Background: Venoms from rare snake species may contain toxins of new structural or/and pharmacological types.
Results: Amino acid sequence of the new polypeptide azemiopsin isolated from Azemiops feae viper venom was established, and its biological activity was determined.
Conclusion: Azemiopsin is the first natural toxin that blocks nicotinic acetylcholine receptors and does not contain disulfide bridges.
Significance: Azemiopsin is the first member of a new toxin group.
Keywords: Cys-loop Receptors, Neurotoxin, Neurotransmitter Transport, Nicotinic Acetylcholine Receptors, Radioreceptor Assays, Snake Venom, Toxins, Azemiops feae
Abstract
Azemiopsin, a novel polypeptide, was isolated from the Azemiops feae viper venom by combination of gel filtration and reverse-phase HPLC. Its amino acid sequence (DNWWPKPPHQGPRPPRPRPKP) was determined by means of Edman degradation and mass spectrometry. It consists of 21 residues and, unlike similar venom isolates, does not contain cysteine residues. According to circular dichroism measurements, this peptide adopts a β-structure. Peptide synthesis was used to verify the determined sequence and to prepare peptide in sufficient amounts to study its biological activity. Azemiopsin efficiently competed with α-bungarotoxin for binding to Torpedo nicotinic acetylcholine receptor (nAChR) (IC50 0.18 ± 0.03 μm) and with lower efficiency to human α7 nAChR (IC50 22 ± 2 μm). It dose-dependently blocked acetylcholine-induced currents in Xenopus oocytes heterologously expressing human muscle-type nAChR and was more potent against the adult form (α1β1ϵδ) than the fetal form (α1β1γδ), EC50 being 0.44 ± 0.1 μm and 1.56 ± 0.37 μm, respectively. The peptide had no effect on GABAA (α1β3γ2 or α2β3γ2) receptors at a concentration up to 100 μm or on 5-HT3 receptors at a concentration up to 10 μm. Ala scanning showed that amino acid residues at positions 3–6, 8–11, and 13–14 are essential for binding to Torpedo nAChR. In biological activity azemiopsin resembles waglerin, a disulfide-containing peptide from the Tropidechis wagleri venom, shares with it a homologous C-terminal hexapeptide, but is the first natural toxin that blocks nAChRs and does not possess disulfide bridges.
Introduction
Snake venoms are complex mixtures of peptides and proteins possessing different types of biological activity (1). Recent proteomic studies showed that, depending on the snake species, each venom may contain from several tens (2) to several hundred (3, 4) proteins. All easily available venoms have been well studied, and, although some minor components with interesting biological and structural features still can be found in them (5, 6), a more promising approach lies in the investigation of venoms from rare or new species. Our studies of the recently established viper species Vipera nikolskii allowed us to identify some new proteins (7, 8). Most viper venom components affect the blood system, but neuroactive compounds are also present, the best known examples of which are neurotoxic phospholipases A2 (9) and waglerins (10). Waglerins are polypeptides, comprising 22–24 amino acid residues, isolated from the venom of the Asian pit viper Tropidechis waglerii. These polypeptides are selective blockers of muscle-type nicotinic acetylcholine receptor (nAChR)3 (11). Until recently, waglerins were the only polypeptides identified in the venoms of the whole Viperidae family that affect neuroreceptors. Studying Bitis arietans venom, we have isolated a protein that blocks nAChRs (12). The Viperidae family includes 295 species that are classified into three subfamilies: Crotalinae (206 species), Viperinae (88 species), and Azemiopinae, the last including one single species, Azemiops feae (see the Reptile data base). Data about venom components of this fairly rare snake are scarce. Early on, the enzymatic activity of the venom was examined, and it was concluded that it had no blood clotting, hemorrhagic, or myolytic activities (13). The presence of phospholipase A2 and plasminogen activator homologues was shown in another study (14). The analysis of A. feae venom by means of mass spectrometry (14) indicated the presence of an intense signal corresponding to a mass of 2540 Da, which is very similar to the masses of waglerins I and II (2522 and 2548 Da, respectively).
Earlier, we purified the 2540-Da polypeptide azemiopsin from A. feae venom (15). In this paper we describe the determination of its amino acid sequence and biological activity of azemiopsin; like waglerins, azemiopsin selectively blocks muscle-type nAChR but has a markedly different primary structure and does not contain disulfide bridges.
EXPERIMENTAL PROCEDURES
Materials
The venom of A. feae was obtained from a snake caught in North Vietnam. Chloramine T was from Serva; acetonitrile, from Kriochrom (Russia); trifluoroacetic acid (TFA), from Merck; dithiothreitol, from Bio-Rad; carboxypeptidase Y and sulfo-NHS-SS-biotin, from Thermo Scientific. nAChR-enriched membranes from the electric organs of Torpedo californica were kindly provided by Prof. F. Hucho (Free University of Berlin, Germany). GH4C1 cells transfected with human α7 nAChR were a gift from Eli-Lilly. The expressed acetylcholine-binding proteins (AChBP) from Lymnaea stagnalis and Aplysia californica were kindly provided by Prof. T. Sixma (The Netherlands Cancer Institute, Amsterdam, The Netherlands). Monoiodinated (3-[125I]iodotyrosyl54)-α-bungarotoxin (∼2000 Ci/mmol) was from GE Healthcare. Other reagents were of the highest quality commercially available.
Isolation of the Azemiopsin
Gel filtration of crude A. feae venom was carried out using a Superdex HR75 column (10 × 300 mm; GE Healthcare) in 0.1 m ammonium acetate buffer, pH 6.2, at a flow rate of 0.5 ml/min. The reverse-phase HPLC was carried out using Jupiter C18 (4.6 × 250 mm; Phenomenex) in a gradient of 15–40% (v/v) acetonitrile in 75 min in the presence of 0.1% (v/v) TFA, at a flow rate of 1.0 ml/min.
MALDI Mass Spectrometry
MALDI-TOF MS and MS/MS analysis was performed on Ultraflex II MALDI-TOF-TOF mass spectrometer (Bruker Daltonik) equipped with a Nd laser. The MH+ molecular ions were measured in reflector mode; the accuracy of mass peak measurement was 0.01%. Aliquots (0.5 μl) of the sample were mixed on a steel target with an equal volume of 2,5-dihydroxybenzoic acid (Aldrich) solution (10 mg/ml in 30% acetonitrile/0.5% TFA), and the droplet was left to dry at room temperature. Every mass spectrum was obtained as a sum of minimum 500 laser shots. Fragment ion spectra were generated by laser-induced dissociation slightly accelerated by low energy collision-induced dissociation using helium as a collision gas. Correspondence of the found masses to the toxin peptides and to MS/MS peptide fragments was interpreted manually with the help of GPMAW 4.04 software (Lighthouse Data, Odense, Denmark) using 0.01–0.02% precision as a criterion. For partial cleavage of possible disulfide bonds, the energy of laser shots was increased from 15 to 25% of maximal value.
Trypsin cleavage was performed according to standard protocols (protease/substrate 1:50 w/w), and recording of MALDI MS of tryptic fragments as well as their MS/MS analysis was as described above.
Circular Dichroism (CD) Spectroscopy
CD spectra were recorded at 22 °C on a JASCO J-810 spectropolarimeter (JASCO International Co., Tokyo, Japan) in the range from 190 to 250 nm. The peptide was dissolved in 50 mm sodium phosphate buffer, pH 7.0, at a concentration of 1 mg/ml. The light path length was 0.1 mm. The results were expressed as molar ellipticity, [ϴ] (deg × cm2 × dmol−1), determined as [ϴ] = ϴ × 100 × MRW/(c × L), where ϴ is the measured ellipticity in degrees at a wavelength, MRW is the mean amino acid residue weight, c is the peptide concentration in mg/ml, and L is the light path length in centimeters. The instrument was calibrated with (+)-10-camphorsulfonic acid, assuming [ϴ]291 = 7820 deg × cm2 × dmol−1 (16). The analysis of the secondary structure was performed by CONTIN/LL algorithm using SMP56 protein reference set.
Edman Degradation
The peptide was subjected to Edman degradation using an ABI model 494 protein sequencer (Applied Biosystems).
Peptide Synthesis
The peptide and its analogues were synthesized by solid phase method using Fmoc (N-(9-fluorenyl)methoxycarbonyl) chemistry as described in Refs. 17, 18. After deblocking, the peptides were purified by reverse-phase HPLC under conditions used for azemiopsin isolation. For the final purification the reverse-phase HPLC on the same column was used; however, a shallower gradient of acetonitrile (from 10 to 35% in 75 min) was applied.
Lethal Effect of Azemiopsin on Mice
The toxicity was determined on adult male mice (18–22 g body weight) of BALB/c line. Animals were kept in 12/12-h light/dark cycle with food and water ad libitum in accordance with the World Health Organization International Guiding Principles for Animal Research (19). The azemiopsin solution in water was injected intraperitoneally at doses of 2, 2.7, 4, 8, and 10 mg/kg body weight.
Receptor Binding Studies
For competition binding assays, suspensions of nAChR-rich membranes from T. californica ray electric organ (1.25 nm α-bungarotoxin binding sites suspended in 20 mm Tris-HCl buffer, pH 8.0, containing 1 mg/ml bovine serum albumin (BSA)), human α7 nAChR-transfected GH4C1 cells (0.4 nm α-bungarotoxin binding sites suspended in 20 mm Tris-HCl buffer, pH 8.0, containing 1 mg/ml BSA), or solutions of expressed AChBPs from L. stagnalis and A. californica (2.4 and 150 nm in phosphate-buffered saline containing 0.7 mg/ml BSA and 0.05% Tween 20 (PBS-T)) were incubated for 90 min with various amounts of the toxins, followed by an additional 5-min incubation with 0.1–0.2 nm 125I-labeled α-bungarotoxin. Nonspecific binding was determined by preliminary incubation of the preparations with 10 μm α-cobratoxin. The membrane and cell suspensions were applied to glass GF/C filters (Whatman) presoaked in 0.25% polyethylenimine, and the unbound radioactivity was removed from the filter by washing (3 × 3 ml) with 20 mm Tris-HCl buffer, pH 8.0, containing 0.1 mg/ml BSA. The AChBP solutions were applied to two layers of DE-81 filters presoaked in PBS-T buffer, and washed (3 × 3 ml) with PBS-T buffer. Almost the same procedures were used to determine the binding parameters of α-cobratoxin. The only difference was in the time of incubation with 125I-labeled α-bungarotoxin, which was increased to 30–60 min.
Radioligand binding data were fitted using Origin 7.5 (MicroCal Software, Northampton, MA) to the equation
 |
where the IC50 is the concentration at which 50% of the sites are inhibited and nH is the Hill coefficient. The Ki values were calculated from the experimentally measured IC50 by the method of Cheng and Prusoff (20): Ki = IC50/(1 + L/Kd), using the dissociation constant for 125I-labeled α-bungarotoxin measured under the similar assay conditions with the same preparations.
Electrophysiology
cDNAs encoding for the human nAChRs α1, β1, γ or ϵ, and δ subunits were injected into the Xenopus oocytes nucleus using a roboinject (Multichannel Systems, Reutlingen, Germany). cDNA concentrations and injection procedures were standard and as previously described (6). Two to 3 days after injection, recordings were carried out with a two-electrode voltage clamp system using a HiClamp (Multichannel Systems). This system offers the advantage of minimal solution sample and multiple recordings from the same solutions. cDNAs for rat GABAA receptor α1, α2, β3, and γ2 subunits were injected into Xenopus oocytes, and two-electrode voltage clamp measurements on recombinant α1β3γ2 or α2β3γ2 receptors were performed as described previously (21). cRNA was transcribed in vitro from human 5-HT3A and 5-HT3B subunit cDNA and injected into oocytes, and currents were recorded 1–4 days after injection as described previously (22).
RESULTS
Isolation of the Azemiopsin
Gel filtration of dried crude A. feae venom on Superdex HR75 resulted in seven polypeptide fractions (I–VII, Fig. 1A). The most abundant fraction VI was further separated by reverse-phase HPLC (Fig. 1B). The fractions obtained were analyzed by MALDI MS. The peptide with a molecular mass of 2540 Da (fraction marked by bar in Fig. 1B) was called azemiopsin and used for further study.
FIGURE 1.
Isolation of azemiopsin. A, separation of crude A. feae venom by gel filtration on Superdex HR75 column (10 × 300 mm) in 0.1 m ammonium acetate buffer, pH 6.2, at a flow rate of 30 ml/h. B, isolation of azemiopsin by reverse-phase HPLC on a Jupiter C18 (4.6 × 250 mm) column in a linear gradient of acetonitrile in water (0.1% TFA). Flow rate is 1 ml/min.
Structural Characterization
The N-terminal eight amino acid residues of azemiopsin were unambiguously determined by Edman degradation: DNWWPKPP. Because of the overlap due to multiple proline residues the sequence could not be followed beyond that point. The sequence of the C-terminal part as given in Fig. 2 was deduced from the fragment spectrum (postsource decay, MALDI MS/MS). The sequence was further confirmed by MS sequencing of tryptic peptides. To eliminate remaining uncertainties we additionally investigated C-terminal truncation by carboxypeptidase Y and determined the number of lysine residues as two by biotin labeling using sulfo-NHS-SS-biotin (data not shown; three biotins attached at the N terminus and two lysine side chains).
FIGURE 2.

Comparison of the amino acid sequences of azemiopsin and waglerins. Identical residues are underlined, conservative substitutions are italicized. The residues essential for biological activity are indicated by gray hatching. The first 5 N-terminal residues in waglerin, the removal of which results in loss of activity (LD50 > 10 μg/g), are shown in outline.
To confirm the determined structure, the peptide was prepared by solid phase peptide synthesis. The synthetic product co-eluted with the natural peptide from the reverse-phase column, had the identical exact mass as determined by high precision MS, and showed the same fragmentation pattern (data not shown).
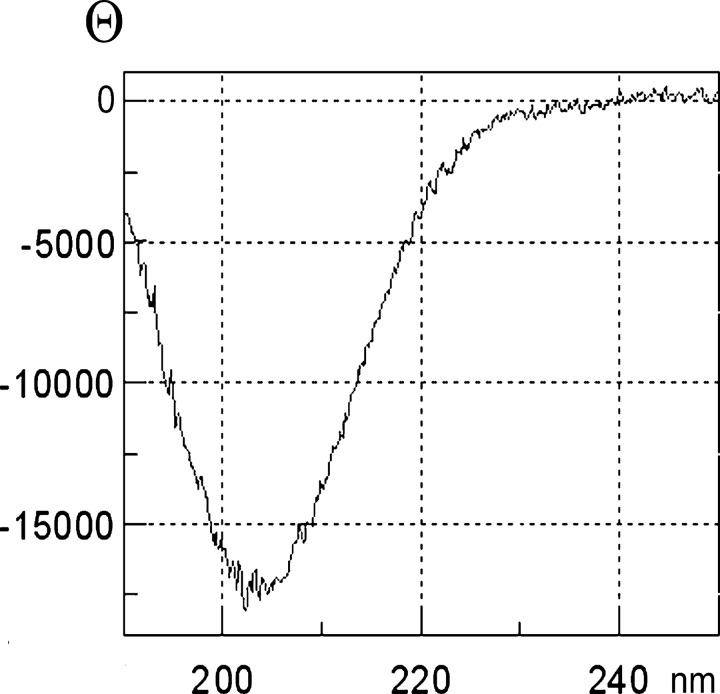
The CD spectrum (Fig. 3) suggests that azemiopsin consists of predominantly β-structure. Calculations using the CONTIN/LL algorithm gave 62% of β-structure (β-sheet + β-turn), 34% of unordered structure, and 4% of α-helix. The structure was not dependent on pH in the range from 4.0 to 8.0 (data not shown).
FIGURE 3.
CD spectrum of azemiopsin at pH 7.0.
Biological Activity of Azemiopsin
Toxicity in Vivo
Azemiopsin displayed pronounced toxicity when injected intraperitoneally into mice. The LD50 was 2.6 ± 0.3 mg/kg.
Binding to Nicotinic Acetylcholine Receptors and Acetylcholine-binding Proteins
Because of its possible relation to the nAChR blocker waglerin, we tested the activity of azemiopsin in competition experiments using the membranes from the electric organ of T. californica as a source of muscle-type nAChR. Although there were data in the literature only on the waglerin interaction with the muscle-type nAChRs, we decided to check the activity of azemiopsin against other putative targets, namely α7 nAChRs and AChBPs. The stably transfected cell line GH4C1 was used as a source of human neuronal α7 nAChR (23). Soluble AChBPs from L. stagnalis and A. californica mollusks which potently bind long chain α-neurotoxins (24, 25) were also used in the competition experiments. Radioactive 125I-labeled α-bungarotoxin was used as a ligand. Azemiopsin competed with α-bungarotoxin for binding to Torpedo nAChR (Table 1). An IC50 value of 0.18 ± 0.03 μm was calculated for a one-site competition model, whereas the calculations using a two-site competition model gave IC50 values of 0.030 ± 0.015 and 0.8 ± 0.4 μm. The two-site model fit (χ2 = 19.8) was better statistically than one-site model fit (χ2 = 25). Azemiopsin was approximately 2 orders of magnitude less active (IC50 22 ± 2 μm; Table 1) on the α7 nAChR, and its interaction with AChBPs was even less potent (IC50 63 ± 6 μm and 230 ± 15 μm for L. stagnalis and A. californica proteins, respectively; Table 1).
TABLE 1.
Inhibition of α-bungarotoxin binding to nAChRs and AChBPs by snake toxins
| nAChR or AChBP | IC50 |
|||
|---|---|---|---|---|
| Azemiopsin | Waglerin II | α-Cobratoxin | Neurotoxin II Naja oxiana | |
| μm | μm | μm | μm | |
| T. californica nAChR | 0.18 ± 0.03 (one site) | 1.2 ± 0.1 | 0.0045 ± 0.0003 | 0.003a |
| 0.030 ± 0.015 and 0.8 ± 0.4 (two sites) | ||||
| Human α7 nAChR | 22 ± 2 | 0.105 ± 0.010 | >4.0b | |
| L. stagnalis AChBP | 63 ± 6 | 7.3 ± 0.4 | 0.0020 ± 0.0002 | |
| A. californica AChBP | 230 ± 15 | 125 ± 11 | 0.058 ± 0.002 | |
Electrophysiology Experiments
When azemiopsin was tested against the acetylcholine-evoked currents in Xenopus oocytes heterologously expressing human muscle-type nAChR a concentration-dependent blockade was observed (Fig. 4). Its affinity for adult α1β1ϵδ and fetal α1β1γδ forms of nAChR was different; it blocked the adult form (EC50 = 0.44 ± 0.1 μm) more potently than the fetal one (EC50 = 1.56 ± 0.37 μm). Although it is known that the embryonic muscle receptors display a higher sensitivity to ACh, the 5 μm concentration is supposed to be below saturation for the two subtypes and yielded currents of comparable amplitude. The toxic effect was readily reversible. After washing out the toxin for 2 min, complete recovery of the response was observed.
FIGURE 4.
Inhibition by azemiopsin of human muscle-type nAChRs expressed in Xenopus oocytes. To evaluate effects of the toxin, cells were challenged at regular intervals (2 min) with brief ACh test pulses (5 μm, 5 s). Following a stabilization period, toxin was applied at different concentrations for at least 1 min. The response to ACh (5 μm) was tested again, and recovery from inhibition was monitored over several minutes. Results obtained with adult α1β1ϵδ nAChR are illustrated in A whereas effects on fetal α1β1γδ form are shown in B. Plot of the peak current as a function of the logarithm of the azemiopsin concentration yielded typical inhibition curves that were fitted with a single Hill equation (continuous curves). Values for the best fit are indicated on the figure.
Azemiopsin was also tested on two GABAA receptor subtypes composed of α1β3γ2 or α2β3γ2 subunits. Azemiopsin showed no significant effects up to 100 or 10 μm, respectively. Similarly, no effects of azemiopsin were observed at 5-HT3A or 5-HT3AB receptors at concentrations up to 10 μm.
Ala Scanning
To dissect which amino acid residues are essential for its biological activity, a series of azemiopsin analogues, containing an alanine residue in different positions of the amino acid sequence, were synthesized. Each analogue was purified by reverse-phase HPLC and its structure confirmed by MALDI MS (Table 1S). Biological activity of the synthetic analogues was determined in competition experiments on Torpedo nAChR (Fig. 5). The data obtained show the decreased affinity of analogues with alanine residues at positions 3–6, 8–11, and 13–14, suggesting that the residues occupying these positions in native azemiopsin are essential for binding to Torpedo nAChR. The analog with C-terminal amide was prepared as well and found to be as active as native underivatized peptide (Fig. 5).
FIGURE 5.
Biological activity of Ala analogues of azemiopsin. Abscissa indicates azemiopsin amino acid residues replaced by alanine. The binding of 125I-labeled α-bungarotoxin (Bgt) was determined after incubation of Torpedo membranes with 2 μm analogue for 1 h. 125I-Labeled α-bungarotoxin binding in the absence of peptide was taken as control (no inhibition).
DISCUSSION
Feae or Burma viper A. feae belongs to the Azemiopinae subfamily, which comprises only this one species. Azemiopinae together with Crotalinae and Viperinae subfamilies form the Viperidae family. However, recent phylogenetic analysis has provided strong support for the position of Azemiops as a sister species to the Crotalines (26). A. feae is a fairly rare snake and inhabits South China, North Myanmar, and North Vietnam. Due to limited availability, the venom of A. feae is not well studied, although the venom of this snake was shown to contain a polypeptide with a molecular mass of 2540 Da (4). The authors suggested that this polypeptide bears similarity to waglerins from T. wagleri venom. In the course of our study of A. feae venom, we have isolated this peptide and determined its primary structure. Interestingly, the sequence contains no cysteine residues, whereas all other neuroactive peptide and protein toxins have at least one disulfide bridge (27), preservation of which is crucial for biological activity. For example, reduction of disulfide bridge in waglerin results in complete loss of activity (28). The C-terminal part of azemiopsin resembles the sequence of waglerins and includes alternating prolines and positively charged residues (Fig. 2). The fragment PRPRPK of azemiopsin is very similar to the C-terminal fragment PRPKPR of waglerins. CD measurements revealed predominantly a β-structure for azemiopsin (Fig. 3). Using the NetTurnP tool (29) for the prediction of β-turn regions in protein sequences, we have found that the central part of the molecule (residues 7–15) has a high probability of β-turn formation. We suggest that azemiopsin may adopt a hairpin-like structure additionally stabilized by cation-π interactions between the N-terminal tryptophan residues and C-terminal arginine or lysine residues. Interestingly, the 1H NMR study of synthetic waglerin I did not reveal any secondary structure in solution (30). However, the central region of waglerin I is well defined as a rigid disulfide-bonded loop, whereas its N and C termini are essentially disordered.
The biological activity of azemiopsin was determined in vivo on mice and in vitro on several types of neurotransmitter receptors using different methods. The peptide was toxic to mice with an LD50 of 2.6 mg/kg. Azemiopsin is therefore less toxic than waglerin I, where the LD50 in mice is in the range 0.33–0.50 mg/kg (10, 28, 31). For waglerin II, which differs from waglerin I by the presence of a tyrosine residue instead of histidine in position 10, this value is 0.58 mg/kg (28).
We observed that azemiopsin interacted efficiently with Torpedo nAChR and with lower affinity with α7 nAChR and AChBPs (Table 1). Compared with so-called short chain α-neurotoxins from snake venoms, the peptide is less active (Table 1). However, it has a similar selectivity profile: both compounds interact more efficiently with muscle-type nAChR. The IC50 values were 0.003 and 4.0 μm for short chain neurotoxin II at muscle-type and α7 neuronal nAChR, respectively, whereas for azemiopsin these values were equal to 0.18 and 22 μm, the differences in the affinity being about 1000 and 100 for neurotoxin II and azemiopsin, respectively. Thus, azemiopsin, a peptide of 21 amino acid residues without disulfide bonds, showed a similar selectivity toward muscle type receptor as neurotoxin II of 61 amino acid residues with four disulfide bridges. For comparison, we used also waglerin II and found that it had an affinity for Torpedo receptor in the micromolar range (Table 1). Surprisingly, there are no published data about the interaction of waglerins with Torpedo nAChR or AChBPs. Compared with waglerin II, azemiopsin showed somewhat higher affinity toward Torpedo nAChR and similar or slightly lower affinity to AChBPs of L. stagnalis and A. californica, respectively (Table 1). Like waglerin I, azemiopsin can discriminate between the two binding sites (α1/γ and α1/δ) on muscle-type (Torpedo) nAChR (Table 1). However, the difference in the affinity between these two sites for azemiopsin (27-fold in Torpedo nAChR) is lower than that for waglerin I, which ranges from 74-fold to 2000-fold for rat and mouse muscle-type nAChRs, respectively (32). Azemiopsin, similarly to waglerin I (11), also can discriminate between the adult and fetal forms of muscle-type nAChRs (Fig. 4), both peptides possessing higher affinity for the adult form.
Blocking acetylcholine-evoked currents in micromolar concentration range, azemiopsin was easily washed out from the receptor. The complete recovery of response was observed after washing for 2 min (Fig. 4). Block of adult mouse nAChR by waglerin-1 (Kd 1.2 nm) was more slowly reversible, approaching about 25% in 8 min (33). Interestingly, WTX (a “weak” toxin from the Naja kaouthia venom having the same three-finger fold as short chain α-neurotoxins and additional disulfide in N-terminal loop) possesses affinity to nAChRs in the micromolar range but is bound almost irreversibly (34). Azemiopsin was inactive on other Cys-loop receptors tested which included GABAA and 5-HT3 receptors.
To identify the amino acid residues essential for the biological activity of azemiopsin, Ala scanning was performed. The biological activity of Ala-substituted analogues reveals several regions that contribute to the high affinity binding to Torpedo nAChR (Fig. 5). These are the residues 3–6, 8–11, and 13-14. It is interesting to compare these data with those obtained for synthetic waglerin I analogues: the removal of the first 5 N-terminal residues in waglerin resulted in almost complete loss of toxicity (35); analogues of azemiopsin in which Trp-4 and Pro-5 are changed to alanine are practically inactive. Waglerin I analogues in which basic amino acids in the central or C-terminal parts of molecule are changed to alanine are only slightly less toxic than native toxin (35); similarly, replacement of each of the seven basic residues in the C-terminal moiety of azemiopsin by alanine does not decrease its activity. The most drastic loss of waglerin toxicity was observed after replacement of His-10 by Ala (36). A corresponding strong decrease of azemiopsin activity was found after changing each of the four central residues PHQG to alanine. These data suggest that the central part of the toxin molecule is absolutely necessary for interaction with the receptor, whereas N-terminal residues are less important and might be involved probably in maintenance of the biologically active conformation. As mentioned above, Trp-3 and Trp-4 may take part in cation-π interaction with basic C-terminal residues. The fact that the removal of single positive charges in the C-terminal part does not strongly affect nAChR binding hints to an interaction network to which several positively charged C-terminal residues contribute. Thus, both in azemiopsin and waglerin the central fragment of the molecule is important for interaction with receptor, whereas the C terminus can be modified almost without loss of activity.
Although azemiopsin lacks disulfide bridges, the main structural features determining the interaction with nAChR are present in the toxin molecule and include positive charges in close vicinity to aromatic residues. Both low molecular weight toxins, like d-tubocurarine, and polypeptide toxins, like α-neurotoxins, possess such moieties. In α-neurotoxins these are invariant tryptophan and lysine residues modifications of which result in dramatic loss of biological activity (37). In azemiopsin, containing many positively charged side chains, their location close to tryptophans 3 and 4 is quite probable.
In summary, we have isolated the peptide azemiopsin from A. feae venom and demonstrated that it is a potent antagonist at adult muscle-type nAChR, with less activity at α7 nAChR and no effect at related GABAA and 5-HT3 receptors. Although azemiopsin resembles waglerins in the C-terminal part, it is the first natural toxin that blocks nAChR and does not contain disulfide bridges. This lack of disulfide bridges means that azemiopsin will be easier to prepare and handle than other peptides possessing nAChR-blocking activity. It is a better template for molecular design and thus has the potential to be a useful tool in neurotransmitter research and in biomedical applications.
Acknowledgment
We thank Irina Kudelina for help with CD measurements.
This work was supported by Neurocypress European Union FP7 Program Grant 202033, Russian Ministry of Education and Science Contract 02.740.11.0865, Russian Foundation for Basic Research Grant 09-04-01061, and Wellcome Trust Grant 01925.
The amino acid sequence reported in this paper has been submitted to the UniProt Knowledgebase under accession number B3EWH2.
- nAChR
- nicotinic acetylcholine receptor
- ACh
- acetylcholine
- AChBP
- ACh-binding protein
- 5-HT
- 5-hydroxytryptamine (serotonin).
REFERENCES
- 1. Utkin Y. N., Osipov A. V. (2007) Nonlethal polypeptide components in cobra venom. Curr. Pharm. Des. 13, 2906–2915 [DOI] [PubMed] [Google Scholar]
- 2. Fernández J., Alape-Girón A., Angulo Y., Sanz L., Gutiérrez J. M., Calvete J. J., Lomonte B. (2011) Venomic and antivenomic analyses of the Central American coral snake, Micrurus nigrocinctus (Elapidae). J. Proteome Res. 10, 1816–1827 [DOI] [PubMed] [Google Scholar]
- 3. Ohler M., Georgieva D., Seifert J., von Bergen M., Arni R. K., Genov N., Betzel C. (2010) The venomics of Bothrops alternatus is a pool of acidic proteins with predominant hemorrhagic and coagulopathic activities. J. Proteome Res. 9, 2422–2437 [DOI] [PubMed] [Google Scholar]
- 4. Fry B. G., Wickramaratna J. C., Hodgson W. C., Alewood P. F., Kini R. M., Ho H., Wüster W. (2002) Electrospray liquid chromatography/mass spectrometry fingerprinting of Acanthophis (death adder) venoms: taxonomic and toxinological implications. Rapid Commun. Mass Spectrom. 16, 600–608 [DOI] [PubMed] [Google Scholar]
- 5. Osipov A. V., Astapova M. V., Tsetlin V. I., Utkin Y. N. (2004) The first representative of glycosylated three-fingered toxins: cytotoxin from the Naja kaouthia cobra venom. Eur. J. Biochem. 271, 2018–2027 [DOI] [PubMed] [Google Scholar]
- 6. Osipov A. V., Kasheverov I. E., Makarova Y. V., Starkov V. G., Vorontsova O. V., Ziganshin R. Kh., Andreeva T. V., Serebryakova M. V., Benoit A., Hogg R. C., Bertrand D., Tsetlin V. I., Utkin Y. N. (2008) Naturally occurring disulfide-bound dimers of three-fingered toxins: a paradigm for biological activity diversification. J. Biol. Chem. 283, 14571–14580 [DOI] [PubMed] [Google Scholar]
- 7. Ramazanova A. S., Zavada L. L., Starkov V. G., Kovyazina I. V., Subbotina T. F., Kostyukhina E. E., Dementieva I. N., Ovchinnikova T. V., Utkin Y. N. (2008) Heterodimeric neurotoxic phospholipases A2, the first proteins from venom of recently established species Vipera nikolskii: implication of venom composition in viper systematics. Toxicon 51, 524–537 [DOI] [PubMed] [Google Scholar]
- 8. Ramazanova A. S., Starkov V. G., Osipov A. V., Ziganshin R. H., Filkin S. Y., Tsetlin V. I., Utkin Y. N. (2009) Cysteine-rich venom proteins from the snakes of Viperinae subfamily: molecular cloning and phylogenetic relationship. Toxicon 53, 162–168 [DOI] [PubMed] [Google Scholar]
- 9. Pungercar J., Krizaj I. (2007) Understanding the molecular mechanism underlying the presynaptic toxicity of secreted phospholipases A2. Toxicon 50, 871–892 [DOI] [PubMed] [Google Scholar]
- 10. Schmidt J. J., Weinstein S. A., Smith L. A. (1992) Molecular properties and structure-function relationships of lethal peptides from venom of Wagler's pit viper, Trimeresurus wagleri. Toxicon 30, 1027–1036 [DOI] [PubMed] [Google Scholar]
- 11. McArdle J. J., Lentz T. L., Witzemann V., Schwarz H., Weinstein S. A., Schmidt J. J. (1999) Waglerin-1 selectively blocks the epsilon form of the muscle nicotinic acetylcholine receptor. J. Pharmacol. Exp. Ther. 289, 543–550 [PubMed] [Google Scholar]
- 12. Vulfius C. A., Gorbacheva E. V., Starkov V. G., Osipov A. V., Kasheverov I. E., Andreeva T. V., Astashev M. E., Tsetlin V. I., Utkin Y. N. (2011) An unusual phospholipase A2 from puff adder Bitis arietans venom: a novel blocker of nicotinic acetylcholine receptors. Toxicon 57, 787–793 [DOI] [PubMed] [Google Scholar]
- 13. Mebs D., Kuch U., Meier J. (1994) Studies on venom and venom apparatus of Fea's viper, Azemiops feae. Toxicon 32, 1275–1278 [DOI] [PubMed] [Google Scholar]
- 14. Fry B. G., Wüster W., Ryan Ramjan S. F., Jackson T., Martelli P., Kini R. M. (2003) Analysis of Colubroidea snake venoms by liquid chromatography with mass spectrometry: evolutionary and toxinological implications. Rapid Commun. Mass Spectrom. 17, 2047–2062 [DOI] [PubMed] [Google Scholar]
- 15. Utkin Y. N., Weise Ch., Hoang N. A., Kasheverov I. E., Starkov V. G., Tsetlin V. I. (2012) The new peptide from the Fea's viper Azemiops feae venom interacts with nicotinic acetylcholine receptors. Dokl. Biochem. Biophys. 442, 33–35 [DOI] [PubMed] [Google Scholar]
- 16. Schippers P. H., Dekkers H. P. (1981) Direct determination of absolute circular dichroism data and calibration of commercial instruments. Anal. Chem. 53, 778–788 [Google Scholar]
- 17. Barlos K., Chatzi O., Gatos D., Stavropoulos G. (1991) 2-Chlorotrityl chloride resin: Studies on anchoring of Fmoc-amino acids and peptide cleavage. Int. J. Pept. Protein Res. 37, 513–520 [PubMed] [Google Scholar]
- 18. Kasheverov I. E., Zhmak M. N., Vulfius C. A., Gorbacheva E. V., Mordvintsev D. Y., Utkin Y. N., van Elk R., Smit A. B., Tsetlin V. I. (2006) α-Conotoxin analogs with additional positive charge show increased selectivity toward Torpedo californica and some neuronal subtypes of nicotinic acetylcholine receptors. FEBS J. 273, 4470–4481 [DOI] [PubMed] [Google Scholar]
- 19. Howard-Jones N. (1985) A CIOMS ethical code for animal experimentation. WHO Chron. 39, 51–56 [PubMed] [Google Scholar]
- 20. Cheng Y., Prusoff W. H. (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50% inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 22, 3099–3108 [DOI] [PubMed] [Google Scholar]
- 21. Ramerstorfer J., Furtmüller R., Sarto-Jackson I., Varagic Z., Sieghart W., Ernst M. (2011) The GABAA receptor α+β interface: a novel target for subtype selective drugs. J. Neurosci. 31, 870–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thompson A. J., Duke R. K., Lummis S. C. (2011) Binding sites for bilobalide, diltiazem, ginkgolide, and picrotoxinin at the 5-HT3 receptor. Mol. Pharmacol. 80, 183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Quik M., Choremis J., Komourian J., Lukas R. J., Puchacz E. (1996) Similarity between rat brain nicotinic α-bungarotoxin receptors and stably expressed α-bungarotoxin binding sites. J. Neurochem. 67, 145–154 [DOI] [PubMed] [Google Scholar]
- 24. Smit A. B., Syed N. I., Schaap D., van Minnen J., Klumperman J., Kits K. S., Lodder H., van der Schors R. C., van Elk R., Sorgedrager B., Brejc K., Sixma T. K., Geraerts W. P. (2001) A glia-derived acetylcholine-binding protein that modulates synaptic transmission. Nature 411, 261–268 [DOI] [PubMed] [Google Scholar]
- 25. Hansen S. B., Talley T. T., Radic Z., Taylor P. (2004) Structural and ligand recognition characteristics of an acetylcholine-binding protein from Aplysia californica. J. Biol. Chem. 279, 24197–24202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wüster W., Peppin L., Pook C. E., Walker D. E. (2008) A nesting of vipers: phylogeny and historical biogeography of the Viperidae (Squamata: Serpentes). Mol. Phylogenet. Evol. 49, 445–459 [DOI] [PubMed] [Google Scholar]
- 27. Tsetlin V., Utkin Y., Kasheverov I. (2009) Polypeptide and peptide toxins, magnifying lenses for binding sites in nicotinic acetylcholine receptors. Biochem. Pharmacol. 78, 720–731 [DOI] [PubMed] [Google Scholar]
- 28. Weinstein S. A., Schmidt J. J., Bernheimer A. W., Smith L. A. (1991) Characterization and amino acid sequences of two lethal peptides isolated from venom of Wagler's pit viper, Trimeresurus wagleri. Toxicon 29, 227–236 [DOI] [PubMed] [Google Scholar]
- 29. Petersen B., Lundegaard C., Petersen T. N. (2010) NetTurnP: neural network prediction of β-turns by use of evolutionary information and predicted protein sequence features. PLoS ONE 5, e15079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chuang L. C., Yu H. M., Chen C., Huang T. H., Wu S. H., Wang K. T. (1996) Determination of three-dimensional solution structure of waglerin I, a toxin from Trimeresurus wagleri, using two-dimensional NMR and molecular dynamics simulation. Biochim. Biophys. Acta 1292, 145–155 [DOI] [PubMed] [Google Scholar]
- 31. Tsai M. C., Hsieh W. H., Smith L. A., Lee C. Y. (1995) Effects of waglerin-I on neuromuscular transmission of mouse nerve-muscle preparations. Toxicon 33, 363–371 [DOI] [PubMed] [Google Scholar]
- 32. Molles B. E., Rezai P., Kline E. F., McArdle J. J., Sine S. M., Taylor P. (2002) Identification of residues at the α- and ϵ-subunit interfaces mediating species selectivity of waglerin-1 for nicotinic acetylcholine receptors. J. Biol. Chem. 277, 5433–5440 [DOI] [PubMed] [Google Scholar]
- 33. Teichert R. W., Garcia C. C., Potian J. G., Schmidt J. J., Witzemann V., Olivera B. M., McArdle J. J. (2008) Peptide-toxin tools for probing the expression and function of fetal and adult subtypes of the nicotinic acetylcholine receptor. Ann. N.Y. Acad. Sci. 1132, 61–70 [DOI] [PubMed] [Google Scholar]
- 34. Utkin Y. N., Kukhtina V. V., Kryukova E. V., Chiodini F., Bertrand D., Methfessel C., Tsetlin V. I. (2001) “Weak toxin” from Naja kaouthia is a nontoxic antagonist of α7 and muscle-type nicotinic acetylcholine receptors. J. Biol. Chem. 276, 15810–15815 [DOI] [PubMed] [Google Scholar]
- 35. Hsiao Y. M., Chuang C. C., Chuang L. C., Yu H. M., Wang K. T., Chiou S. H., Wu S. H. (1996) Protein engineering of venom toxins by synthetic approach and NMR dynamic simulation: status of basic amino acid residues in waglerin I. Biochem. Biophys. Res. Commun. 227, 59–63 [DOI] [PubMed] [Google Scholar]
- 36. Sellin L. C., Mattila K., Annila A., Schmidt J. J., McArdle J. J., Hyvönen M., Rantala T. T., Kivistö T. (1996) Conformational analysis of a toxic peptide from Trimeresurus wagleri which blocks the nicotinic acetylcholine receptor. Biophys. J. 70, 3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Antil S., Servent D., Ménez A. (1999) Variability among the sites by which curaremimetic toxins bind to Torpedo acetylcholine receptor, as revealed by identification of the functional residues of α-cobratoxin. J. Biol. Chem. 274, 34851–34858 [DOI] [PubMed] [Google Scholar]
- 38. Tsetlin V. I., Karlsson E., Arseniev A. S., Utkin Y. N., Surin A. M., Pashkov V. S., Pluzhnikov K. A., Ivanov V. T., Bystrov V. F., Ovchinnikov Y. A. (1979) EPR and fluorescence study of interaction of Naja naja oxiana neurotoxin II and its derivatives with acetylcholine receptor protein from Torpedo marmorata. FEBS Lett. 106, 47–52 [DOI] [PubMed] [Google Scholar]
- 39. Shelukhina I. V., Kryukova E. V., Lips K. S., Tsetlin V. I., Kummer W. (2009) Presence of α7 nicotinic acetylcholine receptors on dorsal root ganglion neurons proved using knockout mice and selective α-neurotoxins in histochemistry. J. Neurochem. 109, 1087–1095 [DOI] [PubMed] [Google Scholar]