Background: In the bacterial heme carrier HasA, an open to closed transition occurs with heme binding.
Results: Axial heme ligand mutants H32A and Y75A are both in a closed conformation.
Conclusion: Simultaneous binding of both axial heme ligands is not required for the closure of loop L1 in HasA.
Significance: The H32A mutant of HasA mimics a proposed structure involved in heme transfer to its partner HasR.
Keywords: Heme, Iron, Metalloproteins, Protein Conformation, Spectroscopy, Heme Iron Spin State, Hemophore HasA, Iron Acquisition
Abstract
The hemophore protein HasA from Serratia marcescens cycles between two states as follows: the heme-bound holoprotein, which functions as a carrier of the metal cofactor toward the membrane receptor HasR, and the heme-free apoprotein fishing for new porphyrin to be taken up after the heme has been delivered to HasR. Holo- and apo-forms differ for the conformation of the two loops L1 and L2, which provide the axial ligands of the iron through His32 and Tyr75, respectively. In the apo-form, loop L1 protrudes toward the solvent far away from loop L2; in the holoprotein, closing of the loops on the heme occurs upon establishment of the two axial coordination bonds. We have established that the two variants obtained via single point mutations of either axial ligand (namely H32A and Y75A) are both in the closed conformation. The presence of the heme and one out of two axial ligands is sufficient to establish a link between L1 and L2, thanks to the presence of coordinating solvent molecules. The latter are stabilized in the iron coordination environment by H-bond interactions with surrounding protein residues. The presence of such a water molecule in both variants is revealed here through a set of different spectroscopic techniques. Previous studies had shown that heme release and uptake processes occur via intermediate states characterized by a Tyr75-iron-bound form with open conformation of loop L1. Here, we demonstrate that these states do not naturally occur in the free protein but can only be driven by the interaction with the partner proteins.
Introduction
Iron is an essential nutrient for commensal and pathogenic bacteria. However, as the ferrous form is highly toxic for cells and the ferric form is scarcely soluble, it is usually sequestered in mammals by proteins such as ferritin, lactoferrin, and transferrin. Iron acquisition represents a challenge for bacteria invading mammalian hosts. Therefore, bacteria have developed different systems to acquire iron from all sources present in their hosts, including heme, either free or bound to hemoproteins. Among them, the heme uptake system Has has been identified in numerous pathogenic Gram-negative bacteria such as Serratia marcescens (1, 2), Pseudomonas aeruginosa (3), Pseudomonas fluorescens (4), Yersinia enterolitica and Yersinia pestis (5), and Yersinia pseudotuberculosis and Erwinia carotorova. Under iron deficiency conditions, these bacteria secrete small soluble heme carrier proteins, the hemophores. In the extracellular medium, hemophores either bind free heme or extract heme from host hemoproteins. Heme is then transported to the bacterial membrane for uptake through a specific receptor, HasR, and subsequent internalization. The hemophore HasA from S. marcescens (19 kDa) was the first to be discovered (1) and characterized (6). Structures of the wild type heme-loaded HasA (holo-HasAWT) (Fig. 1) (7, 8) and heme-free HasA (apo-HasAWT) (9) are available. The protein presents an original α/β fold with a highly curved seven-stranded antiparallel β-sheet on one face and a bundle of four α-helices on the other face. The heme binding pocket is mainly composed of two extended loops, L1 (Gly28–Gly43) and L2 (Tyr75–Thr84) that connect the α and β faces of the molecule. In holo-HasAWT, the pocket assumes a closed conformation, with the two loops holding the heme through an uncommon pair of axial ligands as follows: a histidine (His32) on loop L1 and a tyrosine (Tyr75) on loop L2. In apo-HasAWT the loop L2 adopts a similar conformation as in holo-HasAWT, whereas the loop L1 is displaced by about 30 Å and protrudes toward the exterior of the protein. The heme binding pocket thus adopts an open conformation in the apo-form.
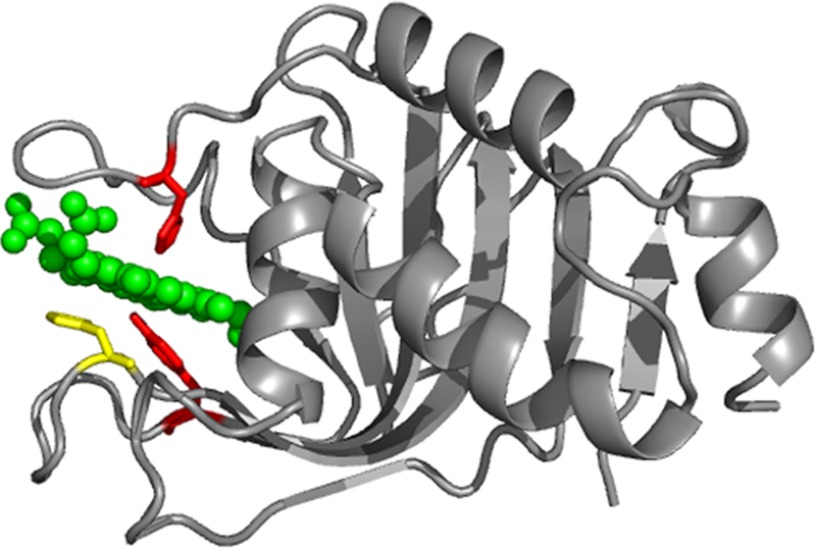
FIGURE 1.
Ribbon representation of the holo-HasAWT structure (Protein Data Bank code 1B2V). The heme is in green. The two axial ligands, His32 and Tyr75, are in red, and residue His83, which is H-bonded to Tyr75, is in yellow.
The heme iron is in the ferric state and presents a thermal high spin (HS)2-low spin (LS) equilibrium, with the two species in fast exchange on the chemical shift NMR time scale (10). The affinity of HasAWT for heme is high (Ka = 5.3 × 1010 m−1), but the two axial ligands make uneven contributions to heme binding. Indeed, whereas the H32A mutation results in a 5-fold decrease in affinity (Ka = 1.0 × 1010 m−1), the Y75A mutation induces a 400-fold decrease (Ka = 1.3 × 108 m−1) (11). Coordination of the Tyr75 ligand is stabilized via a hydrogen bond between its Oη and the Nδ1 of the nearby histidine His83 (12, 13). Removal of His83 causes detachment of the Tyr75 ligand, thus giving rise to a series of pH-dependent equilibria among species with different axial ligation (13); in H83A at physiological pH, a five-coordinated HS species is observed. To decipher the role of His32 and Tyr75 in heme uptake and release processes, we have characterized the electronic properties and the iron coordination sphere of the holo-H32A and holo-Y75A mutants by means of NMR, nuclear magnetic resonance dispersion (NMRD), EPR, resonance Raman (rR), and UV-visible spectroscopies. The resulting picture is that the loss of Tyr75 produces species where the His32 imidazole ring is invariably bound to the heme iron whereas either His83 or H2O bind on the side of the L2 loop, depending on pH. The loss of His32 gives rise, at physiological pH, to Tyr/H2O axial ligation with S = 3/2 spin state. Interestingly, the lack of an amino acid binding in axial position on the L1 loop side does not induce the opening of the loop. These data are discussed in terms of heme uptake and release mechanisms.
EXPERIMENTAL PROCEDURES
Expression and Purification of the H32A and Y75A HasASM Mutant Proteins
H32A and Y75A were constructed by in vitro site-directed mutagenesis as described previously (13). Proteins were obtained from the culture supernatants of Escherichia coli strain Pop3 (pSYC34PAM) grown at 30 °C in M9 minimal medium and purified as reported previously (6). Uniformly labeled 15N and 15N/13C proteins were produced at 30 °C in M9 minimal medium containing 15NH4Cl and [13C]glycerol as sole nitrogen and carbon sources. Purity of the proteins was checked by SDS-PAGE. The protein concentrations were determined using the ϵ277 nm values of 21,700 m−1 cm−1 for H32A mutant and 18,500 m−1 cm−1 for Y75A mutant (14). When necessary, the last C-terminal residues of the HasA proteins were cleaved by the S. marcescens protease PrtSM (15).
NMR Sample Preparation
Bovine hemin or gallium(III) protoporphyrin IX (GaPPIX) was dissolved in a minimal amount of 0.1 n sodium hydroxide (NaOH), centrifuged, and diluted with 20 mm sodium phosphate buffer, pH 7. Concentration of the hemin and GaPPIX solutions were measured in 0.1 m NaOH and in DMSO, respectively, using the ϵ385 nm NaOH and ϵ413 nm DMSO values of 58,440 m−1 cm−1 (16) and 249,000 m−1 cm−1.3 Freshly prepared hemin or GaPPIX solutions were added to the proteins in an ∼1.2:1 ratio. The excess of hemin or GaPPIX was eliminated by gel filtration in 20 mm phosphate buffer at pH 7.0 to obtain 100% loaded proteins. The cyano-complexes were prepared by adding a 15 m excess of potassium cyanide (KCN) to the proteins (0.100 m KCN stock solution in 20 mm phosphate adjusted to the requested pH).
UV-visible Absorbance Spectroscopy
Protein samples for spectrophotometric pH titrations (pH 5.0–10.4) were prepared in a mixture of buffers (20 mm Mes, 20 mm Tris, and 20 mm Ches). The titrations were performed by adjusting pH with dilute hydrogen chloride (HCl) or NaOH solutions and monitoring the UV-visible spectrum. Dilution of each sample over the course of the titration was less than 2%. Twenty two spectra were recorded over the pH range of interest.
Resonance Raman Spectroscopy
Room temperature rR spectra were recorded from 25 to 80 μm protein samples in 5-mm NMR tubes spinning at ∼20 Hz. The spectra were obtained with either 413.1 or 514.1 nm excitation from krypton and argon lasers, respectively, using the 135° backscattering geometry. Laser power at the samples ranged from 15 to 20 milliwatts. Sample integrity was checked by UV-visible absorbance spectroscopy before and after the rR spectra were recorded. The spectrometer was calibrated using the Raman bands of toluene, methylene bromide, and N,N-Dimethylformamide as external frequency standards.
The rR samples of H32A and Y75A examined for isotopic sensitivity were prepared by 30-fold dilution of stock protein solutions (2.0 mm Y75A and 1.3 mm H32A, respectively) into 50 mm N-cyclohexyl-3-aminopropanesulfonic acid buffer at pH 10.0, which had been prepared in H218O (96 atom % 18O).
NMR Spectroscopy
NMR experiments were performed on protein samples ranging from 0.6 to 2 mm concentration in 20 mm phosphate buffer adjusted to the desired pH by adding dilute HCl or NaOH solutions. Unless otherwise specified, NMR spectra were recorded at 303 K. One-dimensional 1H NMR spectra tailored to detect fast relaxing signals were acquired at 500 MHz (11.7 T) with a spectral width of 200 ppm and 8 K data points. Water suppression was achieved by fast repetition rate and presaturation during a 30-ms recycle delay. One-dimensional 15N NMR spectra were acquired at 40 MHz (9.4 T) using a 450-ppm spectral width, 2 K data points, and a 10-ms recycle delay. One-dimensional 13C NMR spectra were acquired at 175 MHz (14.4 T) with a spectral width of 500 ppm and 4 K data points. A triple resonance probe tailored for direct detection of 13C signals was used throughout all 13C NMR experiments. Experiments were performed with the 13C-carrier frequency at −200, 150, 567, and 850 ppm to detect signals over wide spectral regions, using 10 or 200 ms as recycle delays. Several series of inversion recovery experiments were performed with the 13C-carrier frequency at different positions to properly invert and excite all 13C resonances and determine their T1 values. Recycle delays were 10 and 100 ms.
Chemical Shift Mapping
To express chemical shift changes of the individual amide groups, the chemical shift change (in ppm) was defined as shown in Equation 1 (17, 18),
where ΔδHN/N are the chemical shift differences of the corresponding amide 1H/15N nuclei between two protein forms, and 0.16 was the weighting factor for 15N.
EPR Spectroscopy
EPR spectra were recorded with a Bruker ESP300E spectrometer fitted to an Oxford Instrument ESR900 helium flow cryostat. Experiments were carried out on protein samples with concentrations ranging from 0.1 to 0.5 mm in 50 mm sodium acetate buffer, pH 5.5, 50 mm phosphate buffer, pH 7.0, and 50 mm Tricine, pH 9.0. For spin intensity measurements of the low spin heme signal, EPR spectra were taken at 15 K in nonsaturating conditions, doubly integrated, and compared with a copper(II) sulfate standard of known concentration. Spin intensity measurements of the high spin heme signals were performed by comparing the double integration of the low field part of the signal to that given by a myoglobin sample of known concentration (19). When necessary, the deviation of the temperature dependence from the Curie's law was taken into account for high spin signals.
Magnetic Susceptibility Measurements
The magnetic susceptibility of WT and H32A HasA was measured by the modified Evans method (20–21) as reported previously (22). Coaxial NMR tubes were used with 1,4-dioxane as internal reference. The two protein samples had exactly the same concentration, i.e. 0.67 mm in 20 mm phosphate buffer, pH 5.7. The inner capillary contained the paramagnetic protein solution, and the outer tube contained the buffer solution only. The shifts of the proton signals of the reference molecule were measured on a Bruker AVANCE 800 spectrometer, operating at 18.7 T, over the 283–308 K temperature range. The inner-outer tube peak separation (Δδ, expressed in ppm) for the standard molecule was measured and was attributed to the bulk susceptibility shift. The paramagnetic contribution to the molar susceptibility of the solute (χMPARA) was related to the bulk susceptibility shift Δδ as indicated in Equation 2,
in which CM is the millimolar concentration of the protein, and χMPARA is given in m3 mol−1. The magnetic moment in solution (μeff) was then calculated according to Equation 3,
where k is the Boltzman constant; T is absolute temperature; NA is Avogadro's constant, and μ0 is the vacuum permeability.
NMRD Measurements
Longitudinal water proton relaxation rates were measured with a prototype of a Stelar fast field cycling relaxometer (0.01–40 MHz proton Larmor frequency range) (23). The instrument provides R1 values with an error smaller than 1%. 1H NMRD profiles were obtained by plotting proton relaxation rates as a function of applied magnetic field. Reported profiles were obtained for WT and H32A samples that had exactly the same concentration (0.67 mm) in 20 mm phosphate buffer, pH 5.7.
RESULTS
Y75A Mutant
Global Fold
To probe the effect on the protein structure of replacing Tyr75 with the noncoordinating amino acid alanine, the 1H-15N heteronuclear single quantum coherence (HSQC) spectrum of the diamagnetic GaPPIX adduct of Y75A was compared with that of GaPPIX-HasAWT, which is known to share the same structural features and the same axial ligand pair with the native paramagnetic iron(III) complex (24).
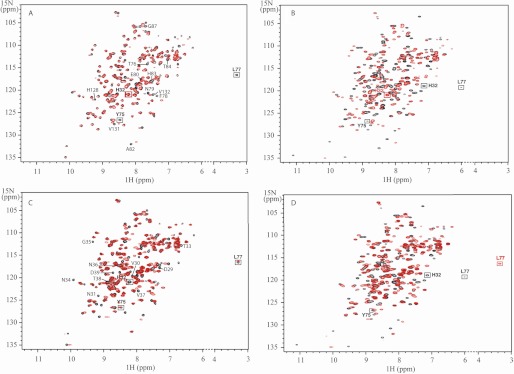
The 1H-15N HSQC spectra of GaPPIX-Y75A and GaPPIX-HasAWT recorded at pH 5.6 are essentially superimposable as shown in Fig. 2A. As expected, the signal of Tyr75 was missing in GaPPX-Y75A. Also the signal of Leu77 disappeared from the position observed in GaPPIX-HasAWT; indeed, the HN of this residue in the wild type protein had a peculiar 1H chemical shift (3.3 ppm) due to ring current effects from the nearby Tyr75 ring, and substitution of Tyr75 with an alanine removed the origin of its upfield shift. Small but significant chemical shift variations were observed for the remaining residues of loop L2, including His83, and for the amino acids belonging to the region facing loop L2, such as 87, 128, 131, and 132. The chemical shift of His32 and of its surrounding residues was only slightly affected by the mutation, indicating that loop L1 did not change its conformation. The spectrum of GaPPIX-Y75A did not vary substantially at increasing pH values up to 8.2.
FIGURE 2.
Superimposition of 15N-1H HSQC spectra. A, GaPPIX-Y75A (red) versus GaPPIX-HasAWT (black). 15N-1H correlations of residues of loop L2 and residues 87, 128, 131, and 132 of GaPPIX-HasAWT are indicated. B, GaPPIX-Y75A (red) versus apo-HasAWT (black). C, GaPPIX-H32A (red) versus GaPPIX-HasAWT (black). 15N-1H correlations of residues localized in the central part of the L1 loop, from Asp29 to Asp39, of GaPPIX-HasAWT are indicated. D, GaPPIX-H32A (red) versus apo-HasAWT (black). Spectra were recorded at 303 K, at pH 5.6 in 20 mm sodium phosphate buffer.
In summary, comparison of the GaPPIX-Y75A and GaPPIX-HasAWT spectra indicated that both proteins shared a very similar backbone conformation, including the two axial ligand bearing loops, independently of the pH. This result indicated that the heme-binding pocket of GaPPIX-Y75A maintained the same closed conformation as its WT counterpart. Accordingly, much larger differences were observed between the 1H-15N HSQC spectra of GaPPIX-Y75A and apo-HasAWT, the latter providing the fingerprint for the open conformation of the hemophore (Fig. 2B).
Chemical shift differences between the 1H-15N HSQC spectra of the paramagnetic heme-loaded holo-Y75A and holo-HasA (supplemental Fig. S1) reflect, in principle, both conformational differences between the two proteins and variations in the hyperfine shift contribution arising from the different coordination state of the iron. However, given the results obtained for the gallium derivatives, the former effect was expected to be very minor. However, substitution of the axial Tyr75 ligand with a nonbonding alanine surely affected the electronic structure of the iron(III) center. Significant chemical shift changes were observed for the signals of Gly35, Asn36, Asn79, and Ala82, which were located in well resolved regions of the spectrum of holo-HasAWT. Furthermore, in holo-Y75A, splitting was observed for most of the signals in the surrounding of the heme. This indicated that two different protein forms, in slow exchange on the chemical shift NMR time scale, exist for the mutant most probably due to two different sets of heme axial ligands. Upon increasing pH, the relative intensity of the two forms changes (supplemental Fig. S1). A pH-dependent transition between protein forms with different heme iron coordinations was also observed by other spectroscopies, as detailed below.
Heme Iron Spin State and Coordination Sphere
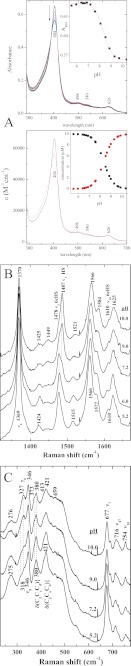
The UV-visible spectrum of holo-Y75A shows meaningful changes over the 5.2–10.4 pH range (Fig. 3A). At pH 5.2, the presence of two Q bands at 533 and 564 nm, typical of LS ferric heme, together with a Q band at 497 nm and a charge transfer band at 629 nm, characteristics for HS ferric heme, was diagnostic for a mixture of the two spin states. The B band was observed at 406 nm. The HS species, predominant at acidic pH, was converted to an LS species with increasing pH values as reported by the B band shifting to 410 nm and the charge transfer (CT) band losing intensity. Determination of pKa values using the absorbance values at a single wavelength is shown in Fig. 3A, inset. Wavelength data for 406 and 629 nm yield a pKa value of 7.2 ± 0.2 for the HS → LS transition. Fitting the intensity of the band at 533 nm data gave a pKa value of <6, which suggested the presence of a further low spin species, as detected by rR (see below).
FIGURE 3.
Holo-Y75A UV-visible and resonance Raman spectra, at pH ranging from 5.1 to 10. A, top panel, UV-visible spectra. Arrows indicate the direction of the absorbance change as the pH is increased. The inset shows all data points at the indicated wavelengths and titration curves calculated by global nonlinear least squares analysis. Bottom panel, component spectra obtained from a global fit of all 22 pH titration spectra of holo-Y75A. The black line is the acidic component; the red line is the intermediate pH component; the blue line is the alkaline species. Inset, species distribution as a function of pH obtained from the global fit. ■, the acidic component; ●, the intermediate pH component; ▴, the alkaline species. B, Soret-excited high frequency; C, low frequency rR spectra at the indicated pH values.
The pH dependence of the spin states of holo-Y75A was also observed by rR spectroscopy. The high frequency rR spectra at pH 5.2 revealed bands at 1477 cm−1 (ν3) and 1557 cm−1 (ν2) that are characteristic of a six-coordinate HS (6cHS) state, together with three bands at 1502 (ν3), 1576 (ν2), and 1633 cm−1 (ν10), typical of a six-coordinated LS (6cLS1) state (Fig. 3B). Upon raising the pH from 5.2 to 6, the intensity of the 6cHS ν3 and ν2 bands decreases significantly while that of the 6cLS ν3 band increases. Moreover, the 6cLS1 ν2 band at 1576 cm−1 shifted to 1579 cm−1, and a second 6cLS ν10 band became observable at 1640 cm−1, consistent with the presence of a second six coordinate LS (6cLS2) species. The LS2 species became predominant at high pH. The low frequency spectrum of holo-Y75A also reflects this pH dependence (Fig. 3C). The most notable changes occurred between pH 5.2 and 6.0 in the vinyl and propionate group bending frequencies. One of the vinyl bending bands δ(CβCaCb) shifted from 421 to 427 cm−1, and the propionate bending band δ(CβCcCd) at 380 cm−1 shifted to 378 cm−1, thus indicating changes in the environment of the peripheral heme substituents. Comparison of the peripheral group bending frequencies for alkaline Y75A with those of HasWT (13) (supplemental Fig. S2) indicated that the vinyl and propionates groups have different environments in these proteins with the frequencies of the δ(CβCcCd) band for Y75A suggesting a weaker hydrogen bonding environment for at least one propionate group relative to that in HasAWT. The possibility of a 6cLS hydroxide complex at alkaline pH was considered by examining Y75A at pH 10.0 in H218O. Heme hydroxides typically exhibit Raman bands between 440 and 560 cm−1. As these bands correspond to normal modes that are dominated by Fe-OH stretching, their frequencies are sensitive to changes in the reduced mass of the FeOH oscillator and shift ∼20 cm−1 to lower frequency in H218O (25 and references therein). No isotope shift was observed suggesting that the 6cLS2 complex was not a hydroxide complex.
The EPR spectrum of Y75A at pH 5.5 indicated the presence of an HS (g⊥ = 5.76 and g‖ = 2.2) and an LS (g = 2.87, 2.28, and 1.54) species. The signal of the HS form decreased with increasing pH up to 9.5, whereas that of the LS species increased (data not shown).
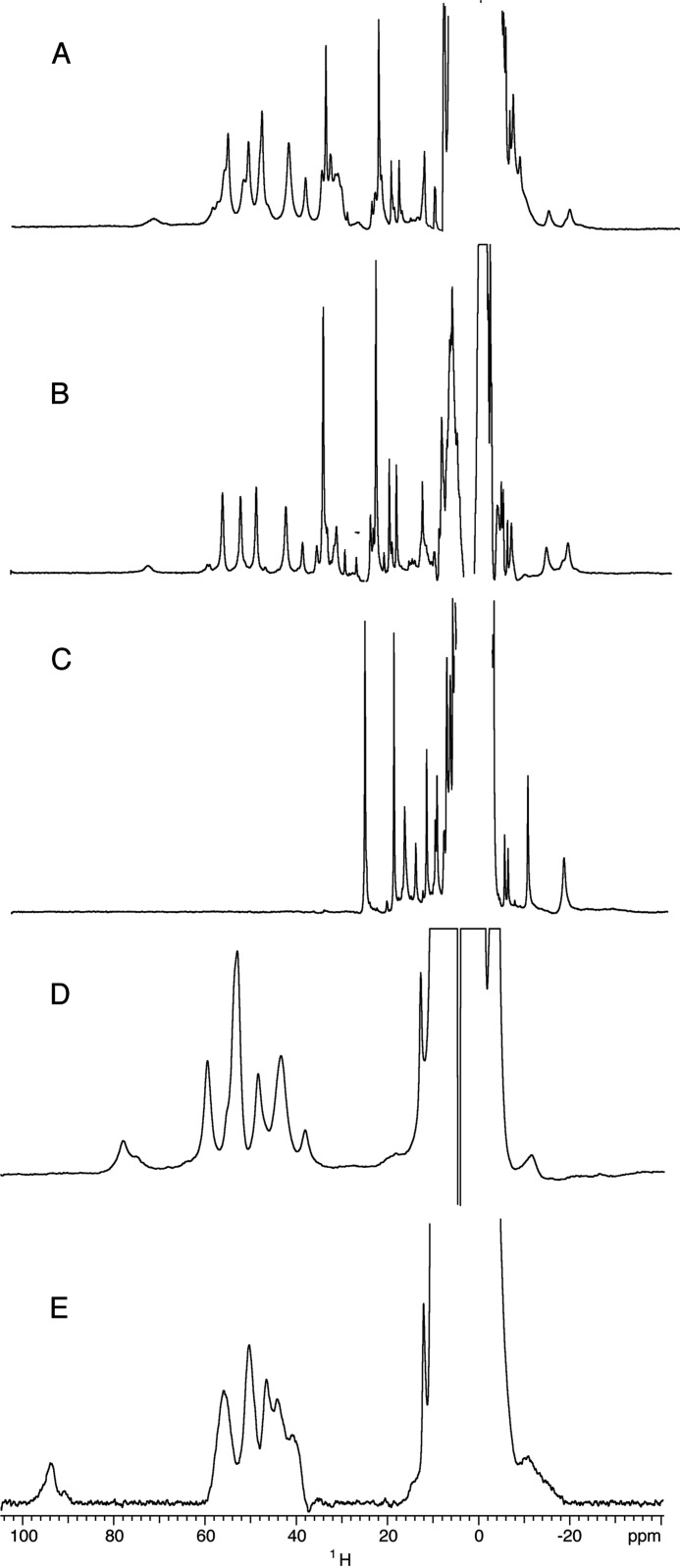
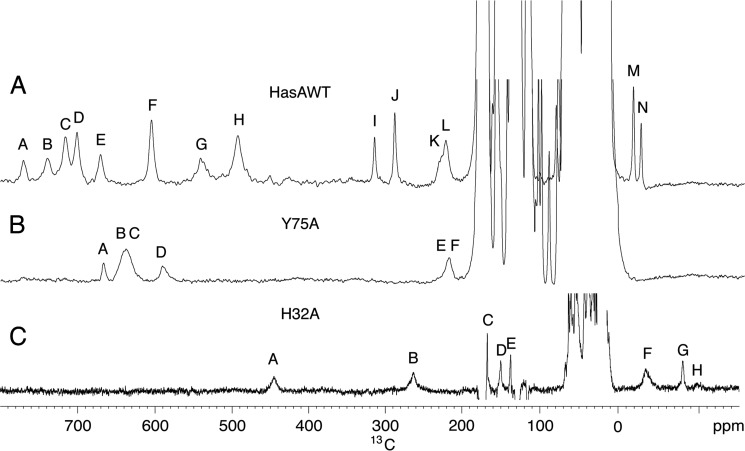
One-dimensional 1H NMR spectra of holo-Y75A, recorded from pH 4.6 to pH 9.9, show two sets of signals in a 80:20 ratio corresponding to the two possible orientations of the heme along the α-γ meso axis, as already observed for holo-HasAWT and holo-H83A mutant (10, 13). Hereafter, only the major set will be considered; however, the observed behavior also holds for the minor set of resonances. The spectrum at pH 5.6 (Fig. 4A) displays two groups of resonances, in agreement with the HSQC results. The first one (form A) was characterized by broad peaks, in the 80 to 40 ppm range. The second (form B) has sharper peaks, in the range 40 to 15 ppm. Upon increasing pH, form A decreases in intensity to the benefit of form B (Fig. 4B). The chemical shift values of the B form showed a slight pH dependence at pH < 6, consistent with the LS1-LS2 equilibrium detected by visible and rR spectroscopies (data not shown). The four most intense signals of form A (57.5, 52.8, 50.1, and 44.6 ppm at pH 5.6 and 303 K) were attributable to the four heme methyls. Their average chemical shift, 51.2 ppm, was intermediate between those expected for purely HS (S = 5/2) and purely LS (S = 1/2) heme iron(III). Line widths of the order of 350 Hz and short T1 values (4–5 ms) of methyl resonances were comparable with those of HasAWT, which experienced a thermal HS/LS equilibrium, fast on the NMR chemical shift time scale. A broad signal, exchangeable in deuterium oxide (D2O), occurred at 73.6 ppm with linewidth of about 1500 Hz and a T1 value shorter than 1 ms. By analogy with the wild type case, it can be attributed to the HNδ1 of the heme-bound His32. The temperature dependence of His32 HNδ1 chemical shift and heme methyl resonances showed a nonlinear behavior indicating a non-pure spin state (supplemental Fig. S3). This is, again, in analogy with previous observations on holo-HasAWT. To further characterize the A form, a one-dimensional 13C spectrum was recorded at pH 5.6, showing three major and three minor hyperfine shifted signals, respectively labeled B, C, F and A, D, E in the 210–670 ppm region (Fig. 5A), with line widths in the range 800–3000 Hz and T1 values shorter than 1 ms (supplemental Table S1). Analogous signals in the WT and H83A proteins were previously attributed to His32 imidazole ring resonances (Fig. 5B) (13). Their shift increases at increasing temperatures. Insights on the axial ligation in Y75A could be gained by comparing the coordination sphere of the previously characterized protein forms and their observed chemical shifts. Average chemical shift values for 13C resonances of His32 and 1H resonances of heme methyls and His32 H Nδ1 measured for holo-HasAWT, holo-H83A, and for the A form of holo-Y75A are reported in Table 1. The pure five-coordinate HS (5cHS) species in holo-H83A, where His32 is the only axial ligand, had the highest chemical shifts. Species with His32 and a sixth ligand (Tyr75 in holo-HasAWT and H2Oδ− in holo-H83A) have somewhat lower shifts, which, with temperature, deviate from a purely Curie behavior. On these bases, and due to the lack of hyperfine shifted signals attributable to a second protein axial ligand, we propose that form A of holo-Y75A was an His32-Fe-H2Oδ− moiety. The preserved conformation for loop L2 upon removal of Tyr75 that emerges from the HSQC fingerprint was consistent with a stabilization of the interaction between the loop and the heme iron mediated by a water molecule that forms an H-bond, probably with His83.
FIGURE 4.
1H NMR spectra. A, holo-Y75A at pH 5.6; B, holo-Y75A at pH 8.2. C, holo-Y75A cyanide adduct at pH 7.3; D, holo-H32A at pH 5.6; E, holo-H32A at pH 9.1. Spectra were recorded at 303 K, in 20 mm phosphate buffer.
FIGURE 5.
13C NMR spectra at pH 5.6. A, 1.6 mm holo-HasAWT (taken from Ref. 10); B, 2.0 mm holo-Y75A; and C, 1.2 mm holo-H32A. Spectra were recorded at a 175 MHz 13C Larmor frequency at 303 K in 20 mm phosphate buffer.
TABLE 1.
Chemical shift fingerprint of the nature of the sixth heme iron ligand in HasAWT and its mutants
Diagnostic values are associated with the average heme-methyl 1H chemical shift, chemical shift of HNδ1 of His32, and 13C average chemical shift for the hyperfine shifted resonances of His32.
| Protein | HasAWT(10) | H83A (13) | Y75A (A form) (Ref. 10 and this work) | |||
|---|---|---|---|---|---|---|
| Axial ligation | His32-Fe-Tyr75 | His32-Fe | His32-Fe-H2Oδ− | His32-Fe-OH− | His32-Fe-H2Oδ− | |
| 1H CH3 | 40.0 | 71.3 | 54.0 | 35.2 | 51.2a | 45.9b |
| His32 HNδ1 | 66.3 | 103 | NDc | ND | 73.6 | 61 |
| 13C H32 | 439.1 | 530.4 | ND | ND | 475.1 | ND |
a Data were at pH 5.6.
b Data were at pH 9.5.
c ND means not determined.
The B form of holo-Y75A included sharp 1H signals for heme methyls (line width ≈150 Hz) with average chemical shift of 45.9 ppm and linear Curie temperature dependence (supplemental Fig. S3). All these NMR parameters suggest a pure LS species. Insight on the nature of the axial ligands of Y75A was further obtained from 15N heteronuclear multiple bond correlation (HMBC) experiments tailored for the detection of imidazole resonances. 15N HMBC spectra of GaPPIX-Y75A and GaPPIX-HasAWT (supplemental Fig. S4) were recorded from pH 5.4 to 9.1. Both GaPPIX-Y75A and GaPPIX-HasAWT spectra gave the same pattern of cross-peaks for His17, His32, His128, and His133, leading to their assignment and to the identification of His32 as an axial ligand in GaPPIX-Y75A (supplemental Table S2). However, two very close sets of cross-peaks corresponding to two different forms were observed for His32 resonances (largest chemical shift differences of 0.05 ppm in proton and 0.6 ppm in nitrogen). One set was predominant at acidic pH and corresponds to form A and the other at basic pH corresponds to form B. The Hϵ1 and Hδ2 protons were highly upfield shifted, and the cross-peak patterns indicate the formation of the Nδ1H tautomer. These data show that His32 was a ligand in both forms, coordinating the metal through its Nϵ2. Two sets of connectivities, corresponding to A and B forms, were also present for His83 in GaPPIX-Y75A spectra. The set dominant at pH 5.4 had a cross-peak pattern resembling that of His83 in GaPPX-HasAWT and was consistent with a protonated imidazole ring. The His83 signals of the B form indicate a neutral imidazole ring and the formation of the Nϵ2H tautomer, with the Nδ1 nitrogen deprotonated and both Hϵ1 and Hδ2 protons further upfield shifted with respect to the A form. These data are consistent with the previous observation that, in holo-Y75A, His83 was a heme iron ligand and suggest an unusual coordination of His83 via its Nδ1 (12). Although bis-His coordination is commonly encountered in heme proteins, binding of His through Nδ1 is rare and occurs only in the presence of specific steric requirements (26). Here, we propose that His83 Nδ1 forms a hydrogen bond with the coordinated water molecule in form A. The binding of its Nδ1 to the heme iron in form B was easier than binding of His83 Nϵ2, which would necessitate a rotation of the imidazole ring.
Finally, addition of cyanide (CN−) induces formation of a new protein form. The UV-visible spectrum of the cyanide adduct was independent over the 7.3–9.5 pH range and showed a B band at 416 nm and Q bands at 536 and 560 nm (data not shown). The 1H NMR spectrum is shown in Fig. 4C. According to their intensities, the two signals at 27.1 and 20.8 ppm were attributable to heme methyl groups. It was clear that both the A and B forms of holo-Y75A converted to the same LS cyanide derivative, where His32 was one of the two axial ligands and CN− the other. Cyanide binding in the sixth position did not occur in the H83A variant, thus indicating that His83 triggered the binding of cyanide in the distal cavity of the protein. Using the heuristic equation proposed by Bertini et al. (27) to calculate the hyperfine shifts of the heme methyls in the cyanide adduct of hemoproteins, the observed heme pattern fits with His32 maintaining the same orientation with respect to the heme plane as in the wild type protein.
H32A Mutant
Global Fold
As for Y75A, the gallium complex of H32A was used to study the effect of the substitution of the His32 axial ligand by a noncoordinating residue on the protein fold. The 1H-15N HSQC spectra of GaPPIX-H32A and GaPPIX-HasAWT, recorded at pH 5.6 (Fig. 2C), are very similar (average chemical shift difference 0.06). The signals of the L2 loop residues, including Tyr75, were essentially superimposable in the two spectra. As expected, chemical shift changes concern residues localized in the central part of the L1 loop, from Asp29 to Asp39 (average chemical shift difference of 0.16 ppm; supplemental Table S3), i.e. the L1 portion surrounding His32. Smaller but meaningful shift changes were also observed for residues 56–65 on β3, β4, and the connecting loop, which face the portion of loop L1 containing His32. Chemical shift differences observed between HSQC spectra of GaPPIX-H32A and apo-HasAWT were larger; chemical shift differences of >0.50 ppm were observed for the entire L1 loop and for the β3, β4, and connecting loop fragment. Differences up to 2.3 ppm were observed for residues 77, 78, and 83–85; these are residues on loop L2 in closest contact with the heme (Fig. 2D and supplemental Table S3). In summary, mutation of His32 did not alter the structure of the protein and did not trigger the opening of the L1 loop even though minor conformational changes likely occur in the central part of this loop. GaPPIX-H32A and consequently heme loaded H32A adopted a closed conformation. The HSQC spectrum of GaPPIX-H32A recorded at pH 9 was similar to the one recorded at pH 5.6 with only a small shift of some resonances (data not shown), thus indicating that the protein structure was not affected by pH.
The comparison of the chemical shift patterns in the 1H-15N HSQC spectra of the paramagnetic heme-loaded H32A and HasAWT (supplemental Fig. S1) at acidic pH highlight some shift differences for residues in both L1 and L2 loops. Obviously, signals of Asp29, Gly35, Asn36, Ala40, Asn79, and Ala82 changed their shift in holo-H32A, consistent with the slight conformational changes observed for the gallium adduct and with differences in the coordination state of the heme iron and therefore in its magnetic properties. As observed for the GaPPIX-H32A derivative, spectral differences in holo-H32A HSQC spectra between pH 5.6 and 9.1 were minor, indicating the same overall backbone conformation for the protein over the investigated pH range.
Heme Iron Spin State and Coordination Sphere
UV-visible spectra of H32A are shown in Fig. 6A as a function of pH. The B band at 404 nm, the Q bands at 498 and 540 nm, and a charge transfer band at 626 nm were observed at pH 5.2. The CT band at 626 nm persisted throughout the pH titration, suggesting that the heme was always HS. The inset in Fig. 6A shows the plot of 404 nm absorbance versus pH, which yields a pKa of 8.3 ± 0.2. The component spectra obtained from the global fit were consistent with the interconversion of two HS species.
FIGURE 6.
Holo-H32A UV-visible and resonance Raman spectra, at pH ranging from ∼5.3 to 10.5. A, top panel, UV-visible spectra (the arrow indicates the direction of the absorbance change as the pH is increased). Inset, Soret absorbance (A404 nm) versus pH; points are experimental absorbances, and the solid line is the titration curve calculated by global nonlinear least squares analysis. Bottom panel, component spectra obtained from a global fit of all 21 pH titration spectra of holo-H32A. The black line is the acidic component; the dotted red line is the alkaline species. Inset, species distribution as a function of pH obtained from the global fit. ■, the acidic component; ●, the alkaline species. B, high frequency; C, low frequency rR spectra (413.1 nm excited) at the indicated pH values.
The rR spectra of ferric holo-H32A recorded over the pH range 5.2 to 10.0 were consistent with the pH-dependent interconversion of two HS species. In the high frequency spectra (Fig. 6B) at pH 5.2, the major component contains 6cHS heme with a ν3 band at 1478 cm−1, a ν2 band at 1560 cm−1, and a ν10 band at 1611 cm−1. As the pH was raised, this ν3 band loses intensity with the appearance of a new band at 1487 cm−1. This frequency falls at the low end of the frequency range expected for 5cHS proteins (28). A similar frequency has been observed for the 5cHS form of holo-H83A mutant (13). The two HS species in equilibrium in holo-H32A were therefore six- and five-coordinated. In the spectra at pH 10, the intensities of the ν3 and ν4 bands were almost equal. This rR signature is characteristic of 5cHS hemes having an axial anionic ligand bound through an oxygen atom such as catalase, ShuT, and CcmE (29–31). These proteins, like some mutant hemoproteins with a tyrosinate as single axial ligand, have I(ν4)/I(ν3) intensity ratios in the 1–1.5 range. Such intensity ratios have also been reported for 5cHS hemoproteins with a coordinated hydroxide ion (32, 33). No isotope shift was observed for H32A in H218O at pH 10.0 indicating that a hydroxide complex was not formed and that Tyr75 was the most likely anionic oxygen heme ligand.
Conversion from an acidic form to a basic form of holo-H32A was also observed in the low frequency rR spectra (Fig. 6C). Although the frequencies of the bands associated with the peripheral substituents (380 and 421 cm−1) do not change, shoulders appear on both of these bands as the pH was increased suggesting that possibly the environment of at least one vinyl and one propionate group was altered. The most dramatic change was the appearance of a band at 327 cm−1. This band is tentatively assigned to an out-of-plane mode γ6 (pyrrole tilt) based on the frequency similarities with metMb (34), CCP (35), and TcAPII (36). Resonance enhancement of this mode was activated by doming of pentacoordinated hemes.
The EPR spectrum of holo-H32A recorded at 15 K in the pH range 5.5–9.5 was dominated by an HS species with g⊥ = 5.75 and g‖ = 2.00 (supplemental Fig. S5). The g⊥ value of 5.75 was too low for a pure high spin state. Based on the pH-independent g⊥ value of 5.75, the spin state is best described as a quantum spin admixture having 12% of an S = 3/2 iron spin state with unresolved g1 and g2 (37, 38).
1H NMR one-dimensional spectra of holo-H32A were recorded from pH 4.5 to pH 10.3. As for holo-Y75A, two sets of signals due to different heme orientations were observed (70:30 ratio), and only the major one was considered hereafter. The spectrum at pH 5.6 and 303 K shows signals in the 35–60 ppm range, with line widths of about 900 Hz and Tl values around 1–2 ms (Fig. 4D). Peaks at 59.4, 53.5, 52.8, and 48.3 ppm were attributable to the four heme methyls. Their observed chemical shifts and relaxation rates were smaller than those observed for other HS heme iron(III) hemoproteins. A signal, not exchangeable in D2O even after a few months at 280 K or after heating the sample at 318K, accounted for a single proton, with a linewidth of about 1400 Hz and T1 values smaller than 0.5 ms occurred at 77.8 ppm. An additional unexchangeable signal, detectable only upon increasing the temperature to 318 K, was observed at 84.1 ppm. Attribution of these two resonances to meta protons of the tyrosine ligand is consistent with previous literature data on catalase (39) and on the H93Y mutant of myoglobin (40). Increasing the pH results in a small shift of all resonances (Fig. 4E), less than 6 ppm over the studied pH range (except for the signal resonating at 77.8 ppm at pH 5.6, which presents a shift of 19 ppm). This behavior was consistent with a rapid equilibrium on the NMR time scale between the two species that rR spectroscopy showed to be 6cHS and 5cHS. The low value of the heme methyl chemical shifts points, however, to a spin state that was lower than S = 5/2 heme.
To further investigate this aspect, Evans measurements were performed in the temperature range 283–308 K and pH 5.7. Table 2 reports the shifts together with the resulting average paramagnetic molar susceptibility and effective magnetic moment; those of the WT protein have been also measured for comparison purposes. The effective magnetic moment for H32A was of the order of ∼3.87 μB. It was essentially invariant over the investigated temperature range and coincided with what was expected for an iron(III) S = 3/2 species (theoretical spin only value of 3.87 μB). At variance, the WT protein has a slightly lower and temperature-dependent μeff, with smaller values at low temperature (Table 2). The magnetic properties of holo-HasAWT were consistent with our previous proposal, based on the temperature dependence of NMR chemical shifts and the rR spectra, that the proteins undergo a spin-dependent equilibrium between a low spin S = 1/2 form (dominant at lower temperature and that would be characterized by μeff values in the 1.9–2.3 μB range due to the large orbital contribution characteristic of this spin state) and a high spin form (dominant at higher temperature and that would be characterized by a μeff of 5.9 μB). At room temperature, the measured μeff of 3.31 μB would account for relative populations of low spin and high spin states of ∼70 and ∼30%, respectively. Effective magnetic moments lower for WT than for H32A were consistent with 1H average chemical shift for heme methyls, which was smaller for WT than for H32A (∼40 ppm versus ∼50 ppm). Structural determinants for the presence of S = 3/2 heme iron(III) have been discussed for other proteins (41). A decreased axial ligand field strength was typically accompanied by a strengthening of the equatorial field and destabilization of the dx2-y2 orbital, which facilitates attainment of the S = 3/2 electron configuration. This could be the case in H32A, where the axial bond with the strong His ligand was removed and possibly replaced by a water molecule (as it results from NMRD measurements, see below).
TABLE 2.
Bulk susceptibility shifts (Δδ, ppm) for H32A and WT HasA measured against 1,4-dioxane by the Evans method in the temperature range 283–308 K at pH 5.7 and 800 MHz
The resulting paramagnetic contribution to the molar susceptibility (χMPARA, m3 mol−1 × 108) and the effective magnetic moment (μeff, μB) of the paramagnetic center are also reported.
| T | H32A |
WT |
||||
|---|---|---|---|---|---|---|
| Δδ | χMPARA | μeff | Δδ | χMPARA | μeff | |
| K | ppm | (m3 mol−1 × 108) | μB | ppm | (m3 mol−1 × 108) | μB |
| 283 | 19.9 | 8.91 | 3.86 | 11.7 | 5.23 | 3.02 |
| 288 | 19.3 | 8.64 | 3.88 | 12.4 | 5.56 | 3.11 |
| 293 | 19.0 | 8.51 | 3.88 | 12.9 | 5.78 | 3.20 |
| 298 | 18.6 | 8.33 | 3.87 | 13.6 | 6.09 | 3.31 |
| 303 | 18.2 | 8.15 | 3.86 | |||
| 308 | 17.8 | 7.97 | 3.85 | 13.9 | 6.22 | 3.40 |
The bond with Tyr75 appears, instead, unaffected. 15N HMBC experiments of GaPPIX-H32A recorded at pH ranging from 5.6 to 9 confirm the H-bonding of His83 with Tyr75. GaPPIX-H32A spectra give the same pattern of cross-peaks as GaPPIX-HasAWT, thus allowing the assignment of the histidines (supplemental Fig. S4). The pattern of His83 was consistent with a protonated imidazole ring over the pH range studied. The Hϵ1 and Hδ2 protons resonances are upfield shifted and similar to those of GaPPIX-HasAWT and of the A form of GaPPIX-Y75A (supplemental Table S2). The one-dimensional 13C NMR spectrum of holo-H32A at pH 5.6 and 303 K (Fig. 5C) shows two broad and fast relaxing hyperfine-shifted signals in the downfield region at 444.2 ppm (A) and 263.2 ppm (B) and three in the upfield region at −34.2 ppm (F), −80.4 ppm (G), and −98.0 ppm (H). An inversion recovery sequence allowed us to identify three additional signals with short T1 and large line widths that were buried in the diamagnetic envelope at 167.4 ppm (C), 150.2 ppm (D), and 137.4 ppm (E), respectively. Signals A–H can be assigned to the axial ligand Tyr75, in both A and B forms. All our data point to the fact that Tyr75 was bound to the metal ion as follows: (a) the number and the relaxation rates of hyperfine shifted signals observed in 13C direct detection; (b) the occurrence of a tyrosinate as a fifth ligand of the heme suggested by rR; (c) the presence of a downfield shifted signal in 1H NMR spectra analogous to that attributed to meta tyrosine protons in catalase and H93Y Mbl, and (d) the fact that His83 was hydrogen bonded to Tyr75 as shown by HMBC spectra. Lack of further hyperfine shifted signals in NMR spectra strongly support the idea that the second axial ligation position in H32A was either empty or occupied by a solvent molecule. This ambiguity has been removed thanks to the use of NMRD measurements. The WT protein has a coordination sphere containing only protein amino acids. Consistently, it has low water proton relaxation rates (supplemental Fig. S6) that correspond to the values expected for systems where only an outer sphere mechanism is operating (42). On the contrary, in H32A solution much faster water relaxation rates are measured (supplemental Fig. S6), which was consistent with the presence of a water molecule coordinated to the heme iron and in fast exchange with bulk solvent.
No spectral changes were observed upon addition of a 15-fold excess of buffered KCN to holo-H32A at pH 7.3 or pH 9.4 (data not shown). Thus, neither the 6c form dominant at neutral pH or the high pH 5c form bind cyanide. Reduced affinity of cyanide for 5c or 6c with a weak axial ligand iron(III) hemes has already been observed in systems in which cyanide binding was not stabilized by H-bonding interactions (43). In addition, the L1 loop site results were largely negative due to amino acid side chains and to the proximity of carboxylate groups from heme propionates, thus creating an electrostatic repulsion between this region of the protein and anionic ligands. Coordinating water in fast exchange with bulk solvent and the inability to bind cyanide are two features both pointing to unrestricted or only slightly restricted mobility of small molecules on the L1 site.
DISCUSSION
The structures of holo- and apo-HasA are largely different due to the conformation of the two loops L1 and L2 that in the holo-form provide the heme-iron(III) axial ligands. The apo-form has an “open” conformation where the two loops are completely disconnected one another. Upon heme uptake, establishment of coordination bonds between iron(III) and His32 Nϵ2 on loop L1 and Tyr75 Oη on loop L2 induces a large conformational rearrangement wherein the loops close onto the heme. Based on the overall shape of HasA in its apo- and holo-forms, the heme binding process has been described as resembling a “fish biting the heme” (7).
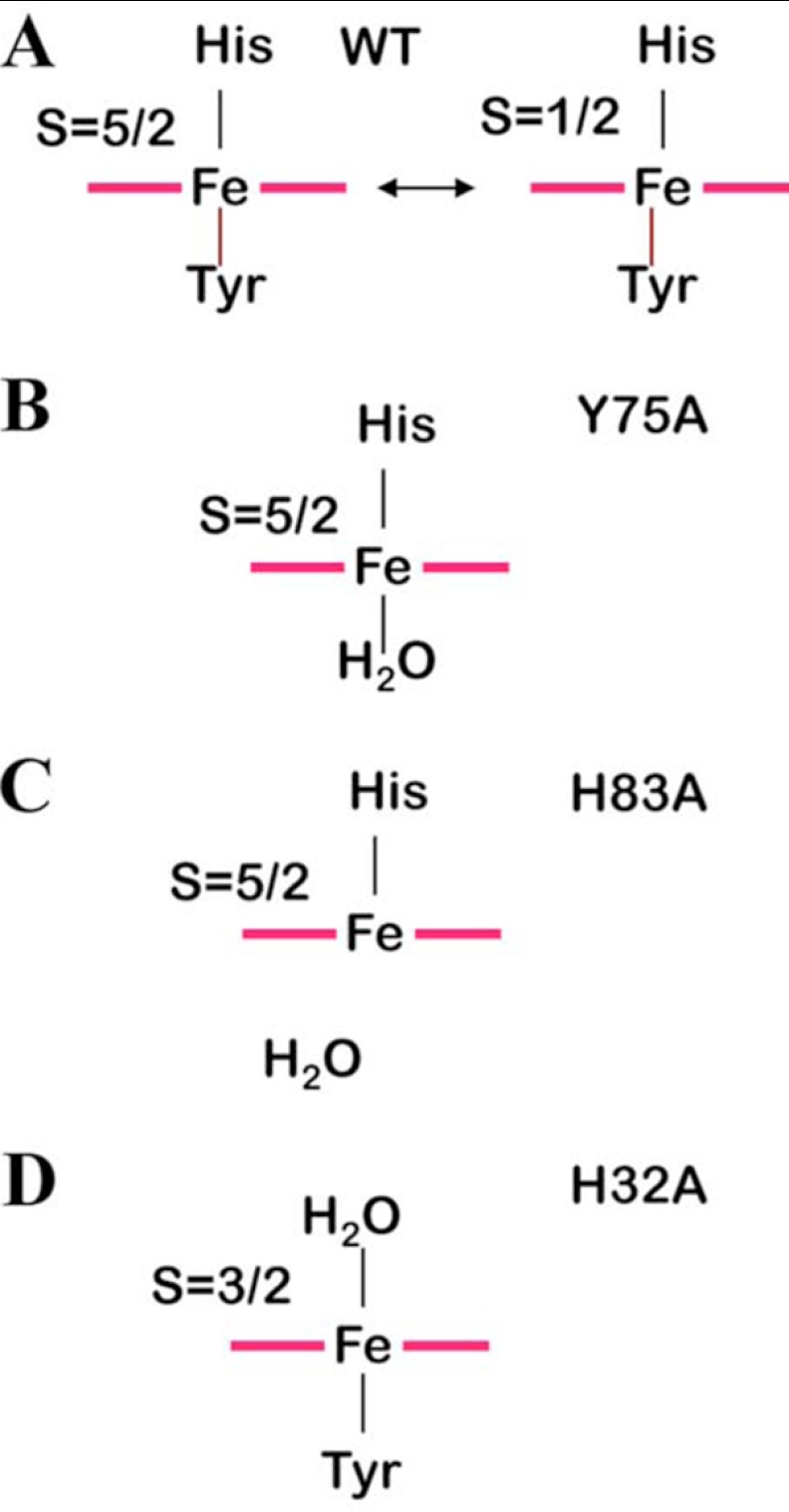
In the WT protein, the heme is linked to the L2 loop on one side via Tyr75. The strength of this bond is modulated by the presence of an H-bond between His83 Nϵ2 and the Tyr75 Oη, which impart a certain degree of tyrosinate character to Tyr75, thus increasing its coordinating ability. Still, the protein undergoes a thermal spin equilibrium between an S = 1/2 state corresponding to His/Tyrδ− axial ligation and an S = 5/2 state where Tyr75 coordination is weakened (Scheme 1A). At room temperature, about 70% of the protein population is in the low spin configuration, as determined here by magnetic susceptibility measurements.
SCHEME 1.
Species present at physiological pH for HasAWT and mutants.
The heme is interacting with loop L1 on the other side via the coordination bond between iron and His32 Nϵ2 and through an H-bond network that involves the solvent-exposed propionates and residues His32, Asn34, and Gly35. The presence of the heme provides a link between L1 and L2. We have demonstrated here that such a link, responsible for the closed conformation of HasAWT, is maintained in the protein variants where either axial ligand is replaced by an alanine.
Tyr is a relatively weak ligand for iron(III) (40, 44), and its presence is not vital for the protein fold. In the Y75A mutant loop L2 remains connected to L1 through the heme thanks to the presence of a water molecule (or of His83 at high pH) in place of Tyr75, as depicted in Scheme 1B. The heme position is probably slightly different from that in the WT protein, as revealed by rR frequencies that are consistent with differences in the H-bonding environment of the propionates. However, similarity among the other rR features and the HSQC fingerprint for the protein backbone are consistent with the closed protein conformation. We have described a similar situation for the H83A variant in which removal of His83 decreases the binding ability of the Tyr and a high spin species is obtained (Scheme 1C) (13).
Mutation of His32 to Ala creates on the L1 heme side a situation similar to that described above for the L2 side; the long L1 loops assumes its closed conformation (as indicated by HSQC data) thanks to the coordination bond between a water molecule and the iron (Scheme 1D). This coordinating H2O molecule, although in relatively fast exchange with bulk solvent, resides in its coordinating position long enough to give rise to measurable paramagnetic effects on water relaxivity, and therefore, it should give rise to non-negligible, albeit weak, H-bond interactions with L1 residues. Other H-bond interactions involving heme propionates are reasonably still operative. All these factors contribute to shift the equilibrium from the extended conformation of L1 in the apoprotein toward its closed conformation. Some functional considerations can be inferred from the above structural data, as described below.
Functional Implications
The whole body of spectroscopic data offers a unique view of the electronic structure and coordination properties of heme in the two mutants, although the comparison with WT gives insights on the role that the two heme-binding residues, as well as the L1 and L2 loops, play during heme uptake and release processes. When discussed together with x-ray data, available for the HasA complex with its membrane receptor HasR, and molecular dynamics studies of holo- and apo-WT protein, our data offer an innovative picture of conformational equilibria of HasA during heme uptake and release.
Heme Uptake
To date, the mechanism of heme binding to HasA has not been delineated. It has been proposed (9) that formation of the holoprotein occurs via a two-step mechanism where the heme first binds to the pre-formed loop L2, and then the presence of the metal cofactor drives the binding of His32 and closure of loop L1. Heme iron binding to His32 and formation of the hydrogen bond network on His32 side stabilize the L1 loop in its final conformation. The H32A variant characterized here represents a possible intermediate in this mechanism, where the H2O-bound form may favor the closed conformation of loop L1 according to the following scheme that adds a further step to the previous mechanism
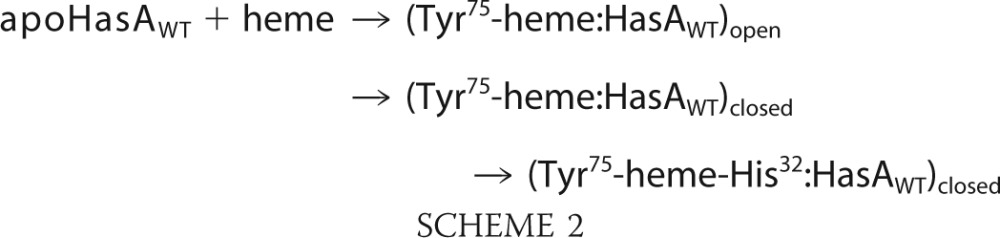 |
As in holo-H32A, a water molecule likely occupies the sixth position in the two intermediate His32-off state species and is displaced by His32 during the closure of the L1 loop.
Heme Transfer to HasR Receptor
HasA hemophore and HasR form a tight complex, dissociable only after heme has been transferred (45). The x-ray structures of holo-HasAWT-HasR and holo-HasAWT-HasRI671G, which display a model of the heme-loaded complex before heme transfer, were recently solved (46). However, loop L1 could not be modeled because of missing electron density. On the basis of x-ray data, the following heme transfer mechanism has been proposed. First, two loops of HasR clash with the L1 loop, causing rupture of the axial coordination between His32 and heme while coordination between Tyr75 and heme persists. Second, displacement of His83 induces the breaking of the Tyr75–His83 hydrogen bond causing rupture of the Tyr75-heme coordination. Finally, residue Ile671 of HasR sterically displaces heme from HasA. This is consistent with the order of axial ligand release predicted from solution rR spectroscopy of WT and mutant HasA-CO complexes (47).
Thus, we propose that the first phase of the holo-HasAWT to HasR docking process is the formation of a closed His32-off state species, similar to H32A. When mapping by NMR the interaction between holo-HasAWT and its receptor (48), we showed that in the holo-HasAWT-HasR complex wherein heme had been transferred to HasR, loop L1 has a conformation resembling that of apo-HasAWT. Thus, the opening of loop L1 must occur before heme transfer to HasR.
In summary, the already available information for the interaction between HasA and HasR suggests that the heme uptake and release processes may occur via a common intermediate state characterized by a Tyr75-iron bound form with open conformation of loop L1. This state is not accessible to the free protein, even upon substitution of the axial His32 ligand with a noncoordinating Ala; we have indeed observed that a water-mediated interaction between iron and L1-loop residues is sufficient to maintain the closed conformation. This finding implies that formation of such an intermediate can only be obtained by the interaction with the partner proteins, which drives the opening of the L1 loop.
Acknowledgments
We thank B. Guigliarelli for EPR data. Bio-NMR Project (Contract 261686) is acknowledged for providing access to NMR and NMRD instrumentations available at Center of Nuclear Magnetic Resonance.
This work was supported, in whole or in part, by National Institutes of Health Grants GM094039 (to G. S. L. R.) and AI072719 (to K. R. R.).

This article contains supplemental Figs. S1–S6 and Tables S1–S3.
V. Kumar and I. Stojiljkovic, personnal communication.
- HS
- high spin
- LS
- low spin
- HSQC
- heteronuclear single quantum coherence
- HMBC
- heteronuclear multiple bond correlation
- GaPPIX
- gallium(III) protoporphyrin IX
- NMRD
- nuclear magnetic resonance dispersion
- rR
- resonance Raman
- Ches
- N-cyclohexyl-2-aminoethanesulfonic acid
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine
- 6cHS
- six-coordinate H
- 6cLS
- six-coordinate LS
- T
- tesla.
REFERENCES
- 1. Létoffé S., Ghigo J. M., Wandersman C. (1994) Iron acquisition from heme and hemoglobin by a Serratia marcescens extracellular protein. Proc. Natl. Acad. Sci. U.S.A. 91, 9876–9880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ghigo J. M., Létoffé S., Wandersman C. (1997) A new type of hemophore-dependent heme acquisition system of Serratia marcescens reconstituted in Escherichia coli. J. Bacteriol. 179, 3572–3579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Létoffé S., Redeker V., Wandersman C. (1998) Isolation and characterization of an extracellular heme-binding protein from Pseudomonas aeruginosa that shares function and sequence similarities with the Serratia marcescens HasA hemophore. Mol. Microbiol. 28, 1223–1234 [DOI] [PubMed] [Google Scholar]
- 4. Idei A., Kawai E., Akatsuka H., Omori K. (1999) Cloning and characterization of the Pseudomonas fluorescens ATP-binding cassette exporter, HasDEF, for the heme acquisition protein HasA. J. Bacteriol. 181, 7545–7551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rossi M. S., Fetherston J. D., Létoffé S., Carniel E., Perry R. D., Ghigo J. M. (2001) Identification and characterization of the hemophore-dependent heme acquisition system of Yersinia pestis. Infect. Immun. 69, 6707–6717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Izadi N., Henry Y., Haladjian J., Goldberg M. E., Wandersman C., Delepierre M., Lecroisey A. (1997) Purification and characterization of an extracellular heme-binding protein, HasA, involved in heme iron acquisition. Biochemistry 36, 7050–7057 [DOI] [PubMed] [Google Scholar]
- 7. Arnoux P., Haser R., Izadi N., Lecroisey A., Delepierre M., Wandersman C., Czjzek M. (1999) The crystal structure of HasA, a hemophore secreted by Serratia marcescens. Nat. Struct. Biol. 6, 516–520 [DOI] [PubMed] [Google Scholar]
- 8. Arnoux P., Haser R., Izadi-Pruneyre N., Lecroisey A., Czjzek M. (2000) Functional aspects of the heme-bound hemophore HasA by structural analysis of various crystal forms. Proteins 41, 202–210 [DOI] [PubMed] [Google Scholar]
- 9. Wolff N., Izadi-Pruneyre N., Couprie J., Habeck M., Linge J., Rieping W., Wandersman C., Nilges M., Delepierre M., Lecroisey A. (2008) Comparative analysis of structural and dynamic properties of the loaded and unloaded hemophore HasA. Functional implications. J. Mol. Biol. 376, 517–525 [DOI] [PubMed] [Google Scholar]
- 10. Caillet-Saguy C., Delepierre M., Lecroisey A., Bertini I., Piccioli M., Turano P. (2006) Direct-detected 13C NMR to investigate the iron(III) hemophore HasA. J. Am. Chem. Soc 128, 150–158 [DOI] [PubMed] [Google Scholar]
- 11. Deniau C., Gilli R., Izadi-Pruneyre N., Létoffé S., Delepierre M., Wandersman C., Briand C., Lecroisey A. (2003) Thermodynamics of heme binding to the HasA(SM) hemophore. Effect of mutations at three key residues for heme uptake. Biochemistry 42, 10627–10633 [DOI] [PubMed] [Google Scholar]
- 12. Wolff N., Deniau C., Létoffé S., Simenel C., Kumar V., Stojiljkovic I., Wandersman C., Delepierre M., Lecroisey A. (2002) Histidine pKa shifts and changes of tautomeric states induced by the binding of gallium-protoporphyrin IX in the hemophore HasA(SM). Protein Sci. 11, 757–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Caillet-Saguy C., Turano P., Piccioli M., Lukat-Rodgers G. S., Czjzek M., Guigliarelli B., Izadi-Pruneyre N., Rodgers K. R., Delepierre M., Lecroisey A. (2008) Deciphering the structural role of histidine 83 for heme binding in hemophore HasA. J. Biol. Chem. 283, 5960–5970 [DOI] [PubMed] [Google Scholar]
- 14. Létoffé S., Deniau C., Wolff N., Dassa E., Delepelaire P., Lecroisey A., Wandersman C. (2001) Hemophore-mediated bacterial heme transport. Evidence for a common or overlapping site for heme-free and heme-loaded hemophore on its specific outer membrane receptor. Mol. Microbiol. 41, 439–450 [DOI] [PubMed] [Google Scholar]
- 15. Izadi-Pruneyre N., Wolff N., Redeker V., Wandersman C., Delepierre M., Lecroisey A. (1999) NMR studies of the C-terminal secretion signal of the heme-binding protein, HasA. Eur. J. Biochem. 261, 562–568 [DOI] [PubMed] [Google Scholar]
- 16. Dawson R., Elliott D., Elliott W., Jones K. (1986) Data for Biochemical Research, pp. 233–236, Oxford University Press, Oxford, UK [Google Scholar]
- 17. Gsponer J., Hopearuoho H., Whittaker S. B., Spence G. R., Moore G. R., Paci E., Radford S. E., Vendruscolo M. (2006) Determination of an ensemble of structures representing the intermediate state of the bacterial immunity protein Im7. Proc. Natl. Acad. Sci. U.S.A. 103, 99–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ayed A., Mulder F. A., Yi G. S., Lu Y., Kay L. E., Arrowsmith C. H. (2001) Latent and active p53 are identical in conformation. Nat. Struct. Biol. 8, 756–760 [DOI] [PubMed] [Google Scholar]
- 19. Bruschi M., Bertrand P., More C., Leroy G., Bonicel J., Haladjian J., Chottard G., Pollock W. B., Voordouw G. (1992) Biochemical and spectroscopic characterization of the high molecular weight cytochrome c from Desulfovibrio vulgaris Hildenborough expressed in Desulfovibrio desulfuricans G200. Biochemistry 31, 3281–3288 [DOI] [PubMed] [Google Scholar]
- 20. Evans D. F. (1959) 400. The determination of the paramagnetic susceptibility of substances in solution by nuclear magnetic resonance. J. Chem. Soc. 2003–2005 [Google Scholar]
- 21. Phillips W. D., Poe M. (1972) Contact shifts and magnetic susceptibilities in iron-sulfur proteins as determined from nuclear magnetic resonance spectra. Methods Enzymol. 24, 304–317 [DOI] [PubMed] [Google Scholar]
- 22. Bertini I., Luchinat C., Turano P., Battaini G., Casella L. (2003) The magnetic properties of myoglobin as studied by NMR spectroscopy. Chemistry 9, 2316–2322 [DOI] [PubMed] [Google Scholar]
- 23. Bertini I., Luchinat C., Parigi G. (2001) Solution NMR of Paramagnetic Molecules, Elsevier, Amsterdam [Google Scholar]
- 24. Deniau C., Couprie J., Simenel C., Kumar V., Stojiljkovic I., Wandersman C., Delepierre M., Lecroisey A. (2001) 1H, 15N, and 13C resonance assignments for the gallium protoporphyrin IX-HasA(sm) hemophore complex. J. Biomol. NMR 21, 189–190 [DOI] [PubMed] [Google Scholar]
- 25. Streit B. R., Blanc B., Lukat-Rodgers G. S., Rodgers K. R., DuBois J. L. (2010) How active-site protonation state influences the reactivity and ligation of the heme in chlorite dismutase. J. Am. Chem. Soc. 132, 5711–5724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chakrabarti P. (1990) Geometry of interaction of metal ions with histidine residues in protein structures. Protein Eng. 4, 57–63 [DOI] [PubMed] [Google Scholar]
- 27. Bertini I., Luchinat C., Parigi G., Walker F. A. (1999) Heme methyl 1H chemical shifts as structural parameters in some low spin ferriheme proteins. J. Biol. Inorg. Chem. 4, 515–519 [DOI] [PubMed] [Google Scholar]
- 28. Jin Y., Nagai M., Nagai Y., Nagatomo S., Kitagawa T. (2004) Heme structures of five variants of hemoglobin M probed by resonance Raman spectroscopy. Biochemistry 43, 8517–8527 [DOI] [PubMed] [Google Scholar]
- 29. Hu S., Kincaid J. R. (1992) Resonance Raman studies of the carbonmonoxy form of catalase. Evidence for and effects of phenolate ligation. FEBS Lett. 314, 293–296 [DOI] [PubMed] [Google Scholar]
- 30. Eakanunkul S., Lukat-Rodgers G. S., Sumithran S., Ghosh A., Rodgers K. R., Dawson J. H., Wilks A. (2005) Characterization of the periplasmic heme-binding protein shut from the heme uptake system of Shigella dysenteriae. Biochemistry 44, 13179–13191 [DOI] [PubMed] [Google Scholar]
- 31. Stevens J. M., Uchida T., Daltrop O., Kitagawa T., Ferguson S. J. (2006) Dynamic ligation properties of the Escherichia coli heme chaperone CcmE to noncovalently bound heme. J. Biol. Chem. 281, 6144–6151 [DOI] [PubMed] [Google Scholar]
- 32. Sun J., Loehr T. M., Wilks A., Ortiz de Montellano P. R. (1994) Identification of histidine 25 as the heme ligand in human liver heme oxygenase. Biochemistry 33, 13734–13740 [DOI] [PubMed] [Google Scholar]
- 33. Franzen S., Bailey J., Dyer R. B., Woodruff W. H., Hu R. B., Thomas M. R., Boxer S. G. (2001) A photolysis-triggered heme ligand switch in H93G myoglobin. Biochemistry 40, 5299–5305 [DOI] [PubMed] [Google Scholar]
- 34. Hu S., Smith K. M., Spiro T. G. (1996) Assignment of Protoheme resonance Raman spectrum by heme labeling in myoglobin. J. Am. Chem. Soc. 118, 12638–12646 [Google Scholar]
- 35. Smulevich G., Hu S., Rodgers K. R., Goodin D. B., Smith K. M., Spiro T. G. (1998) Heme-protein interactions in cytochrome c peroxidase revealed by site-directed mutagenesis and resonance Raman spectra of isotopically labeled hemes. Biospectroscopy 2, 365–376 [Google Scholar]
- 36. Heering H. A., Jansen M. A., Thorneley R. N., Smulevich G. (2001) Cationic ascorbate peroxidase isoenzyme II from tea. Structural insights into the heme pocket of a unique hybrid peroxidase. Biochemistry 40, 10360–10370 [DOI] [PubMed] [Google Scholar]
- 37. Siebel J. F., Kosinsky R. L., Åkerström B., Knipp M. (2012) Insertion of heme b into the structure of the Cys34-carbamidomethylated human lipocalin α1-microglobulin. Formation of a [(heme)2 (α1-microglobulin)]3 complex. ChemBioChem 13, 879–887 [DOI] [PubMed] [Google Scholar]
- 38. Ikezaki A., Nakamura M. (2002) Models for cytochromes c′. Spin states of mono(imidazole)-ligated (meso-tetramesitylporphyrinato)iron(III) complexes as studied by UV-Vis, 13C NMR, 1H NMR, and EPR spectroscopy. Inorg. Chem. 41, 6225–6236 [DOI] [PubMed] [Google Scholar]
- 39. Bondon A., Mouro C. (1998) PASE (paramagnetic signals enhancement). A new method for NMR study of paramagnetic proteins. J. Magn. Reson. 134, 154–157 [DOI] [PubMed] [Google Scholar]
- 40. Hildebrand D. P., Burk D. L., Maurus R., Ferrer J. C., Brayer G. D., Mauk A. G. (1995) The proximal ligand variant H93Y of horse heart myoglobin. Biochemistry 34, 1997–2005 [DOI] [PubMed] [Google Scholar]
- 41. Zeng Y., Caignan G. A., Bunce R. A., Rodríguez J. C., Wilks A., Rivera M. (2005) Azide-inhibited bacterial heme oxygenases exhibit an S = 3/2 (dxz,dyz)3(dxy)1(dz2)1 spin state. Mechanistic implications for heme oxidation. J. Am. Chem. Soc. 127, 9794–9807 [DOI] [PubMed] [Google Scholar]
- 42. Aime S., Fasano M., Paoletti S., Cutruzzolà F., Desideri A., Bolognesi M., Rizzi M., Ascenzi P. (1996) Structural determinants of fluoride and formate binding to hemoglobin and myoglobin. Crystallographic and 1H NMR relaxometric study. Biophys. J. 70, 482–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Motie M., Kassner R. J., Meyer T. E., Cusanovich M. A. (1990) Kinetics of cyanide binding to Chromatium vinosum ferricytochrome c′. Biochemistry 29, 1932–1936 [DOI] [PubMed] [Google Scholar]
- 44. Bertini I., Turano P. (2007) in Biological Inorganic Chemistry (Bertini I., Gray H. B., Stiefel E. I., Valentine J. S., eds) 1st Ed, pp. 31–56, University Science Books, Sausalito, CA [Google Scholar]
- 45. Izadi-Pruneyre N., Huché F., Lukat-Rodgers G. S., Lecroisey A., Gilli R., Rodgers K. R., Wandersman C., Delepelaire P. (2006) The heme transfer from the soluble HasA hemophore to its membrane-bound receptor HasR is driven by protein-protein interaction from a high to a lower affinity binding site. J. Biol. Chem. 281, 25541–25550 [DOI] [PubMed] [Google Scholar]
- 46. Krieg S., Huché F., Diederichs K., Izadi-Pruneyre N., Lecroisey A., Wandersman C., Delepelaire P., Welte W. (2009) Heme uptake across the outer membrane as revealed by crystal structures of the receptor-hemophore complex. Proc. Natl. Acad. Sci. U.S.A. 106, 1045–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lukat-Rodgers G. S., Rodgers K. R., Caillet-Saguy C., Izadi-Pruneyre N., Lecroisey A. (2008) Novel heme ligand displacement by CO in the soluble hemophore HasA and its proximal ligand mutants. Implications for heme uptake and release. Biochemistry 47, 2087–2098 [DOI] [PubMed] [Google Scholar]
- 48. Caillet-Saguy C., Piccioli M., Turano P., Izadi-Pruneyre N., Delepierre M., Bertini I., Lecroisey A. (2009) Mapping the interaction between the hemophore HasA and its outer membrane receptor HasR using CRINEPT-TROSY NMR spectroscopy. J. Am. Chem. Soc 131, 1736–1744 [DOI] [PubMed] [Google Scholar]