Background: Sox2 and Oct1 interact on a variety of promoters to regulate transcription.
Results: Global intermolecular translocation rates in a ternary Sox2·Oct1·FGF4-DNA complex have been analyzed by z-exchange spectroscopy.
Conclusion: Translocation is modulated by protein/protein interactions on the DNA.
Significance: The data suggest a model for the sequence of binding events involved in combinatorial control of gene regulation by Sox2 and Oct1.
Keywords: Gene Regulation, General Transcription Factors, Nuclear Magnetic Resonance, Protein/DNA Interaction, Transcription Regulation, Jumping And Intersegment Transfer, Translocation, z-Exchange Spectroscopy
Abstract
Oct1 and Sox2 synergistically regulate developmental genes by binding to adjacent sites within promoters. We have investigated the kinetics of global intermolecular translocation of Sox2 and Oct1 between cognate sites located on different DNA molecules by z-exchange NMR spectroscopy. In the Hoxb1 promoter, the Sox2 and Oct1 sites are immediately adjacent to one another, and the intermolecular translocation rates are too slow to be measured by z-exchange spectroscopy. By introducing a 3-bp insertion between the Sox2 and Oct1 sites to mimic the spacing in the FGF4 enhancer, the interprotein contact surface is reduced, and the translocation rates are increased. Interaction between Sox2 and the POU-specific domain (POUS) of Oct1 does not affect the translocation mechanism but modulates the rates. Translocation involves only jumping (dissociation and reassociation) for Sox2, but both jumping and direct intersegment transfer (no dissociation into free solution) for Oct1. The dissociation (koff ∼1.5 s−1) and association (kon ∼5.1 × 109 m−1s−1) rate constants for Sox2 are reduced 4-fold and increased 5-fold, respectively, in the presence of Oct1. koff (∼3.5 s−1) for Oct1 is unaffected by Sox2, whereas kon (∼1.3 × 109 m−1s−1) is increased ∼13-fold. The direct intermolecular translocation rate (kinter ∼1.8 × 104 m−1s−1) for the POUS domain of Oct1 is reduced 2-fold by Sox2, whereas that for the POU homeodomain (POUHD) of Oct1 (kinter ∼ 1.7 × 104 m−1s−1) remains unaltered, consistent with the absence of contacts between Sox2 and POUHD. The data suggest a model for the sequence of binding events involved in synergistic gene regulation by Sox2 and Oct1.
Introduction
In eukaryotes, combinatorial control of gene expression involves the formation of multi-transcription factor complexes that effectively integrate a wide range of signaling pathways to provide temporal and cell-specific transcription regulation (1). An example of this phenomenon is provided by members of the Sox and Oct transcription factor families that interact with a variety of DNA promoter/enhancer elements to regulate transcription during embryogenesis and neural development (2, 3). Sox2 is a member of the HMG box family of architectural factors that bind to the minor groove of DNA and bend the DNA by 50–90° (4). Oct1 comprises two major groove DNA binding domains, a POU-specific domain (POUS)3 and a homeodomain (POUHD), connected by a flexible linker (5, 6). Structures of ternary complexes of Sox2 and Oct1 bound to regulatory elements within the Hoxb1 promoter (7) and fibroblast growth factor-4 (FGF4) enhancer (8), differing in the spacing between the Sox2 and Oct1 binding sites, have also been solved by NMR and crystallography, respectively. Although three-dimensional structures of these binary and ternary protein-DNA complexes have yielded a wealth of static information regarding the structural basis of protein-DNA recognition by Sox2 and Oct1, less is known of the mechanisms whereby these transcription factors locate their specific target sites within an overwhelming sea of nonspecific DNA (9–11), especially within the context of multi-transcription factor complexes.
Recently, we have made use of NMR paramagnetic relaxation enhancement measurements (12, 13) to detect and characterize transient sparsely populated, spectroscopically invisible states of protein-DNA complexes that are critical to the target search process (14–19). In the context of a specific complex, these intermediate states, which occupy nonspecific DNA sites and have lifetimes of less than 250–500 μs, are populated at less than 0.5% and are involved in both one-dimensional rotation-coupled sliding along the DNA and direct intersegment transfer from one DNA molecule to another. This methodology has been used to study target searching by the homeodomain transcription factor HoxD9 (15, 16), the bidomain transcription factor Oct1 (17), the minor groove binding architectural factor Sox2 (18), and a ternary complex of Oct1 and Sox2 bound to the regulatory element within the Hoxb1 promoter (18).
In addition to rapid translocation events involving sparsely populated states, global intermolecular translocation of the major spectroscopically visible species between specific sites on different DNA molecules occurs on a much slower time scale (10 ms to 1 s) and can be directly observed and kinetically analyzed using two-dimensional z-exchange NMR spectroscopy (18–21). In the case of HoxD9, global intermolecular translocation occurs exclusively by direct intersegment transfer without necessitating dissociation of the protein into free solution (20). For Sox2, on the other hand, global intermolecular translocation between specific DNA sites proceeds entirely by jumping, a process that entails complete dissociation of Sox2 from the DNA into free solution followed by reassociation (18). Direct intersegment transfer and, to a lesser extent, jumping occur with Oct1 (21). When Oct1 and Sox2 form a ternary complex on the Hoxb1 promoter, the translocation rates between specific DNA sites are reduced by over an order of magnitude and can no longer be studied by z-exchange spectroscopy (18).
To study the mechanism and kinetics of global intermolecular translocation of the protein components within an Oct1·Sox2·DNA ternary complex, we therefore chose to focus on the FGF4 enhancer, where the spacing between the Oct1 and Sox2 recognition sites is increased by 3 bp (22) relative to that within the Hoxb1 regulatory element (23) (see Fig. 1A). The interaction surface between Oct1 and Sox2 in the ternary complex on the FGF4 enhancer (8) is altered and reduced relative to that on Hoxb1 (7), and as a result, the strength of the interaction between the two proteins is weakened, and the rate of translocation is increased sufficiently to permit the application of z-exchange spectroscopy. Here we show how protein/protein interactions between Oct1 and Sox2 on the FGF4 promoter modulate the kinetics of global intermolecular translocation.
FIGURE 1.
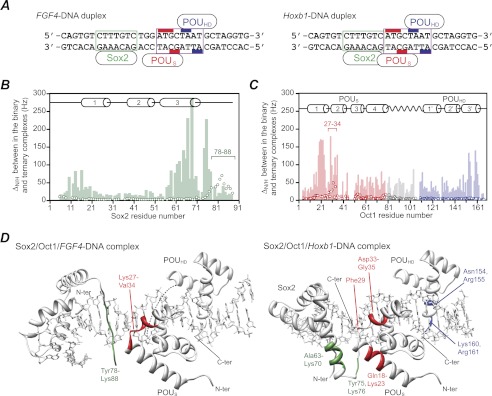
Comparison of chemical shift mapping of the protein/protein interaction surfaces at the Sox2 and Oct1 interfaces in the Sox2·Oct1·FGF4-DNA and Sox2·Oct1·Hoxb1-DNA ternary complexes. A, FGF4 (left) and Hoxb1 (right) DNA duplexes. The Sox2 and Oct1 binding sites are delineated by the boxes in green and purple, respectively. The bases that interact with the POUS and POUHD domains of Oct1 are indicated by the red and blue bars, respectively. The Hoxb1-DNA duplex represents the actual sequence from the Hoxb1 promoter (18). The FGF4-DNA duplex is not the actual sequence present in the FGF4 enhancer but simply represents the Hoxb1 sequence with the 3-bp insertion between the Sox2 and Oct1 sites from the FGF4 enhancer element. As explained under “Results”, this was done to ensure that differences in equilibrium and kinetic rate constants reflect only the different spacing of the Sox2 and Oct1 sites. B and C, profiles of backbone 1HN/15N chemical shift differences (ΔH/N) between the ternary and binary complexes on the FGF4-DNA (open circles) and Hoxb1-DNA (light bar) duplexes for Sox2 (green) (B) and Oct1 (red, POUS domain; blue, POUHD domain; gray, linker) (C). ΔH/N is calculated from ((ΔνH)2 + (ΔνN)2)½ in Hz at a 1H frequency of 600 MHz. D, residues showing significant 1HN/15N chemical shift perturbations mapped onto the structures of the Sox2·Oct1·FGF4-DNA (left, ΔH/N > 20 Hz, Protein Data Bank (PDB) code 1gt0 (8)) and Sox2·Oct1·Hoxb1-DNA (right, ΔN/H > 100 Hz, PDB code 1o4x (7)) ternary complexes. C-ter, C terminus; N-ter, N terminus.
EXPERIMENTAL PROCEDURES
Sample Preparation
The POU region (POUS + POUHD) of human Oct1 (residues 280–442) and the HMG box domain of Sox2 (residues 38–121) were expressed and purified as described previously (7, 18). Uniform 2H/15N isotopic labeling was achieved by growing Escherichia coli BL21-CodonPlus(DE3)-RIPL cells in minimal medium with 99.9% D2O, d7-glucose and 15NH4Cl. Single-stranded unmodified and rhodamine-conjugated DNA oligonucleotides were purchased from Invitrogen and Midland Certified Reagents, respectively, and purified by anion-exchange chromatography on a Mono-Q (GE Healthcare) column with NaCl gradient in a buffer of 50 mm Tris-HCl, pH 7.5, and 1 mm EDTA. After annealing, DNA duplexes were further purified by anion-exchange chromatography to remove any residual single-stranded DNA (24). Fluorescence anisotropy and NMR samples were prepared in 10 mm PIPES, 150 mm NaCl, 94% H2O, 6% D2O, pH 6.5.
Fluorescence Anisotropy
The KD for the binding of Sox2 and Oct1 to FGF4-DNA and of Oct1 to the Sox2·FGF4-DNA complex at 30 °C was determined by fluorescence anisotropy using a Jobin-Yvon FluoroMax-3 spectrometer as described previously (16). The wavelengths for excitation and emission were 550 and 580 nm, respectively. Sox2 (0–154 nm) and Oct1 (0–351 nm) were added to 1.5 and 10 nm rhodamine-conjugated 32-bp FGF4-DNA duplex, respectively. The KD was calculated from the titration data as described previously (14). Because the difference in fluorescence anisotropy for the binding of Sox2 to the Oct1·FGF4-DNA complex is too small to permit an accurate KD determination, the KD for Oct1 binding to FGF4-DNA (10 nm) in the presence of Sox2 (100 nm) was measured. Under these conditions, ∼95% of the DNA is present as a specific Oct1 complex, and <3% is present as a nonspecific complex. The effect of Oct1 on the KD for Sox2 binding was then determined from a thermodynamic cycle.
NMR Spectroscopy
All NMR experiments were carried out at 303 K on Bruker 600-MHz spectrometers equipped with z-gradient triple resonance cryoprobes. Spectra were processed using NMRPipe (25) and analyzed using the program NMRView (26).
Exchange rates were measured using transverse relaxation optimized spectroscopy (TROSY)-based z-exchange (27) with at least eight different mixing times between 20 and 600 ms. Fitting the time dependence of the exchange and auto peaks to derive kinetic rate constants was as described in Refs. 20 and 21.
RESULTS AND DISCUSSION
Interaction of Sox2 and Oct1 on the FGF4 Promoter
In the Hoxb1-DNA promoter, the Sox2 and Oct1 binding sites are immediately adjacent to one another (7, 23), whereas there is a 3-bp insertion between the Sox2 and Oct1 cognate sites in the FGF4 enhancer (8, 22). The sequence of the 32 bp FGF4-like DNA duplex (hereafter referred to as FGF4-DNA) containing the specific binding sites for Sox2 and Oct1 is shown in Fig. 1A (left). This sequence does not represent the actual sequence within the FGF4 enhancer, but rather simply adds the 3-bp insertion (TGG) between the Sox2 and Oct1 binding sites found in the FGF4 enhancer to the Hoxb1 promoter sequence (Fig. 1A, right). The sequences of the Sox2- and Oct1-specific sites as well as the sequences on the 5′ end of the Sox2 and 3′ end of the Oct1 binding sites are thus identical to the Hoxb1 promoter sequence. This ensures that differences in equilibrium dissociation constants and rates of intermolecular translocation between the FGF4 enhancer and Hoxb1 promoter DNA duplexes reflect only the impact of the 3-bp insertion between the Sox2 and Oct1 binding sites.
The different spacing of the Sox2- and Oct1-specific sites on the FGF4-DNA and Hoxb1-DNA duplexes alters the relative orientations of the two proteins and the protein/protein interface in the two ternary complexes (7, 8). In the Hoxb1 ternary complex, the protein/protein interface is formed between residues Lys-59 to Lys-73 (helix 3) of Sox2 and residues Lys-14 to Thr-26 (helix 1) of the POUS domain of Oct1 (7). The protein/protein interface on the FGF4 enhancer, on the other hand, involves only two residues from Sox2 (Arg-81 and Arg-82) and four residues from the POUS domain (Ile-25, Gly-28, Thr-30, and Asp-33) (8). This is in complete agreement with the location and breadth of the corresponding 1HN/15N chemical shift perturbation profiles observed for the two ternary complexes relative to the binary complexes (Fig. 1, B–D). Moreover, the buried accessible surface at the Sox2/POUS interface on the FGF4 enhancer (∼240 Å2) is approximately half that on the Hoxb1 promoter (∼540 Å2) (7, 8), which would predict larger dissociation rate constants for Sox2 and Oct1 in the FGF4-DNA ternary complex (see below).
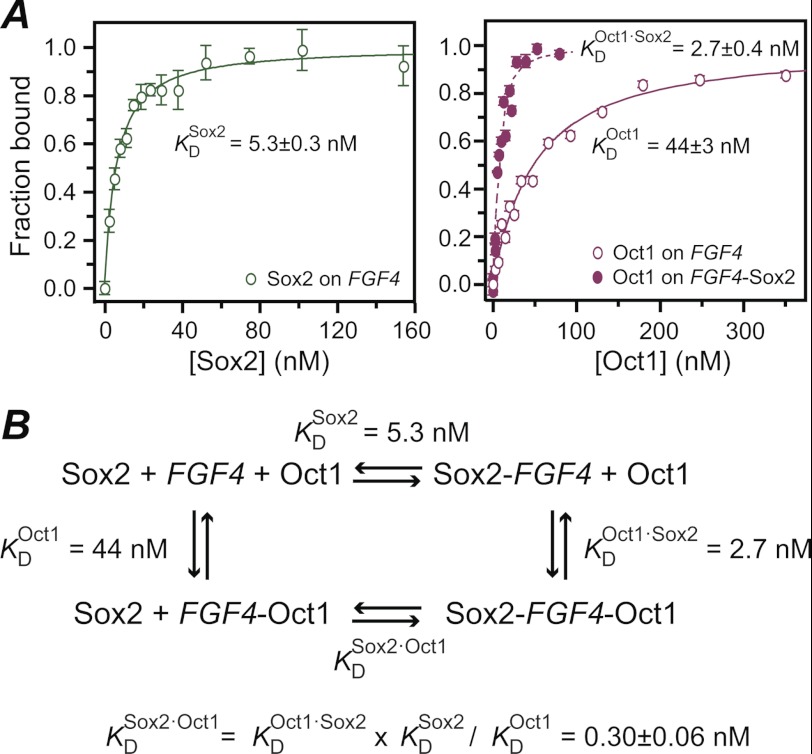
Equilibrium binding of Sox2 and Oct1 to the FGF4-DNA was studied by fluorescence anisotropy. The equilibrium dissociation constants for sequence-specific binding of Sox2 (KDSox2) and Oct1 (KDOct1) are 5.3 ± 0.3 and 44 ± 3 nm, respectively, at 150 mm NaCl, 10 mm PIPES, pH 6.5, and 30 °C (corresponding exactly to the buffer and temperature conditions used in the NMR experiments) (Fig. 2A). The presence of Sox2 bound to the FGF4-DNA duplex increases the sequence-specific affinity of Oct1 ∼15-fold; the equilibrium dissociation constant for sequence-specific binding of Oct1 to the Sox2·FGF4-DNA binary complex (KDOct1·Sox2) determined by fluorescence anisotropy is 2.7 ± 0.4 nm (Fig. 2A, right panel, filled circles). Based on the thermodynamic cycle for the binding of Sox2 and Oct1 to DNA (Fig. 2B), the equilibrium dissociation constant for specific DNA binding of Sox2 in the presence of Oct1 (KDSox2·Oct1) is calculated to be 0.3 ± 0.1 nm (Fig. 2B). By way of comparison, the increase in affinity afforded by protein/protein interactions within the ternary complex on the Hoxb1 promoter is ∼20-fold under slightly different experimental conditions (25 °C, 150 mm NaCl, 10 mm phosphate buffer) (18, 21).
FIGURE 2.
Specific binding of Sox2 to the Oct1·FGF4-DNA binary complex monitored by fluorescence anisotropy. A, titration of Sox2 into FGF4-DNA (left panel) and Oct1 into FGF4-DNA (right panel, open circles) and the Sox2·FGF4-DNA complex (right panel, filled circles). The rhodamine fluorescent label was conjugated to the 5′ end of the bottom strand of the 32-bp FGF4-DNA duplex, and the excitation and emission wavelengths were set to 550 and 580 nm, respectively. The concentration of the FGF4-DNA is 1.5 nm for the Sox2 titration and 10 nm for the Oct1 titration (in the absence and presence of 100 nm Sox2). The temperature is 30 °C, and the buffer conditions are 150 mm NaCl, 10 mm PIPES, pH 6.5, 94% H2O, 6% D2O, identical to those used in the NMR z-exchange experiments. The experimental data points (error bars, ± 1 S.D.) are displayed as circles (open for the binary complexes and filled for the ternary complex), and the best-fit curve are displayed as solid lines (binary complexes) or dashed lines (ternary complex). B, the equilibrium dissociation constant for Sox2 in the presence of Oct1 was calculated using a thermodynamic cycle, and the three experimental KD values shown.
Global Intermolecular Translocation of Sox2 and Oct1 on FGF4-DNA
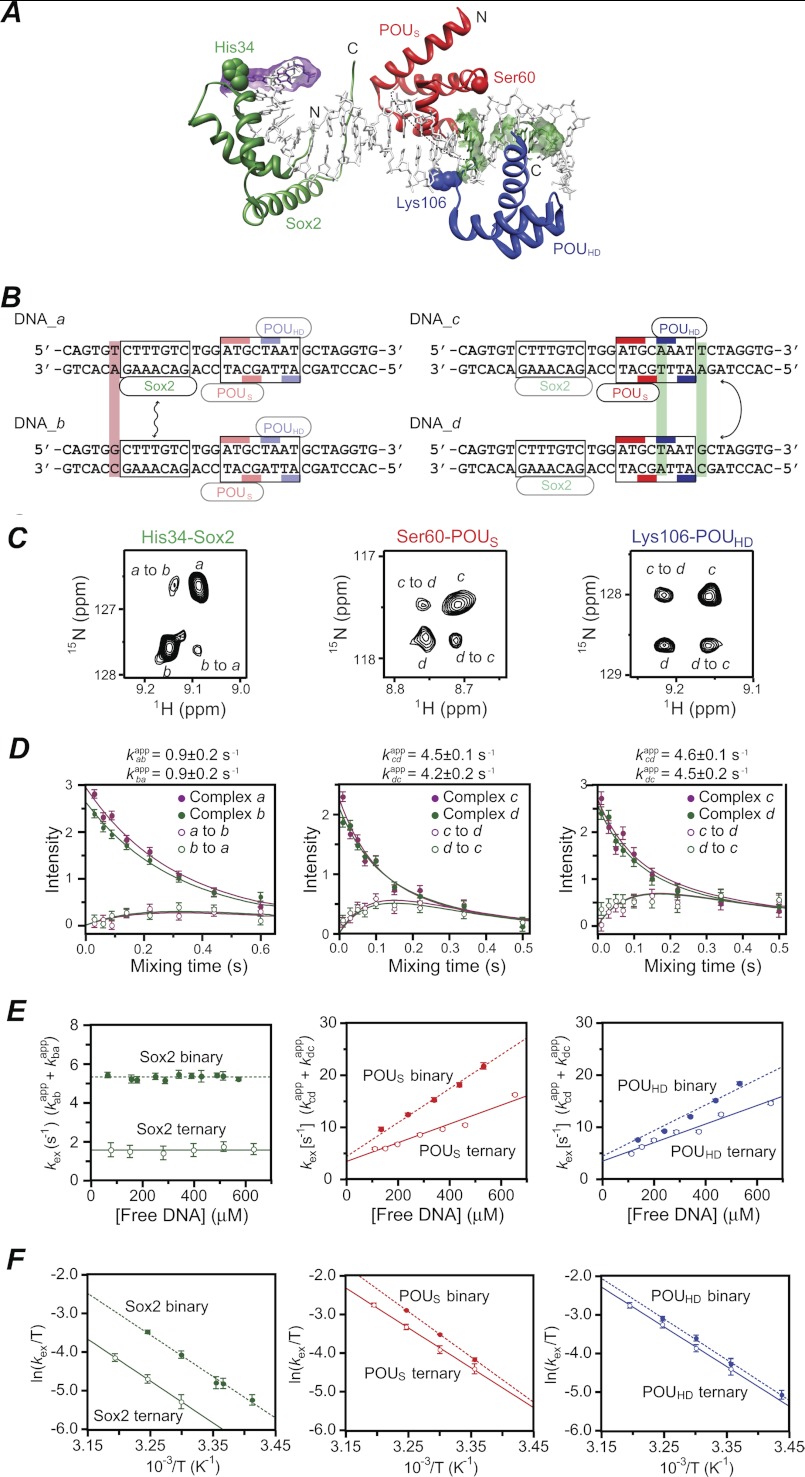
To measure the rate of intermolecular translocation of Sox2 and Oct1 between cognate sites located on different DNA duplexes, we used a similar experimental design to that described in Refs. 18, 20, and 21. Single base pair mutations (Fig. 3A) were introduced in the FGF4-DNA duplex (DNA_a) adjacent to the recognition sites for Sox2 (DNA_b) and the POUHD (DNA_c) and POUS (DNA_d) domains of Oct1 (Fig. 3B). These single point mutations have a minimal effect on the binding affinity of Sox2 or Oct1 (18, 21), but perturb the 1HN/15N chemical shifts for a few backbone amide groups of Sox2 or Oct1 in the ternary complexes with DNA_a and DNA_b or DNA_c and DNA_d (referred to hereafter as complexes a and b or c and d, respectively, Fig. 3C), thereby allowing exchange rates to be measured by monitoring the time dependence of well resolved, isolated exchange and auto cross-peaks in a 15N z-exchange experiment. In the latter experiment, exchange between 15Nz magnetizations from distinct species takes place during the mixing time (which follows the 15N evolution period used to label 15N chemical shifts), giving rise to exchange cross-peaks in a two-dimensional 1H-15N correlation spectrum (28–30). Exchange rates are obtained by simultaneously fitting the time dependence of the intensities of the exchange and auto cross-peaks as a function of the mixing time using the appropriate McConnell equations (31) for the time development of magnetization in a two-site system (20).
FIGURE 3.
Global intermolecular translocation of Sox2 and Oct1 between Sox2·Oct1·FGF4-DNA complexes. A, ribbon diagram of the crystal structure of the Sox2·Oct1·FGF4-DNA complex (PDB code 1gt0 (8)) with Sox2 in green and the POUS and POUHD domains of Oct1 in red and blue, respectively. The purple and green meshes indicate the base pairs changed in DNA_a/DNA_b and DNA_c/DNA_d, respectively. The apparent translocation exchange rates for Sox2, POUS, and POUHD were determined by fitting the time dependence of the exchange and auto cross-peaks in the z-exchange experiment for His-34, Ser-60, and Lys-106, respectively. These three residues exhibit large enough differences in 1HN and 15N chemical shifts between the DNA_a and DNA_b complexes (for His-34 of Sox2) and between the DNA_c and DNA_d complexes (for Ser-60 and Lys-106 of Oct1) to permit accurate quantification of both auto and exchange cross-peak intensities. (C, C terminus; N, N terminus). B, sequences of FGF4-DNA duplexes employed in the z-exchange experiments. DNA_a and DNA_b differ by 1 bp (lilac box) immediately 5′ of the Sox2 binding site and were used to measure Sox2 translocation rates. DNA_c and DNA_d differ by 2 bp (green boxes), one immediately 3′ of the POUS site and the other immediately 3′ of the POUHD site, and were used to measure Oct1 translocation rates. The Sox2 and Oct1 binding sites are delineated by boxes, and the bases that contact the POUS and POUHD domains of Oct1 are indicated by the red and blue bars, respectively. C, examples of z-exchange data for His-34 of Sox2 (left), Ser-60 of POUS (middle), and Lys-106 of POUHD (right) in the Sox2·Oct1·FGF4-DNA ternary complex seen in 1H-15N TROSY-based z-exchange spectra at a mixing time of 220 ms at 30 °C and 150 mm NaCl. For the Sox2 measurements, the concentrations of 2H/15N-labeled Sox2, Oct1 (at natural isotopic abundance), DNA_a, and DNA_b are 0.55, 0.7, 0.35, and 0.35 mm, respectively. For the Oct1 measurements, the concentrations of Sox2 (at natural isotopic abundance), 2H/15N-labeled Oct1, DNA_c, and DNA_d are 0.7, 0.55, 0.35, and 0.35 mm, respectively. D, time dependence of the intensities of auto (filled circles) and exchange (open circles) cross-peaks at 30 °C and 150 mm NaCl together with the best-fit curves (solid lines). The concentrations of proteins and DNA are the same as C. E, dependence of the apparent translocation rates for Sox2 (left), POUS (middle), and POUHD (right) in the ternary complexes (open circles) on the concentration of free DNA at 30 °C and 150 mm NaCl. Also shown are the apparent translocation rates for the corresponding binary complexes (solid circles). The protein concentrations are the same as in C for the ternary complexes; for the binary complexes, the protein at natural isotopic abundance was omitted from the sample. The concentration of free DNA represents the sum of the concentrations of the two DNA duplexes present at a 1:1 ratio in the samples. Association, dissociation, and direct intersegment transfer rate constants derived from the data are summarized in Table 1. F, Eyring plots of the apparent translocation rates at 18, 20, 24, 25, 30, 35, and 40 °C for Sox2 (left), POUS (middle), and POUHD (right) in ternary (open circles) and binary (filled circles) complexes. The protein and DNA concentrations are the same as in C. Linear fits of the apparent translocation rates for the ternary and binary complexes are shown as solid and dashed lines, respectively. Activation enthalpies, entropies, and free energies derived from the data are given in Table 2. Error bars, ± 1 S.D.
Selective observation of Sox2 or Oct1 was achieved by 2H,15N labeling of Sox2 for complexes a and b or Oct1 for complexes c and d. Because both Sox2 and Oct1 bind tightly to their cognate DNA sequences with equilibrium dissociation constants in the nanomolar range at 150 mm NaCl (Fig. 2), the 1H-15N TROSY correlation spectrum for a 1:1 mixture of complexes a and b (signals from Sox2) or complexes c and d (signals from Oct1) contains cross-peaks arising from both ternary complexes (18, 21). The ratios of the cross-peak intensities for each complex in the 1H-15N TROSY spectra of the 1:1 mixtures are very close to 1, indicating that the KD values for both proteins to each DNA duplex are virtually identical. Examples of z-exchange spectra and fits to the time dependence of auto and exchange cross-peak intensities used to determine the exchange rates kabapp and kbaapp for Sox2 or kcdapp and kdcapp for Oct1 are shown in Fig. 3 for His-34 of Sox2, and Ser-60 and Lys-106 of the POUS and POUHD domains, respectively, of Oct1.
The contributions of jumping and intersegment transfer to intermolecular translocation can be dissected from the dependence of the apparent exchange rate constants on the concentration of free DNA (20, 21). The apparent rate constant kABapp for transfer of a protein from site A to site B located on two different DNA molecules is given by the sum of the contributions from jumping and direct intersegment transfer (and similarly for the transfer from sites B to A). With DNA in excess over protein and koff ≪ kon[DNAfree], the rate-limiting step for jumping is governed by the dissociation rate constant (koff). The jumping rate from A to B is therefore independent of the concentration of free DNA and is given by koffA/2, where koffA is the dissociation rate constant from site A (and the statistical factor of 2 arises from the fact that transfer of the protein between DNA molecules of the same sequence is of equal probability to transfer between DNA molecules of differing sequence). The direct intersegment transfer rate from A to B, on the other hand, is linearly dependent on the free concentration of the DNA containing site B and is given by kABinter[DNABfree] where kABinteris the second order rate constant for direct intersegment transfer from A to B.
kexSox2 (= kabapp + kbaapp), and kexPOUS and kexPOUHD (= kcdapp + kdcapp for resonances of POUS and POUHD, respectively) are plotted as a function of free DNA concentration in Fig. 3E. In the context of the FGF4-DNA ternary complex, the presence of Oct1 decreases the translocation rates for Sox2 and vice versa. Although the exchange rates are slower in the ternary complex than those in the binary complexes, the mechanism of translocation is unaffected by the presence of protein interactions on the DNA. kexSox2 is independent of the concentration of free DNA indicative of an exclusive jumping mechanism (Fig. 3E, left panel). kexPOUS (Fig. 3E, middle panel) and kexPOUHD (Fig. 3E, right panel), on the other hand, are linearly dependent on the concentration of free DNA with a measurable intercept at zero free DNA concentration, indicative of the presence of both direct intersegment transfer and jumping mechanisms.
At 30 °C, kexSox2 is reduced from 5.3 ± 0.2 s−1 in the binary complex (18) to 1.5 ± 0.3 s−1 in the ternary complex (Fig. 3E, left panel). kexSox2 is equal to the average dissociation rate constant 〈koffSox2〉 because koffa = 2kappab and koffb = 2kappba (and note that koffa and koffb in this instance are virtually identical). Given the measured equilibrium dissociation constants for the binding of Sox2 to DNA in the context of binary and ternary complexes (Fig. 2), the average association rate constants 〈konSox2 − binary〉 and 〈konSox2 − FGF4·ternary〉 are calculated to be 1.0 (±0.1) × 109 and 5.1 (±1.4) × 109 m−1s−1, respectively (Table 1). Thus, the association rate constant for sequence-specific DNA binding of Sox2 is increased ∼5-fold in the ternary complex relative to the binary one.
TABLE 1.
Intermolecular translocation rates of Sox2 and Oct1 between cognate sites in binary and FGF4 ternary complexes
Intermolecular translocation of Sox2 occurs solely by dissociation followed by reassociation (jumping), while for Oct1 both direct intersegment transfer and jumping occur.
| KD | 〈koff〉 | 〈kon〉 | 〈kinter〉 | |
|---|---|---|---|---|
| nm | s−1 | m−1s−1 | m−1s−1 | |
| Sox2 | ||||
| Binary | 5.3 ± 0.3 | 5.3 ± 0.2 | 1.0 (± 0.1) × 109 | NDa |
| FGF4 ternary | 0.3 ± 0.1 | 1.5 ± 0.3 | 5.1 (± 1.4) × 109 | NDa |
| Oct1 | ||||
| Binary | 44 ± 3 | 4.4 ± 0.2 | 1.0 (± 0.1) × 108 | 3.4 (± 0.2) × 104 (POUS) |
| 1.8 (± 0.4) × 104 (POUHD) | ||||
| FGF4 ternary | 2.7 ± 0.4 | 3.5 ± 0.4 | 1.3 (± 0.2) × 109 | 2.2 (± 0.2) × 104 (POUS) |
| 1.7 (± 0.1) × 104 (POUHD) | ||||
a Not detectable. From the absence of any concentration dependence in the apparent translocation rates for Sox2, one can conclude that translocation of Sox2 does not involve direct intersegment transfer in both binary and ternary complexes.
The average second order rate constants for direct intersegment transfer of the POUS (〈kinterPOUS〉) and POUHD (〈kinterPOUHD〉) domains of Oct1 in the ternary complex are 2.2 (±0.2) × 104 and 1.7 (±0.1) × 104 m−1s−1, respectively. 〈kinterPOUS〉 is reduced by about 50% relative to its value in the binary complex (3.4 × 104 m−1s−1 (21)), whereas 〈kinterPOUHD〉 remains unaltered (1.8 × 104 m−1s−1 in the binary complex (21)). These observations are in complete agreement with the structure of the ternary Sox2·Oct1·FGF4-DNA complex (8) because Sox2 interacts only with the POUS domain of Oct1 (Figs. 1D and 3A). The average dissociation rate constant 〈koffOct1〉 of Oct1 from the ternary complex is 3.5 ± 0.4 s−1, which is comparable with the value of 4.4 ± 0.2 s−1 measured for the binary complex (21). The average association rate constant 〈konOct1〉 for Oct1 binding to the Sox2·FGF4-DNA complex is 1.3 (±0.2) × 109 m−1s−1, which is ∼13-fold larger than the value of 1.0 (±0.1) × 108 m−1s−1 for the binary complex (21).
The increases in the specific association rate constants for both Sox2 and Oct1 in the context of the ternary complex can probably be attributed to electrostatic enhancement of diffusion-controlled association (32, 33) afforded by charge/charge interactions between Sox2 and Oct1 when bound specifically to DNA. The ∼3-fold smaller increase in the specific association rate constant for Sox2 when compared with Oct1 in the ternary complex can be rationalized as follows. First, the ordering of the C-terminal tail of Sox2 upon interaction with the POUS domain of Oct1 in the ternary complex (8) (cf. Fig. 3A) entails an entropic penalty because the C-terminal tail is disordered in the binary Sox2·DNA complex (4, 18). For the POUS and POUHD domains of Oct1, ternary complex formation is not accompanied by any significant backbone conformational change, and therefore does not entail any additional entropic cost. (Note that the flexible linker connecting the POUS and POUHD domains remains largely disordered when bound to DNA (5, 7, 8)). Second, in the context of the DNA duplexes employed (Fig. 3B), the number of available nonspecific sites to which Sox2 can bind and subsequently slide to its specific site is reduced by the presence of Oct1. Although nonspecific binding sites available to Oct1 are occluded by the presence of Sox2, there are still a substantial number of nonspecific DNA binding sites 3′ of the Sox2 binding site (cf. Fig. 3, A and B) from which sliding of Oct1 can occur.
Eyring plots of the temperature dependence of the apparent translocation exchange rates (Fig. 3F) provide estimates of the activation enthalpy (ΔH‡), entropy (TΔS‡ at 30 °C), and by deduction, free energy. The activation free energies (ΔG‡) for intermolecular translocation in the ternary and binary complexes on the FGF4 promoter are comparable (∼16–17 kcal × mol−1; Table 2), indicative of similar energy barriers that are unaffected by the presence of a second protein. These data indicate that protein/protein interactions between Sox2 and Oct1 on the DNA modulate translocation rates without perturbing the activation free energies. By inference, this likely holds true for the Sox2·Oct1·Hoxb1-DNA ternary complex as well, where more extensive protein/protein interactions reduce the translocation rates to levels that are too slow to be measured by z-exchange spectroscopy.
TABLE 2.
Apparent activation enthalpies (ΔH‡), entropies (ΔS‡), and free energies (ΔG‡) for global intermolecular translocation of Sox2 and Oct1 between cognate sites in the binary and FGF4 ternary complexes
| ΔH‡a | ΔS‡a | ΔG‡b | |
|---|---|---|---|
| kcal × mol−1 | cal × mol−1 × K−1 | kcal × mol−1 | |
| Sox2 | |||
| Binary/FGF4 ternary | 21.2 ± 1.9/21.3 ± 1.8 | 15.0 ± 6.5/13.1 ± 5.8 | 16.6 ± 2.8/17.3 ± 2.5 |
| Oct1-POUS domain | |||
| Binary/FGF4 ternary | 23.1 ± 1.8/20.4 ± 0.8 | 22.6 ± 4.0/12.7 ± 2.4 | 16.3 ± 2.2/16.5 ± 1.1 |
| Oct1-POUHD domain | |||
| Binary/FGF4 ternary | 20.8 ± 1.2/20.3 ± 0.5 | 14.7 ± 4.1/12.7 ± 1.6 | 16.4 ± 1.7/16.5 ± 0.7 |
a Values were derived by least squares fitting of the Eyring plots shown in Fig. 3F.
b ΔG‡ is calculated from ΔG‡ = ΔH‡ − TΔS‡ at 303 K.
Concluding Remarks
The kinetic data on global intermolecular translocation of Oct1 and Sox2 between adjacent specific sites located on different DNA molecules presented here complement our previous work that made use of paramagnetic relaxation enhancement measurements (12, 15) to examine the interplay between these two transcription factors in translocation events involving sparsely populated (<1%), highly transient, spectroscopically invisible states (18). The latter comprise an ensemble of nonspecifically bound species in rapid exchange with the specific complex and participate both in one-dimensional sliding along the DNA (intramolecular translocation), as well as the formation of bridged intermediates spanning two DNA molecules that precedes intermolecular translocation. The events probed by paramagnetic relaxation enhancement occur on a time scale less than 250–500 μs, although paramagnetic relaxation enhancement measurements do not afford any further characterization of the kinetics of these processes (12, 13, 15). Global (or bulk) intermolecular translocation between specific sites on different DNA molecules, on the other hand, occurs on a much slower overall time scale (0.1–1 s; cf. Table 1) and involves the major spectroscopically visible species (i.e. the specific complexes), and the rate constants from the z-exchange experiments pertain directly to the rate-limiting steps in this process (20, 21). The interaction of Sox2 and Oct1 on the DNA modulates the translocation mechanisms involving sparsely populated states (18), as well as the kinetics of global intermolecular translocation between specific sites as shown here. The pathways of global intermolecular translocation, however, are unaffected by the interaction between Sox2 and Oct1.
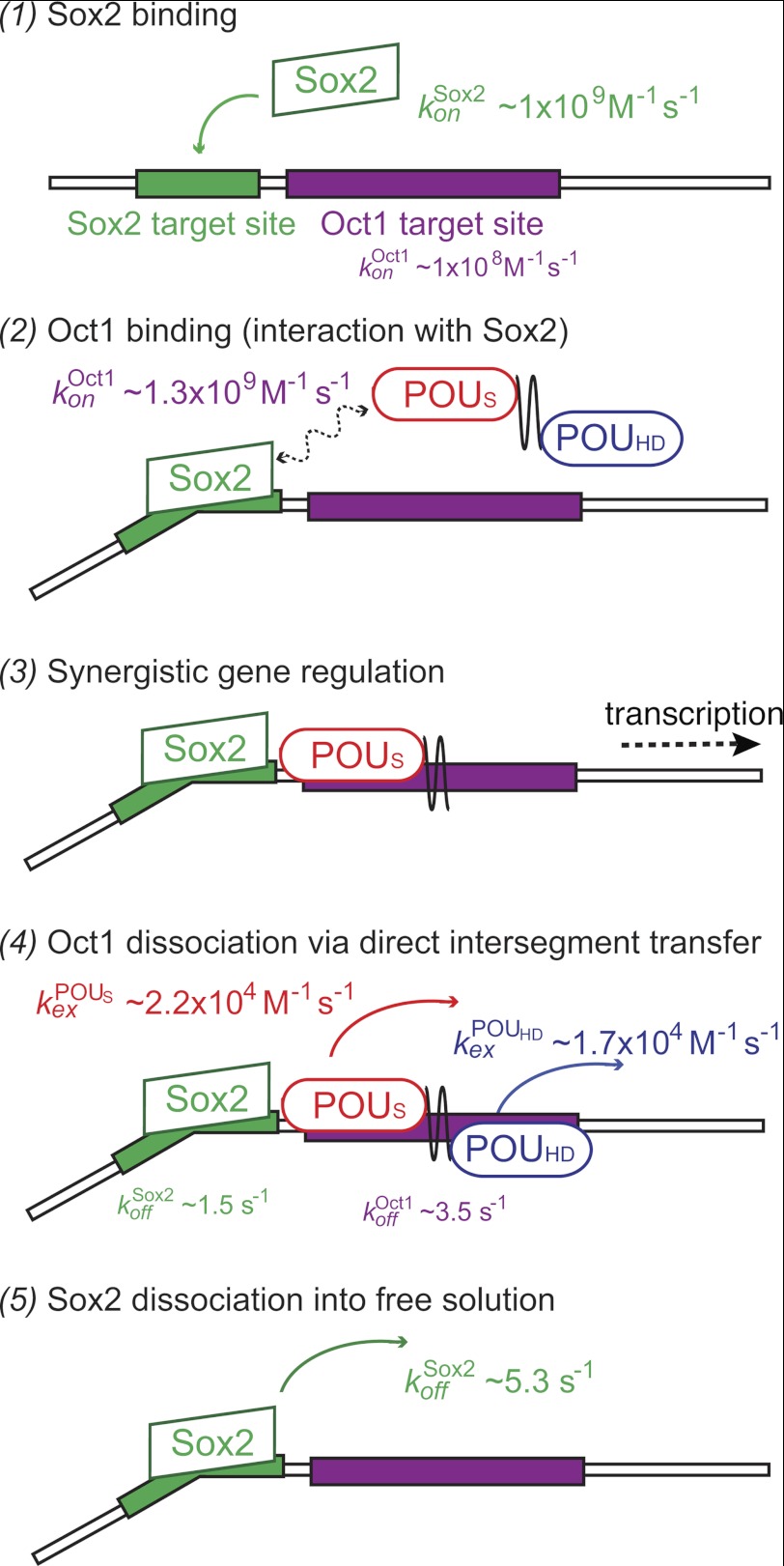
Based on the kinetic data for global intermolecular translocation presented in this study, we propose the following model for the sequence of binding, intersegment transfer and dissociation events involved in combinatorial control of gene regulation by Sox2 and Oct1 (Fig. 4). The initial step involves the binding of Sox2 to its specific DNA target site. This is supported by the observation of a 10-fold larger association rate constant for the formation of the binary Sox2·DNA complex versus the Oct1·DNA complex (Table 1), as well as the fact that translocation of Sox2 is a slow process involving only full dissociation (i.e. jumping), whereas Oct1 can undergo rapid global intermolecular translocation at the high DNA concentrations present in vivo (∼150 mm on a base pair basis). In addition, Sox2 is localized in the cell nucleus (34), whereas Oct1, which is widely expressed in both adult and embryonic tissues (35, 36), is found in both the nucleus and the cytoplasm (37). The presence of Sox2 bound to its specific site on the promoter accelerates the binding of Oct1 by ∼13-fold to a target site adjacent to the Sox2 site, and the ternary complex is further stabilized by protein/protein interactions, predominantly electrostatic in nature, between Sox2 and Oct1. Once the specific Sox2·Oct1·DNA ternary complex is formed, Oct1 and Sox2 activate transcription synergistically. Oct1 subsequently dissociates from the ternary complex largely via direct intersegment transfer, which, at the high DNA concentrations present in vivo will be significantly faster than dissociation into free solution. Intersegment transfer can occur to another specific site on a different promoter or simply to a nonspecific site located either on a different DNA molecule or, if on the same DNA, at a widely separated (>150 bp) location through DNA bridging. Finally, Sox2 dissociates from its specific DNA site slowly, and subsequent DNA binding of Sox2 can only occur via a second order reassociation event either to another specific site or to nonspecific sites.
FIGURE 4.
Model for the sequence of binding, intersegment transfer, and dissociation events involved in synergistic transcription regulation by Sox2 and Oct1. The initial event (step 1) involves the binding of Sox2 to its specific DNA target site followed by binding of Oct1 to form a specific ternary complex (step 2). The latter binding event is accelerated by the presence of specifically bound Sox2. Once formation of the ternary Sox2·Oct1-DNA complex has occurred on the promoter or enhancer, transcription of the relevant gene is activated (step 3). Subsequently, Oct1 dissociates from the DNA largely by direct intersegment transfer to another DNA site (step 4). Finally, Sox2 dissociates from its specific site into free solution (step 5). (See “Results” for more details.)
Acknowledgments
We thank Drs. Garrett, Baber, and Ying for technical support.
This work was supported, in whole or in part, by the intramural program of NIDDK, National Institutes of Health and by the AIDS Targeted Antiviral Program of the Office of the Director of the National Institutes of Health (to G. M. C.).
- POUS
- POU-specific domain
- POUHD
- homeodomain
- TROSY
- transverse relaxation optimized spectroscopy.
REFERENCES
- 1. Wolberger C. (1999) Multiprotein·DNA complexes in transcriptional regulation. Annu. Rev. Biophys. Biomol. Struct. 28, 29–56 [DOI] [PubMed] [Google Scholar]
- 2. Kamachi Y., Uchikawa M., Kondoh H. (2000) Pairing SOX off: with partners in the regulation of embryonic development. Trends Genet. 16, 182–187 [DOI] [PubMed] [Google Scholar]
- 3. Dailey L., Basilico C. (2001) Coevolution of HMG domains and homeodomains and the generation of transcriptional regulation by Sox/POU complexes. J. Cell Physiol. 186, 315–328 [DOI] [PubMed] [Google Scholar]
- 4. Murphy E. C., Zhurkin V. B., Louis J. M., Cornilescu G., Clore G. M. (2001) Structural basis for SRY-dependent 46-X,Y sex reversal: modulation of DNA bending by a naturally occurring point mutation. J. Mol. Biol. 312, 481–499 [DOI] [PubMed] [Google Scholar]
- 5. Klemm J. D., Rould M. A., Aurora R., Herr W., Pabo C. O. (1994) Crystal structure of the Oct1 POU domain bound to an octamer site: DNA recognition with tethered DNA-binding modules. Cell 77, 21–32 [DOI] [PubMed] [Google Scholar]
- 6. Reményi A., Tomilin A., Pohl E., Lins K., Philippsen A., Reinbold R., Schöler H. R., Wilmanns M. (2001) Differential dimer activities of the transcription factor Oct1 by DNA-induced interface swapping. Mol. Cell 8, 569–580 [DOI] [PubMed] [Google Scholar]
- 7. Williams D. C., Jr., Cai M., Clore G. M. (2004) Molecular basis for synergistic transcriptional activation by Oct1 and Sox2 revealed from the solution structure of the 42-kDa Oct1·Sox2·Hoxb1-DNA ternary transcription factor complex. J. Biol. Chem. 279, 1449–1457 [DOI] [PubMed] [Google Scholar]
- 8. Reményi A., Lins K., Nissen L. J., Reinbold R., Schöler H. R., Wilmanns M. (2003) Crystal structure of a POU·HMG·DNA ternary complex suggests differential assembly of Oct4 and Sox2 on two enhancers. Genes Dev. 17, 2048–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berg O. G., von Hippel P. H. (1985) Diffusion-controlled macromolecular interactions. Annu. Rev. Biophys. Biophys. Chem. 14, 131–160 [DOI] [PubMed] [Google Scholar]
- 10. von Hippel P. H., Berg O. G. (1989) Facilitated target location in biological systems. J. Biol. Chem. 264, 675–678 [PubMed] [Google Scholar]
- 11. Halford S. E., Marko J. F. (2004) How do site-specific DNA-binding proteins find their targets? Nucleic Acids Res. 32, 3040–3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clore G. M., Iwahara J. (2009) Theory, practice, and applications of paramagnetic relaxation enhancement for the characterization of transient low population states of biological macromolecules and their complexes. Chem. Rev. 109, 4108–4139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clore G. M. (2011) Exploring sparsely populated states of macromolecules by diamagnetic and paramagnetic NMR relaxation. Protein Sci. 20, 229–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iwahara J., Schwieters C. D., Clore G. M. (2004) Characterization of nonspecific protein/DNA interactions by 1H paramagnetic relaxation enhancement. J. Am. Chem. Soc. 126, 12800–12808 [DOI] [PubMed] [Google Scholar]
- 15. Iwahara J., Clore G. M. (2006) Detecting transient intermediates in macromolecular binding by paramagnetic NMR. Nature 440, 1227–1230 [DOI] [PubMed] [Google Scholar]
- 16. Iwahara J., Zweckstetter M., Clore G. M. (2006) NMR structural and kinetic characterization of a homeodomain diffusing and hopping on nonspecific DNA. Proc. Natl. Acad. Sci. U.S.A. 103, 15062–15067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Takayama Y., Clore G. M. (2011) Intra- and intermolecular translocation of the bidomain transcription factor Oct1 characterized by liquid crystal and paramagnetic NMR. Proc. Natl. Acad. Sci. U.S.A. 108, E169–E176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Takayama Y., Clore G. M. (2012) Interplay between minor and major groove-binding transcription factors Sox2 and Oct1 in translocation on DNA studied by paramagnetic and diamagnetic NMR. J. Biol. Chem. 287, 14349–14363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clore G. M. (2011) Exploring translocation of proteins on DNA by NMR. J. Biomol. NMR 51, 209–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iwahara J., Clore G. M. (2006) Direct observation of enhanced translocation of a homeodomain between DNA cognate sites by NMR exchange spectroscopy. J. Am. Chem. Soc. 128, 404–405 [DOI] [PubMed] [Google Scholar]
- 21. Doucleff M., Clore G. M. (2008) Global jumping and domain-specific intersegment transfer between DNA cognate sites of the multidomain transcription factor Oct1. Proc. Natl. Acad. Sci. U.S.A. 105, 13871–13876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ambrosetti D. C., Basilico C., Dailey L. (1997) Synergistic activation of the fibroblast growth factor 4 enhancer by Sox2 and Oct3 depends on protein/protein interactions facilitated by a specific spatial arrangement of factor binding sites. Mol. Cell Biol. 17, 6321–6329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Di Rocco G., Gavalas A., Popperl H., Krumlauf R., Mavilio F., Zappavigna V. (2001) The recruitment of SOX·OCT complexes and the differential activity of HOXA1 and HOXB1 modulate the Hoxb1 auto-regulatory enhancer function. J. Biol. Chem. 276, 20506–20515 [DOI] [PubMed] [Google Scholar]
- 24. Iwahara J., Anderson D. E., Murphy E. C., Clore G. M. (2003) EDTA-derivatized deoxythymidine as a tool for rapid determination of protein binding polarity to DNA by intermolecular paramagnetic relaxation enhancement. J. Am. Chem. Soc. 125, 6634–6635 [DOI] [PubMed] [Google Scholar]
- 25. Delaglio F., Grzesiek S., Vuister G. W., Zhu G., Pfeifer J., Bax A. (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, 277–293 [DOI] [PubMed] [Google Scholar]
- 26. Johnson B. A., Blevins R. A. (1994) NMR View: A computer program for the visualization and analysis of NMR data. J. Biomol. NMR 4, 603–614 [DOI] [PubMed] [Google Scholar]
- 27. Sahu D., Clore G. M., Iwahara J. (2007) TROSY-based z-exchange spectroscopy: application to the determination of the activation energy for intermolecular protein translocation between specific sites on different DNA molecules. J. Am. Chem. Soc. 129, 13232–13237 [DOI] [PubMed] [Google Scholar]
- 28. Montelione G. T., Wagner G. (1989) Two-dimensional chemical exchange NMR spectroscopy by proton-detected heteronuclear correlation. J. Am. Chem. Soc. 111, 3096–3098 [Google Scholar]
- 29. Perrin C. L., Dwyer T. J. (1990) Application of two-dimensional NMR to kinetics of chemical exchange. Chem. Rev. 90, 935–967 [Google Scholar]
- 30. Farrow N. A., Zhang O., Forman-Kay J. D., Kay L. E. (1994) A heteronuclear correlation experiment for simultaneous determination of 15N longitudinal decay and chemical exchange rates of systems in slow equilibrium. J. Biomol. NMR 4, 727–734 [DOI] [PubMed] [Google Scholar]
- 31. McConnell H. M. (1958) Reaction rates by nuclear magnetic resonance. J. Chem. Phys. 28, 430–431 [Google Scholar]
- 32. Vijayakumar M., Wong K. Y., Schreiber G., Fersht A. R., Szabo A., Zhou H. X. (1998) Electrostatic enhancement of diffusion-controlled protein/protein association: comparison of theory and experiment on barnase and barstar. J. Mol. Biol. 278, 1015–1024 [DOI] [PubMed] [Google Scholar]
- 33. Zhou H. X., Szabo A. (2004) Enhancement of association rates by nonspecific binding to DNA and cell membranes. Phys. Rev. Lett. 93, 178101. [DOI] [PubMed] [Google Scholar]
- 34. Zhang J., Chang D. Y., Mercado-Uribe I., Liu J. (March 9, 2012) Sex-determining region Y-box 2 expression predicts poor prognosis in human ovarian carcinoma. Hum. Pathol. 10.1016/j.humpath.2011.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kang J., Shakya A., Tantin D. (2009) Stem cells, stress, metabolism, and cancer: a drama in two Octs. Trends Biochem. Sci. 34, 491–499 [DOI] [PubMed] [Google Scholar]
- 36. Herr W., Sturm R. A., Clerc R. G., Corcoran L. M., Baltimore D., Sharp P. A., Ingraham H. A., Rosenfeld M. G., Finney M., Ruvkun G., et al. (1988) The POU domain: a large conserved region in the mammalian pit-1, oct-1, oct-2, and Caenorhabditis elegans unc-86 gene products. Genes Dev. 2, 1513–1516 [DOI] [PubMed] [Google Scholar]
- 37. Izadpanah R., Trygg C., Patel B., Kriedt C., Dufour J., Gimble J. M., Bunnell B. A. (2006) Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J. Cell Biochem. 99, 1285–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]