Abstract
In many songbirds, vocal learning-related cellular plasticity was thought to end following a developmental critical period. However, mounting evidence in one such species, the zebra finch, suggests that forms of plasticity common during song learning continue well into adulthood, including a reliance on auditory feedback for song maintenance. This reliance wanes with increasing age, in tandem with age-related increases in fine motor control. We investigated age-related morphological changes in the adult zebra finch song system by focusing on two cortical projection neuron types that a) share a common efferent target, b) are known to exhibit morphological and functional change during song learning, and c) exert opposing influences on song acoustic structure. Neurons in HVC (proper name) and the lateral magnocellular nucleus of the anterior nidopallium (LMAN) both project to the robust nucleus of the arcopallium (RA). During juvenile song learning and adult song maintenance, HVC promotes song syllable stereotypy while LMAN promotes learning and acoustic variability. Following retrograde labeling of these two cell types in adults, there were age-related increases in dendritic arbor in HVC-RA but not LMAN-RA neurons, resulting in an increase in the ratio of HVC-RA:LMAN-RA dendritic arbor. Differential growth of HVC relative to LMAN dendrites may relate to increases in song motor refinement, decreases in the reliance of song on auditory feedback, or both. Despite this differential growth with age, we also show that both cell types retain the capacity for experience-dependent growth. These results may provide insights on mechanisms that promote and constrain adult vocal plasticity.
Keywords: Song System, Dendrites, Age, Social Enrichment
Introduction
Vocal learning in songbirds and humans is similar in many respects. In both cases, the capacity to learn is constrained by innate predispositions, relies on hearing, is influenced by social context and is controlled by specialized brain regions (Doupe & Kuhl, 1999; Kuhl, 2003). In addition, humans and many songbirds demonstrate a much greater capacity for vocal learning early in life, which greatly diminishes with age (Doupe & Kuhl, 2008). Songbirds provide an exceptional opportunity for exploring vocal learning because species vary in their learning trajectories. All oscine songbirds learn their song from a tutor as juveniles. Some species, such as the canary (Serinus canaria) continue to learn new songs throughout life (open-ended learners), while in other species; such as the zebra finch (Taeniopygia guttata) song learning is essentially complete by puberty (close-ended learners) (reviewed by Williams, 2004). An understanding of the mechanisms that permit and constrain adult vocal plasticity may provide insights on species differences in these traits in songbirds that are also relevant for understanding age-related decreases in vocal learning in humans.
While song learning is essentially complete by puberty (3 months) in the zebra finch (reviewed by, Williams, 2004), recent work has demonstrated that learning-related behavioral and cellular changes continue to take place beyond puberty and, in some cases changes continue throughout adulthood (reviewed by Pytte et al., 2008). For example, song maintenance relies on auditory feedback and, in young adults; song degrades rapidly following deafening, suggesting that sensory-motor comparisons similar to those used in vocal learning extend into adulthood for song maintenance (Nordeen & Nordeen, 1992; Wang et al., 1999). Interestingly, the impact of deafening on song decreases with increasing adult age such that between roughly 6–7 months and one year after hatching, there is a dramatic increase in the latency and decrease in the magnitude of post-deafening song change (Lombardino & Nottebohm, 2000; Brainard & Doupe, 2001). Moreover, between 4 and 15 months of age, there is an increase in fine-grained stereotypy (syllable acoustic similarity from rendition to rendition of song) and this increase tapers off between 9 and 15 months (Pytte et al., 2007; also see Brainard & Doupe, 2001; Kao & Brainard, 2006). Thus, age-related changes in both motor refinement and reliance on auditory feedback co-vary and may be functionally related. Combined, these data suggest that even in a so-called “close-ended” learner, adulthood is a very dynamic period, involving vocal learning-related sensory-motor processes and it is also a time where song stability and resistance to change increase with adult age.
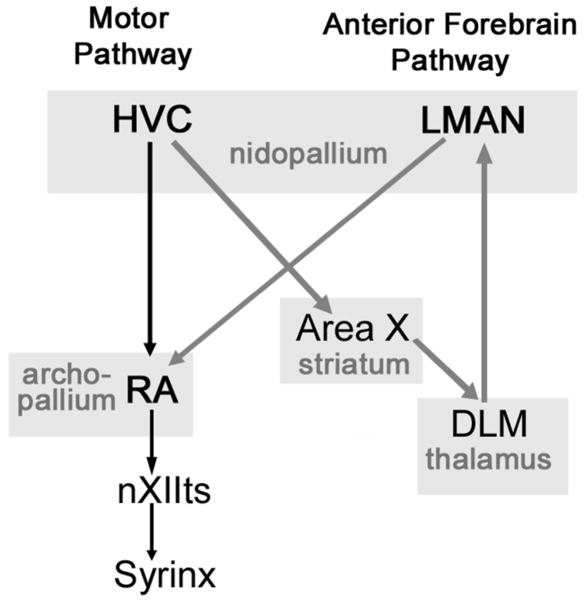
The neural mechanisms controlling these song attributes remain largely unknown. Components of two song control system pathways are of particular relevance to song learning and maintenance (Figure 1). Both pathways begin in HVC (proper name) and project to the robust nucleus of the arcopallium (RA). The avian analogue of a pre-motor/motor cortical circuit (motor pathway), involves direct projections from HVC to RA. The anterior forebrain pathway (analogous to cortical-basal ganglia circuits) begins with a different set of HVC neurons that project to RA following a more indirect route involving the striatum and thalamus. Final inputs to RA from this pathway derive from the lateral magnocellular nucleus of the anterior nidopallium (LMAN) and these cells synapse on the same dendrites in RA as cells in the motor pathway (Herrmann & Arnold, 1991). In turn, RA neurons have direct projections to brainstem motor neurons innervating muscles of the syrinx (the avian vocal organ) (Nottebohm et al., 1976).
Figure 1.
Diagram of key components of the song system involved in song production, acquisition and maintenance. Black arrows represent the motor pathway and grey arrows represent the anterior forebrain pathway. Abbreviations: HVC, (proper name); LMAN, lateral magnocellular nucleus of the anterior nidopallium; RA, robust nucleus of the arcopallium; Area X (proper name); DLM, medial portion of the dorsolateral nucleus of the thalamus; nXIIts, tracheosyringeal part of the nucleus nervi hypoglossi. Based on Nottebohm et al., 1976; Bottjer et al., 1989.
To date, the main focus of research on adult neuroanatomical plasticity in the zebra finch has been on neuron addition, which in HVC also declines dramatically between 4–12 months (Wang et al., 2002). However, there have been no studies of potential changes in neuronal morphology in the song system of the adult zebra finch.
Changes in the morphology of many song system cell types occur in juveniles at times suggesting a functional link to song learning (reviewed by Bottjer, 2004). Changes in the number and weighting of synapses in RA derived from HVC and LMAN may be of particular relevance. Based on lesion work as well as anatomical and neurophysiological studies, the LMAN-RA pathway is essential for early stages of song learning, promoting vocal exploratory behavior, while the HVC-RA pathway is critical for the final stages of song learning, promoting the establishment of song stereotypy (Aronov et al., 2008). In adults, the continued reliance of song on auditory feedback (Nordeen & Nordeen, 1992; Lombardino & Nottebohm, 2000) and progressive increases in vocal motor refinement (Brainard & Doupe, 2001; Kao & Brainard, 2006; Pytte et al, 2007) suggest sensory-motor processes that could be dictated by similar neural mechanisms. Moreover, there is evidence that a similar contrast with respect to contributions of HVC and LMAN to acoustic structure is involved in adult song maintenance. Neural activity within the HVC-RA pathway drives stereotyped adult song (Yu & Margoliash, 1996; Hahnloser et al., 2002) and HVC lesions completely disrupt song acoustic structure (Simpson & Vicario, 1990). In contrast, normal adult variability in song note acoustic structure, as well as the song changes that occur after deafening or experimental alterations to motor function are dependent on LMAN (Williams & Mehta, 1999; Brainard & Doupe, 2000; Brainard & Doupe, 2001; Thompson et al., 2007). These results suggest that the relative weighting and integration of input in RA from HVC and LMAN is important for song acoustic structure throughout life, with HVC promoting stereotypy and LMAN promoting acoustic variability.
The amount of dendritic arbor correlates with amount of afferent and efferent connectivity (Canady et al., 1988; Herrmann & Arnold, 1991; Nixdorf-Bergweiler et al., 1995). Because song refinement continues well into adulthood, we hypothesized that HVC-RA neurons would undergo an age-related increase in dendritic morphological complexity over ages when song stereotypy increases. We also hypothesized that with the age-related decrease in song variability and reliance of song on auditory feedback, LMAN-RA neurons would undergo a decrease in morphological complexity.
Finally, as a step toward assessing the extent to which adult experience influences cell morphology, we housed adults in different social settings. We chose this manipulation in order to maximize our chances of seeing plasticity in this highly social species. Variation in social housing has been shown to influence singing rate (Zann, 1996) and likely affects the perceptual demands of keeping track of colony members based, in part, on vocal attributes (Lipkind et al., 2002). Lesions to HVC and LMAN have been shown to disrupt auditory discrimination, raising the possibility that these regions are important for song perception as well as production (Brenowitz, 1991; Scharff et al., 1998; Burt et al., 2000; Gentner et al., 2000; but see Theunissen et al., 2008). Social factors have also been shown to affect circulating levels of steroids (Vleck & Priedkalns, 1985; Seiler et al., 1992; Christensen & Vleck, 2008) known to influence dendritic arbor or spine densities in mammals (Kurz et al., 1986; Brusco et al., 2008) as well as adult canaries (DeVoogd & Nottebohm, 1981). Finally, variation in social housing has been shown to affect rates of HVC neuron addition in zebra finches (Lipkind et al., 2002) as well as HVC volume in adult canaries (Boseret et al., 2006).
Materials & Methods
Subjects
All experiments were done in accordance with the Wesleyan University Institutional Animal Care and Use Committee and NIH guidelines. Eighty-three male zebra finches born in our lab’s breeder colony were used for analysis. Birds used for examining age-related changes in cell morphology were housed in multiple family groups until approximately ninety days of age at which time they were moved to single sex groups in smaller cages (12–15 birds per cage) with visual and auditory access to both male and female zebra finches, where they remained until they were removed for surgery. A preliminary experiment was done looking for possible age-related morphological changes between the ages of three months and three years (data not shown). Significant changes were only seen in individuals less than one year of age, as a result three age cohorts of 3 months (90–123 days of age; 18 birds, 439 cells), 6 months (166–211 days of age; 18 birds, 436 cells) & 12 months (334–377 days of age; 17 birds, 405 cells) were chosen and used for detailed analyses. Because recent findings from our lab have shown that post-crystallization variability in neuronal incorporation is influenced by nest of origin (Hurley et al., 2008) we also investigated whether variability in neuronal morphology could be related to family of origin. If true, we wanted to control for this variable in our age analyses. Sixteen family groups were analyzed. Family group was defined as all individuals hatched in the same nest box, but not necessarily the same clutch.
As a first step toward assessing the effects of adult experience on dendritic features, we manipulated social housing conditions. For these experiments birds were removed from the general aviary at 90 (±4) days of age and housed in one of four social conditions as follows: 1. GM- group housed males (approximately 12–15 males housed together; 9 birds, 423 cells) 2. GMF- group housed males with females (approximately 12–15 birds housed together, half male, half female; 9 birds, 339 cells) 3. M- one male housed alone per cage (6 birds, 262 cells) 4. MF- one male housed with one female per cage (6 birds, 277 cells). Nest of origin was balanced across the four social settings. Group housed males (GM) and group housed males with females (GMF) were placed in a colony room with other males and females. All singly housed males (M) were housed in a room together where they could hear, but not see one another and no females were in the room. Males housed singly with a female (MF) were in visual but not auditory isolation from neighbors. Nest boxes and nesting material were not provided, however, all females in MF pairs laid eggs in seed cups and eggs were removed within two days of being laid. All birds were provided food and water ad libitum and were supplemented with an egg mixture three times a week. Birds were housed at approximately 22°C on a 14:10 light/dark cycle. For this experiment, birds were maintained in these social housing conditions for approximately 3 months, until roughly 7 months of age (210 ±4 days of age), at which point they underwent tracer injections.
Song Analyses
Twelve of the thirty adult male zebra finches used in the social housing experiment were further used for an analysis of singing rate. Only singly housed males (M) and single males with a female (MF) were recorded for singing rates because accurate counts would be extremely difficult in cages with multiple males, in large part because zebra finch males will often begin singing while other neighboring males are singing. Female zebra finches do not sing, as a result singing rates of MF individuals could be accurately counted. M and MF individuals were recorded for singing rate between ninety (±4 days) and two hundred (±4 days) days of age. All recordings were taken for twenty-four hours once weekly in the subjects home cage. Recordings were started at lights-on the first day and ended at lights-on the following day. Recordings were made with Avisoft- SAS Lab Pro version 4.15 (Raimund Specht, Hauptstr. 52, D-13158 Berlin, Germany) computer software and cardioid condenser microphones (Electro-Voice Inc. Buchanan, MI). Recordings were triggered by sounds that were between 0–12 kHz and a minimum of 2 seconds in duration. Weekly recordings were made under identical conditions. Adult zebra finch song is composed of ~4–10 notes which are produced in a specific sequence. The song is referred to as a motif, and is often repeated several times in a song bout (Williams, 2004). We quantified number of motif repetitions. For each bird, for each of nine twenty-four hour recording sessions, the total number of motifs produced was counted manually, blind to the identity of the individual. Estimates of cumulative singing (number of motifs) during adulthood were calculated by first averaging amount of singing between each pair of consecutive recording sessions and multiplying by the number of days between recording sessions. Values were then adding together to obtain cumulative estimates.
Rhodamine Dextran Amine Surgery
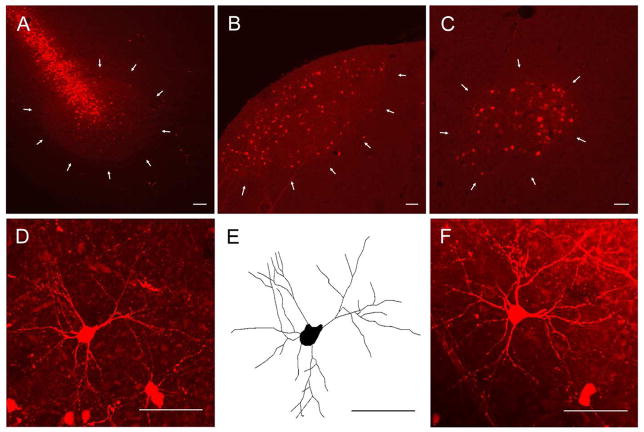
Birds were injected with dextran tetramethylrhodamine (RDA) (3000MW; Invitrogen, Carlsbad, CA.) into RA four days before being sacrificed. A four-day survival allowed for even and extensive backfill of projection neurons. Birds were anesthetized with 0.035 mL intramuscular injection each of ketamine (Ketalar, Parke-Davis, Fort Dodge, IA; working concentration 10 mg/mL) and xylazine (Rompun, Haver, The Butler Co., Columbus, OH; working concentration 20 mg/mL). Subjects were secured in a stereotaxic apparatus and feathers were removed from the top of the head. An incision was made along the anterior-posterior midline and the skin was pulled back to expose the dorsal surface of the skull. Skull and dura above the injection sites (from zero point at the bifurcation of the sinus between telencephalic hemispheres and cerebellum: posterior −1.7 mm, lateral ±2.3 mm, ventral −1.7 mm) were removed to expose the brain. Birds were pressure-injected bilaterally with approximately 0.04 μL of RDA (10% dilution in 0.1M phosphate buffered saline) at a 10° angle from vertical using a glass micropipette. RDA injections into RA backfilled cells throughout HVC and LMAN (Figure 2). Following injection the incision was closed with surgical tape and treated with antiseptic ointment. Birds recovered in a small holding cage under a heat lamp for twenty-four hours. The day after surgery individuals were returned to their home cages and remained there until they were perfused.
Figure 2.
Fluorescence confocal images of seventy-five μm parasagittal sections containing RA, HVC and LMAN. (A) Low power magnification of dextran tetramethylrhodamine (RDA) injection site in RA. White arrows outline the boundary of RA. (B) Low power magnification of RDA backfilled HVC. White arrows outline the ventral boundary of HVC. The hippocampus, normally dorsal to HVC, was removed during sectioning. (C) Low power magnification of RDA backfilled LMAN. White arrows outline the boundary of LMAN. (D) High power magnification merged z-stack of a RDA backfilled cell in HVC. (E) Neurolucida tracing of the RDA backfilled HVC cell pictured in D. (F) High power magnification merged z-stack of a RDA backfilled cell in LMAN. The most prominent cells in D and F are typical for those used in analyses. In all images, dorsal is up and caudal is to the left. All scale bars = 50 μm.
Perfusions and Tissue Processing
Birds were deeply anesthetized with methoxyflurane (Metofane; Mallinckrodt Inc., Mundelgn, IL.). When non-responsive to a toe pinch, the right atrium of the heart was cut and the bird was perfused through the left ventricle with 20 mL of 0.1M phosphate buffer (pH 7.4) followed by 50 mL of 4% formaldehyde (in 0.1M PB; pH 7.4). Brains were stored in 4% formaldehyde at 4°C overnight and then rinsed with deionized water and stored at 4°C in 0.1M phosphate buffered saline until cut. Seventy-five μm sagittal sections were cut on a vibratome. All sections containing HVC and/or LMAN cells were mounted on superfrost slides and cover-slipped with Aqua Mount (Polyscience, Warrington, PA.). Slides were stored at room temperature in slide boxes.
Morphological analyses
All data collection was done blind to identity of individuals. Cells were traced throughout HVC and LMAN and the following criteria were used to select traceable cells: (1) Cells were filled with bright fluorescence. (2) Cells were isolated from neighbors to avoid potential overlap of dendritic arbor from two distinct cells. (3) When scanning through the entire z-plane, arbor from multiple branches did not terminate in the same focal plane, as would happen if arbor were not fully contained within the section. These criteria reduced the number of acceptable cells to between two and five of all labeled cells per section, resulting in twenty-five to forty cells being analyzed per brain region per bird. Before tracing cells the outline of HVC and/or LMAN was traced with a 10X objective with brightfield optics on a computer-yoked microscope system (Olympus BX50; Neurolucida, Microbrightfield Inc., Colchester, VT.). Soma size and dendritic morphology were traced. Tracings were done using a 40X air objective with fluorescence optics. Computer reconstructions of traced cells were rotated before analysis was done to verify that arbor did not terminate in the same plane. The following attributes were measured: maximal cross-sectional area of the soma, total dendritic length, dendritic length by segment order, total number of dendritic segments and number of dendritic segments by segment order. Total dendritic length was calculated by adding the lengths of all dendritic segments, regardless of branch order, that made up the complete network of dendritic trees in each HVC-RA or LMAN-RA cell. We did not attempt to measure dendritic spine densities because individual spines were not easily resolved with our labeling methods. An example of a cell reconstruction is shown in Figure 2. For each bird, mean values for each attribute were calculated from all cells, and these mean values were then averaged (±SEM) for group comparisons. As already mentioned, song acoustic variability results from differential input to RA from HVC and LMAN. We did not measure dendritic arbor for both cell types in all birds, given variability in tracer injection targeting of RA. However, a subset of birds had very good backfills of both cell types, permitting us to address a possible morphological relationship between both cell types within individual birds. We expressed these data as a ratio for each trait in each bird by dividing the average of the HVC-RA cells by the average of the LMAN-RA cells. We were not interested in absolute values obtained with this method, but rather that it providing a within-bird measure of relative change.
Statistics
All statistical comparisons of soma size and total dendritic length as a function of age, family background, and social housing were initially conducted using one- and two-way ANOVAs. Two-tailed t tests were used for all pair-wise comparisons (Microsoft Excel Statistical Analysis Toolpak & JMP, SAS Statistical Software). Further analysis of dendritic segment length by branch order (μm) and dendritic segment number by branch order was done using the same statistical tests, however, a Bonferroni correction was performed to minimize the chance that random statistical significance was obtained as a result of multiple tests on the same data set. A Bonferroni correction on these data adjusted α to equal 0.01. Because age and family background were both found to account for some variability in the data from the experiment on age-related changes in morphology, but not every age was represented in every family and not every family was represented at every age, standard multi-variate analyses could not be performed. As a result three statistical models (Generalized linear model, Nested analysis of variance, Residual variance), which do not require equal representation of the independent variables, were used to determine the extent to which age and family each significantly influenced the distribution (JMP, SAS Statistical Software). All statistical comparisons of singing rate and neuronal measurements were done using two-way ANOVAs and linear regressions (Microsoft Excel Statistical Analysis Toolpak & JMP, SAS Statistical Software). Ratio data where HVC and LMAN attributes were collected from the same individual were analyzed with a mixed model ANOVA (JMP, SAS Statistical Software).
Photomicrograph reproduction
Z-stack confocal image files were opened using Adobe Photoshop CS5. Only brightness and contrast were adjusted to better replicate images seen through the microscope.
Results
Cellular morphology changes as a function of bird age
Preliminary analysis
To determine whether there were systematic morphological changes with bird age a preliminary survey of HVC-RA cells was done on males between the ages of three months and three years. Significant changes in both HVC-RA soma size and HVC-RA total dendritic length were seen between three months and one year of age (soma size- r2= 0.32, p= 0.0002; total dendritic length- r2= 0.27, p= 0.01; data not shown). However, further changes beyond one year were not detected (p ≥ 0.05). Using these preliminary data, three age groups (3 months, 6 months & 12 months) were chosen for subsequent analyses.
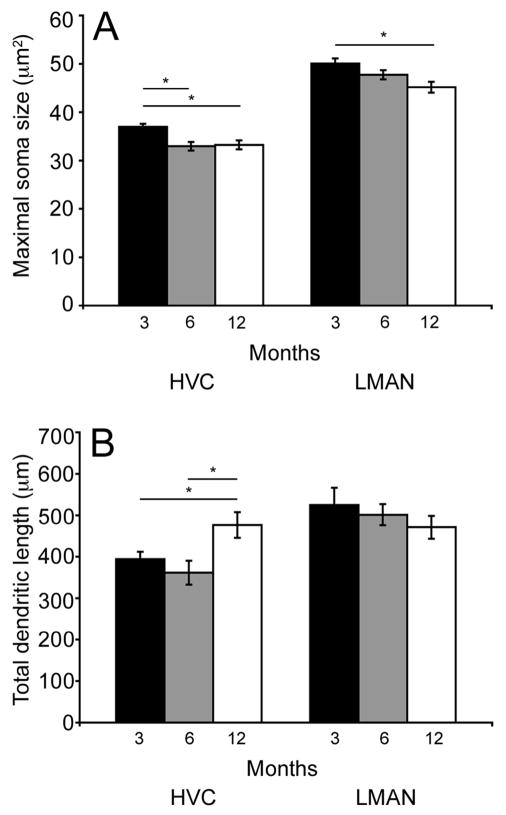
Soma size and total dendritic length
Figure 3A illustrates mean (± SEM) maximal cross-sectional area of the soma for each age cohort for HVC-RA and LMAN-RA cells. There were significant age-related decreases in soma size for HVC-RA cells (overall ANOVA- F(2,25)= 7.91; p= 0.002). HVC-RA cell somas in three-month-old birds were significantly larger when compared to somas of this cell type in the two older age cohorts (p ≤ 0.004), but the two older groups did not differ (p= 0.83). There was a similar decrease in soma size with increasing bird age in LMAN-RA cells (overall ANOVA- F(2, 22)= 5.48; p= 0.01). In pair-wise comparisons, there was no significant difference between mean LMAN-RA soma size in three- and six-month-old bird or six- and twelve-month-old birds (p ≥ 0.09), however there was a significant difference between mean LMAN-RA soma sizes when comparing three- and twelve-month-old birds (p = 0.003).
Figure 3.
Soma size and total dendritic length varied as a function of bird age. (A) Mean (±SEM) cross-sectional soma area of HVC-RA and LMAN-RA cells. In HVC, the average soma size in 3-month-old birds (black bars) was significantly larger compared to 6- (grey bars) or 12-month-old (white bars) individuals (p≤0.004). In LMAN, the average soma size in 3-month-old birds was significantly larger compared to 12-month-old individuals (p= 0.003). (B) Mean (±SEM) total dendritic length of HVC-RA and LMAN-RA cells. In HVC, the average total dendritic length in 12-month-old birds was significantly greater compared to 3- or 6-month-old individuals (p≤0.02). In LMAN, the average total dendritic length did not significantly vary across the three age groups.
Mean total dendritic length for HVC-RA cells but not LMAN-RA cells changed significantly as a function of bird age. Figure 3B illustrates mean (± SEM) total dendritic length for each age cohort for HVC-RA and LMAN-RA cells. An overall ANOVA revealed a significant increase in arbor for HVC-RA cells with increasing age (F(2,25)= 4.8; p= 0.01). In pair-wise comparisons, there was no significant difference in mean HVC-RA total dendritic lengths between three- and six-month-old individuals (p = 0.61), however both age cohorts had significantly less dendritic arbor compared to the twelve-month-old birds (p ≤ 0.02). In contrast, total dendritic length of LMAN-RA cells did not change significantly across the same ages (overall ANOVA- F(2,22)= 0.73; p= 0.49).
Dendritic analysis by segment order
To better resolve the attributes responsible for the increase in total dendritic length in HVC-RA cells, analysis of length and number of segments by branch order was done (Table 1). The data show that in the HVC-RA population segment length by branch order did not vary significantly with age, however segment number increased significantly between six and twelve months. Additionally these changes did not occur at all dendritic segment orders, instead significant changes were only observed at third (F(2,25)= 7.68; p= 0.002), fourth (F(2,25)= 13.63; p< 0.0001), and fifth (F(2,25)= 9.14; p= 0.001) order branches (all pair-wise comparisons between the twelve-month cohort and the two younger cohorts, p ≤ 0.01).
Table 1.
High order branch numbers differed across age cohorts. Mean (±SEM) number and length of dendrites by segment order for HVC-RA and LMAN-RA cells for the three age cohorts. Dendritic lengths are reported in microns. In HVC, the number of third, fourth and fifth order branches was significantly larger (bold font) in 12-month-old birds than in 3- and 6-month-old birds (p≤0.002). There were no significant age-related differences in these attributes for LMAN-RA neurons.
| HVC | 3 Months | 6 Months | 12 Months | |
|---|---|---|---|---|
| 1st Order | Number | 4.2(±0.08) | 4.1(±0.1) | 4.4(±0.18) |
| Length (μm) | 16.2(±0.68) | 16.0(±0.61) | 16.2(±0.8) | |
| 2nd Order | Number | 7.4(±0.17) | 6.9(±0.25) | 7.8(±0.27) |
| Length (μm) | 23.9(±0.59) | 23.8(±1.11) | 23.3(±0.90) | |
| 3rd Order | Number | 4.5(±0.3) | 4.0(±0.44) | 6.3(±0.53) |
| Length (μm) | 24.8(±0.63) | 23.7(±1.34) | 24.2(±1.11) | |
| 4th Order | Number | 0.9(±0.11) | 1.0(±0.2) | 2.4(±0.29) |
| Length (μm) | 25.5(±0.96) | 22.5(±1.56) | 24.0(±0.96) | |
| 5th Order | Number | 0.07(±0.02) | 0.2(±0.04) | 0.4(±0.09) |
| Length (μm) | 26.4(±4.2) | 22.4(±1.3) | 24.5(±2.06) | |
|
| ||||
| LMAN | 3 Months | 6 Months | 12 Months | |
|
| ||||
| 1st Order | Number | 5.1(±0.13) | 4.8(±0.10) | 4.9(±0.11) |
| Length (μm) | 18.2(±0.3) | 17.4(±0.88) | 18.2(±0.63) | |
| 2nd Order | Number | 8.7(±0.24) | 8.8(±0.34) | 8.6(±0.21) |
| Length (μm) | 29.2(±1.02) | 31.0(±1.06) | 27.6(±1.29) | |
| 3rd Order | Number | 4.7(±0.6) | 4.1(±0.27) | 4.3(±0.54) |
| Length (μm) | 30.2(±1.37) | 30.7(±0.93) | 27.5(±1.47) | |
| 4th Order | Number | 0.7(±0.16) | 0.6(±0.13) | 0.7(±0.18) |
| Length (μm) | 29.5(±1.87) | 29.6(±1.82) | 27.2(±1.30) | |
| 5th Order | Number | 0.06(±0.03) | 0.1(±0.07) | 0.04(±0.02) |
| Length (μm) | 26.9(±2.12) | 32.2(±4.88) | 30.0(±0.82) | |
Although no changes in total dendritic length were seen in the LMAN-RA neurons, an analysis of dendritic length and number by segment order was performed because it is possible that the absence of overall length changes could mask changes at a small subset of segments. However, consistent with the data for total dendritic length for LMAN-RA cells, there were no significant age-related changes in the number or length of specific dendritic segments across the ages studied (p ≥ 0.42).
Analysis of the ratio of HVC-RA:LMAN-RA morphology
Because LMAN and HVC projection neurons synapse on the same dendrites in RA (Herrmann & Arnold, 1991) and appear to promote contrasting patterns of motor activity (Yu & Margoliash, 1996; Hahnloser et al., 2002; Kao & Brainard, 2006; Kao et al., 2008), we wished to explore with-in bird differences in arbor for these two cell types to assess changes in one cell type relative to the other, and so in a subset of birds with strong labeling of both cell types (n=15), we expressed morphological attribute values as ratios of HVC:LMAN.
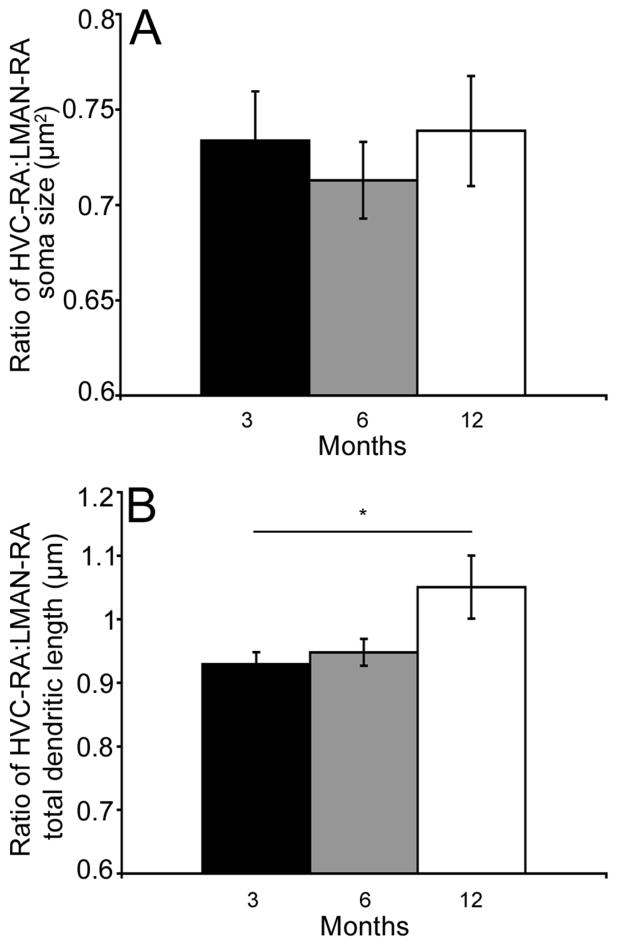
Soma size and total dendritic length ratios
The ratio of HVC-RA soma size to LMAN-RA soma size did not change as a function of bird age (overall ANOVA- F(2,12)= 0.16, p= 0.84; Figure 4A). Analysis of the ratio of HVC-RA total dendritic length by LMAN-RA total dendritic length revealed that, unlike soma size, there was a significant change as a function of bird age (overall ANOVA- F(1,13)= 5.63, p= 0.03; Figure 4B). More specifically, the ratio of HVC-RA:LMAN-RA increased with age (3 month vs. 12 month cohort p= 0.05; all other individual pair-wise comparisons p ≥ 0.14).
Figure 4.
The ratio of total dendritic length varied as a function of bird age. (A) Mean (±SEM) ratio of HVC-RA:LMAN-RA soma size. Average ratio of HVC-RA:LMAN-RA soma size did not vary significantly between the 3- (black bars), 6- (grey bars) and 12-month-old (white bars) individuals. (B) Mean (±SEM) ratio of HVC-RA:LMAN-RA total dendritic length. Average ratio of HVC-RA:LMAN-RA total dendritic length was significantly larger in 12-month-old birds compared to 3-month-old individuals (p= 0.05).
Dendritic ratio analysis by segment order
A more detailed analysis of the ratio of HVC-RA:LMAN-RA dendritic morphology found no significant change with age at any specific segment order for either number or length of the branches (p ≥ 0.10; data not shown). These results suggest the change in the ratio of the total dendritic length (Figure 4B) cannot be attributed to any specific branch order.
Analysis of cellular morphology and nest of origin
Previous work in our lab has demonstrated a relationship between nest of origin and adult HVC neuron addition rates (Hurley et al., 2008). While it was not a primary goal of the present work to determine whether such a relationship extends to dendritic arbor, we wanted to control for potential sources of variation that might otherwise contaminate our results. Therefore, cell morphology in HVC-RA and LMAN-RA populations was analyzed against family background to determine whether there were any significant correlations. Collapsing across ages, LMAN-RA morphology (soma size and all dendritic measures) was not affected by family background (p ≥ 0.14; data not shown). HVC-RA soma size was also not affected by family background (F(15,47)= 1.46; p= 0.15). However, there was a significant relationship between HVC-RA total dendritic length and nest of origin (F(15,47)= 2.48; p= 0.009; data not shown). As a result, morphology of the HVC-RA cells, which was influenced by both age and nest of origin when analyses were done separately, was further analyzed to clarify the relative influence of each factor on the distribution of the data. Three distinct statistical models were used (Generalized linear model, Nested analysis of variance & residual variance). All three indicated that both age and family significantly influenced dendritic morphology in HVC-RA cells (p ≤ 0.05). All three models also indicated that when combined, age and family explained more of the variability then either factor alone.
Cellular morphology changes as a function of social housing
We do not know whether the observed age-related changes are the result of adult experience or part of an experience-independent, protracted developmental process. To determine whether adult cell morphology changes as a result of experience, we housed males in one of four social settings from ninety days to seven months of age.
Soma size and total dendritic length
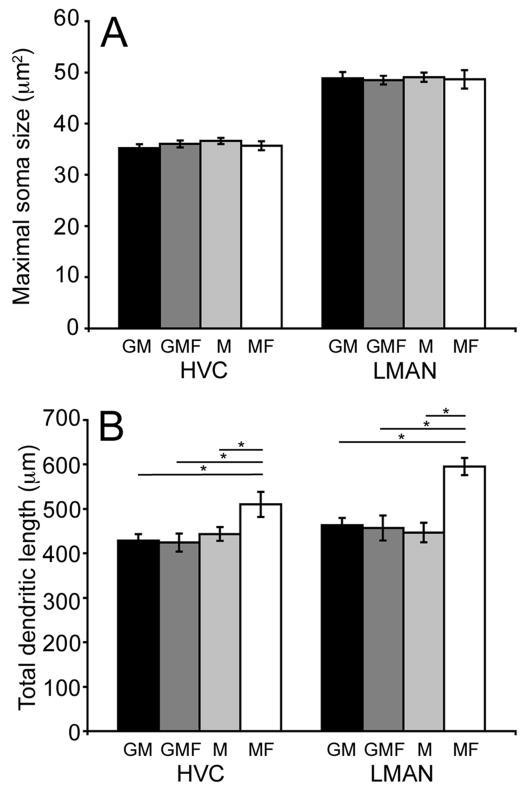
No significant differences were seen in either neuron population when average maximal soma size was compared across housing conditions (overall ANOVA- HVC-RA: F(3,26)= 0.62, p= 0.60; LMAN-RA: F(3,22)= 0.04, p= 0.98; Figure 5A). In contrast, dendritic morphology varied as a function of social environment. Figure 5B shows that variability in social housing conditions resulted in significant differences in total dendritic length for both HVC-RA and LMAN-RA cell populations (overall ANOVA- HVC-RA: F(3,26)= 3.54, p= 0.02; LMAN-RA: F(3,22)= 8.08, p= 0.0008). The significant difference in total dendritic length for both cell types was the result of a significant increase in males housed singly with a female (MF) compared to all other groups (p ≤ 0.04 for HVC-RA; p ≤ 0.0005 for LMAN-RA). In comparisons of the other three social settings (group housed males, group housed males with females and singly housed males) there were no significant differences in total dendritic length for either neuron type (overall ANOVA- HVC-RA: F(2,21)= 0.27, p= 0.76; LMAN-RA: F(2,18)= 0.13, p= 0.87).
Figure 5.
Total dendritic length varied as a function of social housing condition. (A) Mean (±SEM) cross-sectional soma area of cells in HVC-RA and LMAN-RA. In HVC, average soma size did not vary significantly across the four social groups: group housed males (GM; black bars), group housed males with females (GMF; dark grey bars), individually housed males (M; light grey bars), males housed in male-female pairs (MF; white bars). In LMAN, average soma size did not vary significantly across the four social housing conditions. (B) Mean (±SEM) total dendritic length of cells in HVC-RA and LMAN-RA. In HVC, average total dendritic length was significantly larger in MF individuals compared to the three other social housing conditions (GM, GMF & M) (p≤0.04). In LMAN, average total dendritic length was significantly larger in MF individuals compared to the three other social housing conditions (GM, GMF & M) p≤0.0005).
Dendritic analysis by segment order
In both the HVC-RA and LMAN-RA populations, segment length did not vary significantly across social settings at any order (p ≥ 0.60; data not shown). A comparison of HVC-RA segment number across social housing conditions found that MF individuals on average had more high-order dendritic segments compared to individuals in the other three social settings (p ≤ 0.02). There was a significant main effect of social housing on fourth and fifth order branches (overall ANOVA- 4th order: F(3,26)= 6.71, p= 0.001; 5th order: F(3,26)= 5.75, p= 0.003). Pair-wise comparisons among the remaining housing conditions failed to reveal any significant differences (p ≥ 0.76). Similar to the HVC-RA neurons, segment number in the LMAN-RA population was significantly influenced by social housing. There were significantly more third, fourth and fifth order branches in MF individuals compared to individuals in the three other social housing conditions (overall ANOVA- 3rd order: F(3,22)= 11.41, p= 0.0001; 4th order: F(3,22)= 5.10, p= 0.007; 5th order: F(3,22)= 10.74, p= 0.0002; pair-wise comparisons between MF males and males from other housing conditions, p ≤ 0.004). There were no significant pair-wise comparisons for the other housing conditions (p≥0.08).
Relationship between social housing and the morphological ratio of HVC-RA:LMAN-RA
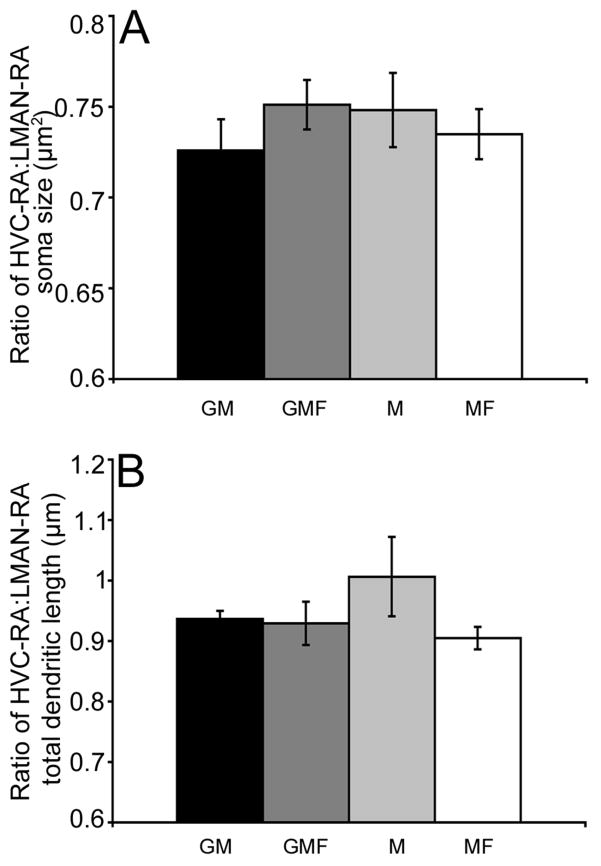
Analysis of the ratio of HVC-RA:LMAN-RA soma size and dendritic arbor failed to reveal significant differences across the four social housing groups. Figure 6A illustrates that all four social groups had an average ratio of approximately 0.75 for soma size (overall ANOVA- F(3,22)= 0.53, p= 0.66; pair-wise comparisons p ≥ 0.2). Figure 6B illustrates that all four social groups had an average ratio of approximately 0.93 for total dendritic length (overall ANOVA- F(3,22)= 1.21, p= 0.32; pair-wise comparisons p ≥ 0.09). Although there were significant differences in total dendritic length for both song system cell populations across the social housing conditions, the overall age-specific ratio was maintained. There were no significant differences between the total dendritic ratio of six-month-old birds from the age experiment (Figure 4B) and the total dendritic ratio of seven-month-old birds in any of the social groups (pair-wise comparisons p ≥ 0.37; Figure 6B). Yet when these groups were pooled, the arbor ratio was significantly lower than in the twelve-month-old group from the age experiment (F(1,38)= 7.65, p= 0.008).
Figure 6.
Ratio of soma size and total dendritic length did not vary as a function of social housing condition. Neither the mean (±SEM) ratio of HVC-RA:LMAN-RA soma size (A) or the ratio of total dendritic length (B) varied significantly across the four social groups: GM (black bars), GMF (dark grey bars), M (light grey bars), MF (white bars).
Social housing affects singing rate
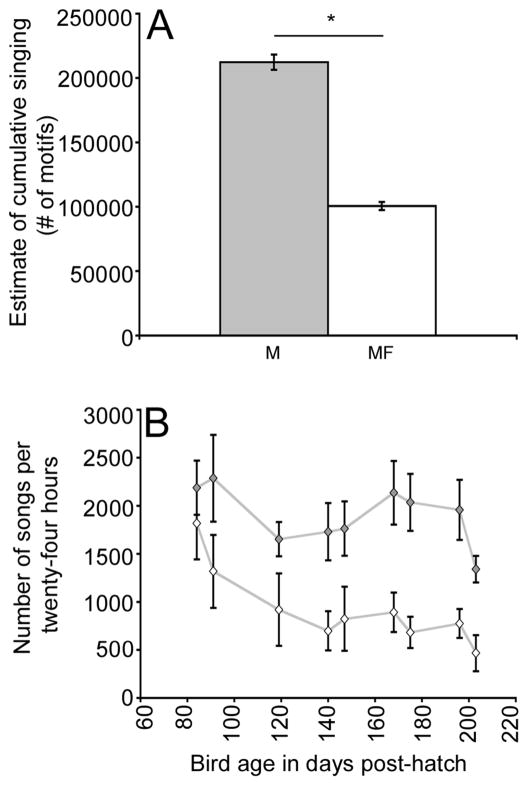
Amount of singing was recorded for singly housed males (M) and Males housed singly with a female (MF). Consistent with data from other labs (Dunn & Zann, 1997) the addition of a female to the cage of individual males caused adult singing rate to become significantly decreased (Figure 7A; ANOVA- F(1,9)= 14.1, p= 0.004). MF individuals as a group sang an estimated average of 100,549 (±3170) songs between three and seven months post-hatch while M individuals as a group were estimated to have sung more than twice as much, averaging 212,303 (±5980) songs. During the first two weeks of the experiment there was no significant difference in the average number of motifs produced per twenty-four hours between M and MF individuals (F(1,12)= 2.55, p= 0.13). However, during subsequent weeks singly housed males (M) sang significantly more compared to males singly housed with a female (MF) (p ≤ 0.04; Figure 7B).
Figure 7.
Spontaneous singing rate varied across social housing condition. (A) Mean (±SEM) estimated cumulative singing rate (number of motifs). M individuals (grey bar) sang significantly more compared to MF individuals (white bar) (p= 0.004). (B) Mean (±SEM) singing rate per twenty-four hours across the 9 ages sampled. Mean singing rates of M individuals (grey diamonds) compared to MF individuals (white diamonds) during the first two recording sessions (84 & 91 days post-hatch) were not significantly different. Singing rates for MF birds from subsequent recording sessions were significantly lower than for M males (p≤0.04).
Cellular morphology changes as a function of adult singing rate
At the group level, decreases in singing rate between singly housed males and males paired with a female were associated with differences in dendritic growth. To further investigate a potential relationship between singing and neural plasticity, we compared each bird’s singing rate to measures of each cell type independently, as well as to measures of relative change in neuronal attributes, expressing the latter as ratios of HVC:LMAN.
Soma size and total dendritic length
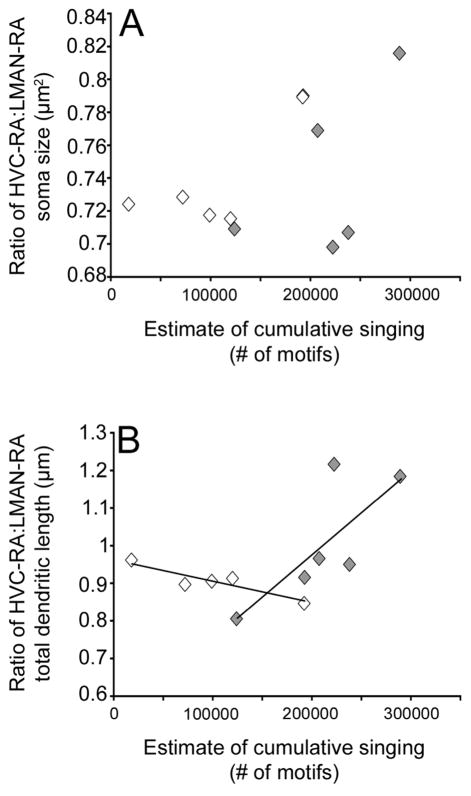
Pooling animals from the two social housing conditions, no significant relationships were found in soma size between HVC-RA, LMAN-RA or the ratio of HVC-RA:LMAN-RA and adult singing rate (p ≥ 0.1, data not shown). Comparisons between singing rate and either HVC-RA or LMAN-RA total dendritic length also failed to show any significant relationship (p ≥ 0.2, data not shown).
A two-way ANOVA of the dendritic ratio, with social condition and adult singing rate as independent factors, uncovered a significant interaction (p= 0.03). There was no significant correlation between the singing rate of M individuals and the ratio of total dendritic length (r2= 0.55, p= 0.09; Figure 8B). Interestingly, MF individuals, showed a modest but significant negative correlation between the ratio of total dendritic length and estimated cumulative adult singing rate (r2= 0.78, p= 0.04; Figure 8B). Thus, while there were no overall group differences in the arbor ratio, social housing significantly altered the relationship between the arbor ratio and amount of singing.
Figure 8.
The relationship between singing rate and the ratio of HVC-RA:LMAN-RA total dendritic length depended on social housing condition. (A) Mean (±SEM) ratio of HVC-RA:LMAN-RA soma size plotted against estimated cumulative singing rate (number of motifs) between 84 and 203 days post-hatch. The ratio of HVC-RA:LMAN-RA soma size did not significantly correlate with singing rate (MF individuals- white diamonds; M individuals- grey diamonds). (B) Mean (±SEM) ratio of HVC-RA:LMAN-RA total dendritic length plotted against estimated cumulative singing rate (number of motifs) between 84 and 203 days post-hatch. The ratio of HVC-RA:LMAN-RA total dendritic length showed a significant negative correlation with amount of singing in MF individuals (p= 0.04). The correlation between the ratio of total dendritic length and singing rate in M individuals was non-significant (p= 0.09).
Dendritic analysis by segment order
We also analyzed possible relationships between dendritic segment number and length by order for HVC-RA and LMAN-RA cells as a function of estimated cumulative adult singing rate in M and MF birds. None of the comparisons showed a significant correlation (p ≥ 0.08, data not shown).
Discussion
We explored the effects of age and experience on morphological attributes of two neuron types that have a common target and have opposing influences on song acoustic structure during juvenile song development and in adulthood. HVC-RA neurons are part of a motor pathway that promotes song stability and stereotypy, whereas LMAN is part of a basal ganglia pathway that promotes acoustic variability (Thompson & Johnson, 2006; Thompson et al., 2007; Thompson et al., 2011). We found that neuronal morphology changes significantly with age, experience, or both in adulthood. However, the magnitude of change was not always the same for the two song system neuron types examined.
Age-related changes
We explored changes in dendritic morphology between 3 months after hatching and 3 years of age. Measurable changes were found up to 12 months but not at older ages. Between 3 and 12 months, there was a decrease in soma size for both cell types and an increase in total dendritic arbor was found for HVC-RA neurons. There were no significant changes in LMAN-RA dendritic arbor over the same age range. Within-bird comparisons of both neuron types revealed a similar result; with increasing bird age there was a significant increase in the ratio of HVC-RA:LMAN-RA arbor. Thus, despite the fact that these two cell types make synapses on dendrites of the same neurons in RA, they are differentially affected by increasing age.
Changes in HVC-RA dendritic morphology were not seen at all branch orders, only higher order branch numbers were affected. Previous research by other labs using different methods of labeling dendritic arbor found that when changes were observed either during development in zebra finches or as a result of adult hormone treatment in canaries, they were greatest at higher order branches (DeVoogd & Nottebohm, 1981; Nixdorf-Bergweiler et al., 1995). Our work compliments and extends these findings by showing similar changes in two neuron types with known projection patterns.
Previous work has shown that the amount of dendritic arbor co-varies with amount of afferent and efferent connectivity. For example, steroid-induced growth of RA dendrites and the number of dendritic spines in adult canaries is associated with an increase in the number of synaptic inputs from both HVC and LMAN (Canady et al., 1988). Moreover, decreases in amount of LMAN arbor during development are associated with decreases in the number of LMAN-RA synapses (Herrmann & Arnold, 1991; Nixdorf-Bergweiler et al., 1995). Dendritic arbor may, therefore, provide an indirect measure of synaptic strength.
Increases in the ratio of HVC-RA:LMAN-RA dendritic arbor occurred over the same age-range when song stereotypy increases in adult finches (Brainard & Doupe, 2001; Kao & Brainard, 2006; Pytte et al., 2007). An increase in the ratio of HVC-RA:LMAN-RA dendritic field size, if accompanied by more synaptic input to HVC arbor, could lead to an increased influence of HVC, relative to LMAN, on RA activity. HVC neurons are highly interconnected, and HVC-RA neurons send axon collaterals to other neurons of the same type (Mooney & Prather, 2005). Small random temporal error in the firing of HVC-RA neurons is produced during each song rendition (Hahnloser et al., 2002) and increased connectivity between HVC-RA cells could decrease variability in pre-motor commands, leading to a more stereotyped song.
Alternatively, or in addition, the age-related and selective increases in the dendritic arbor of HVC-RA cells could be related to age-related increases in the stability of song after deafening. The effects of adult deafening are most pronounced when deafening occurs between the ages of 4–7 months, and thereafter, song stability is maintained for much longer periods of time (Lombardino & Nottebohm, 2000). This non-linear age effect is similar to what we observed with respect to HVC-RA dendritic growth, which appeared to be limited to 3–12 months of age. Deafening-induced changes in song are dependent on LMAN (Brainard & Doupe, 2000; Brainard & Doupe, 2001). While we did not see a significant decrease in LMAN-RA dendritic arbor as originally predicted, the ratio of HVC:LMAN arbor increased. If arbor correlates with input strength, it is possible that the increase in HVC-RA arbor is associated with a delay or attenuation of the effects of deafening. It has also been recently shown that the total number of HVC-RA neurons in zebra finches continues to increase over the age range we studied, potentially providing further strengthening of this input to a fixed number of RA neurons in a way that might impact song stability and stereotypy (Walton et al., 2012).
It would be interesting to look at dendritic spine number and turnover rates over the age range we explored. Recent work has shown that spine stability and increases in spine density are associated with increases in synaptic strength in the HVC of juvenile zebra finches (Roberts et al., 2010). Moreover, there is an increase in dendritic spine stability across different cortical regions between young and older adult mice (reviewed by Alvarez & Sabatini, 2007).
We do not know whether the age-related changes we observed are regulated by experience or are part of a protracted developmental program that is experience-independent. If the latter, the morphological changes we observed may represent part of the mechanism that limits adult vocal plasticity in this species. Indeed, we unexpectedly found that the extent of adult HVC-RA dendritic growth with age could be partially accounted for by nest of origin. Because our birds came from a large breeding colony with multiple nest boxes and, therefore, opportunities for extra-pair copulations, we cannot determine the extent to which shared early environment and shared genes contributed to our findings. It is worth noting that there are now several studies suggesting that other neuronal attributes, including HVC volume, neuron number and rates of neuron addition, are either heritable or shaped by early developmental events (Airey et al., 2000; Ward et al., 2001; Williams et al., 2003; Hurley et al., 2008). It is particularly intriguing that we found co-variation among nest mates for HVC-RA arbor but not LMAN-RA arbor. Previous work has shown that heritability estimates for HVC volume are much higher than for LMAN (Airey et al., 2000). Regardless, this finding suggests that constraints on the extent of adult dendritic growth are put in place early in development.
Effects of variation in adult social housing
In an attempt to see whether these two neuron types have the capacity for morphological changes as a result of adult experience, we manipulated adult social housing. We found that in contrast to the selective growth of HVC-RA dendrites with age, both neuron types showed dramatic growth in males housed in male-female pairs from 4–7 months of age, compared to males housed singly or in larger same- or mixed sex groups. We chose this manipulation in order to maximize our chances of seeing plasticity in this highly social species. As described earlier, variation in social complexity can lead to changes in song motor activity and demands on perceptual memory, as well as endocrine factors known to affect dendritic arbor. Thus, there are many potential ways in which social housing could have affected dendritic growth.
We chose to measure singing rate in males housed alone and males housed singly with a female and found a dramatic decrease in singing in males from the latter condition in this comparison, consistent with previous reports (Dunn & Zann, 1997). Yet, individual correlations between amount of singing and dendritic growth for either cell type and for the ratio of HVC-RA:LMAN-RA arbor were modest or non-significant. Thus, the major association between singing and arbor was found at the group level, where lower singing rates were associated with growth of arbor in both cell types. Perhaps the act of singing in adulthood is associated with the pruning of dendritic arbor. It is well established that regressive events are a hallmark of some stages of neural development (Cowan et al., 1984; Finlay et al., 1987). This idea is not necessarily incompatible with our finding of age-related increases in dendritic arbor. Despite the fact that growth of arbor for both neuron types was affected by social housing, the age-specific ratio of HVC-RA:LMAN-RA arbor was maintained. We also found a trend for amount of singing to decrease with age even in males housed alone, consistent with a previous report showing that singing rate decreases between 3 and 12 months (Johnson et al., 2002).
Regardless, the important finding is that under at least some conditions, neuronal morphology in adult zebra finches can respond to a changing environment with dendritic growth. Moreover, in contrast to age-related changes in dendrites, which occurred selectively for HVC neurons, the experience-dependent growth was found for both cell types. When taken together, our results suggest at least two levels of regulation, one that promotes differential growth favoring neurons in the motor pathway over the anterior forebrain pathway with increasing age, and another that promotes growth in neurons of both pathways. Our results indicate that dynamic changes at the level of sensory-motor control of song co-vary with changes in dendritic morphology in the adult zebra finch. This work sets the stage for testing causal links between cell morphology and song behavior.
Acknowledgments
Supported by NIH: DC004724
Literature Cited
- Airey DC, Castillo-Juarez H, Casella G, Pollak EJ, DeVoogd TJ. Variation in the volume of zebra finch song control nuclei is heritable: developmental and evolutionary implications. Proc Biol Sci. 2000;267:2099–2104. doi: 10.1098/rspb.2000.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- Aronov D, Andalman AS, Fee MS. A specialized forebrain circuit for vocal babbling in the juvenile songbird. Science. 2008;320:630–634. doi: 10.1126/science.1155140. [DOI] [PubMed] [Google Scholar]
- Boseret G, Carere C, Ball GF, Balthazart J. Social context affects testosterone-induced singing and the volume of song control nuclei in male canaries (Serinus canaria) J Neurobiol. 2006;66:1044–1060. doi: 10.1002/neu.20268. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Halsema KA, BROWN SA, Miesner EA. Axonal connections of a forebrain nucleus involved with vocal learning in zebra finches. J Comp Neurol. 1989;279:312–326. doi: 10.1002/cne.902790211. [DOI] [PubMed] [Google Scholar]
- Bottjer SW. Developmental regulation of basal ganglia circuitry during the sensitive period for vocal learning in songbirds. Ann NY Acad Sci. 2004;1016:395–415. doi: 10.1196/annals.1298.037. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Interruption of a basal ganglia-forebrain circuit prevents plasticity of learned vocalizations. Nature. 2000;404:762–766. doi: 10.1038/35008083. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Postlearning consolidation of birdsong: stabilizing effects of age and anterior forebrain lesions. J Neurosci. 2001;21:2501–2517. doi: 10.1523/JNEUROSCI.21-07-02501.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz EA. Altered perception of species-specific song by female birds after lesions of a forebrain nucleus. Science. 1991;251:303–305. doi: 10.1126/science.1987645. [DOI] [PubMed] [Google Scholar]
- Brusco J, Wittmann R, de Azevedo MS, Lucion AB, Franci CR, Giovenardi M, Rasia-Filho AA. Plasma hormonal profiles and dendritic spine density and morphology in the hippocampal CA1 stratum radiatum, evidence by light microscopy, of virgin and postpartum female rats. Neurosci Lett. 2008;438:346–350. doi: 10.1016/j.neulet.2008.04.063. [DOI] [PubMed] [Google Scholar]
- Burt JM, Lent KL, Beecher MD, Brenowitz EA. Lesions of the anterior forebrain song control pathway in female canaries affect song perception in an operant task. J Neurosci. 2000;42:1–13. doi: 10.1002/(sici)1097-4695(200001)42:1<1::aid-neu1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Canady RA, Burd GD, DeVoogd TJ, Nottebohm F. Effect of testosterone on input received by an identified neuron type of the canary song system: a Golgi/electron microscopy/degeneration study. J Neurosci. 1988;8:3770–3784. doi: 10.1523/JNEUROSCI.08-10-03770.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen D, Vleck CM. Prolactin release and response to vasoactive intestinal peptide in an opportunistic breeder, the zebra finch (Taeniopygia guttata) Gen Comp Endocriol. 2008;157:91–98. doi: 10.1016/j.ygcen.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Cowan WM, Fawcett JW, O’Leary DDM, Stanfield BB. Regressive events in neurogenesis. Science. 1984;225:1258–1265. doi: 10.1126/science.6474175. [DOI] [PubMed] [Google Scholar]
- DeVoogd T, Nottebohm F. Gonadal hormones induce dendritic growth in the adult avian brain. Science. 1981;214:202–204. doi: 10.1126/science.7280692. [DOI] [PubMed] [Google Scholar]
- Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Ann Rev Neurosci. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. In: Zeigler HP, Marler P, editors. Neuroscience of Birdsong. New York: Cambridge University Press; 2008. pp. 5–31. [Google Scholar]
- Dunn AM, Zann RA. Effects of pair bond and presence of conspecifics on singing in captive zebra finches. Behaviour. 1997;134:127–114. [Google Scholar]
- Finlay BL, Wikler KC, Sengelaub DR. Regressive events in brain development and scenarios for vertebrate brain evolution. Brain Behav Evol. 1987;30:102–117. doi: 10.1159/000118640. [DOI] [PubMed] [Google Scholar]
- Gentner TQ, Hulse SH, Bentley GE, Ball GF. Individual vocal recognition and the effect of partial lesions to hvc on discrimination, learning, and categorization of conspecific song in adult songbirds. J Neurobiol. 2000;42:117–133. doi: 10.1002/(sici)1097-4695(200001)42:1<117::aid-neu11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Hahnloser RH, Kozhevnikov AA, Fee MS. An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature. 2002;419:65–70. doi: 10.1038/nature00974. [DOI] [PubMed] [Google Scholar]
- Herrmann K, Arnold AP. The development of afferent projections to the robust archistriatal nucleus in male zebra finches: a quantitative electron microscopic study. J Neurosci. 1991;11:2063–2074. doi: 10.1523/JNEUROSCI.11-07-02063.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley P, Pytte C, Kirn JR. Nest of origin predicts adult neuron addition rates in the vocal control system of the zebra finch. Brain Behav Evol. 2008;71:263–270. doi: 10.1159/000127046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson F, Soderstrom K, Whitney O. Quantifying song bout production during zebra finch sensory-motor learning suggests a sensitive period for vocal practice. Behav Brain Res. 2002;131:57–65. doi: 10.1016/s0166-4328(01)00374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao MH, Brainard MS. Lesions of an avian basal ganglia circuit prevent context-dependent changes to song variability. J Neurophysiol. 2006;96:1441–1455. doi: 10.1152/jn.01138.2005. [DOI] [PubMed] [Google Scholar]
- Kao MH, Wright BD, Doupe AJ. Neurons in a forebrain nucleus required for vocal plasticity rapidly switch between precise firing and variable bursting depending on social context. J Neurosci. 2008;28:13232–13247. doi: 10.1523/JNEUROSCI.2250-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK. Human speech and birdsong: communication and the social brain. Proc Natl Acad Sci. 2003;100:9645–9646. doi: 10.1073/pnas.1733998100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz EM, Sengelaub DR, Arnold AP. Androgens regulate the dendritic length of mammalian motorneurons in adulthood. Science. 1986;232:395–398. doi: 10.1126/science.3961488. [DOI] [PubMed] [Google Scholar]
- Lipkind D, Nottebohm F, Rado R, Barnea A. Social change affects the survival of new neurons in the forebrain of adult songbirds. Behav Brain Res. 2002;133:31–43. doi: 10.1016/s0166-4328(01)00416-8. [DOI] [PubMed] [Google Scholar]
- Lombardino AJ, Nottebohm F. Age at deafening affects the stability of learned song in adult male zebra finches. J Neurosci. 2000;20:5054–5064. doi: 10.1523/JNEUROSCI.20-13-05054.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R, Prather JF. The HVC microcircuit: the synaptic basis for interactions between song motor and vocal plasticity pathways. J Neurosci. 2005;25:1952–1964. doi: 10.1523/JNEUROSCI.3726-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixdorf-Bergweiler BE, Wallhausser-Franke E, DeVoogd TJ. Regressive development in neuronal structure during song learning in birds. J Neurobiol. 1995;27:204–215. doi: 10.1002/neu.480270207. [DOI] [PubMed] [Google Scholar]
- Nordeen KW, Nordeen EJ. Auditory feedback is necessary for the maintenance of stereotypyed song in adult zebra finches. Behav Neural Biol. 1992;57:58–66. doi: 10.1016/0163-1047(92)90757-u. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary Serinus canaries. J Compar Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Pytte CL, Gerson M, Miller J, Kirn JR. Increasing stereotypy in adult zebra finch song correlates with a declining rate of adult neurogenesis. Dev Neurobiol. 2007;67:1699–1720. doi: 10.1002/dneu.20520. [DOI] [PubMed] [Google Scholar]
- Pytte CL, Wilbrecht L, Kirn JR. Regulation and function of neuronal replacement in the avian song system. In: Zeigler HP, Marler P, editors. Neuroscience of Birdsong. New York: Cambridge University Press; 2008. pp. 350–366. [Google Scholar]
- Roberts TF, Tschida KA, Klein ME, Mooney R. Rapid spine stabilization and synaptic enhancement at the onset of behavioral learning. Nature. 2010;463:948–952. doi: 10.1038/nature08759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff C, Nottebohm F, Cynx J. Conspecific and heterospecific song discrimination in male zebra finches with lesions in the anterior forebrain pathway. J Neurobiol. 1998;36:81–90. [PubMed] [Google Scholar]
- Seiler HW, Gahr M, Goldsmith AR, Guttinger HR. Prolactin and gonadal steroids during the reproductive cycle of the Bengalese finch (Lonchura striata var. domestica, Estrildidae), a nonseasonal breeder with biparental care. Gen Comp Endocrinol. 1992;88:83–90. doi: 10.1016/0016-6480(92)90196-q. [DOI] [PubMed] [Google Scholar]
- Simpson HB, Vicario DS. Brain pathways for learned and unlearned vocalizations differ in zebra finches. J Neurosci. 1990;10:1541–1556. doi: 10.1523/JNEUROSCI.10-05-01541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theunissen FE, Amin N, Shaevitz SS, Woolley SMN, Fremouw T, Hauber AE. Song selectivity and the songbird brain. In: Zeigler HP, Marler P, editors. Neuroscience of birdsong. Cambridge: Cambridge University Press; 2008. pp. 157–173. [Google Scholar]
- Thompson JA, Johnson F. HVC microlesions do not destabilize the vocal patterns of adult male zebra finches with prior ablation of LMAN. Dev Neurobiol. 2006;67:205–218. doi: 10.1002/dneu.20287. [DOI] [PubMed] [Google Scholar]
- Thompson JA, Wu W, Bertram R, Johnson F. Auditory-dependent vocal recovery in adult male zebra finches is facilitated by lesion of a forebrain pathway that includes the basal ganglia. 2007;27:12308–12320. doi: 10.1523/JNEUROSCI.2853-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JA, Basista MJ, Wu W, Bertram R, Johnson F. Dual pre-motor contribution to songbird syllable variation. 2011;31:322–330. doi: 10.1523/JNEUROSCI.5967-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleck CM, Priedkalns J. Reproduction in zebra finches: Hormone levels and effects of dehydration. The Condor. 1985;87:37–46. [Google Scholar]
- Walton C, Pariser E, Nottebohm F. The zebra finch paradox: song is little changed, but number of neurons doubles. J Neurosci. 2012;32:761–774. doi: 10.1523/JNEUROSCI.3434-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Aviram R, Kirn JR. Deafening alters neuron turnover within the telencephalic motor pathway for song control in adult zebra finches. J Neurosci. 1999;19:10554–10561. doi: 10.1523/JNEUROSCI.19-23-10554.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Hurley P, Pytte C, Kirn JR. Vocal control neuron incorporation decreases with age in the adult zebra finch. J Neurosci. 2002;22:10864–10870. doi: 10.1523/JNEUROSCI.22-24-10864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BC, Nordeen EJ, Nordeen KW. Anatomical and ontongenetic factors producing variation in HVc neuon number in zebra finches. Brain Res. 2001;904:318–326. doi: 10.1016/s0006-8993(01)02488-x. [DOI] [PubMed] [Google Scholar]
- Williams H. Birdsong and singing behavior. Ann N Y Acad Sci. 2004;1016:1–30. doi: 10.1196/annals.1298.029. [DOI] [PubMed] [Google Scholar]
- Williams H, Connor DM, Hill JW. Testosterone decreases the potential for song plasticity in adult male zebra finches. Horm Behav. 2003;44:402–412. doi: 10.1016/j.yhbeh.2003.06.005. [DOI] [PubMed] [Google Scholar]
- Williams H, Mehta N. Changes in adult zebra finch song require a forebrain nucleus that is not necessary for song production. J Neurobiol. 1999;39:14–28. [PubMed] [Google Scholar]
- Yu AC, Margoliash D. Temporal hierarchical control of singing in birds. Science. 1996;273:1871–1875. doi: 10.1126/science.273.5283.1871. [DOI] [PubMed] [Google Scholar]
- Zann RA. The Zebra Finch: A synthesis of field and laboratory studies. New York: Oxford University Press; 1996. Vocalisations; pp. 139–156. [Google Scholar]