Background: Arrestin recruitment to the κ-opioid receptor (KOR) has been linked to several adverse effects of analgesics, such as dysphoria and tolerance.
Results: We identified 6′-guanidinonaltrindole (6′-GNTI) as a potent KOR agonist for G protein activation that fails to recruit arrestin.
Conclusion: 6′-GNTI is an extreme G protein-biased KOR ligand.
Significance: 6′-GNTI is a lead toward analgesics with fewer arrestin-mediated adverse effects.
Keywords: Arrestin, G Protein-coupled Receptors (GPCR), Opiate Opioid, Pain, Tolerance, G Protein Bias, Analgesics, Dysphoria, Functional Selectivity, Kappa-Opioid Receptor
Abstract
κ-Opioid receptor (KOR) agonists do not activate the reward pathway stimulated by morphine-like μ-opioid receptor (MOR) agonists and thus have been considered to be promising nonaddictive analgesics. However, KOR agonists produce other adverse effects, including dysphoria, diuresis, and constipation. The therapeutic promise of KOR agonists has nonetheless recently been revived by studies showing that their dysphoric effects require arrestin recruitment, whereas their analgesic effects do not. Moreover, KOR agonist-induced antinociceptive tolerance observed in vivo has also been proposed to be correlated to the ability to induce arrestin-dependent phosphorylation, desensitization, and internalization of the receptor. The discovery of functionally selective drugs that are therapeutically effective without the adverse effects triggered by the arrestin pathway is thus an important goal. We have identified such an extreme G protein-biased KOR compound, 6′-guanidinonaltrindole (6′-GNTI), a potent partial agonist at the KOR receptor for the G protein activation pathway that does not recruit arrestin. Indeed, 6′-GNTI functions as an antagonist to block the arrestin recruitment and KOR internalization induced by other nonbiased agonists. As an extremely G protein-biased KOR agonist, 6′-GNTI represents a promising lead compound in the search for nonaddictive opioid analgesic as its signaling profile suggests that it will be without the dysphoria and other adverse effects promoted by arrestin recruitment and its downstream signaling.
Introduction
κ-Opioid receptors (KOR)2 are widely expressed in the periphery, the dorsal root ganglia, the spinal cord, and the supraspinal regions associated with pain modulation. KOR agonists have been shown to activate pain inhibitory pathways in the central nervous system, and peripherally restricted KOR agonists have been developed to target KOR located on visceral and somatic afferent nerves for relief of inflammatory, visceral, and neuropathic chronic pain (1, 2). The analgesic properties of KOR agonists are attributed to their ability to activate G proteins in the Gi/o family (3, 4) as the subsequent inhibition of cAMP production (5), as well as the activation of inward rectifier potassium channels (6) and blockade of calcium channels (7), has an inhibitory effect in neurons. In contrast to MOR agonists, KOR agonists are unable to activate the reward pathway and have therefore attracted considerable attention for their ability to exert potent analgesic effects without high abuse potential (1, 8).
Unfortunately, KOR agonists have been found to produce other significant adverse effects, such as dysphoria (9). Interestingly, their dysphoric effects require activation of the p38 MAPK pathway mediated by arrestin recruitment to the activated KOR (4, 10, 11). KOR-induced p38 MAPK activation has been demonstrated in heterologous expression systems, striatal neurons and astrocytes, spinal cord astrocytes, and in vivo (12–14) and requires KOR phosphorylation by the G protein-coupled receptor kinase 3 (GRK3) and subsequent arrestin3 recruitment (12). Based on such findings, Chavkin (11) has proposed that KOR-selective G protein-biased partial agonists that do not efficiently recruit arrestin would not cause dysphoria but would retain sufficient analgesic activity for the treatment of pain-related disorders.
As is evident from the above, KOR and many other G protein-coupled receptors (GPCRs) couple to a number of different downstream signaling cascades (4). It is now well accepted that ligands can display different efficacies and/or potencies for different signaling pathways. This phenomenon, referred to as functional selectivity, biased agonism, or ligand-directed signaling/receptor trafficking, has been widely observed (15, 16). For example, at the level of the DOR and MOR receptors, several compounds have been identified as agonists for G protein coupling but as antagonists (DOR) or partial agonists (MOR) for arrestin (17).
Interestingly, agonist-induced interactions with arrestin have also been shown to influence opioid-induced antinociception and tolerance in vivo. Although still an area of controversy (see also the “morphine paradox” (17, 18)), it has been proposed that the ability of an opioid agonist to promote arrestin-dependent desensitization and internalization in vitro is related to the degree of tolerance that develops after long term treatment in vivo (19, 20). Arrestin recruitment to KOR has also been proposed to play a role in antinociceptive tolerance (20, 21), making the development of a G protein-biased agonist an important goal for achieving analgesic efficacy without the adverse effects triggered by arrestin (4, 11).
In exploring the pharmacological properties of 6′-guanidinonaltrindole (6′-GNTI), previously proposed to be a DOR-KOR heteromer-selective ligand (22), we have identified it as a functionally selective G protein-biased KOR ligand, and thus, as a promising lead compound for treating pain without dysphoria and other arrestin-mediated side effects.
EXPERIMENTAL PROCEDURES
Constructs for Expression Vectors and Transfection
The cDNA for human KOR (hKOR) was obtained from the Missouri S&T cDNA Resource Center. For arrestin recruitment experiments, full-length Renilla luciferase 8 (RLuc8, provided by S. Gambhir) was fused in-frame to the C terminus of hKOR in the pcDNA3.1 vector. The following human G protein constructs used were provided by C. Gales (23, 24): untagged GαoA; GαoB with RLuc8 inserted at position 91 (GαoB-RLuc8); untagged Gβ1 (β1); untagged Gγ2 (γ2). The human γ2 subunit was fused to full-length mVenus at its N terminus (mVenus-γ2), and we used the fusion construct human arrestin3-mVenus previously described (25). All the constructs were confirmed by sequencing analysis. A total of 20 μg of plasmid cDNA (e.g. 0.2 μg of hKOR-RLuc8, 15 μg of arrestin3-mVenus, and 4.8 μg of pcDNA3.1) was transfected into HEK-293T cells using polyethylenimine (Polysciences Inc.) in a 1:3 ratio in 10-cm dishes. Cells were maintained in culture with DMEM supplemented with 10% FBS. The transfected ratio among receptor, Gα, β1, and γ2, or arrestin was optimized by testing various ratios of plasmids encoding the different sensors. Experiments were performed 48 h after transfection.
BRET
BRET was performed as described (26). Briefly, cells were harvested, washed, and resuspended in a phosphate-buffered saline (PBS) solution. Approximately 200,000 cells/well were distributed in 96-well plates, and 5 μm coelenterazine H (luciferase substrate) was added to each well. Five minutes after the addition of coelenterazine H, ligands were added to each well, and after 2 min for G protein activation or 5 min for arrestin recruitment, the BRET signal was determined by quantifying and calculating the ratio of the light emitted by mVenus, the energy acceptor (510–540 nm), over that emitted by RLuc8, the energy donor (485 nm). The drug-induced BRET signal was normalized, taking the Emax of the ethylketocyclazocine (EKC)-induced response as 100%. For measuring cAMP accumulation, we used a BRET-based cAMP sensor using the YFP-Epac-RLuc (CAMYEL) assay previously described (27). GαoA, β1, and γ2 were co-expressed to enhance the signal-to-noise ratio, and the cells were treated for 5 min with 100 μm forskolin prior to stimulation (27). The data were normalized and represented as the percentage of forskolin-stimulated cAMP accumulation with 0 defined as the maximal inhibition triggered by EKC.
Surface Expression Determination by FACS Analysis
FACS was performed to determine the surface expression of FLAG-tagged hKOR stably expressed in CHO cells with a C6 flow cytometer (BD Accuri Cytometers). After stimulation of the cells with the corresponding drugs for 30 min at 37 °C, the cells were placed on ice and resuspended in PBS containing 0.5% BSA and 0.1% NaN3. The cells were then incubated with a mouse FLAG-specific M2 (Sigma) antibody (dilution 1:500) for 30 min, washed, and incubated with secondary mouse-specific antibody labeled with Alexa Fluor 647 (Invitrogen). The fluorescence signal at 647 nm was determined using the appropriate filter set (675 ± 25 nm) on the C6 flow cytometer.
Drugs
Pharmacological reagents were obtained from the National Institute on Drug Abuse Drug Supply Program (U50488), from Sigma (6′-GNTI), and from James Woods (EKC).
RESULTS
In a recent study, 6′-GNTI was shown to produce a prolonged antinociceptive response in a rat behavioral model of thermal allodynia (28). The effect was even more robust than that previously reported for another selective KOR agonist, U50488 (29). Although 6′-GNTI has been proposed to act as a potent DOR-KOR heteromer-selective ligand (22) and only as a weak agonist at KOR (22), in vivo studies showed only a small decrease in its potency in a radiant heat tail-flick assay of nociception in DOR knock-out mice (30). Thus, 6′-GNTI can activate KOR in the absence of DOR, consistent with its original description as a KOR agonist (31). We sought to investigate this controversy by assessing the ability of the compound to activate KOR when it is expressed alone.
6′-GNTI Is a Potent Partial Agonist at KOR for G Protein Activation
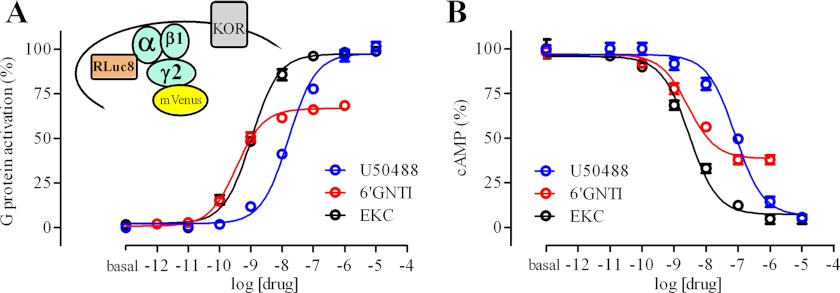
To measure G protein activation, we used a BRET-based assay (23). The hKOR receptor was co-expressed in HEK293T cells with GαoB-RLuc8, β1, and mVenus-γ2 (Fig. 1A). The drug-induced BRET signal corresponds to a dissociation of and/or conformational change within the Gαβγ complex, and thus, to the activation of the co-expressed G protein. EKC and U50488 were found to be full agonists at KOR (Fig. 1A) with potencies of 2.5 ± 1.6 and 43 ± 24 nm, respectively (n = 6). Interestingly, 6′-GNTI robustly activated G protein with a potency of 1.6 ± 1.3 nm and an Emax of 64 ± 6% that of EKC (n = 6) (Fig. 1A). The BRET results with the G protein biosensor were essentially identical to our results measuring drug-induced inhibition of cAMP in a BRET-based CAMYEL sensor assay (27) (Fig. 1B). EKC and U50488 were similarly efficacious (IC50 values of 1.4 ± 1.1 and 38 ± 30 nm, respectively), whereas 6′-GNTI had a relative Emax of 69 ± 4% (IC50 of 1.1 ± 1.2 nm) (n = 3). Although the co-expression of DOR was previously reported to enhance the efficacy of 6′-GNTI for activation of a chimeric Gqi5 protein (22), we did not observe an increase in the efficacy or potency of 6′-GNTI for Gαoβγ activation or cAMP inhibition by KOR upon co-expression of DOR (supplemental Fig. 1, A and B).
FIGURE 1.
6′-GNTI is a potent partial agonist at hKOR for G protein activation and for inhibition of cAMP accumulation. A, the hKOR receptor was co-expressed with GαoB-RLuc8, β1, and mVenus-γ2 to assay G protein activation (23). B, the hKOR receptor was co-expressed with a BRET-based CAMYEL sensor to assay inhibition of forskolin-stimulated cAMP accumulation. Error bars in A and B indicate S.E.
6′-GNTI Is a Functional Antagonist for the Arrestin Pathway
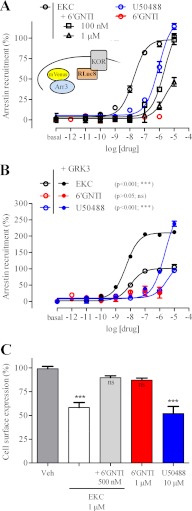
We used a BRET-based assay to measure the recruitment of arrestin3 fused to mVenus to KOR fused to RLuc8 (25). In contrast to EKC and U50488 (Fig. 2A), which both robustly recruited arrestin to KOR (EC50 of 17 ± 10 nm and 2.0 ± 1.2 μm, respectively), 6′-GNTI was without significant effect (Fig. 2A), even after 30 min (supplemental Fig. 2A). To ensure that the absence of signal was not due to a sensitivity limit of the assay given that 6′-GNTI was shown to be a partial agonist for G protein activation, we co-expressed the G protein-coupled receptor kinase, GRK3, to enhance the arrestin BRET signal. GRKs, and more specifically GRK2 and GRK3, are known to enhance arrestin recruitment to various GPCRs, thereby promoting desensitization and internalization of the receptors (32). In the presence of GRK3, we observed a more than 2-fold increase in maximal EKC- and U50488-induced arrestin recruitment to KOR (Fig. 2B). In contrast, the signal triggered by 6′-GNTI was not significantly increased by GRK3, confirming the virtual absence of arrestin3 recruitment to KOR by this compound. 6′-GNTI was also unable to recruit arrestin2 (supplemental Fig. 2B), and the co-expression of DOR with KOR also did not lead to 6′-GNTI-induced recruitment of arrestin3 (supplemental Fig. 1B). These results suggested that 6′-GNTI, although a potent agonist for G protein activation and the cAMP pathway, has such low efficacy for arrestin recruitment that it functions as an antagonist for this pathway. Indeed, 6′-GNTI effectively inhibited EKC-induced arrestin3 recruitment to KOR (Fig. 2A). To confirm the lack of arrestin recruitment triggered by 6′-GNTI by probing its downstream effects, we measured drug-induced internalization. Both EKC and U50488 led to robust receptor internalization, whereas 6′-GNTI led to minimal internalization that did not reach significance (Fig. 2C). Consistent with our arrestin recruitment measurements, 6′-GNTI also potently inhibited EKC-induced KOR internalization (Fig. 2C).
FIGURE 2.
6′-GNTI is a functional antagonist for the arrestin pathway. A and B, the hKOR receptor, fused to RLuc8, was co-expressed with arrestin3 (Arr3) fused to mVenus to assay arrestin recruitment to the receptor (25). A, 6′-GNTI does not induce arrestin3 recruitment to hKOR, in contrast to EKC and U50488, but can inhibit the recruitment induced by EKC. B, GRK3 dramatically enhanced arrestin recruitment to KOR induced by EKC and U50488 but did not affect the signal triggered by 6′-GNTI. A two-way analysis of variance test followed by Bonferroni's post tests was performed to determine the significance of the GRK3 effect on the Emax of each drug. C, after 30 min of incubation with the different drugs, the expression of the FLAG-tagged hKOR receptor, stably expressed in a CHO stable cell line, was measured by flow cytometry using an anti-FLAG antibody (n = 3). A one-way analysis of variance test with 99.9% confidence intervals (p < 0.001) followed by a Tukey's multiple test comparison was performed to determine the significance of each column as compared with vehicle (Veh). Error bars in A–C indicate S.E. ns, not significant.
DISCUSSION
We have found that the naltrindole derivative 6′-GNTI is a potent partial agonist for G protein activation at the KOR receptor. Moreover, 6′-GNTI is extremely G protein-biased as it failed to recruit arrestin and functioned as an antagonist for arrestin recruitment by other KOR agonists. This functional selectivity was manifested downstream as well because 6′-GNTI potently inhibited cAMP activation but functioned as an antagonist of agonist-mediated internalization. This extreme biased agonism is consistent with the potent and efficacious analgesic efficacy of 6′-GNTI observed in vivo (22, 28, 30) and also suggests that 6′-GNTI would not cause dysphoria because of its inability to activate the arrestin pathway. Thus, 6′-GNTI seems to be an ideal lead candidate that establishes the feasibility of an extremely G protein-biased KOR agonist satisfying the design features hypothesized by Chavkin (11). To our knowledge, no other such extreme G protein-biased KOR agonists have been identified to date, although there has been a report of an arrestin-biased KOR ligand (33). Interestingly, although not KOR-selective, etorphine and levorphanol were shown to reduce KOR internalization (34), and their potential functional selectivity at the level of arrestin recruitment merits further investigation.
It is also possible that the extreme G protein bias exhibited by 6′-GNTI would lead to less desensitization and tolerance at KOR. As mentioned above, although not without controversy (17, 18), the KOR-mediated arrestin pathway has been suggested to influence the development of antinociceptive tolerance in vivo (20, 21). It is noteworthy that arrestin3 KO mice display enhanced and prolonged antinociception after short term treatment with morphine and heroin in the hot plate test, a model of thermal antinociception (35). Thus, we hypothesize that after prolonged activation with 6′-GNTI, the KOR receptor would undergo less arrestin-mediated desensitization, which would result in enhanced rather than diminished efficacy over time through persistent G protein activation. In fact, the antiallodynic effect of U50488 was shown to decrease at high concentrations (29), and it was suggested that this might result from rapid desensitization of KOR. Interestingly, we found that EKC and U50488 were both more potent at G protein activation than at recruiting arrestin (7- and 47-fold, respectively) (see above). This could explain the dramatically blunted antinociceptive response to U50488 observed in vivo at high concentrations of the drug as at high concentrations of agonist, arrestin recruitment would be expected to severely limit G protein signaling and hence to limit the analgesic response. Based on our findings, we would predict the absence of such a U-shaped curve for 6′-GNTI analgesia in vivo. Nonetheless, further experiments investigating the desensitization properties of KOR will be necessary to test this hypothesis and to establish that KOR remains functional on the cell surface after prolonged activation by 6′-GNTI.
Our data challenge the notion that 6′-GNTI is DOR-KOR heteromer-selective as we observe robust activation of G protein and inhibition of cAMP in HEK cells in which we only expressed KOR. This is consistent with the quite small alteration in the potency of 6′-GNTI described in a tail-flick assay in DOR KO mice (30). The original study suggesting decreased efficacy of 6′-GNTI in KOR relative to DOR-KOR used a chimeric Gqi5 aequorin signaling assay (22), which may read out differently for G protein activation. It is also possible that enhanced cell surface expression of KOR by co-expression of DOR impacted these results. Although it seems clear that DOR is not essential for 6′-GNTI function, either in HEK cells or in vivo (30), we do not rule out a role of DOR in modulating KOR signaling in vivo, through either a heteromer or a downstream cross-talk mechanism, as proposed by recent studies (28). Nonetheless, the profound functional selectivity of the compound seems likely to dominate its pharmacological properties.
Although functional selectivity/biased agonism was first described almost 20 years ago (36), it has emerged only recently as a key concept for the development of therapeutic drugs (37). GPCRs ligands can activate both G protein-dependent and G protein-independent signaling pathways, which differentially regulate their therapeutic actions and side effects. Understanding which signaling pathways contribute to therapeutic efficacy and which contribute to side effects will enable the design of better drug candidates and lead to safer and more effective therapies. For example, several novel compounds exhibiting antipsychotic-like activity in vivo were shown recently to be antagonists at dopamine D2 receptor of Gi-mediated inhibition of cAMP production but partial agonists for recruitment of arrestin3 to the dopamine D2 receptor (38). These functionally selective arrestin-biased ligands represent valuable chemical probes for investigation of the signaling pathways that are associated with antipsychotic efficacy and side effects. Similarly, the extreme G protein-biased functional selectivity of 6′-GNTI has great potential for effective analgesia with reduced liability for dysphoria and tolerance.
Acknowledgments
We thank Celine Gales and Sam Gambhir for providing cDNA constructs, James Woods for the EKC, and Marta Filizola and Prashant Donthamsetti for helpful comments on the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants DA022413 and MH054137 (to J. A. J.).

This article contains supplemental Figs. 1 and 2.
- KOR
- κ-opioid receptor
- hKOR
- human KOR
- MOR
- μ-opioid receptor
- DOR
- δ-opioid receptor
- BRET
- bioluminescence resonance energy transfer
- 6′-GNTI
- 6′-guanidinonaltrindole
- EKC
- ethylketocyclazocine
- GPCR
- G protein-coupled receptor
- RLuc8
- Renilla luciferase 8
- CAMYEL
- cAMP sensor using YFP-Epac-RLuc
- GRK
- G protein-coupled receptor kinase.
REFERENCES
- 1. Vanderah T. W. (2010) δ- and κ-opioid receptors as suitable drug targets for pain. Clin. J. Pain 26, (Suppl. 10) S10–S15 [DOI] [PubMed] [Google Scholar]
- 2. Basbaum A. I, Jessell T. M. (2000) The perception of pain in Principles of Neural Science, 4th Ed. (Kandel E. R., Schwartz J. H., Jessell T. M., eds) pp. 472–479, The McGraw-Hill Companies, New York, NY [Google Scholar]
- 3. Taussig R., Iñiguez-Lluhi J. A., Gilman A. G. (1993) Inhibition of adenylyl cyclase by Giα. Science 261, 218–221 [DOI] [PubMed] [Google Scholar]
- 4. Bruchas M. R., Chavkin C. (2010) Kinase cascades and ligand-directed signaling at the κ-opioid receptor. Psychopharmacology (Berl) 210, 137–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schoffelmeer A. N., Rice K. C., Jacobson A. E., Van Gelderen J. G., Hogenboom F., Heijna M. H., Mulder A. H. (1988) μ-, δ-, and κ-Opioid receptor-mediated inhibition of neurotransmitter release and adenylate cyclase activity in rat brain slices: studies with fentanyl isothiocyanate. Eur. J. Pharmacol. 154, 169–178 [DOI] [PubMed] [Google Scholar]
- 6. Henry D. J., Grandy D. K., Lester H. A., Davidson N., Chavkin C. (1995) κ-Opioid receptors couple to inwardly rectifying potassium channels when co-expressed by Xenopus oocytes. Mol. Pharmacol. 47, 551–557 [PubMed] [Google Scholar]
- 7. Tallent M., Dichter M. A., Bell G. I., Reisine T. (1994) The cloned κ-opioid receptor couples to an N-type calcium current in undifferentiated PC-12 cells. Neuroscience 63, 1033–1040 [DOI] [PubMed] [Google Scholar]
- 8. Wang Y. H., Sun J. F., Tao Y. M., Chi Z. Q., Liu J. G. (2010) The role of κ-opioid receptor activation in mediating antinociception and addiction. Acta Pharmacol Sin 31, 1065–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Land B. B., Bruchas M. R., Lemos J. C., Xu M., Melief E. J., Chavkin C. (2008) The dysphoric component of stress is encoded by activation of the dynorphin κ-opioid system. J. Neurosci. 28, 407–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Land B. B., Bruchas M. R., Schattauer S., Giardino W. J., Aita M., Messinger D., Hnasko T. S., Palmiter R. D., Chavkin C. (2009) Activation of the κ-opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc. Natl. Acad. Sci. U.S.A. 106, 19168–19173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chavkin C. (2011) The therapeutic potential of κ-opioids for treatment of pain and addiction. Neuropsychopharmacology 36, 369–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bruchas M. R., Macey T. A., Lowe J. D., Chavkin C. (2006) κ-Opioid receptor activation of p38 MAPK is GRK3- and arrestin-dependent in neurons and astrocytes. J. Biol. Chem. 281, 18081–18089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bruchas M. R., Land B. B., Aita M., Xu M., Barot S. K., Li S., Chavkin C. (2007) Stress-induced p38 mitogen-activated protein kinase activation mediates κ-opioid-dependent dysphoria. J. Neurosci. 27, 11614–11623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu M., Bruchas M. R., Ippolito D. L., Gendron L., Chavkin C. (2007) Sciatic nerve ligation-induced proliferation of spinal cord astrocytes is mediated by κ-opioid activation of p38 mitogen-activated protein kinase. J. Neurosci. 27, 2570–2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Urban J. D., Clarke W. P., von Zastrow M., Nichols D. E., Kobilka B., Weinstein H., Javitch J. A., Roth B. L., Christopoulos A., Sexton P. M., Miller K. J., Spedding M., Mailman R. B. (2007) Functional selectivity and classical concepts of quantitative pharmacology. J. Pharmacol. Exp. Ther. 320, 1–13 [DOI] [PubMed] [Google Scholar]
- 16. Lane J. R., Powney B., Wise A., Rees S., Milligan G. (2007) Protean agonism at the dopamine D2 receptor: (S)-3-(3-hydroxyphenyl)-N-propylpiperidine is an agonist for activation of Go1 but an antagonist/inverse agonist for Gi1, Gi2, and Gi3. Mol. Pharmacol. 71, 1349–1359 [DOI] [PubMed] [Google Scholar]
- 17. Molinari P., Vezzi V., Sbraccia M., Grò C., Riitano D., Ambrosio C., Casella I., Costa T. (2010) Morphine-like opiates selectively antagonize receptor-arrestin interactions. J. Biol. Chem. 285, 12522–12535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bohn L. M., Dykstra L. A., Lefkowitz R. J., Caron M. G., Barak L. S. (2004) Relative opioid efficacy is determined by the complements of the G protein-coupled receptor desensitization machinery. Mol. Pharmacol. 66, 106–112 [DOI] [PubMed] [Google Scholar]
- 19. Raehal K. M., Schmid C. L., Groer C. E., Bohn L. M. (2011) Functional selectivity at the μ-opioid receptor: implications for understanding opioid analgesia and tolerance. Pharmacol. Rev. 63, 1001–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu-Chen L. Y. (2004) Agonist-induced regulation and trafficking of κ-opioid receptors. Life Sci. 75, 511–536 [DOI] [PubMed] [Google Scholar]
- 21. McLaughlin J. P., Myers L. C., Zarek P. E., Caron M. G., Lefkowitz R. J., Czyzyk T. A., Pintar J. E., Chavkin C. (2004) Prolonged κ-opioid receptor phosphorylation mediated by G protein receptor kinase underlies sustained analgesic tolerance. J. Biol. Chem. 279, 1810–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Waldhoer M., Fong J., Jones R. M., Lunzer M. M., Sharma S. K., Kostenis E., Portoghese P. S., Whistler J. L. (2005) A heterodimer-selective agonist shows in vivo relevance of G protein-coupled receptor dimers. Proc. Natl. Acad. Sci. U.S.A. 102, 9050–9055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Galés C., Van Durm J. J., Schaak S., Pontier S., Percherancier Y., Audet M., Paris H., Bouvier M. (2006) Probing the activation-promoted structural rearrangements in preassembled receptor-G protein complexes. Nat. Struct. Mol. Biol. 13, 778–786 [DOI] [PubMed] [Google Scholar]
- 24. Saulière A., Bellot M., Paris H., Denis C., Finana F., Hansen J. T., Altié M.-F., Seguelas M.-H., Pathak A., Hansen J. L., Sénard J. M., Galés C. (2012) Deciphering biased-agonism complexity reveals a new active AT1 receptor entity. Nat. Chem. Biol. 8, 622–630 [DOI] [PubMed] [Google Scholar]
- 25. Klewe I. V., Nielsen S. M., Tarpø L., Urizar E., Dipace C., Javitch J. A., Gether U., Egebjerg J., Christensen K. V. (2008) Recruitment of β-arrestin2 to the dopamine D2 receptor: insights into antipsychotic and antiparkinsonian drug receptor signaling. Neuropharmacology 54, 1215–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guo W., Urizar E., Kralikova M., Mobarec J. C., Shi L., Filizola M., Javitch J. A. (2008) Dopamine D2 receptors form higher order oligomers at physiological expression levels. EMBO J. 27, 2293–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jiang L. I., Collins J., Davis R., Lin K. M., DeCamp D., Roach T., Hsueh R., Rebres R. A., Ross E. M., Taussig R., Fraser I., Sternweis P. C. (2007) Use of a cAMP BRET sensor to characterize a novel regulation of cAMP by the sphingosine 1-phosphate/G13 pathway. J. Biol. Chem. 282, 10576–10584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Berg K. A., Rowan M. P., Gupta A., Sanchez T. A., Silva M., Gomes I., McGuire B. A., Portoghese P. S., Hargreaves K. M., Devi L. A., Clarke W. P. (2012) Allosteric interactions between δ- and κ-opioid receptors in peripheral sensory neurons. Mol. Pharmacol. 81, 264–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Berg K. A., Rowan M. P., Sanchez T. A., Silva M., Patwardhan A. M., Milam S. B., Hargreaves K. M., Clarke W. P. (2011) Regulation of κ-opioid receptor signaling in peripheral sensory neurons in vitro and in vivo. J. Pharmacol. Exp. Ther. 338, 92–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ansonoff M. A., Portoghese P. S., Pintar J. E. (2010) Consequences of opioid receptor mutation on actions of univalent and bivalent κ- and δ-ligands. Psychopharmacology 210, 161–168 [DOI] [PubMed] [Google Scholar]
- 31. Sharma S. K., Jones R. M., Metzger T. G., Ferguson D. M., Portoghese P. S. (2001) Transformation of a κ-opioid receptor antagonist to a κ-agonist by transfer of a guanidinium group from the 5′- to 6′-position of naltrindole. J. Med. Chem. 44, 2073–2079 [DOI] [PubMed] [Google Scholar]
- 32. Reiter E., Lefkowitz R. J. (2006) GRKs and β-arrestins: roles in receptor silencing, trafficking, and signaling. Trends Endocrinol Metab. 17, 159–165 [DOI] [PubMed] [Google Scholar]
- 33. Béguin C., Potuzak J., Xu W., Liu-Chen L. Y., Streicher J. M., Groer C. E., Bohn L. M., Carlezon W. A., Jr., Cohen B. M. (2012) Differential signaling properties at the κ-opioid receptor of 12-epi-salvinorin A and its analogues. Bioorg. Med. Chem. Lett. 22, 1023–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li J. G., Zhang F., Jin X. L., Liu-Chen L. Y. (2003) Differential regulation of the human κ-opioid receptor by agonists: etorphine and levorphanol reduced dynorphin A- and U50,488H-induced internalization and phosphorylation. J. Pharmacol. Exp. Ther. 305, 531–540 [DOI] [PubMed] [Google Scholar]
- 35. Raehal K. M., Bohn L. M. (2011) The role of β-arrestin2 in the severity of antinociceptive tolerance and physical dependence induced by different opioid pain therapeutics. Neuropharmacology 60, 58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kenakin T. (1995) Agonist-receptor efficacy. II. Agonist trafficking of receptor signals. Trends Pharmacol. Sci. 16, 232–238 [DOI] [PubMed] [Google Scholar]
- 37. Whalen E. J., Rajagopal S., Lefkowitz R. J. (2011) Therapeutic potential of β-arrestin- and G protein-biased agonists. Trends Mol. Med. 17, 126–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Allen J. A., Yost J. M., Setola V., Chen X., Sassano M. F., Chen M., Peterson S., Yadav P. N., Huang X. P., Feng B., Jensen N. H., Che X., Bai X., Frye S. V., Wetsel W. C., Caron M. G., Javitch J. A., Roth B. L., Jin J. (2011) Discovery of β-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc. Natl. Acad. Sci. U.S.A. 108, 18488–18493 [DOI] [PMC free article] [PubMed] [Google Scholar]