Abstract
θ-Defensins, the only cyclic peptides of animal origin, have been isolated from the leukocytes of rhesus macaques and baboons. Their biogenesis is unusual because each peptide is an 18-residue chimera formed by the head-to-tail splicing of nonapeptides derived from two separate precursors. θ-Defensins have multiple arginines and a ladder-like tridisulfide array spanning their two antiparallel β-strands. Human θ-defensin genes contain a premature stop codon that prevents effective translation of the needed precursors; consequently, these peptides are not present in human leukocytes. Synthetic θ-defensins with sequences that correspond to those encoded within the human pseudogenes are called retrocyclins. Retrocyclin-1 inhibits the cellular entry of HIV-1, HSV, and influenza A virus. The rhesus θ-defensin RTD-1 protects mice from an experimental severe acute respiratory syndrome coronavirus infection, and retrocyclin-1 protects mice from infection by Bacillus anthracis spores. The small size, unique structure, and multiple host defense activities of θ-defensins make them intriguing potential therapeutic agents.
Keywords: AIDS, Anthrax Toxin, Antiviral Agents, Defensins, Drug Discovery, Influenza Virus, Innate Immunity, Leukocyte, Peptide Biosynthesis
Antimicrobial Peptides
A complex immune system enables vertebrate animals to resist challenges from potential pathogens. Some immune system components, such as antibodies and T-cells, show exquisite specificity. Others, like antimicrobial peptides (AMPs),2 act more broadly. Most AMPs are small (1–5 kDa), positively charged, and amphipathic. Two types of AMPs are pertinent to this minireview: cathelicidins and defensins. Both have ancient roots, with cathelicidins having been traced back to hagfish and β-defensins to bony fish.
Cathelicidins
These structurally diverse peptides share a conserved prodomain called “cathelin” (1). Whereas cattle and pigs express 10 or more different cathelicidins, mice and humans express only one. Porcine protegrins are cathelicidins with many structural similarities to θ-defensins (Fig. 1). These include having 18 residues, a β-hairpin backbone, intramolecular disulfides, and multiple arginines. Unlike the θ-defensins, protegrins lack a cyclic backbone, unless one is imparted in the laboratory (2).
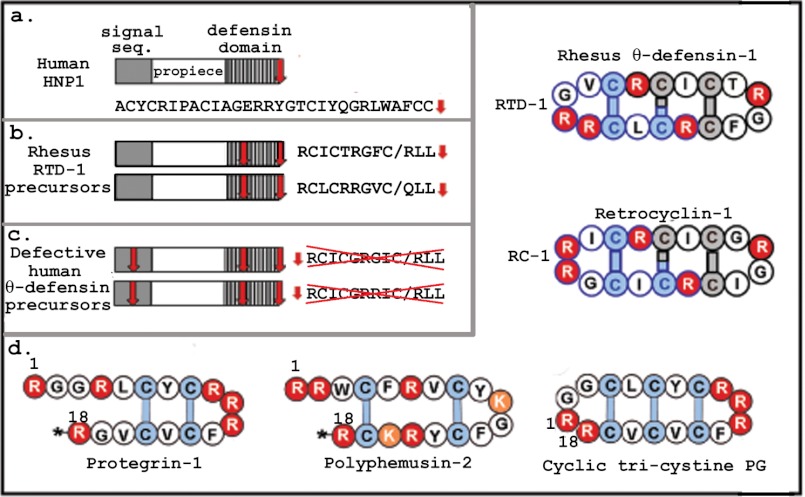
FIGURE 1.
Panel a shows the layout of the prepropeptide of a human α-defensin, HNP-1, as well as the sequence (seq.) of its C-terminal defensin domain. The red arrow represents a stop codon. Panel b shows the corresponding layout and sequences for RTD-1. An additional stop codon (red arrow) limits the initial defensin domain peptides to 12 residues, three of which will be removed during subsequent post-translational processing. The mature cyclic RTD-1 peptide is composed of two different nine-residue precursors. Panel c shows the human counterparts of RTD-1. The transcripts contain an additional stop codon in the signal sequence domain. This mutation aborts translation of the θ-defensin peptide precursors. Panel d shows simplified structural diagrams of five structurally related antimicrobial peptides. In these diagrams, arginines are red, lysines are orange, and cysteines are blue. Asterisks signify amidated C-terminal arginines. Polyphemusin-2 was isolated from the white blood cells of the horseshoe crab Limulus polyphemus. It has impressive antimicrobial properties and is active against HIV-1 (50, 51). The other four peptides are discussed in text.
Defensins
In vertebrates, these peptides comprise three subfamilies called α-, β, and θ-defensins. All of these defensins have six conserved cysteines, three intramolecular disulfide bonds, a net positive charge, and β-sheet regions. The cysteines in α- and β-defensins differ in their spacing and pairing (3), and some β-defensins (but no α-defensins) contain a short α-helical region.
Other peptides have also been called defensins based on their structural and functional similarities to those of vertebrates. Plectasin, from the saprophytic fungus Pseudoplectania nigrella, shows remarkable homology to the defensins of dragonflies (Aeschna cyanea) and mussels (Mytilus galloprovincialis), lineages that diverged over 1 billion years ago (4). It is possible (5), but unproven, that fungal defensins are ancestral to vertebrate β-defensins.
The human genome contains >30 different β-defensin genes, and mice have even more (6). However, research has focused mostly on human β-defensin-1–3, which are expressed by epithelial cells throughout the body. Certain other human and rodent β-defensins are expressed in localized regions of the male genitourinary tract and participate in events related to reproduction (7).
Polymorphonuclear neutrophils (PMNs) are white blood cells that can ingest and kill intruding pathogens. PMNs from cattle (8) and chickens (9) contain multiple β-defensins, but primate PMNs contain α-defensins instead. The presence of α-defensins in basal mammals and marsupials indicates that these genes arose before the groups diverged, some 130 million years ago (10).
Human α-Defensins
Humans express six different α-defensin peptides. Three of these, human neutrophil peptides (HNPs) 1–3, constitute 5–7% of total PMN protein. The DEFA1 and DEFA3 genes for HNP-1 (Fig. 1a) and HNP-3 have duplicated, and extensive copy number polymorphism exists. Consequently, some people carry four copies of both genes (i.e. two on each chromosome), whereas others have 11 copies of both (11). Human PMNs also have small amounts of another α-defensin, HNP-4, which is identical to HNP-1–3 in only 11 of 29–30 residues. Human α-defensin-5 and -6 are secreted primarily by Paneth cells in the small intestine.
HNP-1 prepropeptides contain a 19-residue signal sequence, a 45-residue anionic propiece, and a 30-residue defensin domain (Fig. 1). Removing the N-terminal residue from either HNP-1 or HNP-3 creates HNP-2, whose first and last residues are both cysteines that are joined by a disulfide bond, a common mode of cyclization. One more evolutionary event led to the backbone cyclic peptides described below.
Rhesus θ-Defensins
Much of our knowledge about these peptides appeared in the report describing rhesus θ-defensin-1 (RTD-1) (20). The authors purified an extract of rhesus macaque PMNs and tested its components for bactericidal activity against Escherichia coli, a common urinary tract pathogen, and the clinically temperate 502A strain of Staphylococcus aureus. They found eight AMPs, seven α-defensins, and one smaller (2082 Da) peptide. Biochemical characterization of the small AMP led to the cyclic structure illustrated in Fig. 1. Further studies demonstrated that this 18-residue peptide was heterodimeric, formed by the fusion of two nine-residue peptide fragments. Each of its initial monomers was the product of a mutated α-defensin gene containing a premature stop codon (Fig. 1b, red arrow) in its defensin domain. Because of the codon's position, the initial defensin domain products had only 12 residues and required proteolytic removal of a C-terminal tripeptide to create a nonapeptide “building block” with three cysteines, including one at its C terminus (Fig. 1b). Two such nonapeptides were converted into a θ-defensin by two peptide bonds that spliced them in a head-to-tail manner.
Rhesus macaques have three θ-defensin (DEFT) genes that encode different nonapeptides. For simplicity, we will call the different nonapeptides A, B, and C. Because the nonapeptides in a θ-defensin can be identical (AA, BB, and CC) or different (AB, AC, and BC) (12, 13), two different DEFT genes can produce three different peptides (AA, AB, and AC), and three different DEFT genes can produce six (AA, BB, CC, AB, AC, and BC). All six potential θ-defensin peptides exist in rhesus PMNs (14), but their relative amounts differ greatly, with RTD-1 being the most abundant. Because n different DEFT genes could produce (n/2)(n + 1) peptides (12), the four DEFT genes of olive baboons (Papio anubis) could produce 10 different peptides. Five of these were identified at the peptide level (15).
Human Retrocyclins
These humanized θ-defensins resulted from a combination of cloning, peptide synthesis, and molecular archeology. One decade before RTD-1 was described, Vladimir Kokryakov and colleagues at the Institute for Experimental Medicine in St. Petersburg, Russia, and UCLA discovered protegrins (PGs) (Fig. 1d) in extracts of porcine PMNs (16). The peptide had unusually potent and broad antimicrobial properties, and an analog named IB367 (17) entered human trials as a topical agent intended to prevent oral mucositis associated with cancer treatment. A phase III trial of IB367 failed (18), partly due to the peptide's inherent cytotoxicity, a property that might have been mitigated by cyclizing its backbone (2), as shown in Fig. 1d.
The same Russian-UCLA group later found a small AMP in rhesus macaque blood. Because its mass and composition resembled those of PG-1, cDNA cloning studies were done to identify it. Rhesus bone marrow expressed only one cathelicidin, but as it was too large (37 residues) and lacked cysteines, it was unrelated to the rhesus PG-like peptide (19). Subsequently, six rhesus α-defensin-like genes were cloned, including three whose C-terminal defensin domains would have ended after only 12 residues had been translated. The putative dodecapeptides were called “demidefensins,” but the investigators were unable to figure out how 12 + 12 might equal 18. After the epiphany (20), the investigators followed the advice of Efraim Racker, a distinguished biochemist (1913–1991), who said, “It doesn't matter if you fall down as long as you pick something up from the floor when you get up.”
Although human PMNs lacked any θ-defensin peptides, human bone marrow contained mRNA that closely resembled the mRNA of rhesus θ-defensin precursors. However, the human mRNA contained an additional stop codon in its signal sequence, indicating its derivation from an expressed pseudogene. Although this mRNA seemed useless for in vivo θ-defensin production, it provided sequence information that allowed the investigators to recreate the lost θ-defensin by solid-phase peptide synthesis. They christened the resurrected peptide “retrocyclin-1” (Fig. 1d), from “retro,” meaning backwards in time, and “cyclin,” referring to its cyclic backbone.
Antimicrobial Properties
Retrocyclin-1 (RC-1) and RTD-1 (21) killed E. coli in the same way as human α-defensins (22), by permeabilizing its membranes. Rhesus θ-defensins were expressed in the PMNs and monocytes of macaques and baboons, but more abundantly in the former (16, 21). RTD-1 killed E. coli ML-35 in medium with physiological concentrations of NaCl, Ca2+, or Mg2+ that inhibited α-defensins. The antimicrobial effects of RTD-1–3 and PG-1 were tested against E. coli, S. aureus 502A, and Candida albicans (an opportunistic fungus) to determine the minimal microbicidal concentration (MMC), i.e. the peptide concentration that killed at least 99.9% of the organisms in a 2-h incubation period in low salt medium. RTD-1 and RTD-2 had MMCs of 1–2 μg/ml against all three organisms. RTD-3 had somewhat higher MMCs (1.5–3.0 μg/ml), and PG-1 had lower ones (0.3–1.0 μg/ml). Addition of 154 mm NaCl to the medium increased the MMCs of RTD-1–3 against S. aureus above 10 μg/ml, without increasing the MMC of PG-1. Whereas PG-1 had significant cytotoxic and hemolytic properties, RTDs caused little cytotoxicity, and even at 100 μg/ml did not hemolyze human red blood cells.
Antitoxic Properties
Just as human α-defensins can inhibit various bacterial exotoxins (23), θ-defensins can do this as well. The susceptible toxins include anthrax lethal factor (24) and cholesterol-dependent lytic toxins, such as listeriolysin O from Listeria monocytogenes and anthrolysin from Bacillus anthracis (25, 26). Listeriolysin O permits ingested Listeria to escape confinement and destruction in the phagocytic vacuoles of macrophages by entering the more congenial cytoplasmic space, where they can replicate and hitchhike to adjoining cells. Inactivating listeriolysin traps Listeria in the vacuole and helps control the infection (25). Although the pathogenic role of anthrolysin is less certain, similar events may take place with B. anthracis because RC-1 not only prevents the germination of its spores (24), but also facilitates their destruction by macrophages (27).
Activity against HIV-1
RC-1 has an impressive ability to protect human target cells from infection by HIV-1 in vitro (28). It is hardly necessary to explain why this effect could be important, so instead we will explain its mechanism. The process whereby the AIDS virus enters a cell usually involves gp120, a viral surface glycoprotein (carbohydrates account for 55% of its mass) that engages two receptors on surface of the target cell. One receptor is CD4, and the second can be either CXCR4 or CCR5. Following this initial binding, another viral surface glycoprotein, gp41, undergoes conformational changes to form a six-helix bundle structure that mediates viral entry.
Mechanism of Anti-HIV Activity
RC-1 neither damaged target cells nor directly inactivated the virus, so cytotoxic or virotoxic effects were unlikely mechanisms. RC-1 did inhibit the formation of proviral DNA, indicating that it acted early, most likely by preventing viral entry (29). RC-1 was not effective against HIV-2 and simian immunodeficiency virus type 1, related retroviruses that infect blood mononuclear cells or primary fibroblasts independently of CXCR4 or CCR5 (30). Because RC-1 protected human CD4-positive lymphocytes from infection by HIV-1 strains that used either co-receptor, the investigators initially speculated that it might bind gp120 or CD4.
Surface plasmon resonance experiments revealed that RC-1 bound both, with dissociation constants (Kd) of 35.4 nm for gp120 and 31 nm for CD4. RC-1 also showed high affinity binding to galactosylceramide (Kd = 24.1 nm), a surface glycolipid also implicated in HIV-1 binding. This high affinity binding of RC-1 required both its cyclic backbone and intact disulfide bonds. A cyclic analog of RC-1 whose three disulfide bonds were reduced and alkylated bound gp120 and CD4 ineffectively, as did a noncyclic analog that contained three disulfide bonds. Neither analog protected cells from HIV-1 infection.
RC-1 also bound with high affinity to fetuin, an extensively glycosylated protein in fetal calf serum, but showed much less effective binding to bovine serum albumin or to nonglycosylated gp120. Experiments with glycosidase-treated fetuin, gp120, and CD4 suggested that both O-linked and N-linked sugars provided binding sites for gp120. In a panel of 18 retrocyclin variants, binding to immobilized gp120 and CD4 was highly correlated to each other and to the ability to protect human peripheral blood mononuclear cells from infection by HIV-1. These experiments suggested that the ability of RC-1 to bind carbohydrate-containing surface molecules was integrally related to its ability to protect cells from HIV-1 infection.
To further elucidate the mechanism, fusion assays were done using susceptible target cells and effector cells that expressed envelope glycoproteins of HIV-1 (32). RC-1 completely blocked fusion mediated by HIV-1 envelopes that used CXCR4 or CCR5, but had little effect on cell fusion mediated by HIV-2 or simian immunodeficiency virus envelope proteins. RC-1 inhibited HIV-1 envelope-mediated fusion without impairing the lateral mobility of CD4. It also inhibited the fusion of CD4-deficient cells with cells bearing CD4-independent HIV-1 envelopes. Thus, RC-1 could prevent HIV-1 entry without either cross-linking membrane proteins or inhibiting gp120-CD4 interactions (32).
Timing studies indicated that RC-1 acted late in the HIV-1 fusion process, but before six-helix bundle formation (31). Binding experiments showed that retrocyclin bound the ectodomain of gp41 with high affinity in a glycan-independent manner and also that it bound selectively to the C-terminal heptad repeat of gp41 (31). Other analyses revealed that RC-1 prevented six-helix bundle formation. This mode of action resembles that of peptidic entry inhibitors containing portions of the gp41 sequence (30) and enfuvirtide, a clinically used peptide agent that blocks HIV-1 entry. Most single site mutations of the viral heptad repeat residues predicted to bind retrocyclin decreased or abolished the intrinsic infectivity of HIV-1 (32, 33).
In another study, the anti-HIV-1 activity of RTD-1 was compared with those of human α-defensin HNP-1 and β-defensin. All showed activity, but their mechanisms differed. All three defensins decreased CXCR4 expression on the target cell surface, but only RTD-1 inhibited viral entry. HNP-1 and human β-defensin-2 inactivated the X4 and R5 strains of HIV-1, but RTD-1 inactivated only strains that used the CXCR4 co-receptor (34).
Activity against Influenza A Virus
RC-1 neutralized influenza A virus (IAV) by impairing hemagglutinin-mediated viral entry (35) and by inducing viral aggregation that enhanced ingestion of IAV by neutrophils (36). Retrocyclin-2 (RC-2), which differs from RC-1 only by having an additional arginine, was more effective than RC-1, as was RC-101, which differs from RC-1 by having a single Arg-to-Lys substitution. RC-2 inhibited IAV infection by blocking hemagglutinin-mediated membrane fusion. It could act late in the fusion process, even after hemagglutinin-induced membrane hemifusion had occurred. Like RC-1, RC-2 is also a multivalent lectin, and it prevented IAV entry by creating a network of cross-linked immobilized surface glycoproteins. RC-2 also inhibited fusion mediated by Sindbis virus and baculovirus. The former is an enveloped mosquito-borne virus, and the latter is an insect pathogen (35). Although the therapeutic effects of retrocyclins on IAV have yet to be tested in mammals, RC-2 was reported to protect chicken embryos from infection by the avian H5N1 strain of influenza A (37).
Activity Versus Herpes Simplex Viruses
Twenty θ-defensins were tested in vitro to determine their ability to prevent infection of cervical epithelial cells by HSV-1 and HSV-2 (38). RC-1, RC-2, and RTD-3 were all protective, but only RC-2 did not require preincubation with the virus. RC-2 bound HSV-2 glycoprotein B with a Kd of 13.3 nm and did not bind deglycosylated HSV-2 glycoprotein B. Temperature shift experiments indicated that RC-2 and human α-defensins HNP-1–3 protected cells from HSV-2 by different mechanisms. RC-2 blocked HSV attachment, but HNP-1–3 had little effect on binding, and they were effective if added later.
Structural Studies
The three-dimensional structures of RTD-1 and its open chain analog were determined by two-dimensional NMR (39). RTD-1 and the noncyclic analog had similar extended β-hairpin structures in water, with well defined flexible turns at one or both ends. Their β-sheet strands showed some flexibility, despite the three disulfide bonds that connected them. Unlike more typical AMPs, RTD-1 lacked an amphiphilic character. Some amide protons of RTD-1 that should have been solvent-exposed in monomeric β-sheet structures had low temperature coefficients, consistent with the presence of weak intermolecular hydrogen bonds. From biophysical studies with model membranes, it was concluded that cyclic RTD-1 induced stabilized lipid peptide domains more efficiently than did its open chain analog (40). In SDS micelles, the two-dimensional NMR structure of RC-2 showed a well defined β-hairpin structure braced by three disulfide bonds (41). Analytical ultracentrifugation and NMR data indicated that RC-2 self-associated and formed trimers in a concentration-dependent manner. By increasing the valence of retrocyclins, such self-association may contribute to their high affinity binding to glycoproteins and also enable them to cross-link these and other structures that they bind. The orientation (tilt and rotation) of RC-2 molecules within model membranes has also been studied by advanced solid-state NMR techniques (42). Among the findings were that RC-2 selectively disrupts the orientational order of anionic membranes (which exist in most bacteria) while leaving intact zwitterionic membranes (which are more representative of mammalian membranes).
Phylogeny
Among the world's 7 billion people, might some human groups have retained intact DEFT genes? Rather than testing this directly, a phylogenetic study was performed (43), revealing that humans and their closest ape relatives (gorillas, chimpanzees, and bonobos) all had θ-defensin genes with an identical stop codon mutation in the signal sequence. In the orangutan, six DEFT genes were intact, and one was defective. Only intact DEFT genes were found in several Old World monkeys and in the siamang (Hylobates syndactylus), a lesser ape. No DEFT genes were found in DNA from six New World monkeys and five prosimians. This evidence suggests that DEFT genes and θ-defensins arose in Old World monkeys and that intact genes lasted in our own lineage until it diverged from orangutans, some 7.5 million years ago. These considerations make it unlikely that any human populations express retrocyclins spontaneously.
However, human cells can express θ-defensins when cultured with an aminoglycoside that enables the translation apparatus to misread and bypass the premature stop codon (44). When human cells or cervicovaginal tissues were so treated in vitro, they produced intact RC-1, showing that the trimming and splicing steps needed to produce θ-defensins are available (44). In addition to finding θ-defensin mRNA transcripts in human bone marrow and spleen, smaller amounts were detected in thymus, testis, and skeletal muscle (43). In contrast, Northern blot analysis of RTD-1 expression in rhesus macaque tissues demonstrated abundant transcripts only in bone marrow, but not in these other sites (3). Because different methods were used in these studies, these interspecies differences may be more apparent than actual.
Musings
Did humans lose θ-defensins because an ancestor was dealt only the pseudogene cards from the gene deck? Were they made redundant by the multiply duplicated α-defensin genes? Although RTDs may be more potent than α-defensins, their mode of production is less efficient than the standard assembly line production of α-defensins. “Quantity has a quality all its own” was Stalin's rationale for urging production of massive numbers of low quality tanks to confront the more potent German tanks. This aphorism and the abundance of α-defensins in human PMNs may be relevant to the absence of θ-defensins in these cells, despite the many desirable qualities of these “lost” peptides.
Possibilities
At present, a main focus has been developing θ-defensins as topical microbicides to prevent sexually transmitted HIV-1 and HSV infections (44–47). If their production can be scaled up (48, 49), systemic applications will deserve testing to determine how best to benefit from their impressive array of host defense properties.
This is the third article in the Thematic Minireview Series on Circular Proteins.
- AMP
- antimicrobial peptide
- PMN
- polymorphonuclear neutrophil
- HNP
- human neutrophil peptide
- RTD
- rhesus θ-defensin
- PG
- protegrin
- RC
- retrocyclin
- MMC
- minimal microbicidal concentration
- IAV
- influenza A virus.
REFERENCES
- 1. Tomasinsig L., Zanetti M. (2005) The cathelicidins–structure, function, and evolution. Curr. Protein Pept. Sci. 6, 23–34 [DOI] [PubMed] [Google Scholar]
- 2. Tam J. P., Wu C., Yang J. L. (2000) Membranolytic selectivity of cystine-stabilized cyclic protegrins. Eur. J. Biochem. 267, 3289–3300 [DOI] [PubMed] [Google Scholar]
- 3. Tang Y. Q., Selsted M. E. (1993) Characterization of the disulfide motif in BNBD-12, an antimicrobial β-defensin peptide from bovine neutrophils. J. Biol. Chem. 268, 6649–6653 [PubMed] [Google Scholar]
- 4. Mygind P. H., Fischer R. L., Schnorr K. M., Hansen M. T., Sönksen C. P., Ludvigsen S., Raventós D., Buskov S., Christensen B., De Maria L., Taboureau O., Yaver D., Elvig-Jørgensen S. G., Sørensen M. V., Christensen B. E., Kjaerulff S., Frimodt-Moller N., Lehrer R. I., Zasloff M., Kristensen H. H. (2005) Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature 437, 975–980 [DOI] [PubMed] [Google Scholar]
- 5. Zhu S. (2008) Discovery of six families of fungal defensin-like peptides provides insights into origin and evolution of the CSαβ defensins. Mol. Immunol. 45, 828–838 [DOI] [PubMed] [Google Scholar]
- 6. Schutte B. C., Mitros J. P., Bartlett J. A., Walters J. D., Jia H. P., Welsh M. J., Casavant T. L., McCray P. B., Jr. (2002) Discovery of five conserved β-defensin gene clusters using a computational search strategy. Proc. Natl. Acad. Sci. U.S.A. 99, 2129–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tollner T. L., Venners S. A., Hollox E. J., Yudin A. I., Liu X., Tang G., Xing H., Kays R. J., Lau T., Overstreet J. W., Xu X., Bevins C. L., Cherr G. N. (2011) A common mutation in the defensin DEFB126 causes impaired sperm function and subfertility. Sci. Transl. Med. 3, 92ra65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Selsted M. E. (2004) θ-Defensins: cyclic antimicrobial peptides produced by binary ligation of truncated α-defensins. Curr. Protein Pept. Sci. 5, 365–371 [DOI] [PubMed] [Google Scholar]
- 9. Harwig S. S., Swiderek K. M., Kokryakov V. N., Tan L., Lee T. D., Panyutich E. A., Aleshina G. M., Shamova O. V., Lehrer R. I. (1994) Gallinacins: cysteine-rich antimicrobial peptides of chicken leukocytes. FEBS Lett. 342, 281–285 [DOI] [PubMed] [Google Scholar]
- 10. Lynn D. J., Bradley D. G. (2007) Discovery of α-defensins in basal mammals. Dev. Comp. Immunol. 31, 963–967 [DOI] [PubMed] [Google Scholar]
- 11. Aldred P. M., Hollox E. J., Armour J. A. (2005) Copy number polymorphism and expression level variation of the human α-defensin genes DEFA1 and DEFA3. Hum. Mol. Genet. 14, 2045–2052 [DOI] [PubMed] [Google Scholar]
- 12. Leonova L., Kokryakov V. N., Aleshina G., Hong T., Nguyen T., Zhao C., Waring A. J., Lehrer R. I. (2001) Circular minidefensins and post-translational generation of molecular diversity. J. Leukoc. Biol. 70, 461–464 [PubMed] [Google Scholar]
- 13. Tran D., Tran P. A., Tang Y. Q., Yuan J., Cole T., Selsted M. E. (2002) Homodimeric θ-defensins from rhesus macaque leukocytes: isolation, synthesis, antimicrobial activities, and bacterial binding properties of the cyclic peptides. J. Biol. Chem. 277, 3079–3084 [DOI] [PubMed] [Google Scholar]
- 14. Tongaonkar P., Tran P., Roberts K., Schaal J., Osapay G., Tran D., Ouellette A. J., Selsted M. E. (2011) Rhesus macaque θ-defensin isoforms: expression, antimicrobial activities, and demonstration of a prominent role in neutrophil granule microbicidal activities. J. Leukoc. Biol. 89, 283–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garcia A. E., Osapay G., Tran P. A., Yuan J., Selsted M. E. (2008) Isolation, synthesis, and antimicrobial activities of naturally occurring θ-defensin isoforms from baboon leukocytes. Infect. Immun. 76, 5883–5891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kokryakov V. N., Harwig S. S., Panyutich E. A., Shevchenko A. A., Aleshina G. M., Shamova O. V., Korneva H. A., Lehrer R. I. (1993) Protegrins: leukocyte antimicrobial peptides that combine features of corticostatic defensins and tachyplesins. FEBS Lett. 327, 231–236 [DOI] [PubMed] [Google Scholar]
- 17. Chen J., Falla T. J., Liu H., Hurst M. A., Fujii C. A., Mosca D. A., Embree J. R., Loury D. J., Radel P. A., Cheng Chang C., Gu L., Fiddes J. C. (2000) Development of protegrins for the treatment and prevention of oral mucositis: structure-activity relationships of synthetic protegrin analogs. Biopolymers 55, 88–98 [DOI] [PubMed] [Google Scholar]
- 18. Giles F. J., Rodriguez R., Weisdorf D., Wingard J. R., Martin P. J., Fleming T. R., Goldberg S. L., Anaissie E. J., Bolwell B. J., Chao N. J., Shea T. C., Brunvand M. M., Vaughan W., Petersen F., Schubert M., Lazarus H. M., Maziarz R. T., Silverman M., Beveridge R. A., Redman R., Pulliam J. G., Devitt-Risse P., Fuchs H. J., Hurd D. D. (2004) A phase III, randomized, double-blind, placebo-controlled study of iseganan for the reduction of stomatitis in patients receiving stomatotoxic chemotherapy. Leuk. Res. 28, 559–565 [DOI] [PubMed] [Google Scholar]
- 19. Zhao C., Nguyen T., Boo L. M., Hong T., Espiritu C., Orlov D., Wang W., Waring A., Lehrer R. I. (2001) RL-37, an α-helical antimicrobial peptide of the rhesus monkey. Antimicrob. Agents Chemother. 45, 2695–2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tang Y. Q., Yuan J., Osapay G., Osapay K., Tran D., Miller C. J., Ouellette A. J., Selsted M. E. (1999) A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated α-defensins. Science 286, 498–502 [DOI] [PubMed] [Google Scholar]
- 21. Tran D., Tran P., Roberts K., Osapay G., Schaal J., Ouellette A., Selsted M. E. (2008) Microbicidal properties and cytocidal selectivity of rhesus macaque θ-defensins. Antimicrob. Agents Chemother. 52, 944–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lehrer R. I., Barton A., Daher K. A., Harwig S. S., Ganz T., Selsted M. E. (1989) Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J. Clin. Invest. 84, 553–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim C., Kaufmann S. H. (2006) Defensin: a multifunctional molecule lives up to its versatile name. Trends Microbiol. 14, 428–431 [DOI] [PubMed] [Google Scholar]
- 24. Wang W., Mulakala C., Ward S. C., Jung G., Luong H., Pham D., Waring A. J., Kaznessis Y., Lu W., Bradley K. A., Lehrer R. I. (2006) Retrocyclins kill bacilli and germinating spores of Bacillus anthracis and inactivate anthrax lethal toxin. J. Biol. Chem. 281, 32755–32764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arnett E., Lehrer R. I., Pratikhya P., Lu W., Seveau S. (2011) Defensins enable macrophages to inhibit the intracellular proliferation of Listeria monocytogenes. Cell. Microbiol. 13, 635–651 [DOI] [PubMed] [Google Scholar]
- 26. Lehrer R. I., Jung G., Ruchala P., Wang W., Micewicz E. D., Waring A. J., Gillespie E. J., Bradley K. A., Ratner A. J., Rest R. F., Lu W. (2009) Human α-defensins inhibit hemolysis mediated by cholesterol-dependent cytolysins. Infect. Immun. 77, 4028–4040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Welkos S., Cote C. K., Hahn U., Shastak O., Jedermann J., Bozue J., Jung G., Ruchala P., Pratikhya P., Tang T., Lehrer R. I., Beyer W. (2011) Humanized θ-defensins (retrocyclins) enhance macrophage performance and protect mice from experimental anthrax infections. Antimicrob. Agents Chemother. 55, 4238–4250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cole A. M., Hong T., Boo L. M., Nguyen T., Zhao C., Bristol G., Zack J. A., Waring A. J., Yang O. O., Lehrer R. I. (2002) Retrocyclin: a primate peptide that protects cells from infection by T- and M-tropic strains of HIV-1. Proc. Natl. Acad. Sci. U.S.A. 99, 1813–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Münk C., Wei G., Yang O. O., Waring A. J., Wang W., Hong T., Lehrer R. I., Landau N. R., Cole A. M. (2003) The θ-defensin retrocyclin inhibits HIV-1 entry. AIDS Res. Hum. Retroviruses 19, 875–881 [DOI] [PubMed] [Google Scholar]
- 30. Simmons G., Reeves J. D., Hibbitts S., Stine J. T., Gray P. W., Proudfoot A. E., Clapham P. R. (2000) Co-receptor use by HIV and inhibition of HIV infection by chemokine receptor ligands. Immunol. Rev. 177, 112–126 [DOI] [PubMed] [Google Scholar]
- 31. Gallo S. A., Wang W., Rawat S. S., Jung G., Waring A. J., Cole A. M., Lu H., Yan X., Daly N. L., Craik D. J., Jiang S., Lehrer R. I., Blumenthal R. (2006) θ-Defensins prevent HIV-1 Env-mediated fusion by binding gp41 and blocking six-helix bundle formation. J. Biol. Chem. 281, 18787–18792 [DOI] [PubMed] [Google Scholar]
- 32. Cole A. L., Yang O. O., Warren A. D., Waring A. J., Lehrer R. I., Cole A. M. (2006) HIV-1 adapts to a retrocyclin with cationic amino acid substitutions that reduce fusion efficiency of gp41. J. Immunol. 176, 6900–6905 [DOI] [PubMed] [Google Scholar]
- 33. Fuhrman C. A., Warren A. D., Waring A. J., Dutz S. M., Sharma S., Lehrer R. I., Cole A. L., Cole A. M. (2007) Retrocyclin RC-101 overcomes cationic mutations on the heptad repeat 2 region of HIV-1 gp41. FEBS J. 274, 6477–6487 [DOI] [PubMed] [Google Scholar]
- 34. Seidel A., Ye Y., de Armas L. R., Soto M., Yarosh W., Marcsisin R. A., Tran D., Selsted M. E., Camerini D. (2010) Cyclic and acyclic defensins inhibit human immunodeficiency virus type 1 replication by different mechanisms. PLoS ONE 5, e9737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leikina E., Delanoe-Ayari H., Melikov K., Cho M. S., Chen A., Waring A. J., Wang W., Xie Y., Loo J. A., Lehrer R. I., Chernomordik L. V. (2005) Carbohydrate-binding molecules inhibit viral fusion and entry by cross-linking membrane glycoproteins. Nat. Immunol. 6, 995–1001 [DOI] [PubMed] [Google Scholar]
- 36. Doss M., White M. R., Tecle T., Gantz D., Crouch E. C., Jung G., Ruchala P., Waring A. J., Lehrer R. I., Hartshorn K. L. (2009) Interactions of α-, β-, and θ-defensins with influenza A virus and surfactant protein D. J. Immunol. 182, 7878–7887 [DOI] [PubMed] [Google Scholar]
- 37. Liang Q. L., Zhou K., He H. X. (2010) Retrocyclin-2: a new therapy against avian influenza H5N1 virus in vivo and n vitro. Biotechnol. Lett. 32, 387–392 [DOI] [PubMed] [Google Scholar]
- 38. Yasin B., Wang W., Pang M., Cheshenko N., Hong T., Waring A. J., Herold B. C., Wagar E. A., Lehrer R. I. (2004) θ-Defensins protect cells from infection by herpes simplex virus by inhibiting viral adhesion and entry. J. Virol. 78, 5147–5156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Trabi M., Schirra H. J., Craik D. J. (2001) Three-dimensional structure of RTD-1, a cyclic antimicrobial defensin from rhesus macaque leukocytes. Biochemistry 40, 4211–4221 [DOI] [PubMed] [Google Scholar]
- 40. Abuja P. M., Zenz A., Trabi M., Craik D. J., Lohner K. (2004) The cyclic antimicrobial peptide RTD-1 induces stabilized lipid peptide domains more efficiently than its open chain analog. FEBS Lett. 566, 301–306 [DOI] [PubMed] [Google Scholar]
- 41. Daly N. L., Chen Y. K., Rosengren K. J., Marx U. C., Phillips M. L., Waring A. J., Wang W., Lehrer R. I., Craik D. J. (2007) Retrocyclin-2: structural analysis of a potent anti-HIV θ-defensin. Biochemistry 46, 9920–9928 [DOI] [PubMed] [Google Scholar]
- 42. Tang M., Waring A. J., Lehrer R. I., Hong M. (2006) Orientation of a β-hairpin antimicrobial peptide in lipid bilayers from two-dimensional dipolar chemical shift correlation NMR. Biophys. J. 90, 3616–3624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nguyen T. X., Cole A. M., Lehrer R. I. (2003) Evolution of primate θ-defensins: a serpentine path to a sweet tooth. Peptides 24, 1647–1654 [DOI] [PubMed] [Google Scholar]
- 44. Venkataraman N., Cole A. L., Ruchala P., Waring A. J., Lehrer R. I., Stuchlik O., Pohl J., Cole A. M. (2009) Reawakening retrocyclins: ancestral human defensins active against HIV-1. PLoS Biol. 7, e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cole A. M., Patton D. L., Rohan L. C., Cole A. L., Cosgrove-Sweeney Y., Rogers N. A., Ratner D., Sassi A. B., Lackman-Smith C., Tarwater P., Ramratnam B., Ruchala P., Lehrer R. I., Waring A. J., Gupta P. (2010) The formulated microbicide RC-101 was safe and antivirally active following intravaginal application in pig-tailed macaques. PLoS ONE 5, e15111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sassi A. B., Cost M. R., Cole A. L., Cole A. M., Patton D. L., Gupta P., Rohan L. C. (2011) Formulation development of retrocyclin-1 analog RC-101 as an anti-HIV vaginal microbicide product. Antimicrob. Agents Chemother. 55, 2282–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sassi A. B., Bunge K. E., Hood B. L., Conrads T. P., Cole A. M., Gupta P., Rohan L. C. (2011) Preformulation and stability in biological fluids of the retrocyclin RC-101, a potential anti-HIV topical microbicide. AIDS Res. Ther. 8, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Aboye T.L., Li Y., Majumder S., Hao J., Shekhtman A., Camarero J.A. (2012) Efficient one-pot cyclization/folding of rhesus θ-defensin-1 (RTD-1). Bioorg. Med. Chem. Lett. 22, 2823–2826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gould A., Li Y., Majumder S., Garcia A.E., Carlsson P., Shekhtman A., Camarero J.A. (2012) Recombinant production of rhesus θ-defensin-1 (RTD-1) using a bacterial expression system. Mol. Biosyst. 8, 1359–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Miyata T., Tokunaga F., Yoneya T., Yoshikawa K., Iwanaga S., Niwa M., Takao T., Shimonishi Y. (1989) Antimicrobial peptides, isolated from horseshoe crab hemocytes, tachyplesin II, and polyphemusins I and II: chemical structures and biological activity. J. Biochem. 106, 663–668 [DOI] [PubMed] [Google Scholar]
- 51. Nakashima H., Masuda M., Murakami T., Koyanagi Y., Matsumoto A., Fujii N., Yamamoto N. (1992) Anti-human immunodeficiency virus activity of a novel synthetic peptide, T22 ([Tyr5,12,Lys7]polyphemusin II): a possible inhibitor of virus-cell fusion. Antimicrob. Agents Chemother. 36, 1249–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]