Abstract
Objectives
To evaluate the effect of depression and cognition on function in older adults with amnestic and nonamnestic Mild Cognitive Impairment (aMCI; nonaMCI).
Design
The study uses baseline data from the National Alzheimer’s Coordinating Center.
Setting
Data was collected at multiple Alzheimer’s Disease Centers in the United States.
Participants
The sample included a total of 3117 individuals with MCI, mean age = 74.37 years, SD = 9.37 (aMCI n =2488; nonaMCI n = 629).
Measurements
The 10-item Pfeffer Functional Activities Questionnaire (FAQ) assessed function.
Results
Depressive symptoms (Geriatric Depression Scale), memory impairment (Logical Memory II), and processing speed decrements (Digit Symbol Substitution Test) were significantly associated with functional impairment (p < .001). Processing speed partially mediated the effect of depression on function and fully mediated the effect of executive dysfunction on function (p < .001) in the total MCI and aMCI subsample, while in the nonaMCI subsample processing speed mediated the effect of executive function but not the effect of depression (p = .20) on function.
Conclusions
The findings show that processing speed is central to the effect that depression and executive dysfunction have on functional impairment in cognitively impaired older adults. Future studies are needed to better understand the physiological underpinnings in age-related and disease-specific decrements in processing speed, and to address the problems in the assessment of processing speed in clinical samples.
Objective
Older people have high rates of psychiatric, cognitive, and physical problems that cause or contribute to increased deficits in function including problems with mobility, handling finances, remembering appointments, or managing medications.1 These functional deficits, or disabilities, lead to increased health care expenditures and assisted living or nursing home placements.2 Thus, it is important that we develop interventions to improve function, thereby improving the quality of life of older adults. To do so, we need to understand the specific relationships between psychiatric symptomatology, cognitive deficits, and functional impairment.
Depression and cognitive disorders are some of the most common neuropsychiatric disorders in elderly people. Depression and functional impairment have a bidirectional relationship, with depression causing impaired function,3 and vice versa.4 Although pharmacologic interventions effectively decrease depressive symptomatology, the effect of antidepressant treatment on impaired function in geriatric patients is not established and is often ignored.5, 6 Recently, Problem Solving Therapy was reported to improve function in depressed older adults over the course of 12-months.7 Taken together, these mixed findings underscore the need to promote a better understanding of the effectiveness of interventions on improving function in older adults with late life neuropsychiatric disorders such as depression.
The relationship between cognition and deficits in function is well established.1, 8 In older adults with Mild Cognitive Impairment (MCI), functional impairment is common, with a recent study1 showing that 72% of 394 older adults with amnestic MCI (aMCI) have at least one informant-reported deficit in function, and that these deficits were associated with greater processing speed and episodic memory problems. Depression and cognitive impairment both affect function, yet they are rarely studied simultaneously; depression is excluded in studies focused on MCI, and vice versa. Information on the combined effect of depression and cognitive impairment on function in older adults is important. By determining the domains of cognition that affect function and their relationship with depression, it may be possible to develop interventions targeted to improve function in older patients.
A recent study by Yen and colleagues9 tried to address this issue and showed that the effect of depression on function is both direct and indirect, operating through its effects on learning, memory, reasoning, and speed of processing, but they included a healthy older adult sample without depression (mean CES-D < 6),10 making it difficult to generalize their findings to depressed, cognitively impaired people. The National Alzheimer’s Coordinating Center (NACC) compiles data from a consortium of Alzheimer’s Disease Centers (ADCs) studying a large cohort of older adults with MCI and AD, a subset of whom report significant depressive symptoms. From these data we are able to address the relative effect of depression and cognitive impairment on function and explore whether these relationships remained in older adults with aMCI compared with nonamnestic MCI (nonaMCI). Based on previous work,1, 9 we hypothesized that processing speed would be central to the relationship between depression and function.
Methods
Participants
4,382 adults completed the Initial NACC packet between 2005 and 2009 at ADC’s across the country and were categorized as having MCI. Criteria for the classification of MCI were made across all ADC’s using guidelines set forth by the International Working Group on Mild Cognitive Impairment.11 After it was determined that participants did not have normal memory or dementia, MCI classification was made. If memory impairment was present based on clinical judgment and/or neuropsychological tests, the person was categorized as aMCI; A similar approach was used to determine whether other cognitive domains are impaired. If memory problems did not exist but other cognitive domains were impaired, the patient was categorized as nonaMCI. Classification was made based by a consensus conference or a single clinician decision using clinical judgment and/or neuropsychological tests.
Because missing data, particularly for the primary variables of interest in this study, is prevalent in the NACC dataset, a conservative approach to missing data was taken. If participants had any missing data on the variables that were used in the path analysis, they were excluded from the study. This included 469 participants who had missing data on the Geriatric Depression Scale (GDS) or 3 or more missing items on the Pfeffer Functional Activities Questionnaire (FAQ; 253 of these cases were missing the entire GDS or FAQ, and 216 had 3 or more missing items on the FAQ).12 Of the remaining 3,913 participants who had completed the Initial NACC packet, 796 were excluded based on missing FAQ items used to constitute the latent construct of Function, or missing demographic (education, physical health variables) or neuropsychological variables used in the path analysis. Of these 3,117 participants, MCI classification was made by consensus conference in 2,644 of the cases (2074 were aMCI, 570 were nonaMCI), and by a single clinician in the remaining 473 cases (414 were aMCI, 59 were nonaMCI). Table 1 includes summary statistics for variables from the 3,117 participants included in the data analyses.
Table 1.
Baseline characteristics of older adults with Mild Cognitive Impairment.
| Variable | Total MCI N = 3117 |
aMCI n = 2488 |
nonaMCI n = 629 |
p-value |
|---|---|---|---|---|
| Demographic | Mean (SD) | Mean (SD) | Mean (SD) | |
| Age | 74.37 (9.37) | 74.83 (9.33) | 72.57 (9.33) | < .001 |
| Education | 14.91 (3.34) | 14.97 (3.30) | 14.67 (3.45) | .045 |
| Gender M/F (% F) | 1407/1710 (54.9%) | 1132/1356 (54.5%) | 275/354 (56.3%) | .423 |
| Race | ||||
| Caucasian | 2477 (79.5%) | 2017 (81.1 %) | 460 (73.1%) | < .001 |
| African American | 490 (15.7%) | 360 (14.5%) | 130 (20.7%) | |
| Neuropsychological | ||||
| MMSE | 27.27 (2.32) | 27.14 (2.35) | 27.80 (2.13) | < .001 |
| Logical Mem Delayed | 7.04 (4.76) | 6.32 (4.61) | 9.90 (4.21) | < .001 |
| Trailmaking Test A (seconds) | 45.16 (23.48) | 45.13 (23.76) | 45.30 (22.32) | .869 |
| Trailmaking Test B (seconds) | 142.66 (80.34) | 140.41 (78.92) | 151.57 (85.19) | .003 |
| Digit Symbol | 36.90 (12.27) | 37.02 (12.34) | 36.43 (12.01) | .279 |
| CDR Scores | ||||
| CDR sum of boxes | 1.28 (1.13) | 1.33 (1.11) | 1.11 (1.20) | < .001 |
| Median (min/max) | 1.00 (0/9) | 1.00 (0/8) | 0.50 (0/9) | |
| Physical Health | ||||
| % Independent (n) | 77% (2399) | 75.8% (1887) | 81.4% (512) | .003 |
| Hachinski Ischemic Scale | 1.05 (1.46) | 0.99 (1.39) | 1.28 (1.67) | < .001 |
| Psychiatric | ||||
| GDS | 2.49 (2.70) | 2.44 (2.62) | 2.65 (2.98) | .116 |
| GDS ≥ 5, % (n) | 17.3% (538) | 16.6 % (414) | 19.7 % (124) | .068 |
| Function | ||||
| Functional Activities Questionnaire | 3.31 (4.97) | 3.46 (5.04) | 2.71 (4.66) | .002 |
Note. MCI = mild cognitive impairment; aMCI = amnestic MCI; nonaMCI = nonamnestic MCI; MMSE = Mini Mental State Exam; Logical Mem = Logical Memory II (delayed recall); CDR = Clinical Dementia Rating Scale; GDS = Geriatric Depression Scale.
Measures
Neuropsychological Assessment
Participants underwent a standard neuropsychological battery as part of the evaluation. We selected specific cognitive measures a priori because they assess cognitive functions shown to correlate with function: the Trailmaking Test A and B (executive function),13 the Digit Symbol Substitution Test of the Wechsler Adult Intelligence Scale-Revised (processing speed),14 and the Logical Memory II, Story A (delayed recall) from the Wechsler Memory Scale-Revised.14
Pfeffer Functional Activities Questionnaire (FAQ)
The FAQ12 is a 10-item measure of instrumental activities of daily living. Informant reports were collected for all participants. Each item is rated from 0 (no difficulty/independent) to 3 (dependent). Items include the ability to pay bills (1), complete tax/business records (2), shop (3), play games/hobbies (4), make a cup of coffee (5), prepare a balanced meal (6), track current events (7), attend to books/TV/magazines (8), remember appointments/manage medications (9), and travel outside of the neighborhood (10).
Geriatric Depression Scale (GDS)
Depressive symptoms were assessed using the 15-item GDS15–16, 17 which uses a yes/no answer format. Scores range from 0 to 15. For the preliminary subgroup analysis, individuals who scored 5 or greater17 treated as a depressed group. The continuous total GDS score however was used in the path analysis.
Statistical Analysis
Summary statistics were calculated to describe characteristics of the MCI participants. Two-sample t tests and χ2 tests were used to detect differences in continuous and categorical variables between aMCI and nonaMCI patients. Linear models including analyses of variance/covariance were used to examine the relationships between depression and cognitive measures and whether the associations differ between aMCI and nonaMCI patients.
Because of missing data from the FAQ items (of the 3117, data was missing in 9.1% of the sample for item 2, 11.0% for item 4, and 9.7% for item 6), use of the total FAQ score as an outcome would exclude a large number of cases from the models. No differences were found in percentage of missing items across sites, gender, or cognitive impairment. To maximize data used in the models, a confirmatory factor analysis was conducted for the specification of Function, with items chosen from previous research showing specific FAQ items impaired in older adults with MCI.1 The robust weighted least squares estimation (WLSMV)18 in MPlus (Version 3.11)19 was used to fit the model to a matrix of polychoric correlations for categorical response variables of individual FAQ items. Global fit for the measurement model was evaluated using the root mean square error of approximation (RMSEA), the Tucker-Lewis index (TLI),20 and the Comparative Fit index (CFI).21 The RMSEA is an index of discrepancy between the model and the data per degree of freedom, with a value ≤ .05 indicative of good fit.22, 23 Hu and Bentler’s22 suggestion that TLI and CFI should be above .95 for good fit has also been found reasonable for categorical outcomes.19 The procedure gives consistent test statistics, parameter estimates and standard errors under both normal and non-normal latent response distributions.18
To examine the relationships of the predictors with Function, path analysis with the latent outcome variable was conducted in MPlus.19 Because global model fit indices for the path analyses with both observed and latent variables may be affected by the error in the observed data and model misspecification in latent constructs, global model fit will not be presented for the path models. The path analyses were constructed in the 3,117 patients with MCI using covariates shown to correlate with function, depression, or cognition, including age, gender, education, and physical health (specifically, the total score on the modified Hachinski ischemic scale,24 a scale that assesses hypertension, stroke, and/or neurologic signs/symptoms). All paths that included Trailmaking Test B included Trailmaking Test A as a covariate, a standard procedure for analyzing Trailmaking Test B as a measure of executive abilities. The continuous independent variables and covariates were standardized to have zero mean and unit standard deviation for comparable scales in the analysis. To help understand the complex relationship between depression and function, mediation analysis was conducted as it allows researchers to “explain the process or mechanism by which one variable affects another” (pg. 593)25. A mediator is a variable on the path between predictor and outcome that attenuates the association of the predictor with the outcome when included into the model.
In this study, we hypothesized that depression leads to deficits in processing speed, which in turn increase impairment in functioning. To assess this possible mediation effect, certain conditions must be met:25 a) depression and function (direct effect of depression), and b) depression and processing speed (relationship with potential mediator) must be associated; c) processing speed must be significantly related to function with depression included in the model (indirect effect of depression), and d) within this model, the magnitude of the relationship between depression and function must be less (in absolute value terms) than the relationship between depression and function without processing speed included. As with the model recently examined by Yen and colleagues,9 we also included other cognitive domains that have been shown to affect functioning to test whether or not they too help explain the process by which depression affects function; these cognitive domains include memory and executive function and were chosen based on prior research showing impairment in these domains in both patients with depression,26–28 and an association between these domains and functional impairment.1, 29–32
Probit regression coefficients are reported for main effects and indirect effects. A mediation effect is computed as the product of the effect of the predictor (depression) on the mediator (processing speed) times the effect of the mediator (processing speed) on the outcome (function) with standard errors computed via the Delta method19, 33 and p-values are derived from the Wald test. The effect size of the mediation is calculated as the reduction in percentage of the direct effect due to the mediation, (τ-τ′)/τ34 using standardized regression coefficients for direct (τ) and indirect effects (τ′) of the predictor (depression).
Results
Table 1 includes the baseline characteristics for the MCI sample. Of the 3,117 participants with MCI, 79.5% were Caucasian, 15.7% were African American, and 91.9% of them spoke English as their primary language. According to NACC criteria 629 of the 3,117 older adults with MCI were categorized as nonaMCI. The aMCI group had a greater proportion of Caucasians (p < .001), was older (p < .001), more educated (p = .05), more functionally impaired (FAQ; p = .002), less independent (p = .003), and had fewer ischemic problems (Hachinski; p < .001) than the nonaMCI group. The nonaMCI group had better memory (Logical Memory Delayed; p < .001) but poorer executive function (Trailmaking Test B; p = .003).
To understand the relationship between depression status, MCI classification, and demographic variables, patients were categorized into three groups in terms of depression: no depressive symptoms (GDS = 0; n = 736), mild depressive symptoms (GDS between 1–4; n = 1843), and significant depressive symptoms (GDS ≥ 5; n = 538). Analyses of covariance revealed that the two-way interaction between diagnosis (aMCI, nonaMCI) and depression (nondepressed, mildly depressed, depressed) was not statistically significant for any of the cognitive or function variables covarying for age, gender, and education.
Using analysis of variance and post hoc comparisons for the three depressed groups, we found that the depressed group was younger, less educated, had more vascular impairment, and was significantly more impaired on the FAQ, Digit Symbol, and Trailmaking Test B than both the nondepressed and mildly depressed groups (ps ≤ .004); the depressed group had more impairment in Logical Memory Delayed than the nondepressed group (p < .001), although there was no significant difference between the depressed and mildly depressed groups (p = .82).
Confirmatory factor analysis
The analyses classified a latent construct using seven of the 10 items (Items 1, 3, 5, 7–10). These seven items had no missing data values on the 3,117 participants. This model fit the data well, RMSEA = .091, CFI = .959, TLI = .976. Each of the variables had a communality estimate (R2) with Function ≥ .54. Standardized factor loadings for the measurement model were .81 for Item 1, .84 for Item 3, .73 for Item 5, .78 for Item 7, .73 for Item 8, .79 for Item 9, and .81 for Item 10.
Examination of mediation effects
Linear models were used to assess the effect of potential mediators on the association between the continuous total raw scores for depression and cognition, with the latent construct, Function, which led to the simplification of the paths between these variables. We found that the significant effect of GDS on Trailmaking Test B (estimated regression coefficient b = .05, p < .001) attenuated (b = .02, p = .07) when Digit Symbol (b = −3.26, p < .001) was entered into the model, indicating that the effects of depression and executive function on Function were mediated by processing speed. Logical Memory Delayed however was not associated with GDS after covarying for age, gender, education, and total Hachinski score (r = −.03, p = .11). These findings eliminated pathways from depression to both memory and executive function in the final model.
Path analysis was used to investigate the relationships between the observed independent variables and the latent construct, Function, measured by seven of the ten FAQ items. Both individual direct effects of Logical Memory Delayed (probit coefficient b = − .20, SE = .02, p < .001) and Digit Symbol (probit coefficient b = −.20, SE = .02, p < .001) on Function decreased slightly when entered into the model together (Logical Memory: probit coefficient b = − .19, SE = .01, p < .001; Digit Symbol: probit coefficient b = − .17, SE = .02, p < .001) indicating no mediating effect; rather, a significant partial correlation (after covarying for age, gender, education, and Hachinski) exists between Logical Memory Delayed and Digit Symbol (rpartial = .14, p < .001).
Two mediating relationships were observed with Function. The direct effect of GDS on Function (probit coefficient b = .16, SE = .02, p < .001) decreased (probit coefficient b = .14, SE = .02, p < .001) when Digit Symbol (probit coefficient b = − .17, SE = .02, p < .001) entered the model, indicating a partial mediation. The significant direct effect of Trailmaking Test B on Function (probit coefficient b = .06, SE = .02, p = .003) became null (probit coefficient b = .01, SE = .02, p = .81) as Digit Symbol (probit coefficient b = −.15, SE = .02, p < .001) entered the model, indicating a full mediation.
Final Model
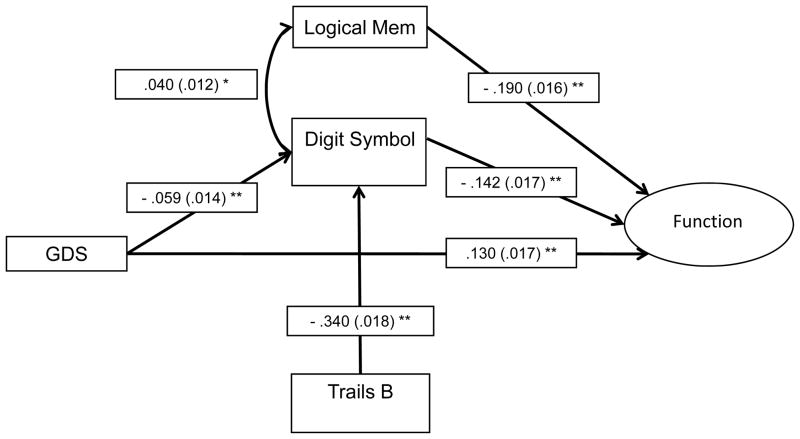
The parsimonious final model (Figure 1, Table 2) includes three main effects, GDS, Digit Symbol, and Logical Memory Delayed (ps < .001), on Function after controlling for education (p = .07) and Hachinski score (p = .054); age and gender did not contribute (ps > .74) and were subsequently dropped from the model. Digit Symbol partially mediated the effect of GDS on Function (p < .001; 5.8% of the direct effect is explained by Digit Symbol) and mediated the effect of Trailmaking Test B on Function after controlling for Trailmaking Test A (p < .001; 91.7% of the direct effect is explained by Digit Symbol). This model and each individual pathway and indirect effect remained comparable and significant when covariates were removed (each pathway increased in magnitude with two exceptions: the coefficient of pathways from GDS to Digit Symbol and from Logical Memory Delayed to Function decreased slightly in magnitude).
Figure 1.
Path analysis model depicting the relationships between depressive symptomatology, cognition and functional impairment in older adults with mild cognitive impairment.
Note. Standardized probit regression coefficients (SEs) are included in the model. Covariates age, gender, education, and Hachinski score were included in each component of the model (with the exception of the path with Function as the outcome, where age and gender did not contribute to the model), but are not depicted in the figure. Function is a latent construct estimated using seven items from the Pfeffer FAQ (Items 1, 3, 5, 7–10), although model fit statistics and items are removed from figure for simplicity. GDS = 15-item Geriatric Depression Scale, Logical Mem = Logical Memory Delayed, Trails B = Trailmaking Test B (with Trailmaking Test A included as a covariate).
* p < .001
** p < .0001
Table 2.
Probit regression coefficients and standard errors for path analysis evaluating the interrelationships between depression and cognition and their effect on function in older adults with Mild Cognitive Impairment.
| Variables | B | SE | B/SE | p- value |
|---|---|---|---|---|
| Outcome: Function | ||||
| Education | .034 | .019 | 1.81 | 0.070 |
| Hachinski | .032 | .017 | 1.93 | 0.054 |
| Geriatric Depression Scale | .130 | .017 | 7.47 | <0.001 |
| Logical Memory | −.190 | .016 | − 11.81 | <0.001 |
| Digit Symbol | −.142 | .017 | − 8.26 | <0.001 |
| Indirect effects | ||||
| Geriatric Depression Scale through Digit Symbol | .008 | .002 | 3.79 | <0.001 |
| Trailmaking Test B through Digit Symbol | .048 | .006 | 7.66 | <0.001 |
| Outcome: Digit Symbol | ||||
| Age | −.156 | .014 | − 10.82 | <0.001 |
| Gender | .194 | .029 | 6.72 | <0.001 |
| Education | .176 | .014 | 12.23 | <0.001 |
| Hachinski | −.045 | .013 | − 3.39 | <0.001 |
| Trailmaking Test A | −.268 | .018 | − 15.15 | <0.001 |
| Geriatric Depression Scale | −.059 | .014 | − 4.27 | <0.001 |
| Trailmaking Test B | −.340 | .018 | − 19.15 | <0.001 |
| Correlation | ||||
| Logical Memory Delayed with Digit Symbol | .040 | .012 | 3.41 | <0.001 |
Note. B: Probit Coefficient. SE: Standard Error, Standardized scores; high scores on Digit Symbol and Logical Memory Delayed indicate better performance, whereas lower scores on Function, Trailmaking Test A & B, Geriatric Depression Scale, and Hachinski indicate better health/performance, N = 3,117.
Amnestic vs. Nonamnestic MCI
To examine whether the identified relationships held in the subsamples of older adults with aMCI and nonaMCI, exploratory path analyses were conducted for the two groups separately. For the model with the aMCI group (n = 2,488), the relationships between variables were comparable to the model depicted in Figure 1. For the model with nonaMCI group (n = 629), the effect of Logical Memory Delayed on Function was less significant (probit coefficient b = −.09, SE = .05, p = .06), although the correlation between Logical Memory Delayed and Digit Symbol was still significant (p < .001), and no evidence supported the relationship between GDS and Digit Symbol (probit coefficient b = − .04, SE = .03, p = .20).
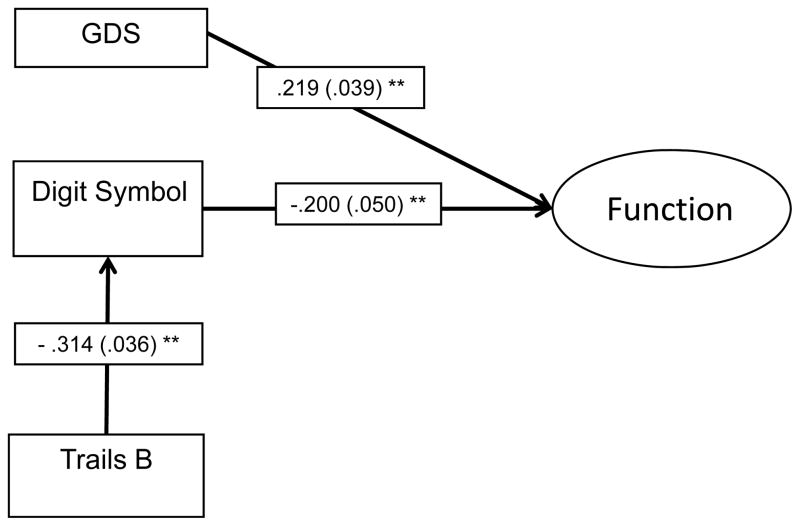
A simplified model was applied to the nonaMCI sample to illustrate these differences. In the model, Function is predicted by GDS (probit coefficient b = .22, SE = .04, p < .001), and a mediated relationship of Trailmaking Test B through Digit Symbol (p < .001; 29.2% of the direct effect is explained by Digit Symbol).
Conclusions
The findings from this study show that processing speed is central to the effect that depression and executive dysfunction have on functional impairment in cognitively impaired older adults. In fact, in both the aMCI and nonaMCI subsamples, processing speed substantially accounted for the relationship between executive dysfunction and functional impairment, explaining 91.7% of the direct effect of executive dysfunction on functional impairment in the total MCI sample, and 29.2% of the direct effect in the nonaMCI sample. Although depression had independent effects on function, it was partially mediated by processing speed.
Geriatric patients with depressive symptoms or impairment in memory experience more functional impairment.31, 35 The lack of a significant association between memory and function in patients with nonaMCI as well as the lack of a relationship between depression and memory provide evidence that the memory deficits experienced by patients with aMCI, the group most likely to convert to Alzheimer’s Disease,36 lead to functional impairments independent of depressive symptomatology. The latter finding contrasts those of a recent study9 in which memory mediated the relationship between depressive symptoms and function. This disparity may be due to the healthy older sample studied by Yen and colleagues,9 which had minimal depressive symptoms; because of this, the relationships they observed may not generalize to clinical populations. In contrast, this study used a sample of cognitively impaired older adults, 17.3% of whom had significant depressive symptoms. As such these relationships may be better represented in this study where depression and memory impairment appear to effect function independently of one another.
Processing speed partially mediates the relationship between depression and function, and fully mediates the relationship between executive dysfunction and function. At its most basic definition, processing speed is the rate at which a task is completed.37 Decrements in processing speed result from disease and/or age-related changes. Salthouse38 and others39 have speculated that age-related slowing is the result of diffuse cell loss, causing neural impulses to travel lengthier pathways to reach the goal. Others38, 40 have postulated that the slowing of neural impulses is the result of a reduction of dendritic branching, fewer active synapses, or decreased myelin, and that slowing may be the result of a reduction of particular neurotransmitters such as dopamine. A recent review37 cited evidence that the dorsal attention system characterized by changes in the morphology of frontal and cerebellar regions might be responsible for age-related cognitive slowing.
Disorders such as depression and MCI/dementia have been shown to be associated with decrements in processing speed.1, 9, 27 A recent study found a relationship between greater medial temporal atrophy, decreased processing speed, and increased functional impairment in patients with aMCI, suggesting that atrophy of the hippocampus and/or entorhinal cortex may lead to decreased processing speed and increased functional impairment. The effect of decreased processing speed and memory impairments on function in the aMCI group in this study fit with this mechanistic picture of the relationship between the progressive pathology of dementia and processing speed decrements.
The link between depression and processing speed decrements is less well characterized. Both depression and slowed processing speed are related to cerebral white matter hyperintensities,37, 41, 42 which are signs of cerebral small vessel disease associated with vascular risk factors such as hypertension, diabetes, and coronary disease.27, 43 These white matter hyperintensities have also been implicated in the etiology of the vascular depression-subtype44 although it is likely that there is a bidirectional relation between depression and the cortical damage caused by cerebrovascular disease.45 Cerebrovascular disease may also cause a reduction in processing speed as a result of loss or decreased efficiency of neuronal connectivity. In other words, it could be that decrements in processing speed result from cerebral small vessel disease in older adults, and that these decrements worsen along with the risk of developing a depressive illness.
The assessment of processing speed as a specific domain of cognition remains enmeshed with motor speed and measures of executive dysfunction. For example, the Digit Symbol task is a measure of processing speed27, 46 that also requires attention and task switching, aspects that fall under the rubric of executive function or more generally fluid intelligence.47 Similarly, poor performance on the Trailmaking Test B may be the result of a variety of cognitive factors. This task, mostly used as a test of visuomotor tracking, divided attention, and cognitive flexibility,47 is also a speeded task with a substantial motor component.
It has been hypothesized1 that processing speed may be a precursor to more severe executive or attentional decrements in cognitively impaired elders marking the early stages of what will progress into more severe cognitive deficits and, as a result, increased dependence in function. Because of the impurity of the assessments, however, we are left with an unclear picture of what processing speed is, the mechanisms by which these decrements occur, how it differentiates (if at all) with psychomotor slowing48 (particularly important in depressive disorders in which psychomotor retardation is a classic symptom and recent research has shown a strong association between depressive symptoms and gait performance49), and how it relates to executive dysfunction. Future research needs to focus on deconstructing these relationships through the use of imaging techniques and purer measures of processing speed and executive dysfunction to better understand the mechanistic properties of the decline in processing speed that is associated with older age and late life neuropsychiatric disorders.
Despite its strengths, there are limitations that need to be taken into account when interpreting the results of this study. This is a cognitively impaired sample presenting for evaluation of cognitive deficits at specialized memory disorder centers, making it difficult to generalize these relationships to a community sample or a purely depressed geriatric sample. Also, while 84.8% of the MCI classifications were made via consensus conference, which lends credence to the decision to classify the patients as MCI, a single clinician classified 15.2% of the 3,117 patients with MCI. As such, the definition of MCI across ADC’s utilized in this study is not a uniform process. While this can lead to inconsistencies in defining the samples, it does reflect a more realistic representation of how a MCI classification would be made in clinical practice. Missing data is also a problem in the NACC database, particularly for the GDS and FAQ, leading to the exclusion of a large number of cases from the analyses. Also, while the FAQ is a widely used measure, it is focused on cognitively mediated activities and does not assess physical or social functioning, two domains likely to also be impaired in depressed elders; also, the FAQ relies on informant-reports of functional impairment, lacking the objectivity of a performance-based measure of function which is not used in the NACC dataset. Finally, the neuropsychological domains are broadly sampled in the NACC dataset. The measures of executive function, episodic memory, and processing speed (the domains chosen for this project because of their relationship to functional impairment) are limited. As such, path models with observed and latent variables rather than a full model of latent constructs were used. Ideally, a more thorough assessment within each cognitive domain would allow for a latent construct of each individual cognitive domain to be used within the framework of a full structural equation model. That was not the case however with this dataset.
In conclusion, this study showed that deficits in function in older adults with MCI are associated with more depressive symptoms, memory problems, and processing speed decrements. These deficits in processing speed fully mediated the relationship between executive function and function and partially mediated the relationship between depression and function in this sample of patients with MCI. Deconstruction studies are needed to better understand the physiological underpinnings in age-related and disease-specific decrements in processing speed.
Figure 2.
Path model depicting the relationships between depressive symptomatology, cognition and functional impairment in older adults with nonamnestic mild cognitive impairment.
Note. Standardized probit regression coefficients (SEs) are included in the model. Covariates age, gender, education, and Hachinski score were included in each component of the model, but are not depicted in the figure; Function is a latent construct estimated using seven items from the Pfeffer FAQ (Items 1, 3, 5, 7–10), although model fit statistics and items are removed from figure for simplicity. GDS = 15-item Geriatric Depression Scale, Trails B = Trailmaking Test B (with Trailmaking Test A included as a covariate).
* p < .001
** p < .0001
Acknowledgments
This research was supported by grants from the National Alzheimer’s Coordinating Center (grant numbers U01 AG016976 and 2010-JI-01), and a National Institute of Mental Health (grant number T32 MH20004).
Footnotes
Disclosure: Drs. Brown, Liu, Sneed, and Ms. Pimontel have nothing to disclose. Dr. Devanand has received research support from Novartis AG and Eli Lilly and Company, and has served as a consultant to Bristol-Myers Squibb and Sanofi-Aventis. Dr. Roose has received research support from Forest Laboratories and serves as a consultant for Medtronics. These sponsors’ had no role in this current manuscript.
References
- 1.Brown PJ, Devanand DP, Liu X, Caccappolo E. Functional impairment in elderly patients with mild cognitive impairment and mild Alzheimer disease. Arch Gen Psychiatry. 2011 Jun;68(6):617–626. doi: 10.1001/archgenpsychiatry.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolinsky FD, Callahan CM, Fitzgerald JF, Johnson RJ. Changes in functional status and the risks of subsequent nursing home placement and death. J Gerontol. 1993 May;48(3):S94–101. [PubMed] [Google Scholar]
- 3.Bruce ML, Seeman TE, Merrill SS, Blazer DG. The impact of depressive symptomatology on physical disability: MacArthur Studies of Successful Aging. Am J Public Health. 1994 Nov;84(11):1796–1799. doi: 10.2105/ajph.84.11.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kennedy GJ, Kelman HR, Thomas C. The emergence of depressive symptoms in late life: the importance of declining health and increasing disability. J Community Health. 1990 Apr;15(2):93–104. doi: 10.1007/BF01321314. [DOI] [PubMed] [Google Scholar]
- 5.Roose SP, Sackeim HA, Krishnan KR, et al. Antidepressant pharmacotherapy in the treatment of depression in the very old: a randomized, placebo-controlled trial. Am J Psychiatry. 2004 Nov;161(11):2050–2059. doi: 10.1176/appi.ajp.161.11.2050. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds CF, 3rd, Butters MA, Lopez O, et al. Maintenance Treatment of Depression in Old Age: A Randomized, Double-blind, Placebo-Controlled Evaluation of the Efficacy and Safety of Donepezil Combined With Antidepressant Pharmacotherapy. Arch Gen Psychiatry. 2011 Jan;68(1):51–60. doi: 10.1001/archgenpsychiatry.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexopoulos GS, Raue PJ, Kiosses DN, et al. Problem-solving therapy and supportive therapy in older adults with major depression and executive dysfunction: effect on disability. Arch Gen Psychiatry. 2011 Jan;68(1):33–41. doi: 10.1001/archgenpsychiatry.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tabert MH, Albert SM, Borukhova-Milov L, et al. Functional deficits in patients with mild cognitive impairment: prediction of AD. Neurology. 2002;58(11889240):758–764. doi: 10.1212/wnl.58.5.758. [DOI] [PubMed] [Google Scholar]
- 9.Yen YC, Rebok GW, Gallo JJ, Jones RN, Tennstedt SL. Depressive symptoms impair everyday problem-solving ability through cognitive abilities in late life. Am J Geriatr Psychiatry. 2011 Feb;19(2):142–150. doi: 10.1097/JGP.0b013e3181e89894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willis SL, Tennstedt SL, Marsiske M, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006 Dec 20;296(23):2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004 Sep;256(3):240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 12.Pfeffer RI, Kurosaki TT, Harrah CH, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37(7069156):323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 13.Reitan R. Validity of the Trail-Making Test as an indication of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 14.DAW . Manual for the Wechsler Adult Intelligence Scale–Revised. New York: Psychological Corporation; 1981. [Google Scholar]
- 15.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 16.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clinical Gerontologist. 1986;5:165–172. [Google Scholar]
- 17.Brown PJ, Woods CM, Storandt M. Model stability of the 15-item Geriatric Depression Scale across cognitive impairment and severe depression. Psychol Aging. 2007 Jun;22(2):372–379. doi: 10.1037/0882-7974.22.2.372. [DOI] [PubMed] [Google Scholar]
- 18.Flora DB, Curran PJ. An empirical evaluation of alternative methods of estimation for confirmatory factor analysis with ordinal data. Psychol Methods. 2004 Dec;9(4):466–491. doi: 10.1037/1082-989X.9.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muthen LK, Muthen BO. Mplus user’s guide. Statistical analysis with latent variable. 3. Los Angeles, CA: Muthen & Muthen; 1998–2006. [Google Scholar]
- 20.Tucker LR, Lewis C. A reliability coefficient for maximum likelihood factor analysis. Psychometrica. 1973;38:1–10. [Google Scholar]
- 21.Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990 Mar;107(2):238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- 22.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- 23.Browne MW, Cudeck R. Alternative ways of assessing model fit. Newbury Park, CA: Sage; 1993. [Google Scholar]
- 24.Hachinski VC, Iliff LD, Zilhka E, et al. Cerebral blood flow in dementia. Arch Neurol. 1975;32(1164215):632–637. doi: 10.1001/archneur.1975.00490510088009. [DOI] [PubMed] [Google Scholar]
- 25.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sneed JR, Culang ME, Keilp JG, Rutherford BR, Devanand DP, Roose SP. Antidepressant medication and executive dysfunction: a deleterious interaction in late-life depression. Am J Geriatr Psychiatry. 2010 Feb;18(2):128–135. doi: 10.1097/JGP.0b013e3181c796d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheline YI, Pieper CF, Barch DM, et al. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010 Mar;67(3):277–285. doi: 10.1001/archgenpsychiatry.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheline YI, Barch DM, Garcia K, et al. Cognitive function in late life depression: relationships to depression severity, cerebrovascular risk factors and processing speed. Biol Psychiatry. 2006 Jul 1;60(1):58–65. doi: 10.1016/j.biopsych.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 29.Farias ST, Mungas D, Reed BR, Harvey D, Cahn-Weiner D, Decarli C. MCI is associated with deficits in everyday functioning. Alzheimer Dis Assoc Disord. 2006 Oct-Dec;20(4):217–223. doi: 10.1097/01.wad.0000213849.51495.d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okonkwo OC, Wadley VG, Griffith HR, Ball K, Marson DC. Cognitive correlates of financial abilities in mild cognitive impairment. J Am Geriatr Soc. 2006;54(17087703):1745–1750. doi: 10.1111/j.1532-5415.2006.00916.x. [DOI] [PubMed] [Google Scholar]
- 31.Wadley VG, Okonkwo O, Crowe M, Ross-Meadows LA. Mild cognitive impairment and everyday function: evidence of reduced speed in performing instrumental activities of daily living. Am J Geriatr Psychiatry. 2008 May;16(5):416–424. doi: 10.1097/JGP.0b013e31816b7303. [DOI] [PubMed] [Google Scholar]
- 32.Wadley VG, Okonkwo O, Crowe M, et al. Mild cognitive impairment and everyday function: an investigation of driving performance. J Geriatr Psychiatry Neurol. 2009;22(19196629):87–94. doi: 10.1177/0891988708328215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bollen KA. Structural Equations with Latent Variables. 1. New York: Wiley-Interscience; 1989. [Google Scholar]
- 34.MacKinnon DP, Dwyer JH. Estimating mediated effects in prevention studies. Eval Rev. 1993;17(2):144–158. [Google Scholar]
- 35.Reynolds CF., 3rd Assessing the capacity to make everyday decisions about functional problems: where does the field go from here? Am J Geriatr Psychiatry. 2007 Feb;15(2):89–91. doi: 10.1097/JGP.0b013e31802e7074. [DOI] [PubMed] [Google Scholar]
- 36.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999 Mar;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 37.Eckert MA. Slowing down: age-related neurobiological predictors of processing speed. Front Neurosci. 2011;5:25. doi: 10.3389/fnins.2011.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salthouse TA. Aging and measures of processing speed. Biol Psychol. 2000;54(11035219):35–54. doi: 10.1016/s0301-0511(00)00052-1. [DOI] [PubMed] [Google Scholar]
- 39.Cerella J. Aging and information-processing rate. In: Birren JE, Schale KW, editors. Handbook of the Psychology of Aging. 3. San Diego: Academic Press; 1990. pp. 201–221. [Google Scholar]
- 40.Miller EM. Intelligence and brain myelination: a hypothesis. Personality Individual Differences. 1994;17:803–832. [Google Scholar]
- 41.de Groot JC, de Leeuw FE, Oudkerk M, Hofman A, Jolles J, Breteler MM. Cerebral white matter lesions and depressive symptoms in elderly adults. Arch Gen Psychiatry. 2000 Nov;57(11):1071–1076. doi: 10.1001/archpsyc.57.11.1071. [DOI] [PubMed] [Google Scholar]
- 42.Taylor WD, Steffens DC, MacFall JR, et al. White matter hyperintensity progression and late-life depression outcomes. Arch Gen Psychiatry. 2003 Nov;60(11):1090–1096. doi: 10.1001/archpsyc.60.11.1090. [DOI] [PubMed] [Google Scholar]
- 43.Brickman AM, Reitz C, Luchsinger JA, et al. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch Neurol. 2010 May;67(5):564–569. doi: 10.1001/archneurol.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sneed JR, Rindskopf D, Steffens DC, Krishnan KR, Roose SP. The vascular depression subtype: evidence of internal validity. Biol Psychiatry. 2008 Sep 15;64(6):491–497. doi: 10.1016/j.biopsych.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas AJ, O’Brien JT, Davis S, et al. Ischemic basis for deep white matter hyperintensities in major depression: a neuropathological study. Arch Gen Psychiatry. 2002 Sep;59(9):785–792. doi: 10.1001/archpsyc.59.9.785. [DOI] [PubMed] [Google Scholar]
- 46.Verhaeghen P, Salthouse TA. Meta-analyses of age-cognition relations in adulthood: estimates of linear and nonlinear age effects and structural models. Psychol Bull. 1997 Nov;122(3):231–249. doi: 10.1037/0033-2909.122.3.231. [DOI] [PubMed] [Google Scholar]
- 47.Lezak M, Howieson D, Loring D, Hannay H, Fischer J. Neuropsychological assessment. 4. New York: Oxford University Press; 2004. [Google Scholar]
- 48.Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997 Apr;154(4):497–501. doi: 10.1176/ajp.154.4.497. [DOI] [PubMed] [Google Scholar]
- 49.Brandler TC, Wang C, Oh-Park M, Holtzer R, Verghese J. Depressive Symptoms and Gait Dysfunction in the Elderly. Am J Geriatr Psychiatry. 2011 Mar 17; doi: 10.1097/JGP.0b013e31821181c6. [DOI] [PMC free article] [PubMed] [Google Scholar]