than have light drinkers, despite equivalent task performance (Tapert et al., 2004b). In an fMRI study of 95 adolescents, heavy drinking was linked to greater spatial working-memory response for males and less response for females, compared with gender-matched nondrinkers, in frontal and other regions, and less activation was linked to poorer neuropsychological test performance (Squeglia et al., 2011). Similarly, young adult females with adolescent-onset alcohol dependence showed less frontal and parietal activation and poorer performance on the spatial working-memory task and other tests of working memory and executive functioning (Tapert et al., 2001), particularly those females with histories of alcohol withdrawal. Together, these findings suggest that the brain may be able to initially compensate for subtle neural abnormalities following heavy alcohol exposure with increased activation, but continued heavy alcohol use may eventually interfere with the capacity for neural compensation as evidenced by reduced activation.
Activation differences may also precede substance use. Among adolescents imaged before ever using substances, those who transitioned into heavy alcohol use over a 4-year follow-up had shown significantly less activation in frontal, parietal, temporal, and basal ganglia areas during an inhibition task compared with those who remained nonusers, despite intact performance (Norman et al., 2011), suggesting that some cross-sectional fMRI findings could be attributed to premorbid differences. In summary, the literature suggests lower activation with intact performance during cognitive challenges in youth who later develop heavy drinking compared with those who do not, more activation with intact performance in youth with brief histories of heavy drinking compared with nondrinkers, and less activation and impaired neurocognitive performance in heavy-drinking adolescents and young adults after several years of adolescent-onset heavy drinking. The chronicity of these abnormalities is unknown because all imaging studies examining alcohol use and adolescent brain development have been cross-sectional.
No studies have examined the effect of adolescent alcohol use on visual working memory (VWM), which is important in the context of brain functioning. First, working memory (i.e., the storage and manipulation of information) is an essential component of executive functioning and information processing (Lezak et al., 2004), crucial to the development of logical thinking and reasoning (Mandler, 2007). Any deficits in working memory accrued from heavy drinking would have substantial negative effects on daily functioning. Second, working-memory capacity has an important influence on decision making and may moderate risk for heavy alcohol use (Finn, 2002; Finn et al., 2009). Third, working memory continues to improve over the course of adolescence to early adulthood, with greater reliance on the more refined and efficient use of right dorsolateral prefrontal and bilateral parietal cortex during task performance (Crone et al., 2006). VWM tasks evoke substantial cortical response, predominantly in prefrontal and parietal regions (Baker et al., 1996; Courtney et al., 1996; Friedman and Goldman-Rakic, 1994; Haxby et al., 2000; Owen et al., 1996; Petrides et al., 1993), areas that appear vulnerable to the effects of adolescent alcohol use (Schweinsburg et al., 2005b; Tapert et al., 2004a). Finally, the neural substrates of VWM have been comprehen sively characterized (Cabeza et al., 2004; Cohen et al., 1997; Courtney et al., 1996; Fougnie and Marois, 2006; Postle and D'Esposito, 1999; Smith and Jonides, 1999; Ungerleider et al., 1998) and are easily probed by manipulating the number of items (i.e., load) presented (Luck and Vogel, 1997).
The goals of this two-part study were to (a) identify brain regions where activation to a VWM task differed between heavy drinkers and controls to ascertain the key regions of interest (ROIs) and (b) use ROIs determined by the cross-sectional study to prospectively examine the influence of alcohol use on brain functioning in a separate sample of adolescents first scanned before alcohol use initiation (ages 12–16), then again on the same scanner approximately 3 years later, after some had begun heavy drinking. We hypothesized that adolescents who transitioned into heavy drinking (“transitioners”) would show lower blood oxygen level– dependent (BOLD) response during a VWM task before initiating heavy drinking (baseline time point), then increased activation after the onset of heavy drinking, compared with adolescents who remained nonusers. This is the first prospective neuroimaging study to examine brain activation before the onset of substance use to help understand differences in neural substrates in adolescents who begin to engage in heavy drinking.
Method
Participants
All participants from Studies 1 and 2 were part of a larger, ongoing neuroimaging study examining neurocognition in youth at risk for substance use disorders (Bava et al., 2010; Squeglia et al., 2009b, 2011, 2012). Participants were recruited through flyers sent to households of students attending local middle schools. Extensive screening and background information were obtained from the youths, a biological parent, and one other parent or close relative. The study protocol was executed in accordance with the standards approved by the University of California, San Diego, Human Research Protections Program.
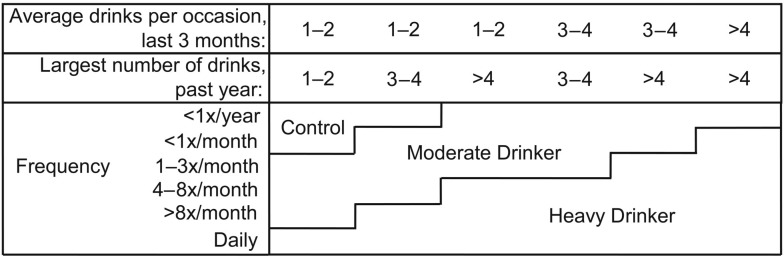
For Study 1, participants ages 15–19 years (N = 40; average age = 17.0 years [SD = 1.0]; 55% female) categorized as heavy drinkers (n = 20; average age = 17.6 years [SD = 1.3]; 39 drinks per month on average; see Figure 1 and Squeglia et al., 2009b, for classification) were individually matched to nonusers (n = 20; no lifetime history of any alcohol or other drug use) on age, pubertal development, gender, and family history of alcoholism. Exclusionary criteria included any suggestion of prenatal alcohol (more than two drinks during a given week) or illicit drug exposure, history of chronic medical illness, any neurological or Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (American Psychiatric Association, 1994), Axis I disorder other than oppositional defiant or conduct disorder, head trauma or loss of consciousness (>2 minutes), learning disability or mental retardation, parental history of psychotic disorder, use of medications potentially affecting the brain or cerebral blood flow, premature birth (i.e., born before 35th gestational week), contraindication to MRI, inadequate comprehension of English, noncorrectable sensory problems, and left-handedness.
Figure 1.
Drinking classification for Studies 1 and 2, based on Cahalan et al. (1969) and modified based on the distribution of drinking characteristics of adolescents (Schweinsburg et al., 2005b, Squeglia et al., 2009; Tapert et al., 2004b).
Participants for Study 2 (N = 40, different individuals than in Study 1) each had a baseline brain scan before any significant alcohol or other drug use and one follow-up scan, approximately 3 years later, for a total of 80 scans. At baseline, all participants were ages 12–16 years (Table 1) and had minimal to no experience with alcohol or other drugs: less than 10 total days in their life on which drinking had occurred, never with more than two drinks in a week; less than one lifetime experience with marijuana and none in the past 3 months; less than five lifetime cigarette uses; and no history of other intoxicant use (Table 2). The same exclusionary criteria from Study 1 were applied for Study 2. After screening, 12% of those who responded to the fliers remained eligible (Table 1). Participants were interviewed every 3 months and then those who started heavy drinking (transitioners: n = 20) and demographically matched, nonusing youth (continuous nondrinkers: n = 20) were scanned for a follow-up.
Table 1.
Study 2 demographic characteristics at baseline and follow-up
| Variable | Continuous nondrinkers (n = 20) M (SD) or % | Heavy drinking transitioners (n = 20) M (SD) or % |
| Baseline | ||
| Age, years | 14.77 (1.14) | 15.07 (1.26) |
| Male gender | 70% | 70% |
| Race, White | 55% | 65% |
| Family history of alcoholism density, range: 0–2 | 0.19 (0.31) | 0.40 (0.63) |
| Conduct disorder positive | 0% | 0% |
| Hollingshead Index of Social Position score | 27.40 (16.30) | 19.10 (12.08) |
| Parental salary, U.S. $* | $109K ($63K) | $171K ($103K) |
| Education, years | 8.10 (1.29) | 8.75 (1.37) |
| Females’ Pubertal Development Scale total | 15.83 (2.48) | 18.00 (2.10) |
| Males’ Pubertal Development Scale total | 13.79 (2.75) | 14.15 (3.16) |
| Beck Depression Inventory total | 2.20 (2.61) | 1.89 (2.62) |
| Spielberger State Anxiety total | 26.15 (6.04) | 26.94 (5.91) |
| CBCL/YSR Internalizing T score | 46.47 (9.28) | 44.25 (10.36) |
| CBCL/YSR Externalizing T score | 39.76 (6.51) | 42.81 (8.38) |
| Sleepiness rating before scan | 4.60 (1.47) | 4.68 (1.83) |
| Sleepiness rating after scan | 5.70 (1.78) | 5.89 (2.13) |
| Grade point average | 3.35 (0.74) | 3.53 (0.45) |
| Follow-up | ||
| Age, years | 17.71 (1.44) | 18.46 (1.90) |
| Years between scans | 2.94 (0.98) | 3.43 (1.07) |
| Conduct disorder positive* | 0% | 15% |
| Females’ Pubertal Development Scale total | 19.43 (0.98) | 20.00 (0.00) |
| Males’ Pubertal Development Scale total | 17.33 (2.35) | 17.29 (3.15) |
| Education, years | 11.00 (1.41) | 11.89 (2.11) |
| Beck Depression Inventory total | 1.35 (1.84) | 2.60 (3.33) |
| Spielberger State Anxiety total | 25.00 (5.01) | 22.68 (2.98) |
| CBCL/YSR Internalizing T score | 40.37 (7.43) | 41.58 (9.47) |
| CBCL/YSR Externalizing T score | 41.74 (8.81) | 46.32 (10.15) |
| Sleepiness rating before scan | 3.90 (1.21) | 4.25 (1.65) |
| Sleepiness rating after scan | 5.60 (1.76) | 6.15 (1.76) |
| Grade point average | 3.48 (0.39) | 3.35 (0.51) |
Notes: Ethnicity was 30% Latino; race was 60% White, 35% multiracial, 3% African-American, 3% Asian (no significant between-group differences). K = 1,000; CBCL = Child Behavior Checklist; YSR = Youth Self-Report.
Continuous nondrinkers ≠ heavy drinkers, p < .05.
Table 2.
Study 2 substance use characteristics at baseline and follow-up
| Variable | Continuous nondrinkers (n = 20) M (SD) or % | Heavy drinking transitioners (n = 20) M (SD) or % |
| Baseline | ||
| Lifetime alcohol use occasions* | 0.05 (0.22) | 1.50 (3.02) |
| Lifetime cannabis use occasions | 0.00 (0.00) | 0.10 (0.31) |
| Lifetime other drug use occasions | 0.00 (0.00) | 0.00 (0.00) |
| Follow-up | ||
| Lifetime alcohol use occasions* | 1.35 (1.90) | 93.50 (79.56) |
| Peak drinks on an occasion, past year* | 0.60 (0.94) | 11.90 (5.61) |
| Peak drinks on an occasion, past 3 months* | 0.25 (0.44) | 8.80 (6.01) |
| Estimated peak BAC, past 3 months* | 0.00 (0.01) | 0.25 (0.14) |
| Drinks per drinking day, past month* | 0.25 (0.55) | 6.10 (4.28) |
| Days since last alcohol use | n.a. | 37.74 (70.75) |
| Tobacco cigarettes per day, past month | 0.00 (0.00) | 0.20 (0.62) |
| Lifetime cannabis use occasions,* range: 0—699 | 0.15 (0.49) | 83.55 (171.81) |
| Used cannabis more than five times | 0% | 50% |
| Cannabis use days/month, past 3 months* | 0.00 (0.00) | 3.75 (6.33) |
| Lifetime other drug use occasions | 0.00 (0.00) | 1.50 (3.55) |
Notes: As expected, significant Time × Drinking Status interactions were observed for lifetime alcohol, cannabis, and other drug use. BAC = blood alcohol concentration; n.a. = not applicable.
Continuous nondrinkers ≠ heavy drinkers, p < .05
Measures (same measures were used in Studies 1 and 2)
Substance use measures.
Using the Customary Drinking and Drug Use Record (Brown et al., 1998), we obtained self-reported quantity and frequency of lifetime and past- 3-month alcohol, tobacco, and other drug use; withdrawal/ hangover symptoms; and abuse and dependence criteria. The Timeline Followback (Sobell and Sobell, 1992) assessed substance use quantity and frequency for 30 days before the scan, and a parental report of the youth's substance use was collected. Breath alcohol analysis and urine toxicology screens confirmed self-report data. For Study 2, substance use information was updated every 3 months after baseline.
Family background.
The Family History Assessment Module (Rice et al., 1995) ascertained familial density of alcohol use disorders and other substance use disorders by adding 0.5 for each biological parent and 0.25 per biological grandparent (Zucker et al, 1994) when endorsed by either youth or parent. Family history information was collected from (a) both parents or (b) one parent and another close relative (the latter occurred in <7% of cases). Socioeconomic background information (i.e., educational attainment, occupation, and salary of each parent) was obtained from parents and converted to a Hollingshead Index of Social Position score, with higher scores indicating lower socioeconomic status (Hollingshead, 1965).
Development.
The Pubertal Development Scale (Petersen et al., 1988) correlates with physician ratings and Tanner Sexual Maturation Scale self-ratings (Miller et al., 1988), providing a reliable and valid four-item (males; score range: 4–16) and five-item (females; score range: 5–20) self-report measure of pubertal maturation, with higher scores indicating greater maturity.
Psychopathology and mood.
The Youth Self-Report (Achenbach and Rescorla, 2001) and the Child Behavior Checklist (Achenbach and Rescorla, 2001) provided a level of adolescent psychopathological syndromes (e.g., internalizing and externalizing behaviors). Beck Depression Inventory–II (21 items; score range: 0–63; Beck et al., 1996) and Spielberger State–Trait Anxiety Inventory (20 items; score range: 20–80; Spielberger et al., 1970) assessed recent depressive symptoms and anxiety on the day of scanning with higher scores indicating greater mood symptoms. The Karolinska Sleepiness Scale (Åkerstedt and Gillberg, 1990) assessed alertness before and after scanning (1 = extremely alert to 9 = extremely sleepy).
Procedures
Imaging.
Imaging data from both studies were collected from the same 3.0 Tesla General Electric (3T GE) short bore Excite-2 system with an eight-channel phase-array head coil. Eight high-bandwidth receivers for ultra-short repetition time (TR) reduced signal distortions and signal dropout. Scan sessions collected a high-resolution 3d T1- weighted sequence obtained using a sagittally acquired spoiled gradient recalled sequence (field of view [FOV] 24 cm, 256 × 256 × 192 matrix, .94 × .94 × 1 mm voxels, 176 slices, repetition time = 20 ms, echo time = 4.8 ms; flip angle 12°, 7:26 minutes). Field maps were acquired to minimize warping and signal dropout (∼4 minutes total) with two different echo times. BOLD signal was measured with T2*-weighted axially acquired echo-planar images (FOV = 24 cm, 64 × 64 matrix, 3.75 × 3.75 × 3.8 mm voxels, 32 slices, echo time = 30 ms, repetition time = 2,000 ms, flip angle 90°). Task stimuli were back-projected to a screen at the foot of the scanner via an angled mirror attached to the head coil. Accuracy and reaction times were logged with a fiber-optic response box (Current Designs, Pittsburgh, PA).
Task.
All participants were administered the same VWM task (Paulus et al., 2006; Tapert et al., 2004a) during fMRI acquisition. Each trial consisted of an array of 2, 4, or 6 colored dots briefly (100 ms) presented against a gray background. Because three to four different items can be held simultaneously in VWM (Luck and Vogel, 1997), this task used the 2-dot array as the low-capacity condition, 4-dot as mid-capacity, and 6-dot as the high- or supra-capacity condition. After a 1,000 ms delay, the subsequent trial (2,000 ms followed by 500 ms timeout) included the same number of dots presented in the same location and were either the same color array or one color different. For each trial, the subjects pressed “1” if the color displays were the same and “2” if they differed; 50% of the trials had identical color arrays, whereas 50% had a one-color dot difference. Each subject completed 30 trials of each type (2, 4, or 6 dots) presented randomly and 69 null trials of 2,000 ms each interspersed to provide an optimized fast-event related sequence (256 repetitions in all; 8 minutes, 32 seconds). The 6-dot condition is considered supra-span (i.e., higher than most people's working-memory span), and the 2-dot condition is sub-span (i.e., well within most people's working-memory load capacity; Luck and Vogel, 1997). BOLD response contrast during the 6-dot array relative to the 2-dot array (high load vs. low load condition) was the outcome measure of interest, interpreted as brain response to increasing working-memory load. A greater BOLD response contrast (i.e., larger fit coefficient) was interpreted as more cognitive energy to complete the supra-span (6-dot) trials. None of the 120 runs in Studies 1 or 2 had performance below chance (50%) on the 2-dot condition.
Follow-up procedures.
The longitudinal study (Study 2) used participants with baseline and follow-up data collected on the same 3T scanner using rigorous procedures (Kleschinsky et al., 2009; Twitchell, 1992) for an overall follow-up rate of 99% through Year 6. Every 3 months after the baseline interview, participants were reassessed on current substance use and psychiatric functioning. Those who met criteria for heavy drinking (see Figure 1 and Squeglia et al., 2009b) were invited to return and complete assessments. Each participant who started heavy drinking during the follow-up period (transitioner) was matched to a demographically similar participant who continued to have no evidence of substance use throughout the follow-up (continuous nondrinker). Moderate drinkers were excluded from analysis in this article.
Matching.
In Study 1, heavy drinkers and controls were equated on age, pubertal development, gender, family history of alcohol and substance dependence, socioeconomic status, and internalizing and externalizing behaviors. In Study 2, transitioners and continuous nondrinkers were matched on all of the same criteria as Study 1, as well as baseline and follow-up age and years since baseline (see Table 1 for Study 2 matching).
Data analysis
Image processing.
All data for Studies 1 and 2 were processed and analyzed using Analysis of Functional NeuroImages (AFNI; Cox, 1996). Artifact and aberrant signal levels were examined in each repetition of each slice using an automated program developed by the University of California, San Diego, Laboratory of Cognitive Neuroimaging. Motion in time series data were corrected by registering each acquisition to the maximally stable base volume with an iterated least squares algorithm (Cox and Jesmanowicz, 1999) to estimate three rotational and three displacement parameters for each participant, which were used in the model to control for spin history effects (Bandettini et al., 1993; Friston et al., 1996). To evaluate task-related motion, the reference vector was correlated with the six motion parameters for each data set. Data sets with significant task-correlated or bulk motion were excluded from analyses. Two trained raters omitted any remaining repetitions with visually discernible motion; if more than 15% of repetitions were discarded, the subject was excluded.
Deconvolution was conducted on time series data with a reference function that convolved the behavioral stimuli with a hemodynamic response model (Cohen et al., 1997) while covarying for linear trends and motion correction and ignoring the first three repetitions, resulting in a functional image in which every voxel contained a fit coefficient representing the change in signal across behavioral conditions, as well as percentage signal change and threshold statistics. Standardization transformations were made for each high-resolution anatomical image (Talairach and Tournoux, 1988), and functional data sets were warped in accordance to manage individual anatomical variability. Functional data were resampled into isotropic voxels (3 mm3), and a spatial smoothing Gaussian filter (full-width half maximum 5 mm) minimized the influence of individual anatomic variability. Co-registration of structural to functional images was performed with a mutual information registration program (Cox and Jesmanowicz, 1999) that robustly handles images with different signal characteristics and of different spatial resolutions.
For Study 2, ROI masks were created of areas that showed a difference in VWM response between drinkers and nondrinkers in Study 1, using brain atlases in AFNI (Lancaster et al., 2000; Talairach and Tournoux, 1988). Masks were applied to the 6-dot (high working-memory load) versus 2-dot (low working-memory load) contrast to extract a fit coefficient averaged across the ROI, which was imported to PASW Statistics 18 (SPSS, Inc., 2009, Chicago, IL, www.spss.com) for each ROI of each participant at both time points.
Statistical analyses.
For both studies, a t test determined differences on VWM task accuracy and reaction time between groups. For Study 1, the AFNI program 3dttest compared heavy drinkers with nonusers on BOLD response to high- to low-load trials of the VWM task in a whole-brain analysis. AlphaSim (Ward, 2000) determined that activations comprising at least 34 contiguous voxels (i.e., 918 μL) each with effect p < .05 would be only 5% likely to occur under the null hypotheses within the brain volume. For Study 2, a repeated-measures analysis of variance with time (baseline and follow-up) as the within-subjects factor and group (transitioner vs. continuous nondrinker) as the between-subjects factor was used to determine main effects of follow-up drinking status and time, as well as the interaction between drinking status and time on VWM high-load relative to low-load BOLD response fit coefficient (6 vs. 2 dot), for each ROI. This examined whether starting heavy alcohol use predicted change in brain activity from baseline to follow-up. An exploratory whole-brain analysis determined if brain regions other than ROIs might show BOLD response contrast change over time with drinking. AlphaSim (Ward, 2000) determined that activations of more than 96 contiguous voxels (2,592 μL) each with an effect p < .05 would be only 5% likely to occur under the null hypotheses. Bonferroni corrections kept family-wise error at .05 (i.e., .01 for each of the five ROIs). Pearson correlations examined the relationship between BOLD response change scores and substance use over the follow-up period.
Post hoc follow-up analyses examined baseline activation predicting future substance use. Hierarchical linear regressions were used, with common predictors of youth drinking (i.e., family history, baseline lifetime drinks, baseline externalizing behaviors, and follow-up age) in Block 1, baseline BOLD response contrast of each ROI in Block 2, and follow-up past-year peak drinks, past-year drinking days, lifetime drinking days, drinks in past month, and hangover/ withdrawal symptoms as dependent variables (a < .05).
Covariates.
For Study 1, no demographic characteristics differed between groups. For Study 2, although overall groups were very well matched, baseline alcohol use (average lifetime use days: continuous nondrinker = 0.5, transitioners = 1.5) and follow-up lifetime cannabis use (average: continuous nondrinker = 0.2, transitioners = 84) differed between groups and, therefore, were treated as covariates in all analyses. Parental salary also significantly differed between groups; however this variable was not used as a covariate because the average salaries for both groups was well above average national income levels (average: controls = $109,000; transitioners = $171,000) and represent similar economic classes (i.e., upper middle class). Furthermore, overall SES was similar between groups, as well as parental education.
Results
Studies 1 and 2
In both studies, the VWM task activated similar parietal and frontal brain regions. Several parietal regions showed greater activation to the 2- relative to 6-dot condition (i.e., more engagement during automatic processing), whereas frontal regions showed greater activation to the 6- relative to 2-dot condition (i.e., more engagement during complex information processing).
Study 1
Task performance data were available for all participants (N = 40) in this cross-sectional study (ages 15–19). The two groups were statistically equivalent on accuracy and reaction time. Heavy drinkers had more BOLD response than nonusers in four areas ([a] left medial frontal gyrus/ supplementary motor area, [b] right middle/superior frontal gyrus, [c] right superior frontal gyrus, and [d] right inferior parietal/supramarginal gyrus) and less BOLD response in left middle occipital gyrus (clusters > 918 μL, corrected p < .05; Table 3). Regions that differed between adolescent drinkers and nondrinkers were used as the ROIs for the longitudinal Study 2.
Table 3.
Study 1 regions of significant BOLD response differences between heavy drinkers and controls (N = 40). These brain regions were used as regions of interest for Study 2.
| Brodmann area (s) | Volume (μL) | Talairach coordinatesa |
|||
| Anatomical region | x | y | z | ||
| L medial frontal gyrus | 6 | 576 | −4.5 | 19.5 | 59.5 |
| R middle/superior frontal gyrus | 8/9 | 621 | −28.5 | −34.5 | 41.5 |
| R superior frontal gyrus | 10 | 459 | −22.5 | −61.5 | 8.5 |
| R inferior parietal lobule/supramarginal gyrus | 40 | 1,395 | −52.5 | 34.5 | 50.5 |
| L middle occipital gyrus | – | 693 | 25.5 | 79.5 | 17.5 |
Notes: L = left; R = right.
Coordinates refer to location of peak group difference in visual working-memory response within the cluster.
Study 2
Task performance.
Study 2 used a different sample of adolescents with longitudinal imaging data to elucidate the chronicity of adolescent alcohol use on brain functioning over time, using the five areas of differences from Study 1 as ROIs. Task performance data were available for 19/20 continuous nondrinkers and 18/20 transitioners at baseline, and 18/20 continuous nondrinkers and 20/20 transitioners at follow-up. A significant time effect (ps < .05) for 2- and 6-dot accuracy and reaction time showed that continuous nondrinkers and transitioners were faster and more accurate at follow-up than at baseline. The two groups were statistically equivalent on task performance at both time points, except at baseline, when transitioners performed slightly faster (p < .05) on the 2-dot condition than did continuous nondrinkers (Table 4). No correlations between performance and 6-dot relative to 2-dot BOLD response contrast were observed at baseline or follow-up.
Table 4.
Study 2 fMRI task-performance data at baseline and follow-up
| Variable | Continuous nondrinkers (n = 20) M (SD) | Heavy drinking transitioners (n = 20) M (SD) |
| Baseline | ||
| 2-dot accuracy, % | 91.01 (8.24) | 91.18 (11.27) |
| 6-dot accuracy, % | 77.93 (12.24) | 79.40 (8.07) |
| 2-dot reaction time, ms* | 2,596.89 (217.96) | 2,436.22 (223.73) |
| 6-dot reaction time, ms | 2,714.00 (230.55) | 2,599.56 (214.30) |
| Follow-up | ||
| 2-dot accuracy, % | 96.11 (4.50) | 94.86 (4.95) |
| 6-dot accuracy, % | 84.04 (8.35) | 80.16 (10.27) |
| 2-dot reaction time, ms | 2,213.89 (125.95) | 2,214.10 (96.40) |
| 6-dot reaction time, ms | 2,369.32 (147.57) | 2,405.50 (104.57) |
Notes: Significant Time × Drinking Status interactions were observed in 2-and 6-dot reaction time. For both interactions, adolescents who transitioned into heavy drinking had attenuated decreases in reaction time at follow-up compared with continuous nondrinkers. fMRI = functional magnetic resonance imaging.
Continuous nondrinkers ≠ heavy drinkers, p < .05.
Group × Time interactions.
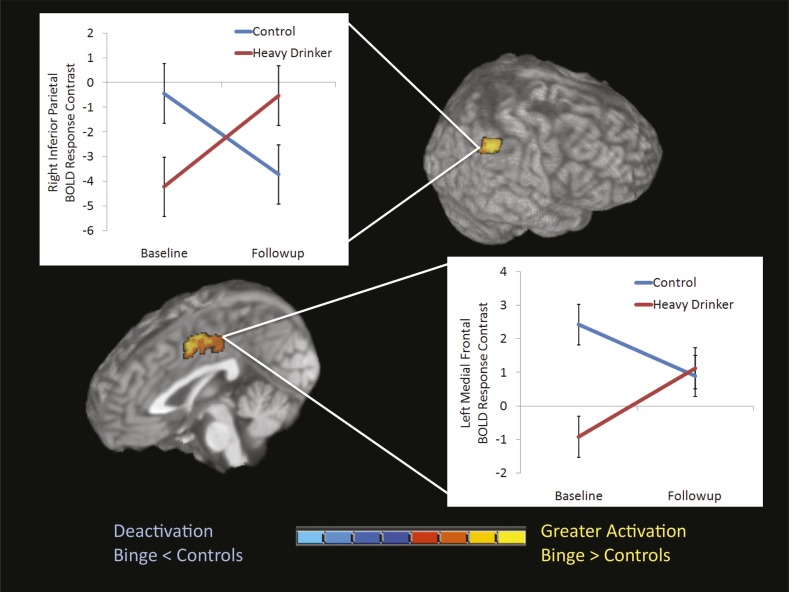
There were no main effects of group or time. However, significant Group × Time interactions emerged in two of the five hypothesized ROIs: the right inferior parietal lobule (cluster size: 810 μL, p = .005; η2 = .23) and the left medial frontal gyrus (cluster size: 1,431 μL, p = .003; η2 = .19) (Table 5 and Figure 2). Independent samples t tests probed significant Group × Time interactions in these two ROIs. At baseline, transitioners showed less activation than continuous nondrinkers in both regions (p < .01). After starting heavy drinking, transitioners showed increased BOLD response contrast, whereas continuous nondrinkers exhibited attenuated activation in both regions, compared with baseline (when heavy drinking had not yet occurred). At follow-up, transitioners showed a trend (p = .10) for greater activation than continuous nondrinkers in the right inferior parietal lobule.
Table 5.
Study 2 significant Group × Time interactions for BOLD response to visual working memory (N = 40)
| Talairach coordinatesa |
Peak activation, M (SD) |
|||||||||
| Anatomical region | Brodmann area | Volume, μL | x | y | z | BL non-drinkers | BL transitioners | FU non-drinkers | FU transitioners | η2 |
| R inferior parietal lobule | 40 | 810 | −52.5 | 46.5 | 38.5 | −0.45 (3.90) | −4.23 (5.11) | −3.73 (6.10) | −0.54 (6.30) | .23 |
| L medial frontal gyrus | 6 | 1,431 | 4.5 | −1.5 | 53.5 | 2.42 (2.95) | −0.92 (3.17) | 0.89 (2.22) | 1.12 (2.47) | .19 |
Notes: R = right; L = left; BL = baseline; FU = follow-up.
Coordinates refer to location of peak group difference in visual working-memory response within the cluster.
Figure 2.
Study 2 significant Group × Time interactions were found in two of the five hypothesized regions of interest: the right inferior parietal lobule (cluster size: 810 μL, p = .005) and left medial frontal gyrus (cluster size: 1,431 μL, p = .003). At baseline, significant differences in BOLD response contrast to high working-memory load relative to low working-memory load were observed in both regions (ps < .01). At follow-up, differences in BOLD response contrast trended toward significance (p = .10) in the right inferior parietal lobule. Although continuous nondrinkers (n = 20) did not change significantly over time, heavy drinkers (n = 20) did in both regions.
In the context of expected activation to the task over time, transitioners exhibited different BOLD response to the VWM task compared with continuous nondrinkers both before and after the onset of heavy drinking; results held after controlling for baseline alcohol use and follow-up lifetime cannabis use. Whole-brain analyses did not show additional interactions (>2,592 μL, corrected p < .05). This interaction was examined in the 4- versus 2-dot and 6- versus 4-dot contrasts to observe the consistency of the findings across contrasts. Both ROIs showed a similar relationship to the 6- versus 2-dot contrast (i.e., increasing activation over time for heavy drinkers, decreasing activation for controls over time). Baseline BOLD response contrast variables did not correlate with baseline drinking or externalizing variables. However, greater past-year peak drinks on an occasion (r = .36, p < .025), more past-month drinking days (r = .36, p < .025), and greater withdrawal/hangover symptoms (r = .46, p < .005) at follow-up correlated with increasing medial frontal gyrus BOLD response from baseline to follow-up, and greater past-year peak drinks on an occasion (r = .32, p < .05) correlated with increasing inferior parietal lobule BOLD response.
Follow-up analyses
Exploratory analyses (N = 40) examined whether baseline left medial frontal or right inferior parietal activation predicted future substance use, greater than commonly observed predictors of youth drinking (family history, baseline drinking, externalizing behaviors, and age at follow-up). Dependent variables were follow-up past year peak drinks, past-year drinking days, lifetime drinking days, and drinks consumed in past month. For the left medial frontal ROI, less BOLD response contrast at baseline predicted higher subsequent peak drinks, F(4, 35) = 7.18, p < .001 (R2Δ = 18%, p = .002; β = -.44), and drinking days, F(4, 35) = 4.58, p = .004 (R2Δ = 10%, p = .04; β = -.32), for the year preceding follow-up and drinks consumed in the month before follow-up, F(4, 35) = 3.18, p = .02 (R2Δ = 9%, p = .05; β = -.31), greater than covariates. Less activation in the right inferior parietal lobule at baseline predicted more peak drinks in the year preceding follow-up, F(4, 35) = 6.17, p = .001 (R2Δ = 15%, p = .007; β = -.39), greater than covariates. Baseline BOLD response did not correlate with family history density scores. Analyses were rerun using transformed alcohol involvement variables, and results remained unchanged.
Discussion
The goals of this study were to use fMRI to (a) identify brain regions used by VWM that differ between heavy-drinking adolescents and controls and (b) to prospectively examine the influence of alcohol use on brain functioning in adolescents before and after transitioning into heavy drinking guided by the results of Study 1. In Study 1, we found that heavy levels of drinking during adolescence were associated with increased activation during high working-memory loads in dorsal networks. Heavy drinkers showed less use of visual and attentional networks yet greater reliance on the dorsal (“where”) stream in comparison with nondrinkers, similar to findings in adult alcoholics (Pfefferbaum et al., 2001). This suggests early compensatory synaptic reorganization after just a few years of heavy adolescent drinking, consistent with the hypothesis that heavy-drinking youth show increased activation with intact cognitive performance.
Because Study 1 was cross-sectional, Study 2 used a different sample of adolescents with longitudinal data to elucidate the chronicity of adolescent alcohol use on brain functioning over time, using the observed five areas of differences from Study 1 as hypothesized ROIs. The purpose was to examine brain activation between adolescents who transitioned into heavy drinking compared with consistently nonusing teens over an approximately 3-year period in mid-adolescence. This longitudinal evaluation permitted controlling for baseline characteristics in neural response to a working-memory challenge, and we found significant predrinking differences in BOLD activation for adolescents who continued to mostly abstain from alcohol compared with those who initiated heavy use at follow-up. The differences indicate that pre-existing frontal and parietal activation could increase adolescents’ susceptibility to engage in heavy drinking, consistent with other findings (Norman et al., 2011), suggesting the utility of fMRI in predicting future behavior.
Over the teen years, adolescents with typical development exhibit less activation to many tasks, as brains begin to process information more efficiently (Luna et al., 2010; Schweinsburg et al., 2005a). This is precisely what was observed in the continuous nondrinking group in both the parietal and frontal regions (i.e., lessening activation over the follow-up period). In contrast, although the transitioners’ brains appeared more efficient before initiating heavy alcohol use, they showed increasing activation in frontal and parietal areas after the onset of drinking. In the parietal region, transitioners required greater activation, whereas in the frontal region, the activation level was equivalent to that of continuous nondrinkers at follow-up, in mid-adolescence. For both regions, increases over time were largest for youths who drank the greatest amounts. This divergent activation pattern over the follow-up suggests that adolescents who initiate heavy drinking show less efficient and less mature processing of information compared with youth who remain relatively substance free. Continued heavy alcohol use over adolescence could result in both activation and behavioral differences. Given that these regions are associated with working memory and other executive functions (Pfefferbaum et al., 2001; Spadoni et al., 2008; Tapert et al., 2004b), adolescent heavy drinkers may have to work harder to complete a demanding task. Together, these findings suggest that heavy drinkers compensate for neural abnormalities, at least initially, by using more brain regions or increasing activation levels of regions specific to the task (Karlsgodt et al., 2007; Kim et al., 2010; Manoach et al., 1999).
Overall, these results fit with an emerging model suggested from prior longitudinal behavioral and cross-sectional imaging studies (Norman et al., 2011; Squeglia et al., 2009b, 2011, 2012; Tapert et al., 2001, 2004b), indicating that youth at risk for heavy drinking show reduced activation to cognitive challenges yet intact performance before the onset of heavy drinking compared with low-risk youth. After heavy drinking started, activation levels tended to be higher, yet performance remained intact, compared with nondrinkers. This argument is further strengthened by the discrepancy between Studies 1 and 2. Specifically, in Study 1, heavy drinkers showed greater activation in the frontal areas when compared with controls; however, in Study 2, heavy drinkers and controls exhibited the same level of activation in the frontal region at follow-up. In Study 1, drinkers were 1 year older and had longer lifetime drinking histories. We would expect that if the transitioners from Study 2 continued to drink, they would follow a similar trajectory to the adolescents from Study 1 and would exhibit even greater activation than the continuous nonusers in these frontal regions (Figure 2). The findings suggest neural activation as potential biomarkers for youth at risk to transition to heavy drinking. Diminished executive cognitive abilities in working memory may contribute significantly to the development and maintenance of alcohol use disorders (Finn, 2002; Finn et al., 2009).
Possible limitations should be considered. Follow-up durations ranged from 1.5 to 5 years; ideally, each individual would be examined after the same interval; groups were matched on baseline and follow-up age and pubertal development and follow-up duration to address this issue, and follow-up duration did not correlate with any substance use variable. Although half of the heavy drinkers had used cannabis less than five times, some had engaged in mild to moderate amounts of cannabis use and were included in the study to increase the generalizability of findings (Johnston et al., 2011). However, the findings remained unchanged after controlling for cannabis use. Statistical power was relatively limited with 20 subjects per group, and gender effects analyses were not possible; future studies with expanded follow-up imaging data will examine gender differences and neurobehavioral correlates.
In summary, this first longitudinal neuroimaging study of adolescents imaged with the same scanner before and after the emergence of heavy drinking showed pre-drinking effects as well as post-drinking effects. Results suggest that less frontal and parietal response to working memory may be a risk factor for future drinking, and those who do initiate particularly heavy drinking show increasing frontal and parietal response over time. Working memory (i.e., the storage and manipulation of information) is an essential component of executive functioning and information processing (Lezak et al., 2004), crucial to the development of logical thinking and reasoning (Mandler, 2007). Any deficits in working memory from heavy drinking would have a substantial negative effect on normal daily functioning of adolescents. Less efficient working memory and attention could deter heavy-drinking adolescents from academic and occupational success and predispose them to alcohol use disorders (Finn et al., 2009). Future directions for this work are to (a) replicate these findings in a larger sample; (b) disseminate findings through adolescent drinking–prevention materials and public service campaigns; and (c) inform intervention and psychoeducational programs on how to optimally intervene with youth engaging in heavy drinking, considering brain response and neurocognitive patterns linked to adolescent alcohol use.
Acknowledgments
We give special thanks to the first author's dissertation committee, including Sarah Mattson, Mark Myers, and Edward Riley and to the Youth at Risk lab: M. J. Meloy, Sonja Eberson, Veronique Boucquey, Norma Castro, and the participating schools and families.
Footnotes
This research was supported by National Institute of Alcohol Abuse and Alcoholism Grants F31 AA18940 (to principal investigator Lindsay M. Squeglia), R21 AA019748 (to principal investigator Carmen Pulido) and R01 AA13419 (to principal investigator Susan F. Tapert). This research was based on portions of the dissertation of the first author under the mentorship of the senior author, Susan F. Tapert.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA schoolage forms & profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- Åkerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. International Journal of Neuroscience. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th edition. Washington, DC: Author; 1994. [Google Scholar]
- Baker SC, Frith CD, Frackowiak RS, Dolan RJ. Active representation of shape and spatial location in man. Cerebral Cortex. 1996;6:612–619. doi: 10.1093/cercor/6.4.612. [DOI] [PubMed] [Google Scholar]
- Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time-course data sets in functional MRI of the human brain. Magnetic Resonance in Medicine. 1993;30:161–173. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- Bava S, Thayer R, Jacobus J, Ward M, Jernigan TL, Tapert SF. Longitudinal characterization of white matter maturation during adolescence. Brain Research. 2010;1327:38–46. doi: 10.1016/j.brainres.2010.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory–II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Brown SA, Tapert SF, Granholm E, Delis DC. Neuro cognitive functioning of adolescents: Effects of protracted alcohol use. Alcoholism: Clinical and Experimental Research. 2000;24:164–171. [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cerebral Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Cahalan D, Cisin I, Crossley H. New Brunswick, NJ: Rutgers Center of Alcohol Studies; 1969. American drinking practices. Monograph No. 6. [Google Scholar]
- Clark DB, Tapert SF. Introduction to alcohol and adolescent brain development. Alcoholism: Clinical and Experimental Research. 2008;32:373–374. doi: 10.1111/j.1530-0277.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, Haxby JV. Object and spatial visual working memory activate separate neural systems in human cortex. Cerebral Cortex. 1996;6:39–49. doi: 10.1093/cercor/6.1.39. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, an International Journal. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magnetic Resonance in Medicine. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue S, van Leijenhorst L, Bunge SA. Neurocognitive development of the ability to manipulate information in working memory. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9315–9320. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn PR. Motivation, working memory, and decision making: A cognitive-motivational theory of personality vulnerability to alcoholism. Behavioral and Cognitive Neuroscience Reviews. 2002;1:183–205. doi: 10.1177/1534582302001003001. [DOI] [PubMed] [Google Scholar]
- Finn PR, Rickert ME, Miller MA, Lucas J, Bogg T, Bobova L, Cantrell H. Reduced cognitive ability in alcohol dependence: Examining the role of covarying externalizing psychopathology. Journal of Abnormal Psychology. 2009;118:100–116. doi: 10.1037/a0014656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fougnie D, Marois R. Distinct capacity limits for attention and working memory: Evidence from attentive tracking and visual working memory paradigms. Psychological Science. 2006;17:526–534. doi: 10.1111/j.1467-9280.2006.01739.x. [DOI] [PubMed] [Google Scholar]
- Friedman HR, Goldman-Rakic PS. Coactivation of prefrontal cortex and inferior parietal cortex in working memory tasks revealed by 2DG functional mapping in the rhesus monkey. Journal of Neuroscience. 1994;14:2775–2788. doi: 10.1523/JNEUROSCI.14-05-02775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RSJ, Turner R. Movement-related effects in fMRI time-series. Magnetic Resonance in Medicine. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Mezzich AC, Tarter RE. Executive cognitive functioning, temperament, and antisocial behavior in conduct-disordered adolescent females. Journal of Abnormal Psychology. 1998;107:629–641. doi: 10.1037//0021-843x.107.4.629. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Rapoport JL. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Cummins K, Tapert SF, Brown SA. Changes in neuropsychological functioning over 10 years following adolescent substance abuse treatment. Psychology of Addictive Behaviors. 2011;25:127–142. doi: 10.1037/a0022350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Petit L, Ungerleider LG, Courtney SM. Distinguishing the functional roles of multiple regions in distributed neural systems for visual working memory. NeuroImage. 2000;11:380–391. doi: 10.1006/nimg.2000.0592. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Two-factor index of social position. New Haven, CT: Yale University Press; 1965. [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2010. Bethesda, MD: National Institute on Drug Abuse; 2011. [Google Scholar]
- Karlsgodt KH, Glahn DC, van Erp TGM, Therman S, Huttunen M, Manninen M, Cannon TD. The relationship between performance and fMRI signal during working memory in patients with schizophrenia, unaffected co-twins, and control subjects. Schizophrenia Research. 2007;89:191–197. doi: 10.1016/j.schres.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Kim MA, Tura E, Potkin SG, Fallon JH, Manoach DS, Calhoun VD, Turner JA the FBIRN. Working memory circuitry in schizophrenia shows widespread cortical inefficiency and compensation. Schizophrenia Research. 2010;117:42–51. doi: 10.1016/j.schres.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleschinsky JH, Bosworth LB, Nelson SE, Walsh EK, Shaffer HJ. Persistence pays off: follow-up methods for difficult-totrack longitudinal samples. Journal of Studies on Alcohol and Drugs. 2009;70:751–761. doi: 10.15288/jsad.2009.70.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Fox PT. Automated Talairach Atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW, Hannay HJ, Fischer JS. Neuropsychological assessment. (4th ed.). New York, NY: Oxford University Press; 2004. [Google Scholar]
- Luck SJ, Vogel EK. The capacity of visual working memory for features and conjunctions. Nature. 1997;390:279–281. doi: 10.1038/36846. [DOI] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, O'Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain and Cognition. 2010;72:101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Sweeney JA. The emergence of collaborative brain function: FMRI studies of the development of response inhibition. Annals of the New York Academy of Sciences. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- Mandler JM. On the origins of the conceptual system. American Psychologist. 2007;62:741–751. doi: 10.1037/0003-066X.62.8.741. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Press DZ, Thangaraj V, Searl MM, Goff DC, Halpern E, Warach S. Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by fMRI. Biological Psychiatry. 1999;45:1128–1137. doi: 10.1016/s0006-3223(98)00318-7. [DOI] [PubMed] [Google Scholar]
- Miller CL, Tucker ML, Pasch L, Eccles JS. Measuring pubertal development: A comparison of different scales and different sources. Alexandria, VA: Paper presented at the biennial meeting of the Society for Research in Child Development; 1988. [Google Scholar]
- Miller JW, Naimi TS, Brewer RD, Jones SE. Binge drinking and associated health risk behaviors among high school students. Pediatrics. 2007;119:76–85. doi: 10.1542/peds.2006-1517. [DOI] [PubMed] [Google Scholar]
- Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, Tapert SF. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug and Alcohol Dependence. 2011;119:216–223. doi: 10.1016/j.drugalcdep.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Morris RG, Sahakian BJ, Polkey CE, Robbins TW. Double dissociations of memory and executive functions in working memory tasks following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Brain. 1996;119:1597–1615. doi: 10.1093/brain/119.5.1597. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Pulido C, Schuckit MA. Alcohol attenuates load-related activation during a working memory task: Relation to level of response to alcohol. Alcoholism: Clinical and Experimental Research. 2006;30:1363–1371. doi: 10.1111/j.1530-0277.2006.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A selfreport measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Evans AC, Meyer E. Dissociation of human mid-dorsolateral from posterior dorsolateral frontal cortex in memory processing. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:873–877. doi: 10.1073/pnas.90.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Desmond JE, Galloway C, Menon V, Glover GH, Sullivan EV. Reorganization of frontal systems used by alcoholics for spatial working memory: An fMRI study. NeuroImage. 2001;14:7–20. doi: 10.1006/nimg.2001.0785. [DOI] [PubMed] [Google Scholar]
- Postle BR, D'Esposito M. “What”-Then-“Where” in visual working memory: An event-related fMRI study. Journal of Cognitive Neuroscience. 1999;11:585–597. doi: 10.1162/089892999563652. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism: Clinical and Experimental Research. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Tapert SF. fMRI reveals alteration of spatial working memory networks across adolescence. Journal of the International Neuropsychological Society. 2005a;11:631–644. doi: 10.1017/S1355617705050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug and Alcohol Dependence. 2005b;79:201–210. doi: 10.1016/j.drugalcdep.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher KJ, Martin ED, Wood PK, Rutledge PC. Alcohol use disorders and neuropsychological functioning in first-year undergraduates. Experimental and Clinical Psychopharmacology. 1997;5:304–315. doi: 10.1037//1064-1297.5.3.304. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-back: A technique for assessing self-reported ethanol consumption. Totowa, NJ: Humana Press; 1992. [Google Scholar]
- Spadoni AD, Norman AL, Schweinsburg AD, Tapert SF. Effects of family history of alcohol use disorders on spatial working memory BOLD response in adolescents. Alcoholism: Clinical and Experimental Research. 2008;32:1135–1145. doi: 10.1111/j.1530-0277.2008.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence: Alcohol sensitivity, tolerance, and intake. Recent Developments in Alcoholism. 2005;17:143–159. [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State–Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- Squeglia LM, Jacobus J, Tapert SF. The influence of substance use on adolescent brain development. Clinical EEG and Neuroscience. 2009a;40:31–38. doi: 10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Schweinsburg AD, Pulido C, Tapert SF. Adolescent binge drinking linked to abnormal spatial working memory brain activation: Differential gender effects. Alcoholism: Clinical and Experimental Research. 2011;35:1831–1841. doi: 10.1111/j.1530-0277.2011.01527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Sorg SF, Schweinsburg AD, Wetherill RR, Pulido C, Tapert SF. Binge drinking differentially affects adolescent male and female brain morphometry. Psychopharmacology. 2012;220:529–539. doi: 10.1007/s00213-011-2500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychology of Addictive Behaviors. 2009b;23:715–722. doi: 10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Coplanar stereotaxic atlas of the human brain. Three-dimensional proportional system: An approach to cerebral imaging. New York, NY: Thieme; 1988. [Google Scholar]
- Tapert SF, Brown GG, Kindermann SS, Cheung EH, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcoholism: Clinical and Experimental Research. 2001;25:236–245. [PubMed] [Google Scholar]
- Tapert SF, Brown SA. Neuropsychological correlates of adolescent substance abuse: Four-year outcomes. Journal of the International Neuropsychological Society. 1999;5:481–493. doi: 10.1017/s1355617799566010. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Granholm E, Leedy NG, Brown SA. Substance use and withdrawal: Neuropsychological functioning over 8 years in youth. Journal of the International Neuropsychological Society. 2002;8:873–883. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Pulido C, Paulus MP, Schuckit MA, Burke C. Level of response to alcohol and brain response during visual working memory. Journal of Studies on Alcohol. 2004a;65:692–700. doi: 10.15288/jsa.2004.65.692. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, Meloy MJ. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2004b;28:1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Twitchell GR, Hertzog CA, Klein JL, Schuckit MA. The anatomy of a follow-up. British Journal of Addiction. 1992;87:1327–1333. doi: 10.1111/j.1360-0443.1992.tb02741.x. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Courtney SM, Haxby JV. A neural system for human visual working memory. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:883–890. doi: 10.1073/pnas.95.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for fMRI data. 2000. Retrieved from http://afni.nimh.nih.gov/afni/doc/manual/AlphaSim.
- Zucker RA, Ellis DA, Fitzgerald HE. Developmental evidence for at least two alcoholisms: I. Biopsychosocial variation among pathways into symptomatic difficulty. New York, NY: The New York Academy of Sciences; 1994. [DOI] [PubMed] [Google Scholar]