Abstract
Objective
Preclinical findings suggest that the over-the-counter supplement N-acetylcysteine, via glutamate modulation in the nucleus accumbens, holds promise as a pharmacotherapy targeting substance dependence. We sought to investigate N-acetylcysteine as a novel cannabis cessation treatment in adolescents, a vulnerable group for whom existing treatments have limited efficacy.
Method
In this 8-week double-blind randomized placebo-controlled trial, treatment-seeking cannabis-dependent adolescents (age 15-21, N = 116) received N-acetylcysteine (1200 mg) or placebo twice daily, each added to a contingency management intervention and brief (≤10 minute) weekly cessation counseling. The primary efficacy measure was the odds of negative weekly urine cannabinoid tests during treatment among participants receiving N-acetylcysteine versus placebo, via intent-to-treat analysis. The primary tolerability measure was frequency of adverse events, compared by treatment group.
Results
N-acetylcysteine was well tolerated with minimal adverse events. N-acetylcysteine participants had more than twice the odds, compared to placebo participants, of submitting negative urine cannabinoid tests during treatment (odds ratio = 2.4, [95% CI: 1.1-5.2], p = 0.029). Exploratory secondary abstinence outcomes numerically favored N-acetylcysteine, but were not statistically significant.
Conclusions
This is the first randomized trial of pharmacotherapy for cannabis dependence in any age group yielding a positive primary cessation outcome via intent-to-treat analysis. Findings support N-acetylcysteine as a pharmacotherapy to complement psychosocial treatment for cannabis dependence in adolescents. Further research is needed to replicate these findings and explore the efficacy of N-acetylcysteine across a variety of treatment contexts and outcomes.
Trial Registration
clinicaltrials.gov identifier: NCT 01005810
INTRODUCTION
Cannabis is the most commonly used illicit substance in adolescents, and rates of use are increasing. One quarter of high school seniors are current cannabis users, and 7% use daily.1 Adolescents are particularly prone to adverse consequences of cannabis use and progression to dependence,2-5 but existing cessation treatments convey low abstinence rates.6-8 A potential strategy to enhance outcomes is pharmacotherapy to complement psychosocial treatment, but there has been little research on this approach in adolescents. Even in adults, examination of pharmacotherapy for cannabis dependence has been limited, and no effective medications have been identified.9
The antioxidant N-acetylcysteine, an N-acetyl pro-drug of the naturally occurring amino acid cysteine, is widely available as an inexpensive over-the-counter supplement. Research interest in N-acetylcysteine has grown amid increasing evidence of the role of the neurotransmitter glutamate in addiction.10-12 Animal models have demonstrated that chronic drug self-administration down-regulates the cystine-glutamate exchanger in the nucleus accumbens, and that N-acetylcysteine administration up-regulates this exchanger, normalizing a drug-induced pathology and reducing reinstatement of drug seeking.10,13 Preclinical and preliminary clinical studies have followed, further supporting a potential treatment role for N-acetylcysteine.14-21 Other clinical studies suggest that N-acetylcysteine, via glutamate modulation and a number of other proposed mechanisms, may be efficacious across a variety of psychiatric conditions.22
Given these findings, and in light of the need for improved adolescent cannabis cessation treatments, we sought to examine N-acetylcysteine as a candidate pharmacotherapy. After completing an encouraging open-label pilot trial,23 we conducted a double-blind randomized placebo-controlled trial of N-acetylcysteine in cannabis-dependent adolescents. To critically judge N-acetylcysteine as a complementary treatment, we evaluated it in the context of an efficacious youth-targeted cannabis cessation psychosocial treatment, contingency management.24,25 We hypothesized that treatment with N-acetylcysteine, relative to placebo, when added to contingency management and brief weekly cessation counseling, would be associated with higher rates of abstinence, measured via the odds of negative urine cannabinoid tests during treatment.
METHOD
Trial Design
Treatment-seeking cannabis-dependent adolescents were randomized, in 1:1 parallel group allocation, to receive a double-blind 8-week course of N-acetylcysteine (1200 mg) or placebo twice daily, added to contingency management and brief weekly cessation counseling. Post-treatment follow-up occurred 4 weeks after treatment conclusion. Urine cannabinoid testing (U.S. Screening Source Inc., Louisville, KY) was conducted at all visits. The FDA approved the Investigational New Drug (IND) application for this study. Procedures were approved by the university institutional review board and were in accord with the Helsinki Declaration of 1975.
Participants
To enroll in the study, adolescents were required to (a) be 13 to 21 years old, (b) use cannabis regularly, (c) meet criteria for cannabis dependence, (d) express interest in cannabis cessation treatment, (e) not be enrolled in substance use treatment, (f) not be pregnant and use birth control to avoid pregnancy, (g) lack current comorbid substance dependence (aside from nicotine), (h) have no acutely unstable psychiatric or medical illness, (i) lack history of adverse reaction to N-acetylcysteine, and (j) not take carbamazepine or nitroglycerin. Recruitment occurred primarily through community media and clinical referrals. If an initial telephone screen suggested potential eligibility, adolescents were scheduled for an informed consent and baseline assessment visit. After complete description of the study, written participant consent was obtained for all adolescents age 18 years or older. Written parental consent and participant assent were obtained for those less than 18 years old.
General Procedures
All procedures were conducted in the university research clinic. At the baseline visit, comprehensive psychiatric and substance use diagnostic assessment,26-28 physical examination, and laboratory testing (urine pregnancy and drug tests) were performed. Timeline Follow-Back methods were used to assess self-reported cannabis and other substance use.29
Eligible participants were given adolescent-targeted cannabis information brochures,30 enrolled in a contingency management intervention (described below), and randomized to treatment group. Participants were seen in clinic weekly during the 8-week medication trial and returned for post-treatment follow-up 4 weeks after treatment conclusion. At all visits, the study physician or physician assistant provided brief individual cessation counseling (≤10 minutes) and adverse event assessment, and participants submitted urine samples for cannabinoid testing.
Interventions
Medication
Enrolled participants were randomized to double-blind treatment assignment (N-acetylcysteine 1200 mg or placebo twice daily), stratified by age (<18 versus ≥18) and baseline cannabis use (using <20 versus ≥20 of last 30 days). The university investigational drug service oversaw randomization, encased medications in identical-appearing capsules, and dispensed them in weekly blister packs with specific instructions on day/time for each dose. Participants, investigators, and clinical staff remained blind to treatment assignment throughout the study. To enhance the blind, a small amount of N-acetylcysteine powder was applied to the inside of all blister packs so that both N-acetylcysteine and placebo packs would contain the scent of N-acetylcysteine. No formal assessment of the integrity of the blind was conducted.
Contingency Management
A twice-weekly contingency management intervention, separately targeting participant retention and cannabis abstinence and modeled after established methods,24 was implemented during the medication trial. An escalating reinforcement schedule, in which participants were able to earn increasing contingent rewards over successive displays of desired behavior (adherence with appointments/procedures, cannabis abstinence as measured by instant urine cannabinoid testing), was used. One weekly evaluation occurred during the week’s scheduled clinic visit, and the other was a “drop-in” on a separate day of the week. For both adherence and abstinence, the initial contingent reward was $5 (cash). For each successive visit at which the participant was adherent or abstinent, the reward increased by $2 ($7, then $9, and so on). If a participant subsequently failed to adhere or tested positive for cannabis use, (s)he did not receive contingent reward at that visit, and the contingent reward value for the next session was “re-set” at the baseline of $5. If, at a given visit, a participant tested positive but adhered with study procedures, (s)he collected the adherence reward as scheduled, but was not eligible for abstinence reward.
Cessation Counseling
The physician or physician assistant, in the context of medication management, provided non-manualized brief (≤10 minute) cessation counseling at all clinic visits, incorporating educational, motivational, and cognitive/behavioral elements.
Outcomes
Efficacy
Urine cannabinoid testing at baseline, during weekly clinic visits, and at post-treatment follow-up, was conducted as the primary biological measure of cannabis use. Self-reported cannabis use was collected via Timeline Follow-Back methods.
Safety/Tolerability
A thorough safety evaluation was conducted at each clinic visit: (a) physician or physician assistant evaluation of adverse events via open-ended interview and comprehensive, structured review of systems,31 (b) urine pregnancy testing (females only), and (c) vital sign measurement.
Adherence
Medication diaries and weekly pill counts (inspection of blister packs and documentation of missed doses) were used to measure adherence.
Statistical Analyses
The primary hypothesis was that N-acetylcysteine participants would have higher odds than placebo participants to submit negative weekly urine cannabinoid tests during treatment. An intent-to-treat approach including all randomized participants was used. In all analyses, participants lost to follow up or absent for visits were coded as having a positive urine cannabinoid test at every missed visit.
The study was powered to detect a 50% rate of negative urine cannabinoid tests in N-acetylcysteine participants, compared with 25% in placebo participants. These estimates were derived from a prior trial of pharmacotherapy to complement contingency management targeting cocaine dependence.32 Setting the type I error rate to 0.05, a sample of 58 participants per treatment group was deemed necessary to yield 80% power. No interim efficacy analyses were conducted.
Standard descriptive statistics were used to summarize the general demographic and clinical data. Group differences in continuous characteristics were assessed using t-tests, while differences in categorical characteristics were assessed using normal (Pearson’s) chi-square tests.
The efficacy of N-acetylcysteine versus placebo, each added to contingency management and weekly brief cessation counseling, on abstinence from cannabis was analyzed over the 8-week treatment and at post-treatment follow-up. A repeated measures logistic regression model using the methods of generalized estimating equations33 was applied to assess the overall treatment effect on urine cannabinoid test results during active treatment. Working correlation structures were independently compared and the final model structure was chosen using the quasilikelihood under the independence model criterion statistic.34 Odds ratios and asymptotic 95% confidence intervals were computed. Additionally, a pre-planned logistic regression model was used to analyze the odds of a negative cannabinoid test at post-treatment follow-up.
Exploratory analyses of select secondary efficacy measures were conducted. Time to first negative urine cannabinoid test was examined using a Cox proportional hazard model with the baseline visit set as the time baseline. The assumption of proportional hazards was assessed by including an interaction between the treatment group assignment variable and the log transformed time to event variable. No violations of the assumption were determined. End of treatment abstinence was assessed via a logistic regression model, using the intent-to-treat sample (n=116) in which participants with missing data were assumed to be non-abstinent. An ANCOVA model was used to test for differences in the proportion of days using throughout treatment.
All study models were adjusted for baseline urine cannabinoid test results and assessed for possible confounding and effect modification of age, weight, gender, race, years of cannabis use, number of previous quit attempts, and presence of psychiatric comorbidities. Baseline demographic and clinical characteristics were independently tested for association with efficacy outcome and those significantly associated were included as predictors in adjusted models. Results are presented as odds ratios with 95% confidence intervals (CI).
Adverse event rates were compared between treatment groups using Pearson’s chi-square tests. Study completion was compared using logistic regression. Cox proportional hazards regression was used to assess the effect of demographics, clinical characteristics, and treatment assignment on time to study dropout. The assumption of proportional hazards was assessed similarly to the aforementioned efficacy model. No violations of the assumption were determined.
No adjustments for multiple testing were made, as they are known to reduce statistical power and increase the probability of accepting a null hypothesis that is truly false. Preliminary analyses leading to a priori hypotheses suggest that differences noted are less likely to be from chance alone. Differences between groups for these hypotheses on multiple related outcome measures would support the treatment effect of N-acetylcysteine on cannabis use. Thus, we specified a priori the primary outcome as well as secondary comparisons, and did not adjust for multiple comparisons of groups on these outcomes.35-37 All statistical analyses were conducted using SAS version 9.2 (SAS Institute Inc. Cary, NC, USA). Significance was set at a 2-sided p-value of 0.05.
RESULTS
Enrollment and Baseline Characteristics
Participants were enrolled between September 2009 and January 2011. One hundred thirty-six adolescents were assessed for eligibility, and 20 (15%) were excluded (Supplemental Figure 1). Demographic and baseline clinical characteristics are presented in Table 1. The randomized cohort (N=116) was an older adolescent sample (mean age 18.9 years [range 15-21]) that was predominantly white (n=96, 83.5%). There were no significant between-group differences in demographics or baseline clinical variables. The groups had similar rates of positive urine cannabinoid tests at baseline (91.4% versus 89.7%, p=0.75).
Table 1.
Baseline demographics and clinical characteristics.
| Variable | Treatment | ||||||
|---|---|---|---|---|---|---|---|
| Overall n=116 |
Placebo n=58 |
N-Acetylcysteine n=58 |
p-value | ||||
| Demographics | Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 18.9 | 1.5 | 18.8 | 1.5 | 18.9 | 1.5 | 0.676 |
| Weight (kg) | 69.8 | 13.9 | 68.4 | 12.0 | 71.2 | 15.7 | 0.312 |
| Heart rate (beats per minute) | 66.6 | 11.4 | 66.6 | 12.0 | 66.7 | 11.0 | 0.936 |
| n | % | n | % | n | % | ||
| Age < 18 yrs | 20 | 17.2 | 10 | 17.2 | 10 | 17.2 | 1.00 |
| Gender, male | 84 | 73.0 | 45 | 77.6 | 39 | 68.4 | 0.286 |
| Race, white | 96 | 83.5 | 51 | 87.9 | 45 | 79.0 | 0.195 |
| Enrolled in school | 85 | 73.9 | 42 | 72.4 | 43 | 75.4 | 0.712 |
| Smoke cigarettes | 65 | 57.0 | 32 | 55.2 | 33 | 58.9 | 0.686 |
| Cannabis Use Characteristics | Mean | SD | Mean | SD | Mean | SD | |
| Years of cannabis use | 4.2 | 1.8 | 4.3 | 2.0 | 4.1 | 1.7 | 0.715 |
| Number of prior cannabis quit attempts | 3.3 | 9.8 | 2.7 | 3.6 | 3.9 | 13.5 | 0.516 |
| Days using cannabis in past 30 days | 22.6 | 7.2 | 22.1 | 7.3 | 23.1 | 7.2 | 0.455 |
| % of Days using cannabis in past 30 days | 75.3 | 24.1 | 73.6 | 24.4 | 77.0 | 23.9 | 0.455 |
| Days since last cannabis use | 2.2 | 3.7 | 2.3 | 3.7 | 2.1 | 3.7 | 0.546 |
| n | % | n | % | n | % | ||
| Positive urine cannabinoid test at baseline | 105 | 90.5 | 52 | 89.7 | 53 | 91.4 | 0.751 |
| Psychiatric Comorbidity | n | % | n | % | n | % | |
| Attention-Deficit/Hyperactivity Disorder | 6 | 5.2 | 4 | 6.9 | 2 | 3.5 | 0.679 |
| Conduct/Oppositional Defiant Disorder | 7 | 6.0 | 5 | 8.6 | 2 | 3.5 | 0.439 |
| Major Depressive Disorder | 9 | 7.8 | 7 | 12.1 | 2 | 3.5 | 0.162 |
| Anxiety Disorder | 9 | 7.8 | 6 | 10.3 | 3 | 5.2 | 0.490 |
| Any Psychiatric Comorbidity | 16 | 13.8 | 11 | 18.9 | 5 | 8.6 | 0.177 |
Efficacy
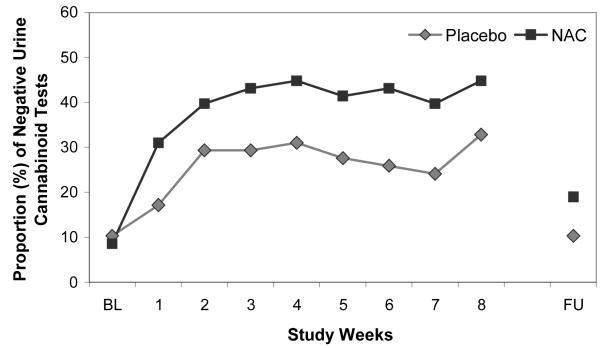
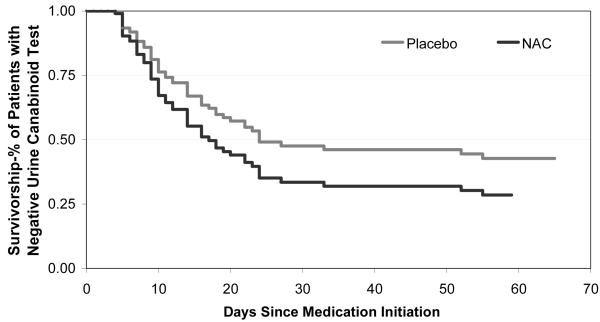
The proportion of negative urine cannabinoid tests in the N-acetylcysteine and placebo groups at each visit (intent-to-treat sample) is shown in Figure 1. Though there were no group differences in baseline years of cannabis use or presence of major depressive disorder, these variables were independent predictors of positive urine cannabinoid tests during treatment (p=0.007 and p=0.066) and were therefore covaried in the primary model along with baseline urine cannabinoid test result. Participants randomized to N-acetylcysteine had more than double the odds of negative urine cannabinoid tests during treatment, compared with those randomized to placebo. In the adjusted model, there was a significant relationship between treatment and the odds of a negative urine cannabinoid test (odds ratio=2.4 [95% CI: 1.1-5.2], χ2=4.72, p=0.029). There was no significant differential drug effect over time (treatment x time interaction p=0.75). Through the final treatment visit, 40.9% (190/464) of the urine cannabinoid tests in the N-acetylcysteine group were negative, compared to 27.2% (126/464) in the placebo group, per intent-to-treat analysis, assuming any missing urine was positive for cannabinoids. At the post-treatment follow-up visit, 19.0% (11/58) of the urine cannabinoid tests in the N-acetylcysteine group were negative, compared to 10.3% (6/58) in the placebo group. While still numerically favoring N-acetylcysteine, the overall treatment effect lost statistical significance at post-treatment follow-up (adjusted odds ratio=2.4 [95% CI: 0.8-7.5], χ 2=2.2, p=0.131). Secondary efficacy measures of time to first negative urine cannabinoid test (hazard ratio=1.5 [95% CI: 0.9-2.5], χ 2=2.1, p=0.146) (Figure 2) and end of treatment abstinence (odds ratio=2.3 [95% CI:1 .0-5.4], χ 2=3.7, p=0.054) revealed a similar magnitude of estimates favoring N-acetylcysteine, though the study was not adequately powered to assess these outcomes (Table 2). There was no significant difference in percentage of self-reported days of cannabis use throughout treatment (p=0.512).
Figure 1.
Proportion of negative urine cannabinoid tests (intent-to-treat analysis including all randomized participants, with urine cannabinoid tests assumed to be positive for all missed visits; n=116); adjusted for years of cannabis use, baseline urine cannabinoid test results, and major depressive disorder. OR=2.4 (95% CI: 1.1-5.2), χ2=4.72, p=0.029
NAC=N-Acetylcysteine, BL=Baseline Visit, FU=Post-Treatment Follow-Up Visit
Figure 2.
Survivorship function for time to first negative urine cannabinoid test. Estimated survival function for N-acetylcysteine versus placebo participants; adjusted for years of cannabis use and baseline urine cannabinoid test results.
NAC=N-Acetylcysteine
Table 2.
Study Outcome Measures.
| Study Outcome Measures | Results | ||||
|---|---|---|---|---|---|
|
Primary Outcome
Measure |
|||||
| Overall Treatment Effect | % Negative Urine Cannabinoid Test |
Odds Ratio | 95% CI | p-value | |
| NAC | 40.9 % | 2.35 | 1.05-5.24 | 0.029 | |
| Placebo | 27.2 % | ||||
|
| |||||
|
Secondary Outcome
Measures |
|||||
| Time to First Negative Urine Cannabinoid Test |
Median Days | Hazard Ratio | 95% CI | p-value | |
| NAC | 17 | 1.48 | 0.87 - 2.49 | 0.146 | |
| Placebo | 24 | ||||
|
| |||||
| 2 Week Urine Cannabinoid Test Confirmed End of Treatment Abstinence |
% Abstinent | Odds Ratio | 95% CI | p-value | |
| NAC | 36.2 % | 2.32 | 0.99 - 5.43 | 0.054 | |
| Placebo | 20.7 % | ||||
| 4 Week Urine Cannabinoid Test Confirmed End of Treatment Abstinence |
% Abstinent | Odds Ratio | 95% CI | p-value | |
| NAC | 27.6 % | 2.14 | 0.85 - 5.42 | 0.108 | |
| Placebo | 15.5 % | ||||
|
| |||||
| Change in days using cannabis during treatment |
% Change | Mean Difference | 95% CI | p-value | |
| NAC | 41.1 ± 4.3 % | −4.0 ± 6.0 | −15.8 – 7.9 | 0.512 | |
| Placebo | 37.0 ± 4.4 % | ||||
Overall Treatment Effect: Generalized estimating equations analysis of negative urine cannabinoid tests during the 8-week treatment. Data are shown as the mean proportion of negative urine cannabinoid tests and associated adjusted odds ratio. Data analysis was conducted on an intent-to-treat sample (n=116) in which those with missing data / lost to follow up were considered non-abstinent. Results shown are adjusted for baseline urine cannabinoid test results, years of reported cannabis use, and major depressive disorder.
Time to First Negative Urine Cannabinoid Test: Data are shown as median number of days to the first negative urine cannabinoid test. Results are shown as adjusted hazard for time to first negative urine cannabinoid test for N-acetylcysteine versus placebo participants (n=116). Results shown are adjusted for baseline urine cannabinoid test results and years of reported cannabis use.
End of Treatment Abstinence (2 and 4 week): Results are shown as the 2 and 4 week urine cannabinoid test confirmed continuous self-reported abstinence at the end of treatment. Results are shown as the odds of abstinence in N-acetylcysteine versus placebo participants. Data analysis was conducted on an intent-to-treat sample (n=116) in which those with missing data / lost to follow up were considered non-abstinent. Results shown are adjusted for baseline urine cannabinoid test results and years of reported cannabis use.
Proportion of Days Using Cannabis: Data are shown as the adjusted change in percent of days with self-reported cannabis use between the treatment phase of the study and the 30 days prior to study entry. Results are shown as the mean difference between the proportion of days used in N-acetylcysteine versus placebo participants and associated standard error (n=89 participants with self-report data available), and are adjusted for baseline urine cannabinoid test results, years of reported cannabis use at entry into the study, and self-reported percent of days used in the 30 days prior to study entry.
NAC=N-Acetylcysteine
In the adjusted primary analysis model, study week and the treatment x study week interaction were not significant. However, participants with a negative baseline urine cannabinoid test had nearly 6 times the odds of negative cannabinoid tests during treatment (odds ratio=5.9 [95% CI: 2.0-17.7], χ 2=5.4, p=0.020). Similarly, those with fewer baseline years of cannabis use had significantly greater odds of negative urine cannabinoid tests during the study (odds ratio=1.4 [95% CI: 1.1-1.7], χ 2=8.0, p=0.047). Participants with major depressive disorder had a trend-level decreased odds of negative urine cannabinoids test during treatment (odds ratio=0.3 [95% CI: 0.1-1.0], χ 2=3.5, p=0.062). Models were additionally examined for possible confounding and effect modification of age, weight, gender, race, other psychiatric comorbidities, and number of previous cannabis quit attempts, revealing no significant confounders or effect modifiers (all p>0.60).
Based on the intent-to-treat sample, in which missing urine samples were assumed to be positive for cannabinoids, the number needed to treat to achieve negative cannabinoid testing during the treatment portion of the study is 7.3 and at the post-treatment follow-up visit is 11.6. These are comparable to numbers needed to treat for several established addiction-targeted pharmacotherapies.38
A post hoc sensitivity analysis was performed on proportion of negative urine cannabinoid tests during treatment, using multiple methods to manage missing data and participant dropouts. In addition to the intent-to-treat approach noted above (n=116), a modified intent-to-treat analysis that examined participants who received at least one dose of study medication (n=106) and a per-protocol analysis using available data (n=varying) were performed. Using a modified intent-to-treat analysis, participants in the N-acetylcysteine group had 2.1 times the odds of submitting negative urine cannabinoid tests, compared to those in the placebo group (adjusted odds ratio=2.1 [95% CI: 1.0-4.5], χ2=4.0, p=0.047) during treatment. When only examining available data (per protocol analysis), participants in the N-acetylcysteine group had 2.4 times the odds of submitting negative urine cannabinoid tests, compared to those in the placebo group (adjusted odds ratio=2.4 [95% CI: 1.1-5.4], χ2=4.4, p=0.036). Finally, combinatorial graphical methods for assessing the impact of missing data on significance of findings were also employed, in which every permutation of missing data assignment was considered, and a subsequent logistic regression performed.39 For the majority of missing data assignments that could be reasonably expected, the odds ratio was still significant. In general, the selection of missing data handling had little effect on analytic outcomes.
Safety/Tolerability
Interim monitoring of adverse events was conducted every 6 months by an independent data and safety monitoring board. There were no FDA-defined serious adverse events, and there were no significant differences between the two treatment groups in the occurrence of any adverse events (38 events in the N-acetylcysteine group [in 24 participants] versus 46 events in the placebo group [in 27 participants]; χ2= 0.32, p=0.57). The most common adverse event was upper respiratory infection, occurring in 19 participants (11 in N-acetylcysteine group versus 8 in placebo group). Adverse events occurring in at least 2 participants and deemed at least possibly treatment-related included vivid dreams (3 in N-acetylcysteine group), insomnia (3 in placebo group), and irritability (2 in placebo group). One participant in the N-acetylcysteine group discontinued medication treatment due to severe heartburn, which resolved upon discontinuation. No other participants in either group discontinued medication due to adverse events.
Retention and Adherence
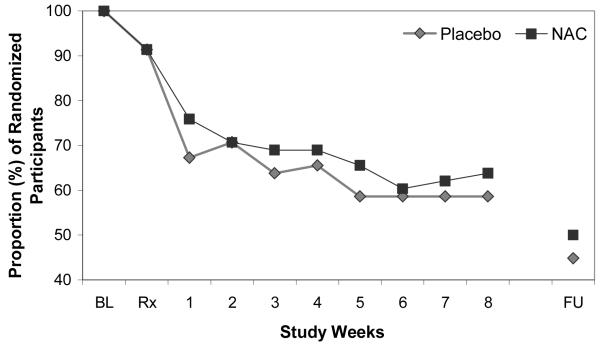
Of the 116 randomized participants, 106 (92%) took at least one dose of study medication, 70 (60%) were retained through treatment completion, and 54 (47%) were retained through post-treatment follow-up (Figure 3). There was no significant between-group difference in retention to treatment completion (37 [64%] in N-acetylcysteine group versus 33 [57%] in placebo group; p=0.45) or post-treatment follow-up (29 [50%] versus 25 [43%]; p=0.46). Time to dropout, assessed using Cox proportional hazards regression models, was not significantly different between treatment groups (adjusted hazard ratio: 1.3 [95% CI: 0.8-2.2]; p=0.23). The median numbers of days retained in the study was 63 days (interquartile range: 13-66) in the N-acetylcysteine group and 62 days (interquartile range: 17-65) in the placebo group. Additionally, time to dropout was not significantly associated with any of the demographic or clinical characteristics (all p>0.20). Review of medication diaries and weekly pill counts indicated that 95% of dispensed N-acetylcysteine doses and 93% of dispensed placebo doses were taken. Via contingency management procedures, N-acetylcysteine participants earned $162±SD 129 (of possible $320) for adherence and $86±106 (of possible $320) for abstinence (total $248±214), while the placebo group earned $141±117 for adherence and $54±98 for abstinence (total $199±190).
Figure 3.
Retention: Proportion of randomized participants (n=116) attending visits.
NAC=N-Acetylcysteine, BL=Baseline Visit, Rx=Medication Initiation, FU=Post-Treatment Follow-Up Visit
DISCUSSION
To our knowledge, this is the first double-blind randomized placebo-controlled trial of pharmacotherapy for cannabis dependence in any age group yielding a positive primary cessation outcome via intent-to-treat analysis. Results support the hypothesis that treatment with N-acetylcysteine, compared to placebo, when added to contingency management and brief cessation counseling, yields improved cannabis abstinence during treatment. N-acetylcysteine more than doubled the odds of submitting negative urine cannabinoid tests as compared to placebo, and differences were detectable within a week of treatment initiation. Exploratory secondary abstinence outcomes also numerically favored N-acetylcysteine, but were not statistically significant. N-acetylcysteine was well tolerated, supporting its safety among cannabis-dependent adolescents. Given the increasing prevalence and adverse consequences of adolescent cannabis use, and the limitations in outcomes with existing treatments, these findings provide a critical addition to the evidence base.
While N-acetylcysteine, via its reversal of drug-induced glutamate dysregulation, has demonstrated significant effects on drug seeking and self-administration in animal models and has been the subject of encouraging preliminary human studies, this is the first randomized trial to demonstrate significant main effects of N-acetylcysteine on substance cessation. This successful translational effort is particularly notable considering that no preclinical N-acetylcysteine studies focused on cannabis or cannabinoid administration. It appears that the neurobiological and behavioral effects of N-acetylcysteine may not be specific to a particular substance, suggesting that N-acetylcysteine may be a promising candidate medication for treatment of other substance use disorders, whether by modulation of glutamate or other potential mechanisms.
This study incorporated contingency management, arguably the most efficacious youth-targeted psychosocial cannabis cessation treatment, which could have potentially created a cessation “ceiling effect” and diminished the opportunity to detect an added medication effect. The finding that co-administration of N-acetylcysteine significantly increased abstinence even in this context is striking. It is thus tempting to argue that, by extension, N-acetylcysteine would enhance cessation outcomes on its own or when added to other psychosocial treatments. However, these remain unanswered questions that can only be addressed by further controlled trials of N-acetylcysteine in other treatment contexts. It may be that N-acetylcysteine and contingency management exert synergistic treatment effects, a possibility that can be investigated via a 2×2 trial (N-acetylcysteine versus placebo and contingency management versus non-contingency management) to detect interaction effects.37 It may alternatively be that N-acetylcysteine requires a non-specific but powerful psychosocial treatment platform to exert its effects.
Findings should be interpreted in light of limitations. This study investigated only one N-acetylcysteine dosing regimen over only 8 weeks, and was conducted at a single university-based research clinic with a relatively small sample of older adolescents within a narrow age range who presented with low rates of psychiatric comorbidity. It was not powered to detect end-of-treatment abstinence or sustained post-treatment effects, or designed to evaluate potential effect mediators, such as N-acetylcysteine-induced changes in psychiatric symptoms. Future work is needed to replicate these findings in other settings and to explore the efficacy of N-acetylcysteine at varying doses, across different age groups, with longer treatment duration and post-treatment follow-up, with a direct test of the integrity of the blind, and with more stringent efficacy measures (e.g., sustained abstinence at end of treatment). Additionally, as noted above, to be optimally implemented as a viable treatment, N-acetylcysteine must be investigated in a variety of psychosocial treatment contexts. While N-acetylcysteine’s over-the-counter availability, low cost, and established safety profile make it highly desirable for eventual dissemination, these characteristics may prompt patients or providers to prematurely consider N-acetylcysteine as a standalone treatment. Nonetheless, the present findings represent a key step in the development of inexpensive, readily available, safe, and efficacious pharmacotherapy for cannabis dependence, and should serve as a springboard to further investigation.
Conclusion
Findings demonstrate that N-acetylcysteine is safe and efficacious when added to contingency management and brief cessation counseling for cannabis-dependent adolescents. N-acetylcysteine should be the focus of further research to confirm these findings and to more broadly and exhaustively investigate its therapeutic role.
Supplemental Figure 1. Recruitment flowchart
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank the adolescents and families who participated in the study, and acknowledge the tremendous contributions of the clinical research team, including Sarah Farber, Jessica Lydiard, and Christine Horne.
Dr. Gray has received research funding from Merck, Inc., and Supernus Pharmaceuticals. Dr. Hartwell has received research funding from Pfizer, Inc. Dr. McRae-Clark has received research funding from Shire Pharmaceuticals. Dr. Brady has received research funding from GlaxoSmithKline.
This study was supported by National Institute on Drug Abuse grant R01DA026777, via the American Recovery and Reinvestment Act of 2009. Administrative and technical support was provided by National Center for Research Resources grant UL1RR029882. Dr. Carpenter’s effort was supported by National Institute on Drug Abuse grant K23DA020482.
Footnotes
A brief (5-min) overview of main findings was presented at Late-Breaking Research session at the College on Problems of Drug Dependence 2011 Annual Meeting, Hollywood, Florida, June 19, 2011
Dr. Carpenter, Mr. Baker, Dr. DeSantis, and Ms. Kryway report no competing interests.
REFERENCES
- 1.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Marijuana use continues to rise among U.S. teens, while alcohol hits historic lows. University of Michigan News Service; Ann Arbor, MI: [Retrieved January 12, 2012]. Dec 14, 2011. from http://www.monitoringthefuture.org. [Google Scholar]
- 2.Chen CY, Anthony JC. Possible age-associated bias in reporting of clinical features of drug dependence: Epidemiological evidence of adolescent-onset marijuana use. Addiction. 2003;98:71–82. doi: 10.1046/j.1360-0443.2003.00237.x. [DOI] [PubMed] [Google Scholar]
- 3.Rey JM, Martin A, Krabman P. Is the party over? Cannabis and juvenile psychiatric disorder: The past 10 years. J Am Acad Child Adolesc Psychiatry. 2004;43:1194–1205. doi: 10.1097/01.chi.0000135623.12843.60. [DOI] [PubMed] [Google Scholar]
- 4.Jager G, Ramsey NF. Long-term consequences of adolescent cannabis exposure on the development of cognition, brain structure and function: An overview of animal and human research. Curr Drug Abuse Rev. 2008;1:114–123. doi: 10.2174/1874473710801020114. [DOI] [PubMed] [Google Scholar]
- 5.Jacobus J, Bava S, Cohen-Zion M, Mahmood O, Tapert SF. Functional consequences of marijuana use in adolescents. Pharmacol Biochem Behav. 2009;92:559–565. doi: 10.1016/j.pbb.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Compton WM, Pringle B. Services research on adolescent drug treatment. Commentary on “The cannabis youth treatment (CYT) study: Main findings from two randomized trials”. J Subst Abuse Treat. 2004;27:195–196. doi: 10.1016/j.jsat.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Dennis M, Godley SH, Diamond G, Tims FM, Babor T, Donaldson J, Liddle H, Titus JC, Kaminer Y, Webb C, Hamilton N, Funk R. The Cannabis Youth Treatment (CYT) Study: Main findings from two randomized trials. J Subst Abuse Treat. 2004;27:197–213. doi: 10.1016/j.jsat.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Waldron HB, Turner CW. Evidence-based psychosocial treatments for adolescent substance abuse. J Clin Child Adolesc Psychol. 2008;37:238–261. doi: 10.1080/15374410701820133. [DOI] [PubMed] [Google Scholar]
- 9.Levin FR, Mariani JJ, Brooks DJ, Pavlicova M, Cheng W, Nunes EV. Dronabinol for the treatment of cannabis dependence: A randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2011;116:142–150. doi: 10.1016/j.drugalcdep.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalivas PW, LaLumiere R, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56(suppl 1):169–173. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry. 2011;16:974–986. doi: 10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olive MF, Cleva RM, Kalivas PW, Malcolm RJ. Glutamatergic medications for the treatment of drug and behavioral addictions. Pharmacol Biochem Behav. 2012;100:801–810. doi: 10.1016/j.pbb.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12:182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LaRowe SD, Myrick H, Hedden S, Mardikian P, Saladin M, McRae A, Brady K, Kalivas PW, Malcolm R. Is cocaine desire reduced by N-acetylcysteine? Am J Psychiatry. 2007;164:1115–1117. doi: 10.1176/ajp.2007.164.7.1115. [DOI] [PubMed] [Google Scholar]
- 15.Mardikian PN, LaRowe SD, Hedden S, Kalivas PW, Malcolm RJ. An open-label trial of N-acetylcysteine for the treatment of cocaine dependence: A pilot study. Prog Neuro-psychopharmacol Biol Psychiatry. 2007;31:389–394. doi: 10.1016/j.pnpbp.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Zhou W, Kalivas PW. N-acetylcysteine reduces extinction responding and induces enduring reductions in cue- and heroin-induced drug-seeking. Biol Psychiatry. 2008;63:338–340. doi: 10.1016/j.biopsych.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, Markou A, Kalivas P. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry. 2009;65:841–845. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amen SL, Piacentine LB, Ahmad ME, Li SJ, Mantsch JR, Risinger RC, Baker DA. Repeated N-acetyl cysteine reduces cocaine seeking in rodents and craving in cocaine-dependent humans. Neuropsychopharmacology. 2011;36:871–878. doi: 10.1038/npp.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moussawi K, Zhou W, Shen H, Reichel CM, See RE, Carr DB, Kalivas PW. Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proc Natl Acad Sci U S A. 2011;108:385–390. doi: 10.1073/pnas.1011265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reichel CM, Moussawi K, Do PH, Kalivas PW, See RE. Chronic N-acetylcysteine during abstinence or extinction after cocaine self-administration produces enduring reductions in drug seeking. J Pharmacol Exp Ther. 2011;337:487–493. doi: 10.1124/jpet.111.179317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray JE, Everitt BJ, Belin D. N-acetylcysteine reduced early- and late-stage cocaine seeking without affecting cocaine taking in rats. Addict Biol. 2011;17:437–440. doi: 10.1111/j.1369-1600.2011.00330.x. [DOI] [PubMed] [Google Scholar]
- 22.Dean O, Giorlando F, Berk M. N-acetylcysteine in psychiatry: Current therapeutic evidence and potential mechanisms of action. J Psychiatry Neurosci. 2011;36:78–86. doi: 10.1503/jpn.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gray KM, Watson NL, Carpenter MJ, LaRowe SD. N-acetylcysteine (NAC) in young marijuana users: An open-label pilot study. Am J Addict. 2010;19:187–189. doi: 10.1111/j.1521-0391.2009.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carroll KM, Easton CJ, Nich C, Hunkele KA, Neavins TM, Sinha R, Ford HL, Vitolo SA, Doebrick CA, Rounsaville BJ. The use of contingency management and motivational/skills—building therapy to treat young adults with marijuana dependence. J Consult Clin Psychol. 2006;74:955–966. doi: 10.1037/0022-006X.74.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanger C, Budney AJ, Kamon JL, Thostensen J. A randomized trial of contingency management for adolescent marijuana abuse and dependence. Drug Alcohol Depend. 2009;105:240–247. doi: 10.1016/j.drugalcdep.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33. [PubMed] [Google Scholar]
- 27.Sheehan DV, Sheehan KH, Shytle RD, Janavs J, Bannon Y, Rogers JE, Milo KM, Stock SL, Wilkiinson B. Reliability and validity of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID) J Clin Psychiatry. 2010;71:313–326. doi: 10.4088/JCP.09m05305whi. [DOI] [PubMed] [Google Scholar]
- 28.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for Axis I Disorders. Patient Edition Biometrics Research, New York State Psychiatric Institute; New York, NY: 2004. [Google Scholar]
- 29.Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. Br J Addict. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 30.U.S. Department of Health and Human Services: Marijuana: Facts for Teens—Revised. 2008. NIH Publication No. 08-4037.
- 31.Kalachnik JE. Standardized Monitoring for Psychopharmacologic Medication Side Effects. Manual for the Monitoring of Side Effects Scale (MOSES) University of South Carolina, School of Medicine, Department of Pediatrics, Center for Disability Resources, Columbia, SC, and the South Carolina Department of Disabilities and Special Needs; Columbia, SC: 2001. [Google Scholar]
- 32.Moeller FG, Schmitz JM, Steinberg JL, Green CM, Reist C, Lai LY, Swann AC, Grabowski J. Citalopram combined with behavioral therapy reduces cocaine use: A double-blind, placebo-controlled trial. Am J Drug Alcohol Abuse. 2007;33:367–378. doi: 10.1080/00952990701313686. [DOI] [PubMed] [Google Scholar]
- 33.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 34.Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57:120–125. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 35.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- 36.Feise R. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol. 2002;2:8. doi: 10.1186/1471-2288-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schulz KF, Grimes DA. Multiplicity in randomized trials I: Endpoints and treatments. Lancet. 2005;365:1591–1595. doi: 10.1016/S0140-6736(05)66461-6. [DOI] [PubMed] [Google Scholar]
- 38.Moore RA, Aubin HJ. Do placebo response rates from cessation trials inform on strength of addictions? Int J Environ Res Public Health. 2012;9:192–211. doi: 10.3390/ijerph9010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hollis S. A graphical sensitivity analysis for clinical trials with non-ignorable missing binary outcome. Stat Med. 2002;21:3823–3834. doi: 10.1002/sim.1276. [DOI] [PubMed] [Google Scholar]
- 40.Gray KM, Carpenter MJ, Baker NL, Hartwell KJ, Lewis AL, Hiott DW, Deas D, Upadhyaya HP. Bupropion SR and contingency management for adolescent smoking cessation. J Subst Abuse Treat. 2011;40:77–86. doi: 10.1016/j.jsat.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.