Background: ADAMTS metalloproteases are multidomain proteins with remarkable substrate specificity.
Results: Swapping noncatalytic domains between ADAMTS13 and ADAMTS5 causes reciprocal changes in the cleavage of their natural substrates.
Conclusion: ADAMTS exosites in noncatalytic domains are portable modifiers of proteolytic activity.
Significance: Shuffling and recombination of ADAMTS ancillary structural domains may be exploited to evolve or engineer new protease functions.
Keywords: ADAM ADAMTS, Metalloprotease, Proteoglycan, Proteolytic Enzymes, von Willebrand Factor, Exosite
Abstract
ADAMTS proteases typically employ some combination of ancillary C-terminal disintegrin-like, thrombospondin-1, cysteine-rich, and spacer domains to bind substrates and facilitate proteolysis by an N-terminal metalloprotease domain. We constructed chimeric proteases and substrates to examine the role of C-terminal domains of ADAMTS13 and ADAMTS5 in the recognition of their physiological cleavage sites in von Willebrand factor (VWF) and aggrecan, respectively. ADAMTS5 cleaves Glu373–Ala374 and Glu1480–Gly1481 bonds in bovine aggrecan but does not cleave VWF. Conversely, ADAMTS13 cleaves the Tyr1605–Met1606 bond of VWF, which is exposed by fluid shear stress but cannot cleave aggrecan. Replacing the thrombospondin-1/cysteine-rich/spacer domains of ADAMTS5 with those of ADAMTS13 conferred the ability to cleave the Glu1615–Ile1616 bond of VWF domain A2 in peptide substrates or VWF multimers that had been sheared; native (unsheared) VWF multimers were resistant. Thus, by recombining exosites, we engineered ADAMTS5 to cleave a new bond in VWF, preserving physiological regulation by fluid shear stress. The results demonstrate that noncatalytic thrombospondin-1/cysteine-rich/spacer domains are principal modifiers of substrate recognition and cleavage by both ADAMTS5 and ADAMTS13. Noncatalytic domains may perform similar functions in other ADAMTS family members.
Introduction
The ADAMTS (a disintegrin-like and metalloprotease domain, with thrombospondin type-1 motif) superfamily contains 19 metalloproteases with a modular structure that includes a reprolysin-like metalloprotease domain (M),3 a disintegrin-like domain (D), a thrombospondin type 1 repeat (T), a Cys-rich domain (C), and a spacer domain (S), and a variable number of additional thrombospondin type 1 repeat and other domains. The ADAMTS superfamily also contains seven ADAMTS-like (ADAMTSL) proteins that lack M and D domains.
ADAMTS proteases, sometimes with assistance from ADAMTSL proteins, participate in many biological processes including procollagen processing, hemostasis, and extracellular matrix proteolysis relating to morphogenesis, angiogenesis, cancer, and osteoarthritis (1). For example, ADAMTS4 and ADAMTS5 degrade the cartilage proteoglycan aggrecan, which contributes to the development of arthritis. ADAMTS5 appears to be the major aggrecanase because it has higher aggrecanolytic activity than ADAMTS4, and genetic deletion of the ADAMTS5 catalytic domain protects mice from cartilage erosion in experimental models of osteoarthritis (2–4).
ADAMTS13 cleaves von Willebrand factor (VWF), which is required for normal platelet adhesion at sites of vascular injury. Interestingly, the susceptible peptide bond is buried in the native VWF A2 domain but is exposed when VWF is stretched as occurs in vivo within platelet-rich thrombi in flowing blood. Shear stress-induced VWF cleavage is an essential feedback inhibitory mechanism: congenital or acquired ADAMTS13 deficiency causes thrombotic thrombocytopenic purpura, which is characterized by life-threatening microvascular thrombosis (5–7).
Different ADAMTS proteases recognize very distinct substrates but employ similar mechanisms to establish strict substrate specificity: the metalloprotease domain determines cleavage site specificity, and C-terminal ancillary domains provide additional binding precision or localization (1). For example, ADAMTS4 and ADAMTS5 bind to the glycosaminoglycan chains of aggrecan and other extracellular matrix proteoglycans through the S domain (8) and C domain (9), respectively, and these interactions can profoundly affect substrate recognition. ADAMTS4 and ADAMTS5 both cleave aggrecan at several sites, including the Glu373–Ala374 bond in the interglobular domain (IGD) and the Glu1480–Gly1481 bond in the chondroitin sulfate-2 (CS-2) domain (bovine aggrecan numbering), and deletion of the ADAMTS4 S domain (10) or the ADAMTS5 CS domains (9) markedly impairs cleavage at these sites. For ADAMTS13, optimal VWF cleavage depends on contacts between successive segments of the VWF A2 domain and corresponding binding sites in the proximal T, C, S, and distal thrombospondin type 1 repeat domains of ADAMTS13 (11–23).
The modular structure of ADAMTS active sites and exosites suggests that substrate specificity could be investigated and intentionally modified by reassorting metalloprotease and noncatalytic domains from different family members. The altered properties of chimeric proteases constructed from ADAMTS4 and ADAMTS5 support this concept (10). We have now created chimeric ADAMTS5 and ADAMTS13 proteases and have characterized their activity toward model aggrecan IGD and VWF substrates. The results confirm the modularity and portability of ADAMTS exosites and show definitively that ADAMTS13 TCS domains specify shear stress-dependent cleavage of VWF by conferring that activity onto ADAMTS5 MD domains.
EXPERIMENTAL PROCEDURES
Plasmid Constructs
An ADAMTS5 cDNA provided by Richard Leduc (Université de Sherbrooke, Sherbrooke, Canada) was used as the template for PCR amplification of a fragment encoding ADAMTS5 Met1–Thr863. The product was cloned into pcDNA3.1/V5-His-TOPO (Invitrogen) to yield plasmid pcDNA3.1/MDTCS5. Chimeric enzymes (see Fig. 1A) were constructed by overlapping PCR using pcDNA3.1/MDTCS13 (24) or pcDNA3.1/MDTCS5 as the template. The PCR products, which included adjacent polylinker sequences from pcDNA3.1/V5-His-TOPO, were digested with HindIII and PmeI and ligated into the HindIII and EcoRV sites of pcDNA4/TO (Invitrogen).
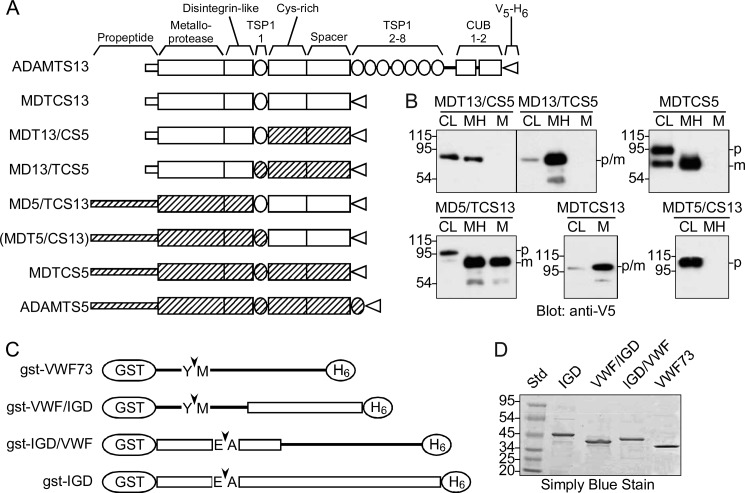
FIGURE 1.
ADAMTS proteases and substrates. A, domains in recombinant ADAMTS proteases are derived from ADAMTS13 (white bars) or ADAMTS5 (hatched bars). Construct MDTCS13 contains Met1–Ala685 of ADAMTS13; MDT13/CS5 contains ADAMTS13 Met1–Glu439 and ADAMTS5 Pro623–Thr863; MD13/TCS5 contains ADAMTS13 Met1–Gly385 and ADAMTS5 Asn569–Thr863. MD5/TCS13 contains ADAMTS5 Met1–Gly568 and ADAMTS13 Arg386–Ala685. MDT5/CS13 contains ADAMTS5 Met1–Glu631 and ADAMTS13 Gln449–Ala685. MDTCS5 contains ADAMTS5 Met1-Thr863. All of the constructs have a C-terminal V5 epitope and His6 tag (V5-H6). These proteases were expressed successfully except for MDT5/CS13 (in parentheses). B, the indicated ADAMTS proteases were expressed in stably transfected T-Rex 293 cell lines without or with the addition of heparin to the culture medium. Samples of cell lysate (CL) and conditioned medium with (MH) or without heparin (M) were separated by SDS-PAGE and visualized by Western blotting with anti-V5 antibody. The molecular mass (kDa) and mobility of standard proteins are indicated at the left. C, all substrates have an N-terminal GST moiety and C-terminal His6 tag that flank segments the VWF A2 domain (lines) and/or aggrecan IGD (boxes). Substrate gst-VWF73 contains VWF residues Asp1596–Arg1668; gst-VWF/IGD contains VWF residues Asp1596–Ile1616 and aggrecan residues Glu394–Gly458; gst-IGD/VWF contains aggrecan residues Thr331–Leu393 and VWF residues Lys1617–Arg1668. gst-IGD contains aggrecan residues Thr331–Gly458. Arrowheads indicate the Tyr1605–Met1606 bond that ADAMTS13 cleaves in VWF or the Glu373–Ala374 that ADAMTS5 cleaves in the IGD of aggrecan. D, substrates expressed in E. coli were purified by sequential chromatography on Ni2+-nitrilotriacetic acid-agarose and glutathione-agarose. Samples of substrates and standard proteins (Std) were separated by SDS-PAGE and stained with SimplyBlue SafeStain. The data shown are representative of at least three independent experiments.
Plasmid pGST-VWF73 was described previously. Plasmid pGST-IGD was constructed similarly in Schistosoma japonicum GST fusion expression vector pGEX-6P-1 (GE Healthcare) using a QuikChange II site-directed mutagenesis kit (Stratagene) with human aggrecan cDNA (MGC-26414, ATCC) as the template and primers 5′-TCTGTTCCAGGGGCCCCTGGGATCCACAGGTGAAGACTTTGTG-3′ (forward) and 5′-CTCAGTGATGGTGATGGTGATGCCCCCCTGGCAAATGCGGCT-3′ (reverse). Megaprimers for chimeric substrate construction (25) were amplified using pGST-IGD as the template and the following primers: for VWF/IGD 5′-CGGAAATCCTGCCTCTGATGAGATC/GAACCCGAGGAGCCCTTC-3′ (forward), and 5′-CTCAGTGATGGTGATGGTGATG/CCCCCCTGGCAAATGCGGCT-3′ (reverse); for IGD/VWF 5′-TCTGTTCCAGGG GCCCCTGGGATCCA/ACAGGTGAAGACTTTGTG-3′ (forward), 5′-CCTGGATGTCTCCAG GCAGCCTCTT/CAGGGGACTGGGGGAGACCT-3′ (reverse). The desired chimeric sequences were amplified using purified PCR products as megaprimers and pGST-VWF73 as the template. Digestion with DpnI was increased to 3 h to completely remove methylated template DNA. Products were electroporated into Escherichia coli XL1-Blue cells, and clones were selected based on restriction digestion, PCR, and DNA sequencing.
Recombinant Proteases
T-Rex 293 cells (Invitrogen) were transfected with plasmids encoding ADAMTS variants (10 μg for transient expression or 1 μg for stable expression) using Lipofectamine 2000 (Invitrogen). Transiently transfected cells were induced to express proteases in Freestyle serum-free medium (Invitrogen) with 1 μg/ml tetracycline. Stable cell lines were maintained in Dulbecco's modified Eagle's medium containing 10% tetracycline-approved fetal bovine serum (Clontech or Invitrogen), 300 μg/ml zeocin, 5 μg/ml blasticidin, 2 mm glutamine, 5 units/ml penicillin, and 5 μg/ml streptomycin. Protein expression was initiated in 70–80% confluent roller bottles with 1 μg/ml tetracycline in Freestyle serum-free medium. This concentration of tetracycline does not inhibit ADAMTS13. Heparin (100 μg/ml) was added to the medium for cell lines expressing MDT13/CS5, MD13/TCS5, or MDTCS5. Conditioned media were centrifuged, and filtered, and serine protease inhibitors were added (0.1 μm d-Phe-Pro-Arg-CH2Cl (FPR-CK), 0.1 μm Phe-Phe-Arg-CH2Cl (FFR-CK), and 144 μm phenylmethylsulfonyl fluoride).
ADAMTS protein concentrations were determined as described (11) by SDS-PAGE with V5-tagged Positope reference protein standards (Invitrogen), Western blotting on PVDF membranes with anti-V5 antibody and peroxidase-conjugated goat anti-mouse IgG (1:10,000 dilution, A3673; Sigma), and chemifluorescence detection (ECL Plus; GE Healthcare). Signals were collected and analyzed with a fluorescence imaging system (Typhoon Trio; GE Healthcare) and ImageQuant TL software (GE Healthcare). The concentration of ADAMTS proteases in conditioned medium typically was 12–36 nm (1–3 μg/ml). The media were concentrated 5–10-fold by ultrafiltration on YM30 membranes (Millipore, Inc.) and dialyzed into an appropriate reaction buffer. Proteases were aliquoted and stored at −30 °C until used without further purification. When assayed with substrate FRETS-VWF73 (26), the activity of 0.6 nm MDTCS13 was equivalent to that of 0.6 nm ADAMTS13 in standard pooled normal plasma.
Substrates
Substrate gst-VWF73 (37.5 kDa), gst-VWF/IGD (39 kDa), gst-IGD/VWF (42 kDa), and gst-IGD (44 kDa) were expressed and purified from the soluble fraction of E. coli lysates as described previously for gst-VWF73 (14). After dialysis against 20 mm Tris·HCl, pH 8.0, 150 mm NaCl, the protein concentration was determined with a BCA protein assay kit (Pierce) and a BSA standard.
Substrate Cleavage by Recombinant ADAMTS Variants
The reactions included 40 μl of reaction buffer (50 mm Hepes, pH 7.4, 150 mm NaCl, 5 mm CaCl2, 0.1 μm ZnCl2), 0.9 μl of BSA (5 mg/ml), 0.1 μl 4-(2-aminoethyl)benzenesulfonyl fluoride (40 mm), 0.1 μg of GST-peptide substrate, and 22.5 nm protease to make a total volume of 45 μl. The reactions were incubated at 37 °C and stopped by adding an equal volume of 2× SDS sample buffer containing 20 mm EDTA. Samples (10 μl) were analyzed by SDS-PAGE on 10–20% gradient gels (Invitrogen), electrotransferred onto PVDF membranes, and incubated with a 1:10,000 dilution of horseradish peroxidase-conjugated rabbit anti-GST antibody (GE Healthcare), and chemiluminescence (ECL Plus; GE Healthcare).
To assess catalytic efficiency (kcat/Km), reactions containing 3–7 nm substrate, 0.5 or 1 nm protease, and 0.05% Tween 20 were incubated at 37 °C for different times (t) and stopped with an equal volume of 50 mm EDTA, and products were analyzed by sandwich ELISA as described (16). Briefly, reactions were adsorbed onto microtiter plates coated with anti-GST antibodies (Pierce), and uncleaved substrates were detected with peroxidase-conjugated anti-His antibodies (Invitrogen). MDTCS13 cleaves GST-gst-VWF73 with Km ≥800 nm (14), and bovine aggrecanase (principally ADAMTS5) cleaves a 40-residue bovine IGD peptide substrate with Km ∼480 μm (27). Therefore, in all reactions the enzyme concentration (E) and initial substrate concentration (S0) are much lower than the Km, and the time courses of product (P) generation were analyzed to obtain kcat/Km (corresponding to the initial rate) by fitting to the following integrated Michaelis-Menten equation.
Cleavage Site Characterization
GST-peptide substrates (5 μg) were incubated with ADAMTS protease (10 nm) in reaction buffer at 37 °C. After 0.5 h, substrate cleavage was confirmed by SDS-PAGE and Western blotting using peroxidase-conjugated anti-His tag antibody (Tetra-His; Qiagen). After incubation for 5 h, the products were separated by SDS-PAGE on a 10–20% gradient gel, transferred onto a PVDF membrane (0.2 μm; Invitrogen), and stained with SimplyBlue SafeStain (Invitrogen). C-terminal product bands (8.8–11 kDa) were excised from the membrane and sequenced by automated Edman degradation (W. M. Keck Foundation Biotechnology Resource Laboratory, Yale University).
Aggrecan Cleavage
ADAMTS variants were incubated with 750 nm bovine aggrecan (Sigma-Aldrich) in reaction buffer at 37 °C, and the reactions were terminated by the addition of an equal volume of 20 mm EDTA. Digestion products were deglycosylated overnight with chondroitinase ABC (0.01 unit/10 μg aggrecan) (Sigma-Aldrich) and keratanase (0.01 unit/10 μg aggrecan) (Sigma-Aldrich). The aggrecan core protein was precipitated with 5 volumes of acetone at −20 °C for 1 h. The pellet was dried, dissolved in reducing SDS sample buffer, and analyzed by 4% SDS-PAGE and Western blotting with mouse monoclonal antibody BC-3 (28) (Abcam; 1:200 dilution) or rabbit polyclonal anti-GELE antibody (8) (provided by Hideaki Nagase; 1:200 dilution) and peroxidase-conjugated goat anti-mouse IgG (1:10,000 dilution, A3673; Sigma) or peroxidase-conjugated goat anti-rabbit IgG (1:10,000 dilution, A0545; Sigma) for detection by chemiluminescence (ECL Plus).
VWF Cleavage
Reactions (20 μl total volume) were performed at room temperature in 0.2-ml PCR tubes (MicroAmp; Applied Biosystems, Inc., Foster City, CA) with purified plasma VWF (15 nm) MDTCS13, MD5/TCS13, or MDTCS5 (20 nm), with or without 10 mm EDTA. Fluid shear stress was applied for 200 s as described (21, 29) using a bench top vortexer (Vortex-Genie 2; Scientific Industries, Inc., Bohemia, NY) at maximal speed (3200 rpm). The reactions were stopped by the addition of an equal volume of SDS sample buffer and analyzed by 4% SDS-PAGE and Western blotting with a 1:2,500 dilution of peroxidase-conjugated rabbit anti-VWF antibody (P226; Dako, Carpinteria, CA) and chemiluminescence (ECL Plus).
RESULTS
ADAMTS Proteases and Extracellular Matrix Binding
Chimeric ADAMTS proteases were designed to assess the ability of noncatalytic domains to determine the cellular localization and substrate specificity of the metalloprotease domain. For ADAMTS13 (Fig. 1A), MDTCS13 was selected as the reference construct because it cleaves model peptide substrates efficiently and is sufficient for shear-dependent cleavage of VWF multimers (11, 13, 30). In addition, the role of ADAMTS13 D, T, C, and S domains in substrate recognition is well supported (11–17, 19, 20, 22, 23).
ADAMTS5 (Fig. 1A) was chosen for domain exchanges because it is at least 20-fold more active than ADAMTS4 toward aggrecan, and ADAMTS5 noncatalytic domains confer increased activity when transferred to ADAMTS4 (10). A truncated ADAMTS5 consisting of only the M domain has little or no proteolytic activity, and sequential addition of DTCS domains progressively increases activity toward many substrates (9). The ADAMTS5 distal thrombospondin type 1 repeat is dispensable for efficient substrate recognition (9), and MDTCS5 was selected as the reference ADAMTS5 construct.
For both ADAMTS13 and ADAMTS5, the smallest known fragment with detectable proteolytic activity consists of MD domains (9, 14), and the crystal structure of ADAMTS5 MD domains indicates that M and D domains are structurally integrated (31). Therefore, the more distal TCS domains were exchanged in chimeric constructs MD13/TCS5 and MD5/TCS13. Additional constructs MDT13/CS5 and MDT5/CS13 were prepared by exchanging the distal CS domains.
Recombinant ADAMTS variants were expressed in stably transfected T-Rex 293 cell lines and analyzed by SDS-PAGE and Western blotting in cell lysates and conditioned medium (Fig. 1B). ADAMTS5 CS domains bind proteoglycans and cause the retention of ADAMTS5 in extracellular matrix unless heparin is included in the cell culture medium (9). The three constructs with distal ADAMT5 domains, MDT13/CS5, MD13/TCS5 and MDTCS5, were released into conditioned medium only in the presence of heparin (Fig. 1B), demonstrating that CS5 domains are sufficient to specify matrix association.
Two constructs with distal ADAMTS13 domains, MD5/TCS13 and MDTCS13, were secreted without added heparin. MDT5/CS13 was retained intracellularly, suggesting that it is misfolded. Another MDT5/CS13 construct with a different junction between the T and C domains (ADAMTS5 Met1–Pro622 and ADAMTS13 Lys440–Ala685) also was not secreted (data not shown).
ADAMTS5 has a 243-amino acid residue propeptide that is processed by furin, and cleavage of the propeptide is required for ADAMTS5 activity (32). Evidence of this proteolytic processing is apparent for MDTCS5 and MD5/TCS13 (Fig. 1B), which were detected in cell lysates mainly (MDTCS5) or entirely (MD5/TCS13) as larger unprocessed ∼100-kDa species but were processed completely to ∼82 kDa species in conditioned medium. ADAMTS13 has a much smaller 41-residue propeptide, and its precursor and processed forms are difficult to resolve by SDS-PAGE (33); size differences were not observed between intracellular and secreted MDT13/CS5, MD13/TCS5, or MDTCS13 (Fig. 1B).
A faint 54-kDa species was detected with anti-V5 antibody in some recombinant proteases that may be a C-terminal proteolytic fragment (Fig. 1B). If so, the size of the fragment is consistent with cleavage in the metalloprotease domain.
ADAMTS Substrates
Chimeric GST-peptide substrates based on the sequences of human VWF and aggrecan (Fig. 1C) were expressed in E. coli and purified to homogeneity (Fig. 1D). Substrate gst-VWF73 corresponds to the 73-residue Asp1596–Arg1668 segment of VWF, which is the smallest VWF fragment that is cleaved efficiently by ADAMTS13 (12). ADAMTS13 D, T, C, and S domains interact with successive segments of this substrate between Asp1614 and Arg1668, and these cooperative interactions accelerate the cleavage of the Tyr1605-Met1606 bond by several orders of magnitude (16, 17, 19, 20).
Substrate gst-IGD contains the Thr331–Gly458 IGD segment of human aggrecan. A similar GST-peptide substrate containing aggrecan Tyr330–Gly457 and a C-terminal FLAG tag (34) is cleaved rapidly at the Glu373–Ala374 bond by ADAMTS5 (10). The gst-VWF/IGD and gst-IGD/VWF substrates exchange N-terminal cleavage sites and C-terminal potential exosite binding sites. gst-VWF/IGD consists of VWF Asp1596–Ile1616 followed by aggrecan Glu394–Gly458 and therefore retains the Tyr1605–Met1606 bond cleaved by ADAMTS13, as well as a Glu1615–Ile1616 bond that might be cleaved by ADAMTS5. Substrate gst-IGD/VWF is the reciprocal construct, with aggrecan Thr331–Leu393 followed by VWF Lys1617–Arg1668. The retained aggrecan sequence includes the entire Gln354–Leu393 peptide previously reported to be cleaved efficiently by bovine aggrecanase (27).
Substrate Specificity of ADAMTS Variants
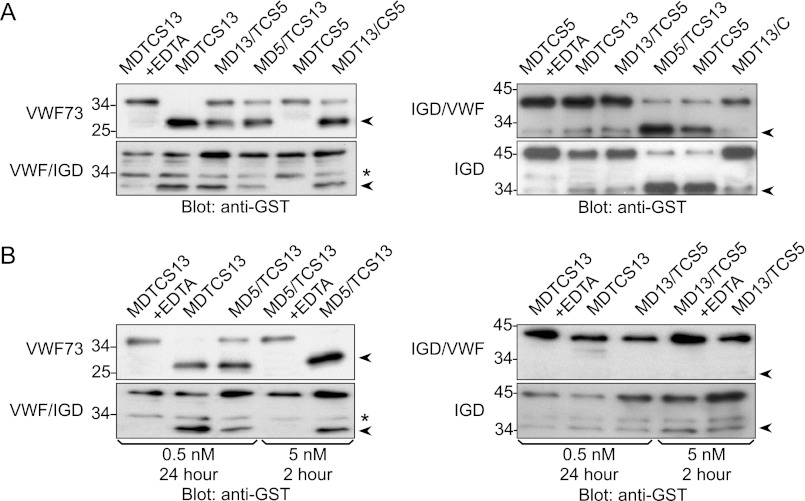
Recombinant proteases in conditioned medium were concentrated by ultrafiltration, dialyzed against reaction buffer, and used without further purification. The activity of ADAMTS proteases toward chimeric substrates was assessed by a semiquantitative Western blot assay method. As expected (16), proteases containing the MD domains of ADAMTS13, MDTCS13, MDT13/CS5, and MD13/TCS5, cleaved gst-VWF73 and gst-VWF/IGD to yield a 28-kDa N-terminal product detected with anti-GST antibody (Fig. 2A). These enzymes cleaved gst-VWF/IGD less completely than gst-VWF73, which is consistent with direct interactions between the Lys1617–Arg1668 segment of VWF and the TCS domains of ADAMTS13 (16). The aggrecan IGD has no obvious potential cleavage sites for ADAMTS13, and MDTCS13, MDT13/CS5, and MD13/TCS5 did not cleave gst-IGD/VWF or gst-IGD to a detectable extent (Fig. 2A), even after increasing the time of digestion from 2 to 24 h or increasing the enzyme concentration 10-fold (Fig. 2B).
FIGURE 2.
Activity of ADAMTS proteases. A, proteases (0.5 nm) and substrates (60–100 nm) without or with EDTA (10 mm) were incubated at 37°C for 2 h. B, proteases (0.5 nm or 5 nm) and substrates (60–100 nm) without or with EDTA (10 mm) were incubated at 37 °C for 24 h or 2 h. Substrates and products (arrowheads) were detected by SDS-PAGE and Western blotting with anti-GST antibody. Cleavage products are 28 kDa for gst-VWF73 and gst-VWF/IGD and 32 kDa for gst-IGD/VWF and gst-IGD. Asterisks indicate nonspecific bands in gst-VWF/IGD. The data shown are representative of at least three independent experiments.
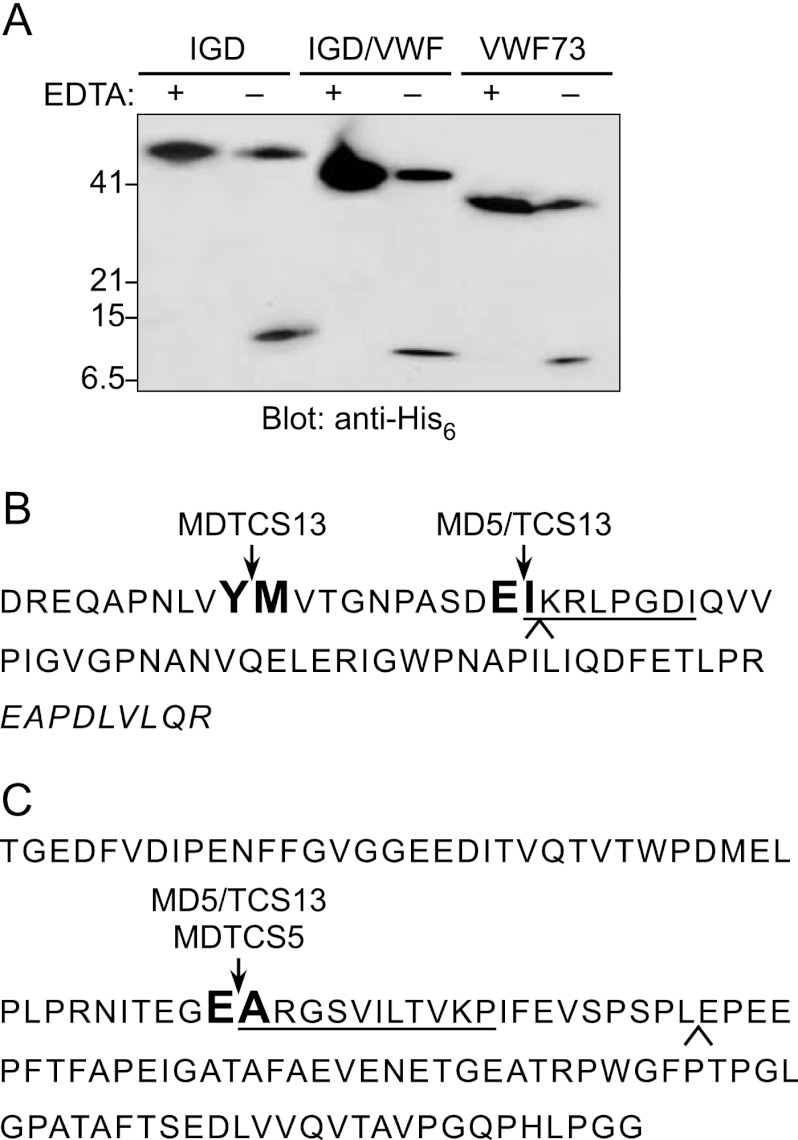
Proteases containing the MD domains of ADAMTS5, MDTCS5 and MD5/TCS13, cleaved gst-IGD and gst-IGD/VWF to generate a 32-kDa product (Fig. 2A). To establish the site of cleavage, MD5/TCS13 was incubated with gst-IGD and gst-IGD/VWF, and C-terminal cleavage products were isolated and sequenced. The N-terminal sequence obtained was ARGSVILTVKP in each case, indicating that MD5/TCS13 cleaves both substrates at the Glu373–Ala374 bond (Fig. 3).
FIGURE 3.
Identification of substrate cleavage sites. A, substrates (5 μg) were incubated with 10 nm MD5/TCS13 at 37 °C for 5 h without (−) or with (+) 10 mm EDTA. At 0.5 h, the reactions were analyzed by SDS-PAGE and Western blotting with anti-His antibody. The size of cleavage products is 7.5 kDa for gst-VWF73, 8.6 kDa for gst-IGD/VWF, and 11 kDa for gst-IGD. B, the sequence of gst-VWF73 is annotated to indicate cleavage sites identified by amino acid sequencing. The Tyr1605–Met1606 bond in gst-VWF73 is cleaved by MDTCS13, MDTC13, MDT13, and MD13 (16, 41). The Glu1615–Ile1616 bond in gst-VWF73 is cleaved by MD5/TCS13. The C-terminal 9 residues indicated by italic letters interact directly with the ADAMTS13 spacer domain. C, the aggrecan IGD sequence included in substrate gst-IGD is shown. The Glu373–Ala374 in gst-IGD/VWF and gst-IGD is cleaved by MD5/TCS13. Amino acid residues identified by Edman degradation are underlined. A caret indicates the boundary between sequences exchanged in substrates gst-VWF/IGD and gst-IGD/VWF.
MDTCS5 could not cleave substrates gst-VWF73 and gst-VWF/IGD, but MD5/TCS13 was able to cleave them (Fig. 2), and the mobility of the N-terminal product on SDS-PAGE was similar to that generated by MDTCS13. Automated Edman degradation of the C-terminal product gave the sequence IKRLPGDI (Fig. 3B), indicating that MD5/TCS13 cleaves the Glu1615–Ile1616 bond of gst-VWF73 or gst-VWF/IGD, 10 residues C-terminal of the Tyr1605–Met1606 bond cleaved by MDTCS13 (16). Prolonged incubation for 24 h or with 10-fold more MD5/TCS13 resulted in complete cleavage of gst-VWF73 but partial cleavage of gst-VWF/IGD (Fig. 2B). Thus, TCS13 domains confer on MD5 the ability to cleave substrates based on the VWF A2 domain, and the chimeric MD5/TCS13 protease prefers gst-VWF73, which contains sequences that interact with TCS13 domains (12, 14, 16, 17).
Catalytic Efficiency of Substrate Cleavage by ADAMTS Variants
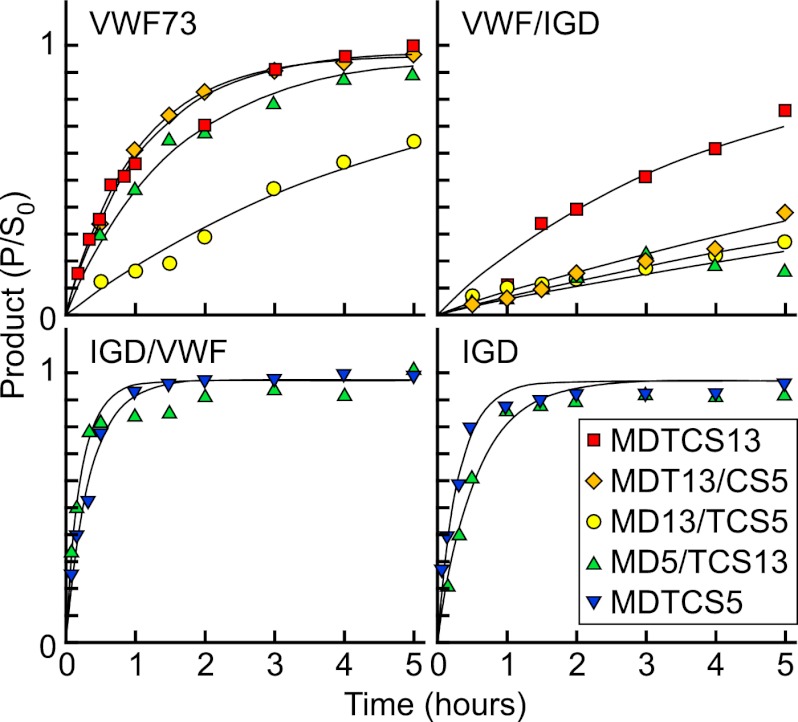
The time course of substrate cleavage (Fig. 4) was analyzed under conditions such that E ≪ Km and S0 ≪ Km. Fitting to the integrated Michaelis-Menten equation yields values for kcat/Km, a measure of catalytic efficiency (Table 1).
FIGURE 4.
Kinetics of substrate cleavage by ADAMTS proteases. The indicated GST-substrates were incubated at 37 °C with MDTCS13 (red squares), MDT13/CS5 (orange diamonds), MD13/TCS5 (yellow circles), MD5/TCS13 (green triangles), or MDTCS5 (blue triangles). The concentration of protease was 1 nm except for the reaction of MDTCS13 (0.5 nm) and VWF73. Cleavage products were quantitated by ELISA, and values for kcat/Km were determined by fitting to the integrated Michaelis-Menten equation (Table 1). The data points represent the mean values for at least two independent experiments.
TABLE 1.
Efficiency of substrate cleavage
Numerical entries are the values ± S.E. for kcat/Km calculated from the time course of GST peptide substrate cleavage (Fig. 4). Substrates not cleaved as determined by Western blotting are indicated by a minus sign (−). Cleavage of bovine aggrecan at Glu373–Ala374 and Glu1480–Gly1481 bonds was analyzed by Western blotting with site-specific antibodies (Fig. 5); a plus sign (+) indicates cleavage, and a minus sign (−) indicates no cleavage.
| Enzyme |
kcat/Km of GST substrate |
Aggrecan site |
||||
|---|---|---|---|---|---|---|
| VWF73 | VWF/IGD | IGD/VWF | IGD | Glu373–Ala374 | Glu1480–Gly1481 | |
| × 104m−1s−1 | ||||||
| MDTCS13 | 49 ± 5 | 11 ± 1 | − | − | − | − |
| MDT13/CS5 | 26 ± 2 | 2.3 ± 0.3 | − | − | − | − |
| MD13/TCS5 | 6.0 ± 0.9 | 1.5 ± 0.3 | − | − | − | − |
| MD5/TCS13 | 15 ± 1 | 1.5 ± 0.4 | 144 ± 16 | 65 ± 5 | + | − |
| MDTCS5 | − | − | 81 ± 6 | 83 ± 5 | + | + |
Substrate gst-VWF/IGD lacks the exosite-binding sequences of gst-VWF73 that are important for VWF recognition (12, 16, 17, 19, 20), and MDTCS13 cleaved gst-VWF73 ∼5-fold more rapidly than gst-VWF/IGD as expected (Table 1). MDT13/CS5 cleaved both substrates more slowly than MDTCS13 but also preferred gst-VWF73 over gst-VWF/IGD, which is consistent with a role for the ADAMTS13 T domain in binding the Gln1624–Val1630 segment that is present in gst-VWF73 but missing from gst-VWF/IGD (16). MD13/TCS5 was less active than MDT13/CS5 and also discriminated less effectively between gst-VWF73 and gst-VWF/IGD; the difference in cleavage rate was ∼4-fold for MD13/TCS5 compared with 10-fold for MDT13/CS5 (Table 1). Thus, replacement of the ADAMTS13 T domain made substrate recognition less dependent on substrate sequences after VWF Ile1616.
Replacing the distal domains of MDTCS5 gave complementary results but with interesting differences. MDTCS5 cleaved substrate gst-IGD with efficiency similar to that with which MDTCS13 cleaved gst-VWF73. MDTCS5 also cleaved gst-IGD and gst-IGD/VWF with similar efficiency, indicating that the C-terminal 65 residues of the IGD domain, Glu394–Gly458, contribute little to substrate recognition by distal domains of MDTCS5. MD5/TCS13 cleaved gst-IGD/VWF ∼2.2-fold more rapidly than gst-IGD (Table 1), indicating that TCS13 domains confer a modest preference for substrates containing their natural binding partners in the Lys1617–Arg1668 segment of VWF. More strikingly, MD5/TCS13 acquired the ability to cleave gst-VWF73 with surprising efficiency. MDTCS5 was inactive toward gst-VWF73, but MD5/TCS13 cleaved gst-VWF73 at nearly one-third the rate of MDTCS13 (Table 1). In addition, MD5/TCS13 cleaved gst-VWF73 ∼10-fold faster than gst-VWF/IGD, indicating that efficient gst-VWF73 recognition was mediated by interactions between distal segments of gst-VWF73 and exosites in TCS13 domains.
Aggrecanase Activity of Recombinant ADAMTS Enzymes
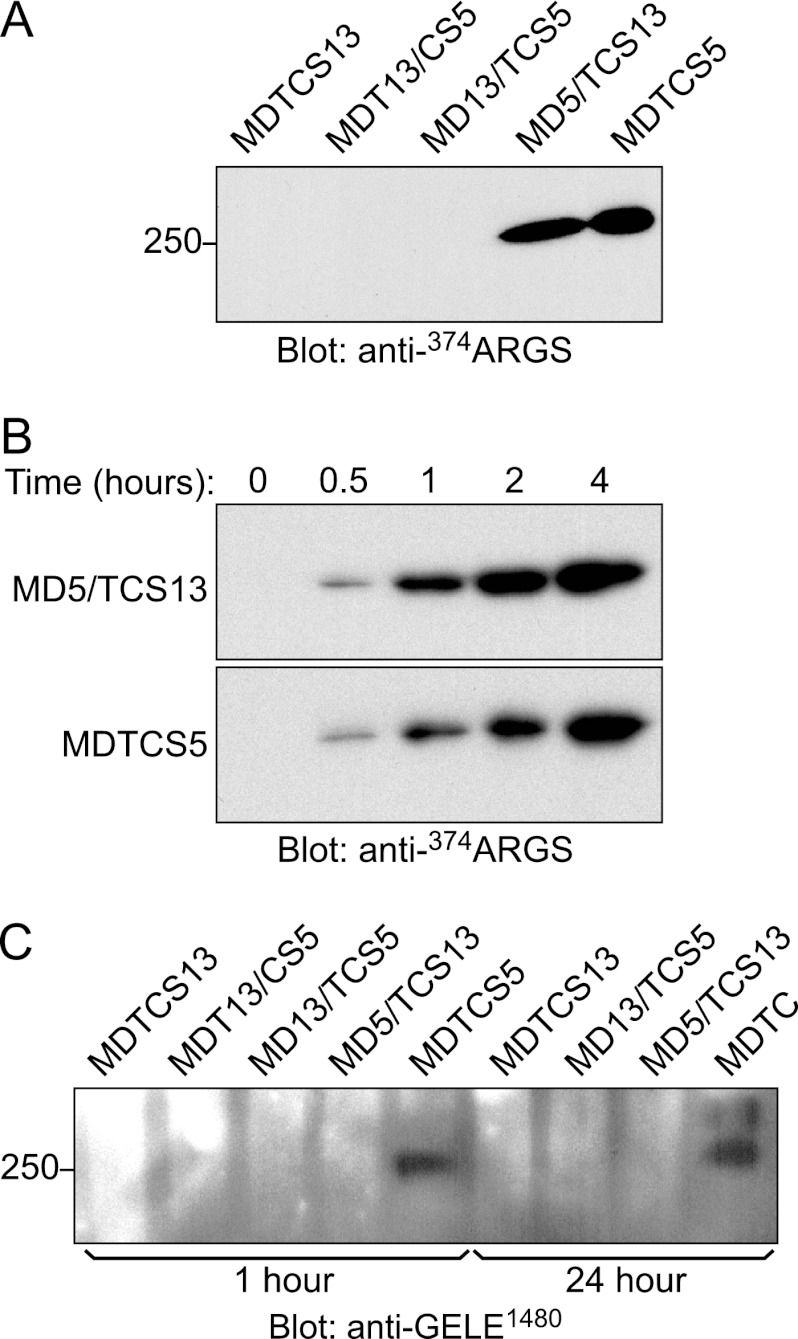
The glycosaminoglycan chains of aggrecan bind ADAMTS5 and influence cleavage by this protease. However, gst-IGD and gst-IGD/VWF substrates (Fig. 1) are not glycosylated and cannot be used to evaluate this relationship. To assess the contribution of glycosylation to substrate recognition, ADAMTS variants were incubated with bovine aggrecan, and specific neoepitopes produced by cleavage were detected by Western blotting (Fig. 5).
FIGURE 5.
Cleavage of aggrecan by ADAMTS proteases. A and B, cleavage at Glu373–Ala374 in the aggrecan IGD was assessed by incubating bovine aggrecan (750 nm) with the indicated enzyme (1 nm) in reaction buffer at 37 °C for 4 h (A) or the indicated time (B). The reactions were terminated with 50 mm EDTA, and samples were deglycosylated and analyzed by SDS-PAGE and Western blotting with antibody BC-3, which recognizes N-terminal 374ARGS (28). C, cleavage at Glu1480–Gly1481 in the aggrecan CS-2 region was assessed by incubating bovine aggrecan (750 nm) with the indicated enzyme (0.2 nm) at 37 °C for 1 or 24 h. After deglycosylation and SDS-PAGE, the products were detected by Western blotting with anti-GELE1480 antibody (8). The data shown are representative of at least three independent experiments.
ADAMTS13 has not been shown to cleave after Glu residues in any substrate, and proteases MDTCS13, MDT13/CS5, and MD13/TCS5 did not generate any products corresponding to cleavage at Glu373–Ala374 (Fig. 5A) or Glu1480–Gly1481 (Fig. 5C). However, both MDTCS5 and MD5/TCS13 cleaved aggrecan at Glu373–Ala374 in the IGD (Fig. 5A), and the time course of cleavage indicated they have approximately equal activity toward this site (Fig. 5B). In contrast to their similar IGD cleaving activity, MDTCS5 cleaved the Glu1480–Gly1481 site in the aggrecan CS-2 domain, but MD5/TCS13 did not (Fig. 5C). Deletion mutagenesis of ADAMTS5 indicates that truncated MD5 cannot cleave either the Glu373–Ala374 or Glu1480–Gly1481 site (9). Appending TCS13 domains in MD5/TCS13 restored cleavage only at Glu373–Ala374 (Fig. 5), indicating that TCS13 exosites discriminate between the aggrecan IGD and CS-2 domains.
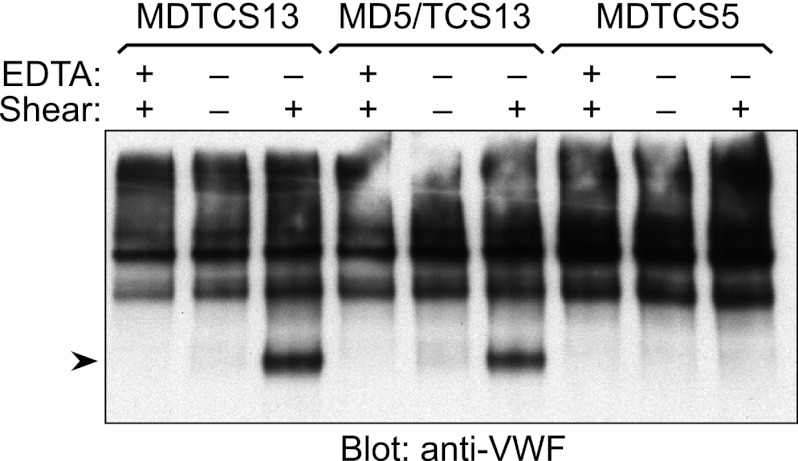
Shear-dependent Cleavage of VWF
MD5/TCS13 acquired the ability to cleave the Glu1615–Ile1616 bond of gst-VWF73, and therefore multimeric VWF was also evaluated as a substrate (Fig. 6). Plasma VWF and ADAMTS proteases were incubated under static conditions or sheared by vortexing for 200 s. The reactions were analyzed by SDS-PAGE and Western blotting to detect VWF cleavage products (21, 29). As a negative control, the reactions were supplemented with EDTA before vortexing. As reported previously (21, 29), plasma VWF was resistant to cleavage by MDTCS13 under static conditions but was cleaved rapidly when the reaction was subjected to fluid shear stress. VWF was resistant to cleavage by MDTCS5, with or without shear stress. However, the results for MD5/TCS13 were similar to those for MDTCS13. VWF was not cleaved by MD5/TCS13 under static conditions but was cleaved under fluid shear stress. Thus, recombining exosites enabled MD5/TCS13 to perform shear-dependent cleavage of VWF multimers.
FIGURE 6.
Cleavage of VWF by ADAMTS proteases. Purified plasma VWF (15 nm) was incubated with 20 nm MDTCS13, MD5/TCS13, or MDTCS5 at room temperature without (−) or with (+) 10 mm EDTA and without (−) or with (+) fluid shear stress. Substrates and the cleavage products (arrowhead) were detected by Western blotting with anti-VWF antibody. The data shown are representative of at least three independent experiments.
DISCUSSION
The noncatalytic domains that characterize the ADAMTS protease family mediate several distinct functions in different family members, including substrate recognition, tissue localization, and angiogenesis (1). We have used a domain substitution approach to investigate the role of noncatalytic domains in ADAMTS5 and ADAMTS13. In addition we prepared chimeric model substrates in which cleavage sites and ancillary binding sites were exchanged between VWF domain A2 and aggrecan IGD. The results show that the catalytic center and noncatalytic domains can cooperate to determine substrate specificity, but the importance of each interaction varies considerably for different enzyme-substrate combinations.
MDTCS5 cleaved gst-IGD efficiently as expected but also cleaved gst-IGD/VWF at essentially the same rate. Furthermore, MD5/TCS13 cleaved gst-IGD rapidly despite the absence of distal ADAMTS5 domains. These results suggest that the C-terminal segment of the nonglycyosylated IGD polypeptide does not interact functionally with TCS5 exosites. In addition, MDTCS5 and MD5/TCS13 both cleaved the Glu373–Ala374 bond of native bovine aggrecan with similar efficiency, indicating that TCS5 exosites that interact with glycosaminoglycans (9) may not contribute to cleavage of the aggrecan IGD.
Although MD5/TCS13 cleaved the aggrecan IGD efficiently, it could not cleave the Glu1480–Gly1481 bond in the aggrecan CS-2 domain. However, studies of ADAMTS5/ADAMTS4 chimeric proteases showed that MD5/TCS4 cleaved both IGD and CS-2 sites normally (10). Thus, when combined with MD5, TCS4 can functionally replace TCS5 for both IGD and CS2 cleavage, whereas MD5/TCS13 only preserves IGD cleavage. These results are consistent with a model proposed by Fushimi et al. (10) in which cleavage of the Glu373–Ala374 site depends mainly on interactions between protease MD domains and the IGD polypeptide, whereas cleavage of the Glu1480–Gly1481 bond requires interactions between the TCS domains of ADAMTS4 or ADAMTS5 and CS-2 glycosaminoglycan chains.
The ADAMTS13 construct MDTCS13 predictably cleaved gst-VWF73 ∼5-fold more rapidly than gst-VWF/IGD, which lacks the exosite binding sequences that promote the efficient recognition of gst-VWF73 (12, 14, 16, 17). Unexpectedly, MD5/TCS13 also cleaved gst-VWF73 very efficiently, ∼10-fold more rapidly than gst-VWF/IGD, although MDTCS5 was completely inactive toward these substrates. Therefore, appending TCS13 dramatically altered the specificity of MD5, enabling it to cleave an otherwise resistant substrate, and cleavage depended on TCS13 exosite interactions with gst-VWF73 that cannot occur for gst-VWF/IGD. These exosite-substrate interactions are consistent with loss of function phenotypes induced by engineered deletion and missense mutations in ADAMTS13 and VWF (11–17, 19, 22, 23). The gain of function produced by transferring functional ADAMTS13 exosites onto ADAMTS5, in construct MD5/TCS13, shows definitively that TCS13 domains are critical for recognizing and cleaving VWF because they bind to a C-terminal segment of the A2 domain.
Although the ADAMTS5 metalloprotease domain could be induced to cleave VWF in construct MD5/TCS13, none of the tested proteases that contained an ADAMTS13 active site was able to cleave aggrecan IGD sequences or native aggrecan core protein, at least at the sites recognized by aggrecanase neoepitope antibodies. This result probably reflects the extraordinarily strict specificity of ADAMTS13 for the sequence Leu-Xaa-Tyr-Met (35, 36), which occurs once in VWF and not at all in aggrecan.
The physiological cleavage of VWF by ADAMTS13 is regulated by fluid shear stress, which unfolds the A2 domain and exposes the buried scissile bond. MDTCS5 did not cleave plasma VWF detectably, but chimeric MD5/TCS13 acquired the ability to cleave VWF in a shear-dependent manner, most likely at the Glu1615–Ile1616 bond that is cleaved in gst-VWF73. In the A2 domain crystal structure, Glu1615–Ile1616 is located on the surface in an extended segment referred to as the α4-less loop (37). The side chain of Glu1615 projects into solvent, but Ile1616 is buried. Exposure of this bond by shear-induced unfolding of VWF was not sufficient to render it sensitive to MDTCS5. However, exosites conferred by TCS13 domains enabled MD5/TCS13 to recognize and cleave VWF with remarkable efficiency and with regulation by fluid shear stress.
The Glu1615–Ile1616 selected by MD5/TCS13 is one of six Glu-Xaa bonds in gst-VWF73, and the predilection for this bond reflects the specificity of the ADAMTS5 metalloprotease domain, as well as geometric constraints on positioning a substrate between the active site and exosites. ADAMTS5 prefers certain Glu-Gly, Glu-Leu, and Glu-Ala bonds in aggrecan (4), but little is known about its specificity for extended sequences adjacent to these sites. It seems likely that modifying the scissile bond environment to better match the specificity of ADAMTS5 would further enhance the shear-dependent cleavage of VWF by MD5/TCS13.
The evolution of ADAMTS proteases has involved many instances of domain duplication and shuffling (38). These proteins have diverged considerably—ADAMTS5 and ADAMTS13 are just 29% identical over the domains they share—and the reshuffling of domains in vitro is a powerful strategy to investigate structure-function relationships. Our studies and others (10, 39, 40) indicate that ADAMTS noncatalytic domains often are portable, easily transferred between family members with retention of structural integrity. In addition, their exosite functions tend to be modular, corresponding to one or a few domains. Finally, the lack of stringent distance constraints allows new combinations of exosites and active sites to manifest new proteolytic activities, which could have practical applications. For example, MD5/TCS13 might be able to restore regulated proteolysis of VWF in patients with thrombotic thrombocytopenic purpura caused by autoantibodies against the MD domains of ADAMTS13.
Noncatalytic domains strongly determine the specificity of ADAMTS5 and ADAMTS13, supporting the principle that ADAMTS proteases require their noncatalytic domains to recognize substrates and localize their proteolytic activity (1). A corollary might be that targeting of noncatalytic domains should inactivate ADAMTS proteases in vivo. In a way, this concept is validated by the observation that autoantibodies against the ADAMTS13 spacer domain can inhibit protease activity enough to cause thrombotic thrombocytopenic purpura (5, 6). This proof of principle experiment of nature suggests that intentionally targeting the exosites of other ADAMTS proteases may be therapeutically useful.
Acknowledgments
We thank Dr. Richard Leduc (Université de Sherbrooke) for an ADAMTS5 cDNA, and Dr. Hideaki Nagase (Imperial College London) for anti-GELE1480 cleavage site antibody.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 HL72917, R01 HL89746, and U54 HL112303.
- M domain
- metalloprotease domain
- D domain
- disintegrin-like domain
- T domain
- thrombospondin-1 domain
- C domain
- cysteine-rich domain
- S domain
- spacer domain
- VWF
- von Willebrand factor
- CS
- chondroitin sulfate
- IGD
- interglobular domain.
REFERENCES
- 1. Apte S. S. (2009) A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily. Functions and mechanisms. J. Biol. Chem. 284, 31493–31497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Glasson S. S., Askew R., Sheppard B., Carito B., Blanchet T., Ma H. L., Flannery C. R., Peluso D., Kanki K., Yang Z., Majumdar M. K., Morris E. A. (2005) Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature 434, 644–648 [DOI] [PubMed] [Google Scholar]
- 3. Stanton H., Rogerson F. M., East C. J., Golub S. B., Lawlor K. E., Meeker C. T., Little C. B., Last K., Farmer P. J., Campbell I. K., Fourie A. M., Fosang A. J. (2005) ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature 434, 648–652 [DOI] [PubMed] [Google Scholar]
- 4. Fosang A. J., Rogerson F. M., East C. J., Stanton H. (2008) ADAMTS-5. The story so far. Eur. Cell Mater. 15, 11–26 [DOI] [PubMed] [Google Scholar]
- 5. Furlan M., Robles R., Galbusera M., Remuzzi G., Kyrle P. A., Brenner B., Krause M., Scharrer I., Aumann V., Mittler U., Solenthaler M., Lämmle B. (1998) von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. N. Engl. J. Med. 339, 1578–1584 [DOI] [PubMed] [Google Scholar]
- 6. Tsai H. M., Lian E. C. (1998) Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N. Engl. J. Med. 339, 1585–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Levy G. G., Nichols W. C., Lian E. C., Foroud T., McClintick J. N., McGee B. M., Yang A. Y., Siemieniak D. R., Stark K. R., Gruppo R., Sarode R., Shurin S. B., Chandrasekaran V., Stabler S. P., Sabio H., Bouhassira E. E., Upshaw J. D., Jr., Ginsburg D., Tsai H. M. (2001) Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature 413, 488–494 [DOI] [PubMed] [Google Scholar]
- 8. Kashiwagi M., Enghild J. J., Gendron C., Hughes C., Caterson B., Itoh Y., Nagase H. (2004) Altered proteolytic activities of ADAMTS-4 expressed by C-terminal processing. J. Biol. Chem. 279, 10109–10119 [DOI] [PubMed] [Google Scholar]
- 9. Gendron C., Kashiwagi M., Lim N. H., Enghild J. J., Thøgersen I. B., Hughes C., Caterson B., Nagase H. (2007) Proteolytic activities of human ADAMTS-5. Comparative studies with ADAMTS-4. J. Biol. Chem. 282, 18294–18306 [DOI] [PubMed] [Google Scholar]
- 10. Fushimi K., Troeberg L., Nakamura H., Lim N. H., Nagase H. (2008) Functional differences of the catalytic and non-catalytic domains in human ADAMTS-4 and ADAMTS-5 in aggrecanolytic activity. J. Biol. Chem. 283, 6706–6716 [DOI] [PubMed] [Google Scholar]
- 11. Zheng X., Nishio K., Majerus E. M., Sadler J. E. (2003) Cleavage of von Willebrand factor requires the spacer domain of the metalloprotease ADAMTS13. J. Biol. Chem. 278, 30136–30141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kokame K., Matsumoto M., Fujimura Y., Miyata T. (2004) VWF73, a region from D1596 to R1668 of von Willebrand factor, provides a minimal substrate for ADAMTS-13. Blood 103, 607–612 [DOI] [PubMed] [Google Scholar]
- 13. Ai J., Smith P., Wang S., Zhang P., Zheng X. L. (2005) The proximal carboxyl-terminal domains of ADAMTS13 determine substrate specificity and are all required for cleavage of von Willebrand factor. J. Biol. Chem. 280, 29428–29434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gao W., Anderson P. J., Majerus E. M., Tuley E. A., Sadler J. E. (2006) Exosite interactions contribute to tension-induced cleavage of von Willebrand factor by the antithrombotic ADAMTS13 metalloprotease. Proc. Natl. Acad. Sci. U.S.A. 103, 19099–19104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zanardelli S., Crawley J. T., Chion C. K., Lam J. K., Preston R. J., Lane D. A. (2006) ADAMTS13 substrate recognition of von Willebrand factor A2 domain. J. Biol. Chem. 281, 1555–1563 [DOI] [PubMed] [Google Scholar]
- 16. Gao W., Anderson P. J., Sadler J. E. (2008) Extensive contacts between ADAMTS13 exosites and von Willebrand factor domain A2 contribute to substrate specificity. Blood 112, 1713–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu J. J., Fujikawa K., McMullen B. A., Chung D. W. (2006) Characterization of a core binding site for ADAMTS-13 in the A2 domain of von Willebrand factor. Proc. Natl. Acad. Sci. U.S.A. 103, 18470–18474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zanardelli S., Chion A. C., Groot E., Lenting P. J., McKinnon T. A., Laffan M. A., Tseng M., Lane D. A. (2009) A novel binding site for ADAMTS13 constitutively exposed on the surface of globular VWF. Blood 114, 2819–2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Akiyama M., Takeda S., Kokame K., Takagi J., Miyata T. (2009) Crystal structures of the noncatalytic domains of ADAMTS13 reveal multiple discontinuous exosites for von Willebrand factor. Proc. Natl. Acad. Sci. U.S.A. 106, 19274–19279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Groot R., Bardhan A., Ramroop N., Lane D. A., Crawley J. T. (2009) Essential role of the disintegrin-like domain in ADAMTS13 function. Blood 113, 5609–5616 [DOI] [PubMed] [Google Scholar]
- 21. Feys H. B., Anderson P. J., Vanhoorelbeke K., Majerus E. M., Sadler J. E. (2009) Multi-step binding of ADAMTS-13 to von Willebrand factor. J. Thromb. Haemost. 7, 2088–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jin S. Y., Skipwith C. G., Zheng X. L. (2010) Amino acid residues Arg659, Arg660, and Tyr661 in the spacer domain of ADAMTS13 are critical for cleavage of von Willebrand factor. Blood 115, 2300–2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pos W., Crawley J. T., Fijnheer R., Voorberg J., Lane D. A., Luken B. M. (2010) An autoantibody epitope comprising residues R660, Y661, and Y665 in the ADAMTS13 spacer domain identifies a binding site for the A2 domain of VWF. Blood 115, 1640–1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Majerus E. M., Anderson P. J., Sadler J. E. (2005) Binding of ADAMTS13 to von Willebrand factor. J. Biol. Chem. 280, 21773–21778 [DOI] [PubMed] [Google Scholar]
- 25. Geiser M., Cebe R., Drewello D., Schmitz R. (2001) Integration of PCR fragments at any specific site within cloning vectors without the use of restriction enzymes and DNA ligase. BioTechniques 31, 88–90, 92 [DOI] [PubMed] [Google Scholar]
- 26. Kokame K., Nobe Y., Kokubo Y., Okayama A., Miyata T. (2005) FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br. J. Haematol. 129, 93–100 [DOI] [PubMed] [Google Scholar]
- 27. Miller J. A., Liu R. Q., Davis G. L., Pratta M. A., Trzaskos J. M., Copeland R. A. (2003) A microplate assay specific for the enzyme aggrecanase. Anal. Biochem. 314, 260–265 [DOI] [PubMed] [Google Scholar]
- 28. Hughes C. E., Caterson B., Fosang A. J., Roughley P. J., Mort J. S. (1995) Monoclonal antibodies that specifically recognize neoepitope sequences generated by “aggrecanase” and matrix metalloproteinase cleavage of aggrecan. Application to catabolism in situ and in vitro. Biochem. J. 305, 799–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang P., Pan W., Rux A. H., Sachais B. S., Zheng X. L. (2007) The cooperative activity between the carboxyl-terminal TSP1 repeats and the CUB domains of ADAMTS13 is crucial for recognition of von Willebrand factor under flow. Blood 110, 1887–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Soejima K., Matsumoto M., Kokame K., Yagi H., Ishizashi H., Maeda H., Nozaki C., Miyata T., Fujimura Y., Nakagaki T. (2003) ADAMTS-13 cysteine-rich/spacer domains are functionally essential for von Willebrand factor cleavage. Blood 102, 3232–3237 [DOI] [PubMed] [Google Scholar]
- 31. Mosyak L., Georgiadis K., Shane T., Svenson K., Hebert T., McDonagh T., Mackie S., Olland S., Lin L., Zhong X., Kriz R., Reifenberg E. L., Collins-Racie L. A., Corcoran C., Freeman B., Zollner R., Marvell T., Vera M., Sum P. E., Lavallie E. R., Stahl M., Somers W. (2008) Crystal structures of the two major aggrecan degrading enzymes, ADAMTS4 and ADAMTS5. Protein Sci. 17, 16–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Longpré J. M., McCulloch D. R., Koo B. H., Alexander J. P., Apte S. S., Leduc R. (2009) Characterization of proADAMTS5 processing by proprotein convertases. Int. J. Biochem. Cell Biol. 41, 1116–1126 [DOI] [PubMed] [Google Scholar]
- 33. Majerus E. M., Zheng X., Tuley E. A., Sadler J. E. (2003) Cleavage of the ADAMTS13 propeptide is not required for protease activity. J. Biol. Chem. 278, 46643–46648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lim N. H., Kashiwagi M., Visse R., Jones J., Enghild J. J., Brew K., Nagase H. (2010) Reactive-site mutants of N-TIMP-3 that selectively inhibit ADAMTS-4 and ADAMTS-5. Biological and structural implications. Biochem. J. 431, 113–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pruss C. M., Notley C. R., Hegadorn C. A., O'Brien L. A., Lillicrap D. (2008) ADAMTS13 cleavage efficiency is altered by mutagenic and, to a lesser extent, polymorphic sequence changes in the A1 and A2 domains of von Willebrand factor. Br. J. Haematol. 143, 552–558 [DOI] [PubMed] [Google Scholar]
- 36. Xiang Y., de Groot R., Crawley J. T., Lane D. A. (2011) Mechanism of von Willebrand factor scissile bond cleavage by a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13). Proc. Natl. Acad. Sci. U.S.A. 108, 11602–11607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang Q., Zhou Y. F., Zhang C. Z., Zhang X., Lu C., Springer T. A. (2009) Structural specializations of A2, a force-sensing domain in the ultralarge vascular protein von Willebrand factor. Proc. Natl. Acad. Sci. U.S.A. 106, 9226–9231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nicholson A. C., Malik S. B., Logsdon J. M., Jr., Van Meir E. G. (2005) Functional evolution of ADAMTS genes. Evidence from analyses of phylogeny and gene organization. BMC Evol. Biol. 5, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Colige A., Ruggiero F., Vandenberghe I., Dubail J., Kesteloot F., Van Beeumen J., Beschin A., Brys L., Lapière C. M., Nusgens B. (2005) Domains and maturation processes that regulate the activity of ADAMTS-2, a metalloproteinase cleaving the aminopropeptide of fibrillar procollagens types I-III and V. J. Biol. Chem. 280, 34397–34408 [DOI] [PubMed] [Google Scholar]
- 40. Luken B. M., Turenhout E. A., Kaijen P. H., Greuter M. J., Pos W., van Mourik J. A., Fijnheer R., Voorberg J. (2006) Amino acid regions 572–579 and 657–666 of the spacer domain of ADAMTS13 provide a common antigenic core required for binding of antibodies in patients with acquired TTP. Thromb. Haemost. 96, 295–301 [DOI] [PubMed] [Google Scholar]
- 41. Wu J. J., Fujikawa K., Lian E. C., McMullen B. A., Kulman J. D., Chung D. W. (2006) A rapid enzyme-linked assay for ADAMTS-13. J. Thromb. Haemost. 4, 129–136 [DOI] [PubMed] [Google Scholar]