Background: A1-subtype of the adenosine receptors (A1AR) is arrhythmogenic.
Results: A1AR activation enhanced Ca2+ entry through TRPC3 channel.
Conclusion: TRPC3 is involved in conduction disturbances induced by A1AR.
Significance: TRPC3 represents a promising target to prevent conduction disturbances.
Keywords: Adenosine Receptor, Calcium Channels, Calcium Imaging, Calcium Signaling, Heart, TRPC3, Conduction, Embryonic Cardiomyocytes
Abstract
Although the activation of the A1-subtype of the adenosine receptors (A1AR) is arrhythmogenic in the developing heart, little is known about the underlying downstream mechanisms. The aim of this study was to determine to what extent the transient receptor potential canonical (TRPC) channel 3, functioning as receptor-operated channel (ROC), contributes to the A1AR-induced conduction disturbances. Using embryonic atrial and ventricular myocytes obtained from 4-day-old chick embryos, we found that the specific activation of A1AR by CCPA induced sarcolemmal Ca2+ entry. However, A1AR stimulation did not induce Ca2+ release from the sarcoplasmic reticulum. Specific blockade of TRPC3 activity by Pyr3, by a dominant negative of TRPC3 construct, or inhibition of phospholipase Cs and PKCs strongly inhibited the A1AR-enhanced Ca2+ entry. Ca2+ entry through TRPC3 was activated by the 1,2-diacylglycerol (DAG) analog OAG via PKC-independent and -dependent mechanisms in atrial and ventricular myocytes, respectively. In parallel, inhibition of the atypical PKCζ by myristoylated PKCζ pseudosubstrate inhibitor significantly decreased the A1AR-enhanced Ca2+ entry in both types of myocytes. Additionally, electrocardiography showed that inhibition of TRPC3 channel suppressed transient A1AR-induced conduction disturbances in the embryonic heart. Our data showing that A1AR activation subtly mediates a proarrhythmic Ca2+ entry through TRPC3-encoded ROC by stimulating the phospholipase C/DAG/PKC cascade provide evidence for a novel pathway whereby Ca2+ entry and cardiac function are altered. Thus, the A1AR-TRPC3 axis may represent a potential therapeutic target.
Introduction
Adenosine (ADO)2 is a widespread modulator of cell function, plays a critical role in regulating heart rate and contractility, and is a crucial regulator of the developing cardiovascular system. ADO derives from intra- and extracellular ATP degradation and accumulates when oxygen is lacking (1, 2). Its action can be exerted through four adenosine receptor (AR) subtypes, namely A1, A2A, A2B, and A3.
Considerable evidence points to an important role for the ARs in protection against various myocardial injuries induced by hypoxia or ischemia in animal models and human, the cardioprotective action occurring mainly through activation of the A1AR (1–6). However, other studies concede that A1AR contributes to arrhythmias including bradycardia, atrial fibrillation, conduction disturbances, and negative inotropy in the developing and adult heart (7–11).
For many years, the A1AR was considered as a Gi protein-coupled receptor leading to inhibition of adenylyl cyclase and cAMP reduction, regulating cardiac function. Recent studies show that A1AR is coupled to dual signaling and stimulates the phospholipase C (PLC)/PKC pathway in smooth muscle cells and neurons and is currently identified as a Gi/o protein-coupled receptor (12–14). It is also well established that A1AR can mediate modulation of cytosolic Ca2+ concentration through two mechanisms such as direct regulation of plasmalemmal Ca2+ channels or PLC-mediated mobilization of intracellular Ca2+ stores via inositol 1,4,5-trisphosphate receptor (IP3R) in neurons and smooth muscle cells (15–18). However, the role played by A1AR in Ca2+ signaling in cardiomyocytes remains to be explored.
In addition to the well characterized mode of Ca2+ entry through voltage-dependent Ca2+ channels (e.g. L- and T-type Ca2+ channels), receptor-mediated Ca2+-permeable cation channels activated by PLC are recognized for their physiological role (19).
In particular, the G protein-coupled receptor-mediated activation of the Gq-PLC results in hydrolysis of phosphatidylinositol 4,5-bisphosphate with generation of the second messengers 1,2-diacylglycerol (DAG) and IP3, leading to IP3-induced release of Ca2+ from endoplasmic and sarcoplasmic reticulum (ER/SR). The combined action of DAG and released Ca2+ activate conventional PKCs whereas novel PKCs require only DAG. This signaling cascade activates plasmalemmal Ca2+-permeable cation channels which are referred to receptor-operated channels (ROCs).
The transient receptor potential canonical (TRPC) channels have been postulated as the pore-forming proteins through which receptor-operated Ca2+ entry (ROCE) occurs (20, 21). There are seven members of the mammalian TRPC family, designated TRPC1–TRPC7, which assemble as homo- or heterotetramers to form cation-permeable channels. The properties of the heterotetramers are distinct from those of homotetramers. Using knocked down or knocked out strategies, TRPC channels have been originally proposed as store-operated channels (SOCs) activated by Ca2+ depletion of stores (22–24). This situation remains highly controversial because of the recent identification of STIM1 (stromal interacting molecule 1) as an ER/SR Ca2+ sensor and the Orai proteins forming the pore of SOC (25–27). In general, TRPC1, the first mammalian TRPC reported, can form heteromeric channels with TRPC4 and/or TRPC5 designated as SOCs, whereas TRPC3, TRPC6, and TRPC7 proteins, which share 75% identity, form ROCs and show activation sensitivity to the membrane-delimited action of DAG (23, 28–31).
All isoforms except TRPC2 have been found at mRNA and/or protein levels in mammalian and avian cardiac muscle cells (32–37) making them candidates for the receptor-operated nonselective cation channel known to exist in this cell type. There is accumulating evidence that TRPC channels mediate many physiological and pathological processes including arrhythmias, hypertrophy, heart failure, and apoptosis via ROCE (38). Indeed, a variety of studies using in vitro assays and transgenic and knock-out mice have suggested that TRPC3/6 proteins may assemble to form DAG-activated cation channels, which mediate Gαq-mediated Ca2+ signaling pathway. These TRPC-dependent pathways play a central role in the development of cardiac hypertrophy or arrhythmias (38–42). We recently demonstrated that dysfunction of TRPC channels leads to second-degree atrioventricular blocks and ventricular arrhythmias in the embryonic chick heart model (37). In this model, the A1AR is expressed, and its activation is transiently arrhythmogenic through NADPH oxidase/ERK- and PLC/PKC-dependent mechanisms whereas specific activation of A2AAR, A2BAR, or A3AR had no effect (11). The present study was designed to characterize the Ca2+ entry pathway associated with the activation of A1AR in embryonic cardiac cells. In particular, the molecular mechanisms by which the TRPC channels could play a role in the A1AR-induced conduction disturbances have been investigated. Our findings reveal for the first time a new mechanism of TRPC3 channel activation dependent on A1AR activation and playing a predominant role in arrhythmogenesis.
EXPERIMENTAL PROCEDURES
Antibodies and Agents
Rabbit polyclonal antibodies used against TRPC1, 3, 4, 5, and 6 were from Alomone Labs (Jerusalem, Israel). Goat polyclonal anti-TRPC7 was from Everest Biotech (Oxfordshire, UK). The monoclonal antibody against cardiac troponin I (cTnI) was from Abcam (Cambridge, UK). Secondary antibodies for Western blotting were horseradish peroxidase-conjugated donkey anti-rabbit IgG (GE Healthcare) and horseradish peroxidase-conjugated goat anti-mouse IgG (Bio-Rad Laboratories). The specific agonist of A1AR CCPA, the L-type calcium channel inhibitor nifedipine, the general TRPC channels inhibitor SKF-96365 (SKF), the specific inhibitor of TRPC3 Pyr3, the PLC inhibitor U73122 and its inactive analog U73343, the DAG analog OAG, the PKC activator PMA, the general PKC inhibitor Ro 31-8220, and the irreversible SERCA inhibitor thapsigargin were from Sigma-Aldrich. The other general PKC inhibitor chelerythrine chloride, the myristoylated PKCζ pseudosubstrate inhibitor (MPI-PKCζ), the CRAC channel inhibitor BTP2, and the Fura-2/AM dye were from Calbiochem. The ER-targeted cameleon probe (D1ER) genetically targeted to the SR was used to determine specifically [Ca2+]SR.
Cardiomyocytes Culture
Atria and ventricles were carefully dissected from the heart of 4-day-old chick embryos and washed in a Ca2+- and Mg2+-free medium (PBS containing 130 mm NaCl, 2.07 mm KCl, 8 mm KH2PO4, and 1.5 mm Na2HPO4, pH 7.4) and dissociated twice for 15 min at 37 °C with continuous agitation in PBS containing trypsin-EDTA 0.05% (AmiMed), 0.45 mg/ml collagenase type 2 (Worthington Biochemical Corp. Lakewood, NJ), and 1 mg/ml pancreatin (Sigma-Aldrich). The suspension was centrifuged (5 min, 1300 rpm at room temperature), and the pellets were resuspended in a growth medium constituted of a 3:1 mixture of DMEM and Medium 199 (Invitrogen) supplemented with 10% fetal bovine serum (FBS, Serotec Ltd.), 100 units/ml penicillin G, 100 μg/ml streptomycin, and 1% HEPES (Invitrogen).
Atrial and ventricular myocytes were seeded on 0.1% gelatin-coated glass coverslips in 35-mm plastic wells and expanded in culture for 3 days in the growth medium. These cultures were nearly pure as all cells showed contractile activity observed with phase-contrast microscope and expressed cTnI.
Cell Transfections
Atrial and ventricular myocytes were transiently transfected with an N-terminal fragment of hTRPC3 (amino acids 1–302 of TRPC3) cloned into pEYFP-C1 creating a N-terminally YFP-tagged fusion protein, 1 day after the seeding on glass coverslips using FuGENE® HD (Promega) according to the manufacturer's instructions. pmaxGFP (Amaxa Biosystems) was used as control to verify whether transfection itself could affect the Ca2+ response. 2 μg of plasmid (dominant negative (DN) TRPC3) was mixed in 250 μl of serum-free medium, and 5 μl of FuGENE® HD transfection reagent was added. The mixture was incubated for 15 min at room temperature and added drop by drop to the serum-free medium (1.75 ml) contained in each cell culture dish. After 24 h (at 37 °C, 5% CO2), the serum-free medium was replaced by the growth medium. The cells were kept in culture for further 24–48 h until expression of the YFP fusion protein (as a marker of successful transfection) was detectable in the cells. Transfection efficiency was typically approximately 5%. Cardiomyocytes transfected with the DN-TRPC3 were selected via the YFP tag and subjected to measurement of CCPA-mediated Ca2+ response.
For the D1ER experiments, cells were transiently transfected with Lipofectamine® 2000 reagent (Invitrogen) by adding 2 μg of cDNA/coverslip encoding the D1ER construct. Cells were imaged 48 h after transfection.
Immunostaining
The cultured cells were fixed and permeabilized in cold 100% methanol for 5 min and washed three times in 1× TBS containing 20 mm Tris, 154 mm NaCl, 2 mm EGTA, 2 mm MgCl2, pH 7.5. A saturation step was executed with TBS plus 1% BSA for 10 min. Samples were incubated for 1 h with primary antibody (monoclonal antibody against cTnI) diluted 1:200 in TBS plus 1% BSA. After washing out in TBS, cells were incubated for 1 h in TBS plus 1% BSA with secondary anti-mouse immunoglobulins labeled with Alexa Fluor 594 (Invitrogen). The cells were mounted using the Vectashield mounting medium with DAPI (Vector Laboratories, Burlingame, CA). The immunolabeled samples were examined using a conventional fluorescence microscope (Leica Microsystems).
Western Blotting
The lysates from atrial or ventricular myocytes and the whole heart were denatured, and 30 μg of protein was loaded per lane, separated on SDS-polyacrylamide gels, and transferred to nitrocellulose membranes. Membranes were blocked and probed overnight with antibodies against TRPC1, 3, 4, 5, 6, and 7 and cTnI. After washes, the membranes were incubated with the secondary anti-rabbit, anti-goat, or anti-mouse IgG. Immunoreactive bands were detected with enhanced chemiluminescent procedure using the ECL Western blotting Analysis System (Amersham Biosciences). See supplemental Experimental Procedures for details.
PCR Amplification
Messenger RNA isolation from the whole embryonic heart or cultured atrial and ventricular myocytes obtained from 4-day-old chick embryos was performed using RNeasy Plus Mini kit (Qiagen). RT-PCR was conducted using SuperScript III one-step RT-PCR with platinum Taq (Invitrogen) in a Biometra TRIO-thermoblock (Lab Extreme Inc., Kent City, MI). See supplemental Experimental Procedures for details.
Measurement of Cytosolic Ca2+ Changes Using Fura-2 Fluorescence
After 72 h in culture, atrial and ventricular myocytes were rinsed with a physiological solution containing 135 mm NaCl, 5 mm KCl, 2 mm CaCl2, 1 mm MgCl2, 10 mm HEPES, 10 mm d-glucose, pH adjusted to 7.4 with NaOH, and then incubated for 30 min in darkness in the same solution supplemented with 2 μm Fura-2/AM (Invitrogen) plus 500 nm pluronic acid. Loaded cells were washed twice with the physiological solution before fluorometry. The changes in cytosolic Ca2+ concentration were measured with Fura-2. Ratiometric images of Ca2+ signal were obtained using an inverted microscope (Axio Observer, Zeiss) equipped with a Lambda DG4 illumination system (Sutter Instrument Company, Novato, CA), which alternatively changed the excitation wavelength between 340 nm (340AF15; Omega Optical) and 380 nm (380AF15). Emission was collected through a 415DCLP dichroic mirror, and a 510WB40 filter (Omega Optical), by a cooled, 12-bit CCD camera (CoolSnap HQ; Ropper Scientific Trenton, NJ) coupled to the microscope (x40 oil immersion fluorescence objective). Image acquisition in selected cells and analysis were performed with the Metafluor 6.3 software (Universal Imaging, West Chester, PA). To study the A1AR-activated Ca2+ entry, the spontaneously beating cardiomyocytes to be explored were first selected in presence of external 2 mm Ca2+. Then, the cells were stimulated with 50 μm CCPA, a specific agonist of A1AR, for 5 min in a Ca2+-free solution containing 1 mm EGTA. Subsequently, 2 mm Ca2+ was re-added to the medium, and the peak amplitude of the fluorometric signal corresponding to the response to re-introduction of Ca2+ was determined.
Measurement of Sarcolemmal Cation Influx Using Mn2+ Quenching of Fura-2 Fluorescence
The procedure for loading cells and the set-up are the same as above. Fura-2 was excited at the isosbestic wavelengh, 360 nm, and emission fluorescence was monitored at 510 nm. The divalent cation influx was evaluated by the quenching of Fura-2 fluorescence when Mn2+ ions enter into the cells. This technique exclusively reflects the cation influx through Ca2+ channels. To study the A1AR-activated Ca2+ entry, the cardiac cells were stimulated with 50 μm CCPA in Ca2+-free medium, and then 500 μm Mn2+ was re-added to the medium with Ca2+. The quenching rate of fluorescence intensity (F) was estimated using linear regression of the initial decaying phase (slope, ΔF/Δt) just after Mn2+ addition and expressed as the decrease of F per min, normalized to the maximal F signal obtained before Mn2+ (100%) to correct for differences in the cell size and/or fluorophore loading. Photobleaching was <0.5%/min during measurements.
Measurement of SR Ca2+ Changes
Atrial and ventricular myocytes were transiently transfected with cDNA encoding the D1ER construct 48 h before the experiments. Cardiomyocytes were illuminated at 440 nm (440AF21; Omega Optical), and emission was collected through a 455DRLP dichroic mirror, alternatively at 480 nm (480AF30; Omega Optical) and 535 nm (535AF26; Omega Optical). Photometric values were corrected for photobleaching and expressed as ratio 535/480. Values were normalized to the signal obtained before CCPA.
Ex Vivo Mounting of the Heart and Experimental Protocol
The isolated spontaneously beating hearts were placed in each well of 24-plastic wells containing 1 ml of the medium (±drugs) and stabilized for 45 min at 37.5 °C on the thermostabilized stage of an inverted microscope (Leica DMI3000 B, Wetzlar, Germany) as described previously (37). The mean atrial beating rate of control and treated hearts was measured every 5 min for 60 min, and all arrhythmias were noted. See supplemental Experimental Procedures for details.
Electrocardiogram of the Whole Heart
Intact spontaneously beating hearts were placed in the culture compartment of a stainless steel air-tight chamber maintained at 37.5 °C. ECG displayed characteristic P, QRS, and T components, which allowed us to determine the beating rate from PP or RR interval (beats/min), PR interval (ms), QT duration (ms), and QRS complex duration (ms) as described previously in details (37, 43). See supplemental Experimental Procedures for details.
Statistical Analysis
All values are reported as mean ± S.E. For all experiments, the significance of any difference between two groups was assessed with one-way analysis of variance (ANOVA) completed by Tukey's post hoc test. The statistical significance was defined by a value of p ≤ 0.05 (*, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001).
RESULTS
Characterization of Atrial and Ventricular Myocytes
After 72 h of culture, cardiomyocytes isolated from atria or ventricle of 4-day-old chick embryos differentiated into spontaneously beating atrial and ventricular myocytes at a rhythm of 106 ± 6 (n = 20) and 57 ± 9 (n = 18) beats/min at 37 °C, respectively. All myocytes displayed the characteristic spindle-shaped morphology (Fig. 1A), with sarcomeric organization. The majority of myocytes was positive for the cTnI immunostaining (Fig. 1B), and Western blotting showed the presence of cTnI in both cultured cells and whole heart (Fig. 1C). Only atrial and ventricular myocytes with spontaneous beats were investigated.
FIGURE 1.
Characterization of cultured embryonic cardiomyocytes. A, atrial and ventricular myocytes isolated from 4-day-old embryonic chick heart after 72 h in culture. Two representative images obtained with phase-contrast microscopy show the morphology of spontaneously beating atrial (left) and ventricular (right) myocytes. Scale bars, 10 μm. B, immunostaining for cTnI in red and the nucleus in blue in atrial (left) and ventricular (right) myocytes showing characteristic sarcomeric striations (n = 3 primary cultures). Stars represent the selected areas enlarged (×2) showing striations. Scale bars, 1 μm. C, Western blotting showing the presence of cTnI (21 kDa) in cultured atrial and ventricular myocytes (n = 3 primary cultures).Whole 4-day-old embryonic heart was used as positive control.
TRPC Channels and A1AR Are Expressed in Atrial and Ventricular Myocytes
In cultured atrial and ventricular myocytes, Western blotting analysis identified protein expression of TRPC1, 3–7 (TRPC2 being expressed only in rodents (44)), and RT-PCR revealed transcript for A1AR (Fig. 2). The whole heart was used as a positive control for TRPC proteins as previously published (37). For each TRPC isoform, a fusion protein was used as control antigen for negative control (data not shown). Western blotting for A1AR was not performed because no antibody matching the chicken is available on the market.
FIGURE 2.
Expression of TRPC isoforms and A1AR. A, TRPC1, TRPC3–7 proteins were expressed in cultured atrial and ventricular myocytes as well as in whole heart as the positive control (n = 3 primary cultures). B, A1AR mRNA was identified by RT-PCR in cultured myocytes and whole heart (n = 2 primary cultures). PCR product of the predicted size was 318 bp. β-Actin was amplified as a positive control. The negative control (Ctrl(−)) contained water instead of DNA.
A1AR Activation Mobilizes Ca2+ Mainly via TRPC3 Isoform
Under control conditions, atrial and ventricular myocytes showed a basal Ca2+ entry (Fig. 3, A and B). Stimulation of A1AR by a specific agonist CCPA (50 μm) (45) did not induce SR Ca2+ depletion in Ca2+-free medium but doubled Ca2+ entry in the presence of extracellular Ca2+ with respect to basal level in both types of cardiomyocytes. Blockade of TRPC channels by a widely used inhibitor at 40 μm SKF (46) strongly reduced the CCPA-induced Ca2+ entry, suggesting that A1AR-induced Ca2+ entry is mediated by TRPCs.
FIGURE 3.
Contribution of TRPC channels to CCPA-induced sarcolemmal Ca2+ influx. A and B, cytosolic Ca2+ changes determined from Fura-2 fluorescence ratio (340/380) as described under “Experimental Procedures.” Left panels, representative traces of the Ca2+ entry in atrial (A) and ventricular (B) myocytes in basal conditions (basal, black traces), after A1AR stimulation by 50 μm CCPA (dark gray traces), and in the presence of CCPA + 40 μm SKF (light gray traces). Right panels, corresponding bar graphs representing the mean maximal amplitude of the Ca2+ response in each condition (n = 3 primary cultures; number of investigated cells ranged from 44 to 232). ***, p < 0.001. C and D, sarcolemmal cation influx determined by adding 500 μm Mn2+ to the medium to quench the Fura-2 fluorescence as described under “Experimental Procedures.” Left panels, Mn2+-induced rapid decrease of Fura-2 fluorescence (F) calculated from the initial slope (ΔF/Δt in %) in atrial (C) and ventricular (D) myocytes in basal conditions (basal, black traces), after A1AR stimulation by 50 μm CCPA (dark gray traces), and in the presence of CCPA + 40 μm SKF (light gray traces). Right panels, corresponding bar graphs representing the cation entry in each condition (n = 3–8 primary cultures; number of investigated cells ranged from 68 to 248). *, p < 0.05. In A–D, the Ca2+ entry was normalized to the respective basal influx.
To confirm the A1AR-induced Ca2+ response and for the rest of the study, we have used an alternative approach that measured sarcolemmal divalent cation entry using Mn2+ quenching of Fura-2 fluorescence method into Fura-2-loaded cells. This technique takes advantage of the fact that essentially all Ca2+-permeable cation channels exhibit permeability to Mn2+. The rate of fluorescence quenching after the addition of Mn2+ showed that, in the absence of A1AR stimulation, there was a basal cation entry inhibitable by SKF in atrial and ventricular myocytes (supplemental Fig. S1). Inhibition of CRAC channel by BTP2 and TRPC3 by the selective antagonist Pyr3 at 10 μm (47) did not affect this unstimulated cation entry. However, blockade of the L-type calcium channel by nifedipine significantly inhibited the basal cation entry in both types of cell. These findings indicate that TRPC channels, except TRPC3, functioning as constitutively activated Ca2+ channel, and the L-type Ca2+ channel, participate in the basal cation influx.
In agreement with the data shown in Fig. 3, A and B, the CCPA-enhanced cation entry in atrial and ventricular myocytes was inhibited by SKF (Fig. 3, C and D). Furthermore, BTP2 and Pyr3 abolished the CCPA-induced cation entry, confirming that TRPC channels are involved in adenosinergic signaling and, in particular, the TRPC3 isoform in atrial myocytes (Fig. 4A). Surprisingly, in ventricular myocytes, although Pyr3 also strongly reduced the cation influx activated by CCPA, BTP2 had no effect. To further support the contribution of TRPC3 in A1AR-enhanced cation entry, we transiently transfected cardiomyocytes to express an N-terminal fragment of TRPC3, which exerts a DN effect on TRPC3 channel function presumably due to disruption of channel assembly (48–50). A sufficient number of atrial and ventricular myocytes were efficiently transfected as shown with the co-staining for the tag-YFP and cTnI to perform the experiment (supplemental Fig. S2). Expression of YFP-DN-TRPC3 significantly suppressed CCPA-induced cation entry in atrial and ventricular myocytes (Fig. 4A), supporting the concept that TRPC3 constitutes a key element in adenosinergic signaling. The empty vector (pmaxGFP) used as control had no effect on A1AR-induced cation influx. It should be noticed that the cation influx is the result of two components: one insensitive to Pyr3 (the basal cation entry in unstimulated cells as shown in supplemental Fig. S1), which represented approximately 40% of the total CCPA-induced cation influx, and the other sensitive to Pyr3 or to overexpression of the DN-TRPC3 (Fig. 4A). These findings show that approximately 85% of A1AR-dependent cation influx is carried by TRPC3 channel in atrial and ventricular myocytes, once basal influx is deduced.
FIGURE 4.
CCPA-induced sarcolemmal cation influx through TRPC3 channel in a store-independent mechanism. A, bar graphs represent the effects of 40 μm SKF, 10 μm BTP2, 10 μm Pyr3, transient transfection of an empty plasmid (pmaxGFP), and DN-TRPC3 on A1AR-stimulated cation entry in atrial and ventricular myocytes (n = 3–6 primary cultures; number of investigated cells ranged from 46 to 194). ns, not significant; **, p < 0.01; ***, p < 0.001 versus CCPA. B, cells were transiently transfected with the SR-targeted cameleon probe D1ER. Images illustrate staining for cTnI (in red) and D1ER fluorescence (in green) in atrial and ventricular myocytes. Scale bars represent 5 μm. Representative traces show the time course of normalized D1ER ratio changes in four atrial and eight ventricular cells in response to 50 μm CCPA and 1 μm thapsigargin (Tg) in Ca2+-free medium. A1AR activation by CCPA did not induce detectable SR Ca2+ depletion.Values were normalized to the signal obtained before CCPA (n = 3 primary cultures; number of investigated cells ranged from 11 to 18).
Additionally, we showed by cytosolic Ca2+ measurement that A1AR activation did not induce Ca2+ release from internal stores. To corroborate this result, we transfected the myocytes with a genetically encoded Ca2+ probe targeted to the SR (D1ER) to detect Ca2+ store depletion after A1AR activation. Accordingly, the SR Ca2+ depletion was almost undetectable with the D1ER after A1AR stimulation by CCPA in Ca2+-free medium, whereas the irreversible SERCA inhibitor thapsigargin strongly depleted the Ca2+ store in atrial and ventricular cells (Fig. 4B). These results suggest that adenosine induced Ca2+ entry through TRPC3 in a store-independent mechanism.
A1AR-dependent Cation Entry through TRPC3 Channel Involves PLC/DAG/PKC Pathway
We first examined whether A1AR activation stimulates PLCs which are known to activate TRPC channels. Inhibition of PLCs by U73122, indeed, significantly reduced the CCPA-induced cation entry in cardiomyocytes. The inactive analog of this inhibitor, U73343, did not affect the cation influx (Fig. 5A). Activation of PLCs is known to induce the cleavage of phosphatidylinositol 4,5-bisphosphate into DAG and IP3. In turn, DAG activates the conventional and novel PKC isoforms. Based on their requirements for activation, three PKC classes are defined: conventional cPKCs (α, β, γ), which are activated by Ca2+ and DAG; novel nPKCs (δ, ϵ, η, θ), which require DAG but are Ca2+-independent; and atypical aPKCs (ζ, λ, τ), which are activated independently of Ca2+ or DAG. The fact that two inhibitors of all PKC isoforms, chelerythrine and Ro 31-8220 (Ro 31) significantly reduced the CCPA-induced cation entry showed that PKCs are also involved in A1AR-mediated Ca2+ influx in atrial and ventricular myocytes (Fig. 5A).
FIGURE 5.
Contribution of PLC/DAG/PKC pathway to CCPA-induced sarcolemmal cation influx. A, bar graphs represent the effects of 5 μm U73122, 5 μm U73343, 10 μm chelerythrine (chele), and 1 μm Ro 31-8220 (Ro 31) on A1AR-stimulated cation entry in atrial and ventricular myocytes (n = 3–6 primary cultures; number of investigated cells ranged from 41 to 179). ns, not significant; ***, p < 0.001 versus CCPA. B, in atrial cells pretreated with the PLCs inhibitor U73122 and stimulated with CCPA, only 100 μm OAG partly restored the cation entry. In pretreated ventricular cells, both OAG and 1 μm PMA restored the cation entry. 10 μm Pyr3 abolished the OAG- and/or PMA-induced restoration of cation entry in atrial and ventricular myocytes (n = 3–4 primary cultures; number of investigated cells ranged from 36 to 183). ns, not significant; *, p < 0.05 versus CCPA+U73122. C, bar graphs represent the effect of 25 μm MPI-PKCζ on A1AR-stimulated cation entry in atrial and ventricular myocytes (n = 3 primary cultures; number of investigated cells ranged from 46 to 90). **, p < 0.01 atrial versus ventricular cells; ***, p < 0.001 versus CCPA. In A–C, cation entry was normalized to that induced by CCPA alone. In A and B, all inhibitors, activators, U73343, and OAG were added 3 min before CCPA. In C, the myocytes were pretreated with the peptide MPI-PKCζ 30 min before CCPA.
When PLCs were inhibited by U73122, preventing the formation of DAG and, in turn, activation of conventional and novel PKCs, the DAG analog (OAG) restored 53% of the cation entry whereas the activator of conventional and novel PKCs (PMA) had no effect in atrial myocytes (Fig. 5B), suggesting that DAG can directly activate TRPC channels independently of PKCs. By contrast, in ventricular myocytes, either OAG or PMA restored A1AR-induced cation entry by 50% or 69%, respectively, suggesting that DAG acts indirectly on TRPC channels via PKCs. Furthermore, SKF (supplemental Fig. S3) and Pyr3 (Fig. 5B) abolished the OAG- and/or PMA-induced restoration of cation entry in both type of cells, indicating that TRPC3 channel activity is mostly regulated by DAG and/or PKCs.
Additionally, the direct DAG-dependent regulation of TRPC3, the absence of SR Ca2+ depletion, and the absence of effect of PMA on A1AR-induced cation entry in atrial myocytes suggest that an atypical PKC isoform could be involved in the adenosine-dependent Ca2+ response. The MPI-PKCζ inhibited the A1AR-mediated Ca2+ entry by 86% in atrial myocytes and only by 56% in ventricular myocytes (Fig. 5C). The ventricular myocytes were less sensitive to MPI-PKCζ than atrial myocytes, suggesting a predominant contribution of PKCζ in A1AR-mediated Ca2+ entry in atrial cells.
TRPC3 Isoform Is Involved in A1AR-induced Conduction Disturbances
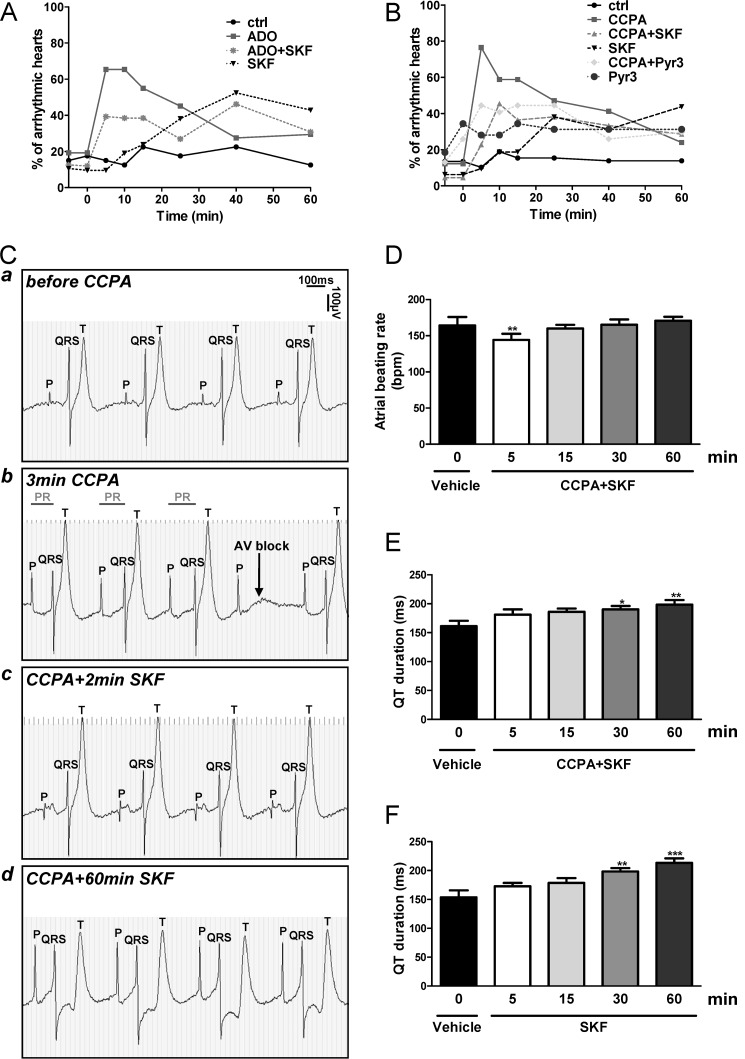
The proportion of spontaneously arrhythmic hearts was always <20% under basal (ctrl) condition (Fig. 6, A and B). ADO and CCPA induced transient arrhythmias after 5 min in 65 and 80% of the hearts, respectively. SKF at 5 μm as well as Pyr3 at 10 μm attenuated ADO- and CCPA-induced arrhythmias (Fig. 6, A and B). These findings indicate that overactivation of TRPC channels, in particular of the TRPC3 isoform contribute to A1AR-induced arrhythmias.
FIGURE 6.
Inhibition of all TRPC isoforms suppressed the A1AR-induced conduction disturbances. A and B, the transient arrhythmogenic effect of ADO (A) or CCPA (B) was reduced by SKF and Pyr3. Time 0 is the time point just before introduction of the 100 μm ADO or 10 μm CCPA after 5 min of pretreatment with 5 μm SKF or 10 μm Pyr3. Controls are untreated hearts. n = 26–54 whole hearts for each condition. C, representative ECG recording shows the P, QRS, and T components of the same embryonic heart spontaneously beating ex vivo before (a) and after 3 min exposure to 10 μm CCPA (b). CCPA mainly provoked second-degree atrioventricular blocks (Wenckebach phenomenon) which were rapidly suppressed by addition of 5 μm SKF (c) for at least 60 min (d) (n = 5 independent experiments, see also supplemental Fig. 4A). D shows time-dependent effect of 10 μm CCPA + 5 μm SKF on atrial beating rate (n = 4). **, p < 0.01 versus vehicle. E and F show time-dependent effects of 10 μm CCPA + 5 μm SKF (E) or 5 μm SKF alone (F) on QT duration (n = 3–4). *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus vehicle.
We also explored the contribution of TRPC channels in CCPA-induced arrhythmias on the basis of electrocardiography (ECG) of the whole heart. The effects of CCPA, SKF, and Pyr3 alone or combined on atrial and ventricular beating rate, atrioventricular conduction (PR interval), ventricular activation (QT duration), and intraventricular conduction (QRS complex width) were determined ex vivo. It should be noticed that the electrical parameters were stable for at least 60 min under control conditions (data not shown). The important changes in ECG morphology and alteration of functional parameters induced by CCPA, CCPA+SKF, and CCPA+Pyr3 are illustrated in Figs. 6 and 7. CCPA rapidly induced second-degree atrioventricular blocks, essentially in the form of Mobitz type I (Wenckebach phenomenon) characterized by a progressive lengthening of the PR interval followed by a dropped QRS without alteration of the atrial rhythm (Figs. 6Cb and 7b). It should be noticed that the period of arrhythmias induced by CCPA varied from one heart to another ranging from 5 to 45 min as we observed previously (11).
FIGURE 7.
Inhibition of TRPC3 isoform suppressed the A1AR-induced conduction disturbances. Representative ECG recording showing that arrhythmias induced by CCPA after 3 min (b) were suppressed within 4 min by Pyr3 (5 μm) (c) for at least 60 min (d) (n = 5 independent experiments, see also supplemental Fig. 4B).
The administration of 5 μm SKF in the presence of CCPA rapidly suppressed the conduction disturbances induced by A1AR stimulation for at least 60 min (Fig. 6C, c and d). As expected, a slight transient bradycardic effect of CCPA was observed after 5 min (Fig. 6D) without affecting PR interval and QRS duration (data not shown) for at least 60 min as we showed previously (11). CCPA+SKF or SKF alone increased the QT duration from 30 min onward (Fig. 6, E and F), which was due to the specific effect of SKF alone on ventricular activation as recently observed (37).
The same pattern of response was obtained in presence of Pyr3 which rapidly suppressed the conduction disturbances in all hearts (Fig. 7, c and d), suggesting that TRPC3 has a major role in A1AR-induced conduction abnormalities. The noticeable protection against these arrhythmias afforded by blockade of all TRPC channels by SKF or by specific inhibition of TRPC3 isoform by Pyr3 is illustrated by ECG tracings shown in supplemental Fig. S4.
DISCUSSION
In the present work, we assessed the role of TRPC3-encoded ROC in the A1AR-induced cardiac conduction disturbances. We showed that activation of A1AR by CCPA increased sarcolemmal Ca2+ entry without SR Ca2+ depletion in embryonic atrial and ventricular myocytes. This A1AR-induced Ca2+ entry was mainly due to upstream activation of PLCs and PKCs because it was prevented by U73122 and chelerythrine or Ro 31-8220, respectively. Although the isoforms IP3R1, 2, and 3 were expressed at mRNA level in atria and ventricle of the embryonic heart (except IP3R2 in atria; data not shown), there was no Ca2+ release from SR measured with Fura-2 fluorescence in Ca2+-free solution and no detectable change of SR intraluminal Ca2+ measured with the D1ER probe after exposure to CCPA, indicating that SR Ca2+ release was not associated with A1AR stimulation. In adult cardiac fibers, the contribution of A1AR to adenosine-induced Ca2+ release from SR is also negligible compared with A2AR (51). Because A1AR is coupled to PLC at the (sub)sarcolemmal level, it is possible that IP3 production was not sufficient to optimally activate the sarcoplasmic IP3Rs and lead to a measurable Ca2+ release and/or that IP3 could not reach IP3Rs as suggested in arterial myocytes (52). It is also conceivable that, at the investigated stage of development, the IP3Rs could not be functional. Therefore, the effects of A1AR activation being strictly dependent on extracellular Ca2+ could be attributed to Ca2+ entry through sarcolemmal ROC by a store-independent mechanism.
Additionally, in atrial and ventricular myocytes pretreated with the inhibitor of PLCs, the DAG analog OAG evoked Ca2+ entry indicating the involvement of a DAG-dependent Ca2+ influx. Interestingly, in atrial myocytes, when PLCs were inhibited, OAG restored the Ca2+ entry induced by CCPA whereas the activator of conventional and novel PKCs (PMA) had no effect. By contrast, in ventricular myocytes, either OAG or PMA restored Ca2+ entry. These results indicate that, in atrial cells, A1AR stimulation induced Ca2+ entry via a G protein coupled to PLC and that DAG formation plays a pivotal role in this phenomenon via a PKC-independent mechanism as suggested in CHO and smooth muscle cells (28, 53, 54). This is in contrast to the Ca2+ entry in ventricular cells which was stimulated by DAG via PKCs, as reported in portal vein myocytes (55).
As PKCs have been postulated to play a key role in adenosine signaling (11, 56, 57), subsequent focus was placed on the PKC pathway. In our model, general PKC inhibitors (Ro 31-8220 and chelerythrine) indeed abolished the effect of A1AR stimulation on Ca2+ entry in both atrial and ventricular myocytes. In ventricular myocytes, the novel PKC isotypes appeared to be principally involved in adenosinergic signaling as DAG but not Ca2+ is required for activation of this PKC family. In adult cardiomyocytes, activation of A1AR promotes targeting of the novel PKC isoforms to caveolin-rich plasma membrane microdomains resulting in their activation (57). These observations are consistent with our findings that A1AR activated novel PKCs which are known as the most abundant Ca2+-independent PKC isoforms in neonatal and adult ventricular cardiomyocytes (58). Surprisingly, in atrial myocytes, PKCs were activated neither by OAG and PMA nor by Ca2+, suggesting that atypical PKC isoforms are involved in adenosinergic response. PKCζ is the most abundant isoform in fetal myocardium, but the mode of activation and its role in cardiac function are not completely understood (59). The selective inhibitor of PKCζ (MPI-PKCζ) strongly decreased the A1AR-induced plasmalemmal Ca2+ influx in atrial cells (−86%) whereas this inhibitor had a slighter effect (p < 0.01) in ventricular cells (−56%), supporting a predominant role for PKCζ in adenosinergic signaling in atrial cells. Adenosine A2A receptor activation has been shown to induce translocation/activation of PKCζ (60, 61), and the Gq protein can be regarded as a scaffold protein capable of recruiting PKCζ into a complex that activates the ERK pathway in neonatal and adult cardiomyocytes and fibroblasts (62). We also recently found that A1AR activation induces pacemaking and conduction disturbances through downstream activation of ERK pathway in the developing heart (11). Thus, these observations and our present data indicate that, in parallel to the DAG-dependent Ca2+ influx, A1AR activation may be able to recruit PKCζ which, in turn, could regulate ROC in atrial myocytes and, to a lesser extent, in ventricular cells.
The closely related candidates for ROC are TRPC3/6/7 channels subfamily which can be activated in response to DAG in a membrane-delimited action independently of Ca2+ store depletion (28, 63, 64). Because Ca2+ mobilization in embryonic atrial and ventricular cells was store-independent, we hypothesized that ROC could be constituted of TRPC proteins, especially TRPC3 subfamily because of its activation by DAG.
All TRPC isoforms at the protein level, except TRPC2, were expressed in cultured atrial and ventricular myocytes. The fact that SKF significantly reduced the A1AR-, PMA-, and/or OAG-induced Ca2+ influx clearly indicates that TRPC channels play a role in the adenosinergic response. The other drug BTP2 used in this study is known to inhibit TRPC3, 5, and 6 (40, 65) and Orai channels (CRAC and SOC), which are activated by Ca2+ store depletion through a STIM-dependent mechanism (66, 67). In our model, BTP2 reduced A1AR-mediated Ca2+ influx in atrial but not in ventricular myocytes, which could be attributable to distinct types of assembly of TRPC isoforms into homo- or heterotetramers ion channels and/or to their differential interactions with STIM/Orai proteins.
TRPC3 isoform is known to be strongly implicated in various pathophysiological processes. Indeed, TRPC3 protein forms DAG-activated Ca2+ channel, which mediates Gq-induced hypertrophy in rat neonatal cardiomyocytes and transgenic mice overexpressing TRPC3 (68–70). In adult ventricular cardiomyocytes submitted to ischemia, activation of the purinergic P2Y2 receptor by ATP/UTP activates heteromeric TRPC3/7 channels leading to cell depolarization, Ca2+ overload, and arrhythmias (39). The fact that A1AR-enhanced Ca2+ entry was completely abolished by selective inhibition of TRPC3 by Pyr3 and significantly reduced by the DN TRPC3 construct preventing TRPC3 channel assembly indicates that TRPC3 predominantly contributed to the ROC pathway in atrial and ventricular cells. Furthermore, Pyr3 significantly reduced the PMA- and/or OAG-mediated Ca2+ influx, clearly indicating that TRPC3 isoform was regulated by DAG and PKCs in atrial and ventricular myocytes. Our hypothesis that TRPC3 isoform is the predominant component of the A1AR-stimulated Ca2+ entry is also strengthened by the fact that TRPC3 is generally activated through activation of PLC and DAG production.
Thus, A1AR, via PLCs activation, triggers a nonselective cationic influx occurring through TRPC3 channel. However, we cannot rule out the possibility that A1AR activation can stimulate other TRPC isoforms because TRPC3 can form functional ROC channels also as heterotetramers.
We showed previously that (i) adenosine induces transient arrhythmias through A1AR whereas specific activation of A2AAR, A2BAR, or A3AR had no effect and (ii) activation of A1AR is pro-arrhythmic through NADPH oxidase/ERK- and PLC/PKC-dependent mechanisms (11). These findings are in accordance with our present data showing the involvement of PLCs, DAG, and PKCs in the TRPC3-dependent Ca2+ entry triggered by A1AR stimulation in atrial and ventricular myocytes. Moreover, it has been reported that Ca2+ entry through TRPC3 channel regulates ERK phosphorylation via PKC activation (71), a mechanism that could occur also in our model via PKCζ isoform. Electrocardiography of the whole heart showed that A1AR activation induced rapidly and transiently second degree atrioventricular blocks (essentially in the form of Wenckebach phenomenon) and that selective inhibition of TRPC3 channel by Pyr3 immediately suppressed these arrhythmias. It should be noticed that the well known transient bradycardia induced by an adenosinergic stimulation after 5 min was exclusively due to A1AR activation in our model and not to TRPC channels. By contrast, the SKF-induced prolongation of the QT duration observed after 30 min was due to inhibition of TRPCs (suppressing the negative regulation of Cav1.2 channel by TRPCs in ventricle as we previously documented (37) by a mechanism independent of adenosine signaling).
As in adult heart, a tight control of intracellular Ca2+ level is required to maintain normal cardiac activity in the developing heart because any activation of transmembrane Ca2+ influx can result in Ca2+ overload associated with arrhythmias and contractile dysfunction (72, 73). Thus, on the basis of our findings, we propose that a subtle activation/inhibition of voltage-independent Ca2+ channels like TRPC3 could play a crucial role in Ca2+ homeostasis and consequently in regulation of electromechanical activity of the developing heart. Any alteration of these channels by activation of G protein-coupled receptors (such as ARs) under pathological conditions could affect Ca2+ entry and promote pro-arrhythmic alterations of membrane potential. For instance, activation of TRPC3/6 channels by Gαq and DAG results in early afterdepolarizations in the failing heart of adult mouse (42).
We demonstrated previously that TRPC channels play a key role in regulating cardiac pacemaking, conduction, and ventricular activity without any stimulation of GPCR. Here, we highlight a novel axis involving specifically A1AR/TRPC3 in conduction abnormalities. The present study provides the first demonstration that A1AR activation in cardiomyocytes elicits ROCE which is mediated by the TRPC3 channel isoform via PLC-, DAG-, and PKC-dependent mechanisms (Fig. 8). Furthermore, an enhanced TRPC3-dependent Ca2+ entry can lead to a rise of intracellular Ca2+ concentration and trigger transient arrhythmias in the developing heart model. Such an A1AR-dependent signal transduction could play a crucial role in rhythm and conduction disturbances observed under hypoxia or ischemia when ADO accumulates in the interstitial fluid of the myocardium. Hence, the TRPC3 channel isoform may be regarded as a new potential therapeutic target to reduce intracellular Ca2+ overload and subsequent arrhythmias in fetal and adult heart.
FIGURE 8.
Proposed model of A1AR-induced ROCE through TRPC3 in atrial (A) and ventricular (B) myocytes. The A1AR-induced Ca2+ entry through TRPC3 channel requires the upstream activation of PLC/DAG pathway in atrial and ventricular myocytes. The atypical PKCζ predominant in atrial myocytes and the novel PKCs predominant in ventricular myocytes are crucial for regulating TRPC3 activity. Increased ROCE via TRPC3 appears to be involved in conduction disturbances induced by A1AR stimulation in the developing heart. PIP2, phosphatidylinositol 4,5-bisphosphate; aPKC, atypical PKC; nPKC, novel PKC.
Acknowledgments
We thank Dr. Klaus Groschner from the Department of Pharmacology and Toxicology, University of Graz, Austria, for the plasmid encoding for dominant negative TRPC3. The cameleon probe D1ER was provided by Drs. Amy Palmer and Roger Tsien from the University of California, San Diego. We thank Anne-Catherine Thomas for skillful technical assistance.
This work was supported by Swiss National Science Foundation Grant 310030-127633.

This article contains supplemental Experimental Procedures and additional references and Figs. S1–S4.
- ADO
- adenosine
- AR
- adenosine receptor
- cTnI
- cardiac troponin I
- DAG
- 1,2-diacylglycerol
- DN
- dominant negative
- ER
- endoplasmic reticulum
- IP3
- inositol 1,4,5-trisphosphate
- IP3R
- IP3 receptor
- MPI-PKCζ
- myristoylated PKCζ pseudosubstrate inhibitor
- PLC
- phospholipase C
- PMA
- phorbol 12-myristate 13-acetate
- ROC
- receptor-operated channel
- ROCE
- receptor-operated Ca2+ entry
- SOC
- store-operated channel
- SKF
- SKF-96365
- SR
- sarcoplasmic reticulum
- STIM1
- stromal interacting molecule 1
- TRPC
- transient receptor potential canonical
- CRAC
- Ca2+ release-activated Ca2+ channel.
REFERENCES
- 1. Görlach A. (2005) Control of adenosine transport by hypoxia. Circ. Res. 97, 1–3 [DOI] [PubMed] [Google Scholar]
- 2. Takahashi T., Otsuguro K., Ohta T., Ito S. (2010) Adenosine and inosine release during hypoxia in the isolated spinal cord of neonatal rats. Br. J. Pharmacol. 161, 1806–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peart J., Headrick J. P. (2000) Intrinsic A1 adenosine receptor activation during ischemia or reperfusion improves recovery in mouse hearts. Am. J. Physiol. Heart Circ. Physiol. 279, H2166–2175 [DOI] [PubMed] [Google Scholar]
- 4. Roscoe A. K., Christensen J. D., Lynch C., 3rd (2000) Isoflurane, but not halothane, induces protection of human myocardium via adenosine A1 receptors and adenosine triphosphate-sensitive potassium channels. Anesthesiology 92, 1692–1701 [DOI] [PubMed] [Google Scholar]
- 5. Cerniway R. J., Morrison R. R., Byford A. M., Lankford A. R., Headrick J. P., Van Wylen D. G., Matherne G. P. (2002) A1 adenosine receptor overexpression decreases stunning from anoxia-reoxygenation: role of the mitochondrial KATP channel. Basic Res. Cardiol. 97, 232–238 [DOI] [PubMed] [Google Scholar]
- 6. Morrison R. R., Teng B., Oldenburg P. J., Katwa L. C., Schnermann J. B., Mustafa S. J. (2006) Effects of targeted deletion of A1 adenosine receptors on postischemic cardiac function and expression of adenosine receptor subtypes. Am. J. Physiol. Heart Circ. Physiol. 291, H1875–1882 [DOI] [PubMed] [Google Scholar]
- 7. Priviero F., De Nucci G., Antunes E., Zanesco A. (2004) Negative chronotropic response to adenosine receptor stimulation in rat right atria after run training. Clin. Exp. Pharmacol. Physiol. 31, 741–743 [DOI] [PubMed] [Google Scholar]
- 8. Juránek I. (2004) On augmentation of adenosine-mediated negative chromotropic effect by K+ released during myocardial ischemia. Cent. Eur. J. Public Health 12, S33–36 [PubMed] [Google Scholar]
- 9. Rose'Meyer R. B. (2010) Adenosine receptor interactions alter cardiac contractility in rat heart. Clin. Exp. Pharmacol. Physiol. 37, 46–50 [DOI] [PubMed] [Google Scholar]
- 10. Hioki M., Matsuo S., Yamane T., Tokutake K., Ito K., Narui R., Tanigawa S., Yamashita S., Tokuda M., Inada K., Date T., Yoshimura M. (2012) Adenosine-induced atrial tachycardia and multiple foci initiating atrial fibrillation eliminated by catheter ablation using a non-contact mapping system. Heart Vessels 27, 221–226 [DOI] [PubMed] [Google Scholar]
- 11. Robin E., Sabourin J., Benoit R., Pedretti S., Raddatz E. (2011) Adenosine A1 receptor activation is arrhythmogenic in the developing heart through NADPH oxidase/ERK- and PLC/PKC-dependent mechanisms. J. Mol. Cell. Cardiol. 51, 945–954 [DOI] [PubMed] [Google Scholar]
- 12. Parsons M., Young L., Lee J. E., Jacobson K. A., Liang B. T. (2000) Distinct cardioprotective effects of adenosine mediated by differential coupling of receptor subtypes to phospholipases C and D. FASEB J. 14, 1423–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rogel A., Bromberg Y., Sperling O., Zoref-Shani E. (2006) The neuroprotective adenosine-activated signal transduction pathway involves activation of phospholipase C. Nucleosides Nucleotides Nucleic Acids 25, 1283–1286 [DOI] [PubMed] [Google Scholar]
- 14. Ansari H. R., Teng B., Nadeem A., Roush K. P., Martin K. H., Schnermann J., Mustafa S. J. (2009) A1 adenosine receptor-mediated PKC and p42/p44 MAPK signaling in mouse coronary artery smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 297, H1032–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Basheer R., Arrigoni E., Thatte H. S., Greene R. W., Ambudkar I. S., McCarley R. W. (2002) Adenosine induces inositol 1,4,5-trisphosphate receptor-mediated mobilization of intracellular calcium stores in basal forebrain cholinergic neurons. J. Neurosci. 22, 7680–7686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fredholm B. B., Assender J. W., Irenius E., Kodama N., Saito N. (2003) Synergistic effects of adenosine A1 and P2Y receptor stimulation on calcium mobilization and PKC translocation in DDT1 MF-2 cells. Cell. Mol. Neurobiol. 23, 379–400 [DOI] [PubMed] [Google Scholar]
- 17. Ethier M. F., Madison J. M. (2006) Adenosine A1 receptors mediate mobilization of calcium in human bronchial smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 35, 496–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gundlfinger A., Bischofberger J., Johenning F. W., Torvinen M., Schmitz D., Breustedt J. (2007) Adenosine modulates transmission at the hippocampal mossy fibre synapse via direct inhibition of presynaptic calcium channels. J. Physiol. 582, 263–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mori Y., Inoue R., Ishii M., Hara Y., Imoto K. (2001) Dissecting receptor-mediated Ca2+ influx pathways: TRP channels and their native counterparts. Jpn. J. Pharmacol. 87, 245–252 [DOI] [PubMed] [Google Scholar]
- 20. Ambudkar I. S., Bandyopadhyay B. C., Liu X., Lockwich T. P., Paria B., Ong H. L. (2006) Functional organization of TRPC-Ca2+ channels and regulation of calcium microdomains. Cell Calcium 40, 495–504 [DOI] [PubMed] [Google Scholar]
- 21. Firth A. L., Remillard C. V., Yuan J. X. (2007) TRP channels in hypertension. Biochim. Biophys. Acta 1772, 895–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu X., Cheng K. T., Bandyopadhyay B. C., Pani B., Dietrich A., Paria B. C., Swaim W. D., Beech D., Yildrim E., Singh B. B., Birnbaumer L., Ambudkar I. S. (2007) Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1−/− mice. Proc. Natl. Acad. Sci. U.S.A. 104, 17542–17547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saleh S. N., Albert A. P., Peppiatt-Wildman C. M., Large W. A. (2008) Diverse properties of store-operated TRPC channels activated by protein kinase C in vascular myocytes. J. Physiol. 586, 2463–2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sabourin J., Lamiche C., Vandebrouck A., Magaud C., Rivet J., Cognard C., Bourmeyster N., Constantin B. (2009) Regulation of TRPC1 and TRPC4 cation channels requires an α1-syntrophin-dependent complex in skeletal mouse myotubes. J. Biol. Chem. 284, 36248–36261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang S. L., Yu Y., Roos J., Kozak J. A., Deerinck T. J., Ellisman M. H., Stauderman K. A., Cahalan M. D. (2005) STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 437, 902–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vig M., Peinelt C., Beck A., Koomoa D. L., Rabah D., Koblan-Huberson M., Kraft S., Turner H., Fleig A., Penner R., Kinet J. P. (2006) CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 312, 1220–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yeromin A. V., Zhang S. L., Jiang W., Yu Y., Safrina O., Cahalan M. D. (2006) Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature 443, 226–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hofmann T., Obukhov A. G., Schaefer M., Harteneck C., Gudermann T., Schultz G. (1999) Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397, 259–263 [DOI] [PubMed] [Google Scholar]
- 29. Dietrich A., Kalwa H., Rost B. R., Gudermann T. (2005) The diacylgylcerol-sensitive TRPC3/6/7 subfamily of cation channels: functional characterization and physiological relevance. Pflugers Arch. 451, 72–80 [DOI] [PubMed] [Google Scholar]
- 30. Yoshida J., Ishibashi T., Imaizumi N., Takegami T., Nishio M. (2005) Capacitative Ca2+ entries and mRNA expression for TRPC1 and TRPC5 channels in human epidermoid carcinoma A431 cells. Eur. J. Pharmacol. 510, 217–222 [DOI] [PubMed] [Google Scholar]
- 31. Alfonso S., Benito O., Alicia S., Angélica Z., Patricia G., Diana K., Vaca L., Luis V. (2008) Regulation of the cellular localization and function of human transient receptor potential channel 1 by other members of the TRPC family. Cell Calcium 43, 375–387 [DOI] [PubMed] [Google Scholar]
- 32. Freichel M., Schweig U., Stauffenberger S., Freise D., Schorb W., Flockerzi V. (1999) Store-operated cation channels in the heart and cells of the cardiovascular system. Cell Physiol. Biochem. 9, 270–283 [DOI] [PubMed] [Google Scholar]
- 33. Ju Y. K., Allen D. G. (2007) Store-operated Ca2+ entry and TRPC expression; possible roles in cardiac pacemaker tissue. Heart Lung Circ. 16, 349–355 [DOI] [PubMed] [Google Scholar]
- 34. Ohba T., Watanabe H., Murakami M., Takahashi Y., Iino K., Kuromitsu S., Mori Y., Ono K., Iijima T., Ito H. (2007) Up-regulation of TRPC1 in the development of cardiac hypertrophy. J. Mol. Cell. Cardiol. 42, 498–507 [DOI] [PubMed] [Google Scholar]
- 35. Seth M., Zhang Z. S., Mao L., Graham V., Burch J., Stiber J., Tsiokas L., Winn M., Abramowitz J., Rockman H. A., Birnbaumer L., Rosenberg P. (2009) TRPC1 channels are critical for hypertrophic signaling in the heart. Circ. Res. 105, 1023–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vassort G., Alvarez J. (2009) Transient receptor potential: a large family of new channels of which several are involved in cardiac arrhythmia. Can. J. Physiol. Pharmacol. 87, 100–107 [DOI] [PubMed] [Google Scholar]
- 37. Sabourin J., Robin E., Raddatz E. (2011) A key role of TRPC channels in the regulation of electromechanical activity of the developing heart. Cardiovasc. Res. 92, 226–236 [DOI] [PubMed] [Google Scholar]
- 38. Eder P., Molkentin J. D. (2011) TRPC channels as effectors of cardiac hypertrophy. Circ. Res. 108, 265–272 [DOI] [PubMed] [Google Scholar]
- 39. Alvarez J., Coulombe A., Cazorla O., Ugur M., Rauzier J. M., Magyar J., Mathieu E. L., Boulay G., Souto R., Bideaux P., Salazar G., Rassendren F., Lacampagne A., Fauconnier J., Vassort G. (2008) ATP/UTP activate cation-permeable channels with TRPC3/7 properties in rat cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 295, H21–28 [DOI] [PubMed] [Google Scholar]
- 40. Kinoshita H., Kuwahara K., Nishida M., Jian Z., Rong X., Kiyonaka S., Kuwabara Y., Kurose H., Inoue R., Mori Y., Li Y., Nakagawa Y., Usami S., Fujiwara M., Yamada Y., Minami T., Ueshima K., Nakao K. (2010) Inhibition of TRPC6 channel activity contributes to the antihypertrophic effects of natriuretic peptides-guanylyl cyclase-A signaling in the heart. Circ. Res. 106, 1849–1860 [DOI] [PubMed] [Google Scholar]
- 41. Nishida M., Watanabe K., Nakaya M., Kurose H. (2010) [Mechanism of cardiac hypertrophy via diacylglycerol-sensitive TRPC channels]. Yakugaku Zasshi 130, 295–302 [DOI] [PubMed] [Google Scholar]
- 42. Hirose M., Takeishi Y., Niizeki T., Nakada T., Shimojo H., Kashihara T., Horiuchi-Hirose M., Kubota I., Mende U., Yamada M. (2011) Diacylglycerol kinase ζ inhibits ventricular tachyarrhythmias in a mouse model of heart failure. Circ. J. 75, 2333–2342 [DOI] [PubMed] [Google Scholar]
- 43. Sarre A., Maury P., Kucera P., Kappenberger L., Raddatz E. (2006) Arrhythmogenesis in the developing heart during anoxia-reoxygenation and hypothermia-rewarming: an in vitro model. J. Cardiovasc. Electrophysiol. 17, 1350–1359 [DOI] [PubMed] [Google Scholar]
- 44. Löf C., Viitanen T., Sukumaran P., Törnquist K. (2011) TRPC2: of mice but not men. Adv. Exp. Med. Biol. 704, 125–134 [DOI] [PubMed] [Google Scholar]
- 45. Amoroso S., Iannotti E., Saggese M. L., Di Renzo G., Annunziato L. (1995) The A1 agonist CCPA reduced bisoxonol-monitored membrane potential depolarization elicited by high K+ in cerebrocortical nerve endings. Biochim. Biophys. Acta 1239, 67–73 [DOI] [PubMed] [Google Scholar]
- 46. Putney J. W., Jr. (2001) Pharmacology of capacitative calcium entry. Mol. Interv. 1, 84–94 [PubMed] [Google Scholar]
- 47. Kiyonaka S., Kato K., Nishida M., Mio K., Numaga T., Sawaguchi Y., Yoshida T., Wakamori M., Mori E., Numata T., Ishii M., Takemoto H., Ojida A., Watanabe K., Uemura A., Kurose H., Morii T., Kobayashi T., Sato Y., Sato C., Hamachi I., Mori Y. (2009) Selective and direct inhibition of TRPC3 channels underlies biological activities of a pyrazole compound. Proc. Natl. Acad. Sci. U.S.A. 106, 5400–5405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xu X. Z., Li H. S., Guggino W. B., Montell C. (1997) Coassembly of TRP and TRPL produces a distinct store-operated conductance. Cell 89, 1155–1164 [DOI] [PubMed] [Google Scholar]
- 49. Eder P., Schindl R., Romanin C., Groschner K. (2007) in TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades (Liedtke W. B., Heller S., eds) CRC Press, Boca Raton, FL: [PubMed] [Google Scholar]
- 50. Poteser M., Graziani A., Eder P., Yates A., Mächler H., Romanin C., Groschner K. (2008) Identification of a rare subset of adipose tissue-resident progenitor cells, which express CD133 and TRPC3 as a VEGF-regulated Ca2+ entry channel. FEBS Lett. 582, 2696–2702 [DOI] [PubMed] [Google Scholar]
- 51. Hleihel W., Lafoux A., Ouaini N., Divet A., Huchet-Cadiou C. (2006) Adenosine affects the release of Ca2+ from the sarcoplasmic reticulum via A2A receptors in ferret skinned cardiac fibres. Exp. Physiol. 91, 681–691 [DOI] [PubMed] [Google Scholar]
- 52. Xi Q., Adebiyi A., Zhao G., Chapman K. E., Waters C. M., Hassid A., Jaggar J. H. (2008) IP3 constricts cerebral arteries via IP3 receptor-mediated TRPC3 channel activation and independently of sarcoplasmic reticulum Ca2+ release. Circ. Res. 102, 1118–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Helliwell R. M., Large W. A. (1997) α1-Adrenoceptor activation of a non-selective cation current in rabbit portal vein by 1,2-diacyl-sn-glycerol. J. Physiol. 499, 417–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Saleh S. N., Albert A. P., Peppiatt C. M., Large W. A. (2006) Angiotensin II activates two cation conductances with distinct TRPC1 and TRPC6 channel properties in rabbit mesenteric artery myocytes. J. Physiol. 577, 479–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Albert A. P., Large W. A. (2002) Activation of store-operated channels by noradrenaline via protein kinase C in rabbit portal vein myocytes. J. Physiol. 544, 113–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Henry P., Demolombe S., Pucéat M., Escande D. (1996) Adenosine A1 stimulation activates δ-protein kinase C in rat ventricular myocytes. Circ. Res. 78, 161–165 [DOI] [PubMed] [Google Scholar]
- 57. Yang Z., Sun W., Hu K. (2009) Adenosine A1 receptors selectively target protein kinase C isoforms to the caveolin-rich plasma membrane in cardiac myocytes. Biochim. Biophys. Acta 1793, 1868–1875 [DOI] [PubMed] [Google Scholar]
- 58. Bogoyevitch M. A., Parker P. J., Sugden P. H. (1993) Characterization of protein kinase C isotype expression in adult rat heart. Protein kinase C-ϵ is a major isotype present, and it is activated by phorbol esters, epinephrine, and endothelin. Circ. Res. 72, 757–767 [DOI] [PubMed] [Google Scholar]
- 59. Rybin V. O., Buttrick P. M., Steinberg S. F. (1997) PKC-λ is the atypical protein kinase C isoform expressed by immature ventricle. Am. J. Physiol. 272, H1636–1642 [DOI] [PubMed] [Google Scholar]
- 60. Huang N. K., Lin Y. W., Huang C. L., Messing R. O., Chern Y. (2001) Activation of protein kinase A and atypical protein kinase C by A2A adenosine receptors antagonizes apoptosis due to serum deprivation in PC12 cells. J. Biol. Chem. 276, 13838–13846 [DOI] [PubMed] [Google Scholar]
- 61. Gardner A. M., Olah M. E. (2003) Distinct protein kinase C isoforms mediate regulation of vascular endothelial growth factor expression by A2A adenosine receptor activation and phorbol esters in pheochromocytoma PC12 cells. J. Biol. Chem. 278, 15421–15428 [DOI] [PubMed] [Google Scholar]
- 62. Garcia-Hoz C., Sanchez-Fernandez G., Garcia-Escudero R., Fernandez-Velasco M., Palacios-Garcia J., Ruiz-Meana M., Diaz-Meco M. T., Leitges M., Moscat J., Garcia-Dorado D., Bosca L., Mayor F., Ribas C. (2012) PKCζ-mediated Gαq stimulation of the ERK5 pathway in cardiomyocytes and cardiac fibroblasts. J. Biol. Chem. 287, 7792–7802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zitt C., Obukhov A. G., Strübing C., Zobel A., Kalkbrenner F., Lückhoff A., Schultz G. (1997) Expression of TRPC3 in Chinese hamster ovary cells results in calcium-activated cation currents not related to store depletion. J. Cell Biol. 138, 1333–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Antigny F., Jousset H., König S., Frieden M. (2011) Thapsigargin activates Ca2+ entry both by store-dependent, STIM1/Orai1-mediated, and store-independent, TRPC3/PLC/PKC-mediated pathways in human endothelial cells. Cell Calcium 49, 115–127 [DOI] [PubMed] [Google Scholar]
- 65. Goel M., Sinkins W. G., Zuo C. D., Hopfer U., Schilling W. P. (2007) Vasopressin-induced membrane trafficking of TRPC3 and AQP2 channels in cells of the rat renal collecting duct. Am. J. Physiol. Renal Physiol. 293, F1476–1488 [DOI] [PubMed] [Google Scholar]
- 66. Steinckwich N., Frippiat J. P., Stasia M. J., Erard M., Boxio R., Tankosic C., Doignon I., Nüsse O. (2007) Potent inhibition of store-operated Ca2+ influx and superoxide production in HL60 cells and polymorphonuclear neutrophils by the pyrazole derivative BTP2. J. Leukoc. Biol. 81, 1054–1064 [DOI] [PubMed] [Google Scholar]
- 67. Yoshino T., Ishikawa J., Ohga K., Morokata T., Takezawa R., Morio H., Okada Y., Honda K., Yamada T. (2007) YM-58483, a selective CRAC channel inhibitor, prevents antigen-induced airway eosinophilia and late phase asthmatic responses via Th2 cytokine inhibition in animal models. Eur. J. Pharmacol. 560, 225–233 [DOI] [PubMed] [Google Scholar]
- 68. Bush E. W., Hood D. B., Papst P. J., Chapo J. A., Minobe W., Bristow M. R., Olson E. N., McKinsey T. A. (2006) Canonical transient receptor potential channels promote cardiomyocyte hypertrophy through activation of calcineurin signaling. J. Biol. Chem. 281, 33487–33496 [DOI] [PubMed] [Google Scholar]
- 69. Onohara N., Nishida M., Inoue R., Kobayashi H., Sumimoto H., Sato Y., Mori Y., Nagao T., Kurose H. (2006) TRPC3 and TRPC6 are essential for angiotensin II-induced cardiac hypertrophy. EMBO J. 25, 5305–5316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Brenner J. S., Dolmetsch R. E. (2007) TrpC3 regulates hypertrophy-associated gene expression without affecting myocyte beating or cell size. PLoS One 2, e802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Numaga T., Nishida M., Kiyonaka S., Kato K., Katano M., Mori E., Kurosaki T., Inoue R., Hikida M., Putney J. W., Jr., Mori Y. (2010) Ca2+ influx and protein scaffolding via TRPC3 sustain PKCβ and ERK activation in B cells. J. Cell Sci. 123, 927–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tenthorey D., de Ribaupierre Y., Kucera P., Raddatz E. (1998) Effects of verapamil and ryanodine on activity of the embryonic chick heart during anoxia and reoxygenation. J. Cardiovasc. Pharmacol. 31, 195–202 [DOI] [PubMed] [Google Scholar]
- 73. Bruchez P., Sarre A., Kappenberger L., Raddatz E. (2008) The L-type Ca+ and KATP channels may contribute to pacing-induced protection against anoxia-reoxygenation in the embryonic heart model. J. Cardiovasc. Electrophysiol. 19, 1196–1202 [DOI] [PubMed] [Google Scholar]