Background: TRPA1 is a calcium channel expressed in polymodal nociceptors sensitive to noxious cold temperature.
Results: Cold triggered calcium influx in cells expressing wild type TRPA1, but not variant Lys-179.
Conclusion: The impaired cold sensitivity of variant TRPA1 protein might result in altered sensory responses of nociceptors.
Significance: The understanding of molecular basis of sensory phenotypes is critical for adequate therapy of pain patients.
Keywords: Calcium Channels, Genetic Polymorphism, Neurophysiology, Pain, TRP Channels
Abstract
The role of genetic modifications of the TRPA1 receptor has been well documented in inflammatory and neuropathic pain. We recently reported that the E179K variant of TRPA1 appears to be crucial for the generation of paradoxical heat sensation in pain patients. Here, we describe the consequences of the single amino acid exchange at position 179 in the ankyrin repeat 4 of human TRPA1. TRPA1 wild type Lys-179 protein expressed in HEK cells exhibited intact biochemical properties, inclusive trafficking into the plasma membrane, formation of large protein complexes, and the ability to be activated by cold. Additionally, a strong increase of Lys-179 protein expression was observed in cold (4 °C) and heat (49 °C)-treated cells. In contrast, HEK cells expressing the variant Lys-179 TRPA1 failed to get activated by cold possibly due to the loss of ability to interact with other proteins or other TRPA1 monomers during oligomerization. In conclusion, the detailed understanding of TRPA1 genetic variants might provide a fruitful strategy for future development of pain treatments.
Introduction
Periodic irritation of nociceptors during inflammation, injury, or a neural lesion may lead to the amplification of pain and development of clinical symptoms such as cold hyperalgesia, allodynia, and/or paradoxical heat sensation (1, 2). The molecular mechanism underlying the aberrant cold sensation in neuropathic states, however, is not yet fully understood. Inflammation and nerve dysfunction have been associated with increased excitability of nociceptors based on their ionic conductance characteristics (3). TRPV1, TRPV3, and TRPV4 are implicated in heat response and two other TRP channels have been described to mediate response to cooling (TRPM8) or cold (TRPA1) (4). TRPA1 is activated at temperatures below 17 °C, making it a candidate to explain transduction of noxious cold in sensory neurons (5). Further behavioral analysis of TRPA1 knock-out mice provided convincing evidence of impaired noxious cold sensation (6). In animal models of neuropathic pain, the altered expression of TRPA1 in injured afferent neurons and uninjured adjacent Aδ fibers was suggested to be an executive mechanism of cold hyperalgesia (7–9). Moreover, neurons that usually co-express TRPA1 and the heat receptor TRPV1 act as polymodal nociceptors; thus, the perception of noxious cold and heat appears to be linked (10).
Kremeyer et al. (11) identified the first pain-associated TRPA1 channelopathy. They described a N855S gain-of-function mutation in the S4 transmembrane segment of TRPA1, which resulted in increased activation of the ion channel by noxious cold temperature underlining the contribution of genetics in pain plasticity (11). Recently, we identified the non-synonymous genetic variant E179K (rs920829) of the human TRPA1 gene to be associated with the occurrence of paradoxical heat sensation (PHS)2 in neuropathic pain patients (12). PHS is defined as a burning heat sensation when a noxious cold stimulus is applied. Patients carrying the Lys-179 variant only rarely exhibited PHS compared with carriers of Glu-179. This observation suggests a diminished sensitivity to noxious cold, resulting in prevention from PHS. We hypothesized that the exchange of glutamate to lysine at position 179 in the ankyrin repeat 4 of the TRPA1 N terminus could result in alterations of expression and/or functionality and characterized both TRPA1 variants under temperature stress in an in vitro expression system.
EXPERIMENTAL PROCEDURES
In Vitro Expression Model
Human TRPA1 cDNA carrying either Glu-179 (WT) or Lys-179 (variant) was synthesized by Geneart (Invitrogen) and subsequently cloned into a pcDNA3.1-V5/His mammalian expression vector (Invitrogen). The conditions for transient transfection with Lipofectamine 2000 (Invitrogen) of cells in serum-free conditions were optimized. The medium was exchanged for FCS-containing DMEM followed 4 h after transfection. The cells were incubated at 37 °C for the next 16 h. Transfection efficiency was controlled through co-transfection with the enhanced GFP vector (Clontech, Takara Bio), coding for green fluorescent protein. TRPA1 expression in HEK293T17 cells reached a maximum 20–26 h after transfection. All temperature stimulations (4 °C, 49 °C) were performed within this time range.
Immunoblotting Analysis
Transfected HEK293T17 cells were lysed in CHAPS-based (0.2%) Tris/NaCl buffer (pH 7.5) followed by three freeze/thaw cycles. After centrifugation, the concentration of proteins in the supernatant was estimated using the BCA protein assay (Thermoscientific, Rockford, IL). Equal amounts of each protein samples were precipitated with cold acetone to facilitate handling. Protein samples dissolved in 2× Laemmli buffer were loaded into 4–20% Bis-tris NuPAGE gels (Invitrogen) for SDS-PAGE and then transferred onto nitrocellulose membranes (Millipore Corp.). The pcDNA3.1-V5/His mammalian expression vector carrying a fragment of the paramyxovirus SV5 V5 epitope allowed the precise immunodetection of heterologously expressed channel proteins using an anti-V5 mouse monoclonal antibody (Invitrogen). Monoclonal rabbit anti-GFP and monoclonal mouse anti-β-actin antibodies as well as all secondary antibodies, conjugated with HRP, were from Sigma-Aldrich, and GFP and β-actin served as controls for transfection efficiency and equal sample loading, respectively. The chemiluminiscence HRP substrate (Millipore Corp.) was used for detection.
Immunofluorescence Staining of TRPA1
Prior to transfection, HEK293T17 cells were seeded onto the glass coverslips coated with poly-d-lysine and laminin for better adhesion of cells. After transfection, cells were incubated for 20 h at 37 °C in FCS-containing DMEM followed by subsequent exposure to 4, 49, or further 37 °C (control) for 10 min. The cells were then fixed with cold acetone for 10 min, washed twice with 10 mm Tris-HCl, 300 mm NaCl buffer (pH 7.5), stained overnight at 4 °C with mouse anti-V5 antibody diluted 1:700 in antibody-diluent reagent solution (Invitrogen), and followed by incubation with Alexa Fluor 488-labeled secondary antibody (Invitrogen) for 1 h at room temperature. The coverslips were washed three times with Tris-HCl/NaCl buffer, and the nuclei staining reagent (bis-benzimid) was applied for 1 min. The mounting medium was dropped onto each coverslip and closed with microscope slide. Apotome-based microscopy was used to prepare photos of stained TRPA1 channels in HEK293T17 cells.
Fluorescence-based detection of intracellular calcium was employed to test the functionality of both Glu-179 and Lys-179 TRPA1. Fluo-4 NW calcium assay (Molecular Probes, Invitrogen) was performed as follows: HEK293T17 cells were cultured in black flat-bottomed 96-well plates prior to Lipofectamine-based transfection. Cells expressing Glu-179 or Lys-179 TRPA1 were maintained under the indicated conditions. Subsequently, cells were washed twice with PBS, and a solution of fluo-4 indicator was added according to the manufacturer's instructions. Fluorescence intensity (excitation wavelength, λ = 485 nm; emission, λ = 525 nm) was measured using an Infinity 200 monochromator reader (Tecan). Each measurement was done twice in eight repeats. The Magelan software (Tecan) was used for evaluation and calculation of mean value and S.D. The TRPA1-specific inhibitor HC030031 (Sigma Aldrich) used in inhibitory study was dissolved in dimethyl sulfoxide, and a concentration of 15 μm was used in our experiments.
Blue Native Gel Electrophoresis
Blue native gel electrophoresis was performed as described previously by Wittig et al. (13). Briefly, the membrane proteins from HEK293 cells expressing either Glu-179 or Lys-179 variant were extracted under native conditions with use of 2% dodecyl-β-d-maltoside (w/v) followed by homogenization step. The mild neutral detergent as dodecyl-β-d-maltoside is suited to isolate protein complexes with high efficiency. Several freeze/thaw cycles in liquid nitrogen facilitated homogenization. Finally, the insoluble cell rests were separated by centrifugation for 10 min at 4 °C at 20,000 × g. Coomassie dye G-250 was added in ratio 1:4 to detergent concentration to each supernatant. This step is crucial because anionic Coomassie dye bind the proteins and enables their migration to the anode buffer at pH 7.5. Coomassie dye was also added in cathode buffer. The preparation of buffers for blue native gel electrophoresis was done according to the manufacturer's instructions (Invitrogen, NativePAGETM Novex® Bis-Tris gel system). Protein complexes were run in the first, native dimension as blue-colored bands and were separated according to their molecular weight. To estimate Mr of complexes, the NativeMarkTM unstained protein standard (Invitrogen) was employed. The single strip containing separated complexes from one sample were excised and equilibrated in SDS buffer with addition of 50 mm dithiothreitol for 30 min. Thus complexes in strip were denatured and could be applied into the second SDS-PAGE. We have used the ZOOM gradient 4–12% Bis-Tris gel (Invitrogen) with one immobilized pH gradient well. We investigated the presence of TRPA1 in complexes after the first (native) and also the second denatured dimension in Western blot with mouse anti-V5 antibody.
RESULTS
Structural Insights into E179K Polymorphism
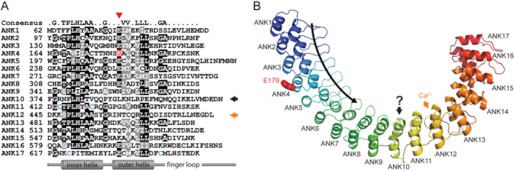
The TRPA1 protein sequence contains a set of 17 ankyrin repeat sequence motifs (ANK1 to ANK17) within is the cytoplasmic N terminus (Fig. 1A). Residue Glu-179 (highlighted in red in Fig. 1A) is located within the fourth ankyrin repeat in TRPA1. Of note, residue 179 is part of a conserved string of negatively charged glutamates that line the N-terminal end of the outer helix in repeats ANK1 through ANK8. Other notable elements include a motif important for calcium sensitivity in the ANK12 finger (orange arrow) (5, 14) and ANK10 (black arrow), which deviates markedly from the ankyrin repeat consensus (10, 15). Cysteines colored blue are important for the activation of TRPA1 by electrophiles (16, 17). Ankyrin repeats are short motifs of ∼33 amino acid residues that are found in a large number of proteins and are typically implicated in ligand interactions (18). Ankyrin repeats form a repeating structural unit consisting of two short helices and a hairpin “finger” loop. Multiple repeats stack side-by-side to form a coiling structure. The concave face of the coiling structure, indicated by the curved arrow in Fig. 1B, is formed by the inner helices and finger loops and has most often been implicated in ligand interactions (19). To obtain further insights into the potential role of the residue at position 179 for the function of human TRPA1, we used the structure of ankyrinR (19) as a basis to construct a structural homology model of the ankyrin repeats of human TRPA1 (Fig. 1B). The sequence similarity between human TRPA1 and ankyrinR provides us with a good level of confidence for several elements of this homology model: (i) the position and sequence register of the helices; (ii) the overall elongated shape of the model; and (iii) the predicted length of the loop regions. Other elements are not predicted with high confidence: (i) the overall coiling angle; (ii) the exact shape of each loop region (especially the ones that are longer than the ankyrin repeat motif consensus); and (iii) the overall structure of repeat ANK10, which, as stated above, deviates significantly from the ankyrin sequence motif consensus.
FIGURE 1.
Structural model of the TRPA1 Glu-179 variant. A, sequence of the human TRPA1 ankyrin repeats aligned to the ankyrin repeat consensus (top). Similar and identical residues are shaded gray and black, respectively. Glu-179 is highlighted in red. Glutamate tends to be conserved at that position (red arrowhead). A motif important for calcium binding is in the ANK12 finger (orange arrow). ANK10 drastically deviates from consensus sequence (black arrow). Cysteines (blue) are important for TRPA1 activation by electrophiles. B, ribbon diagram of human TRPA1, rainbow-colored from the N to C termini. The model contains residues 62–649 predicted to form 17 ANK repeats. The curved black arrow indicates the direction of the concave surface commonly used for protein-protein interactions. Residue Glu-179 (red) is on the convex surface of ANK4. The above-mentioned ANK12 and ANK10 are assigned as well.
Residue 179 (Fig. 1, red) is located on the convex surface on the solvent-exposed face of the outer helix of repeat ANK4. The amino acid residue at position 179 is therefore not predicted to be crucial to the hydrophobic core packing of the ankyrin repeat domain. Thus, it is more likely to affect the surface properties of the ankyrin repeat domain and possibly some yet-to-be determined ligand interactions, although it is not on the commonly used concave interaction surface.
Expression of TRPA1 Variants under Cold and Heat
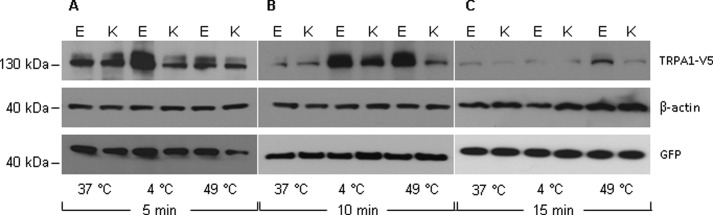
TRPA1 protein expression were investigated at 4 and 49 °C for exposure time of 0, 5, 10, and 15 min, respectively (Fig. 2). Our results demonstrate that TRPA1 Glu-179 and Lys-179 protein are differentially regulated under temperature stimulation and in a time-dependent manner. Short term (5 min) cold treatment was sufficient to increase significantly the amount of wild type Glu-179 protein (Fig. 2A). The increase of Glu-179 protein expression was prolonged up to 10 min of stimulation (Fig. 2B). Finally, after 15 min of exposure to 4 °C, Glu-179 expression declined back to its basal level determined before at 37 °C (Fig. 2C). Interestingly, the wild type Glu-179 TRPA1 protein underwent also dramatic heat-induced up-regulation after 10 min (Fig. 2B). In contrast, the variant Lys-179 TRPA1 protein exhibited only a moderate increase after 10 min of cold stimulation, and there was no clear change upon heat exposure (Fig. 2B).
FIGURE 2.
Effects of temperature on expression of TRPA1 Glu-179 (WT) and Lys-179. Green fluorescent protein (GFP) was used as a control for transfection efficiency, and β-actin was used as marker for equal protein loading. TRPA1 proteins were evaluated at time points 5 min (A), 10 min (B), and 15 min (C) upon cold (4 °C) and heat (37 °C) stimulation. Strong up-regulation of WT Glu-179 was detected at a time point of 10 min of cold and heat treatment, whereas variant Lys-179 protein was increased moderately only by cold. The results were confirmed in three independent experiments. The full Western blot is presented in supplemental Fig. 1.
Temperature-initiated Trafficking of TRPA1 Variants
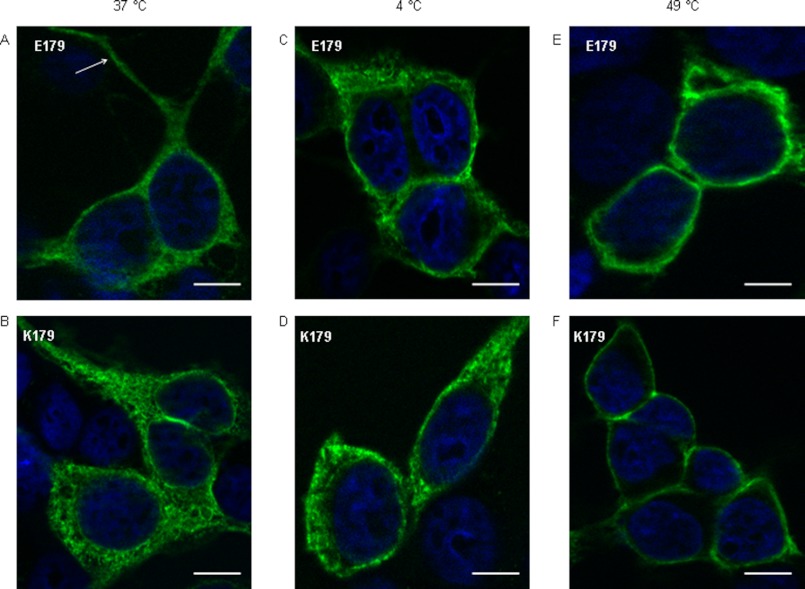
To examine the relationship between thermal stimulation and the translocation of TRPA1 protein into the plasma membrane, an immunofluorescence staining with use of anti-V5 antibody was performed (Fig. 3). The microscopy photographs showed a strong cytoplasmic expression of both Glu-179 and Lys-179 in cells at 37 °C (Fig. 3, A and B). HEK293T17 cells have some features typical of neuronal cells (20) such as neurite outgrowth-like structures, which are important for adhesion and/or cell-cell contact. Interestingly, these structures were TRPA1-positive (see arrow in Fig. 3A) The cells maintained for 10 min at 4 °C started to concentrate TRPA1 channels close to the plasma membrane, whereas a portion of TRPA1 proteins was still localized in the cytoplasm (Fig. 3, C and D). In contrast, sharp membrane-associated TRPA1 staining evoked by heat (49 °C) indicated the complete translocation of channels from the cytoplasm (Fig. 3, E and F). Prolonged exposure at 49 °C had lethal consequences on HEK293 cells, with morphological alterations like rounding and cell death.
FIGURE 3.
Trafficking of variant TRPA1 upon cold and heat. TRPA1 Glu-179 (A) and TRPA1 Lys-179 (B) were localized at 37 °C in the cytoplasm (green fluorescence). Treatment with cold (10 min at 4 °C) initiated partial translocation of Glu-179 (C) and Lys-179 (D) close to the plasma membrane. Heat shock (10 min at 49 °C) caused a complete translocation of Glu-179 (E) and also Lys-179 (F) TRPA1 into the plasma membrane. The nuclei are colored blue. The scale bars represent a length of 50 μm.
Functional Characterization of TRPA1 Variants
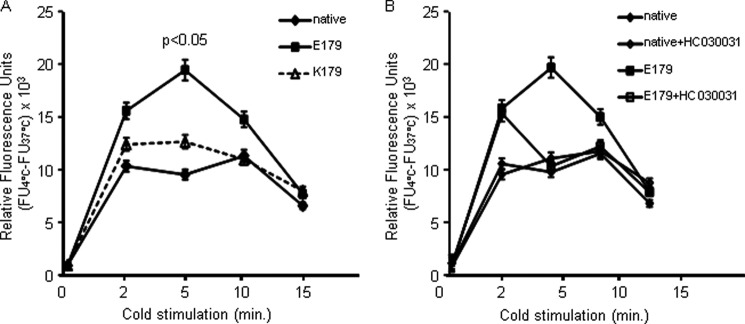
To define the functional differences in the temperature activation between Glu-179 and Lys-179 TRPA1 channels, intracellular calcium flux was determined. HEK293T17 cells heterologously expressing the TRPA1 variants were shifted to temperature of 4 or 49 °C for periods of up to 15 min (Fig. 4). Initially, two control experiments were performed. First, because other endogenous temperature-sensitive Ca2+-channels could not be excluded in HEK293T17 cells, the effects of cold and heat on basal calcium concentrations in native (untransfected) HEK293T17 cells were determined. Second, calcium influx rates in cells expressing either Glu-179 or Lys-179 were estimated at 37 °C, revealing no differences. The relative fluorescence units were calculated as a difference between fluorescence upon cold/heat and fluorescence threshold at 37 °C. The increase of calcium influx in untransfected cells and cells expressing Lys-179 TRPA1 upon cold did not differ significantly. This observation suggests that Lys-179 hast no impact on the cold response of HEK293T17 cells and that there may be other cold-sensitive calcium influx mechanisms in these cells. In contrast, the fluorescence-visualized calcium influx in Glu-179-expressing cells was significantly higher than that of Lys-179 (p < 0.05) upon exposure to noxious cold (Fig. 4A). Although the activity difference between TRPA1 variants reached a maximum after 5 min, corresponding to the peak calcium influx response, Glu-179 TRPA1 showed increased calcium influx levels over the time course of stimulation. The calcium flux of both genetic variants and untransfected cells dropped down to basal level after 15 min of incubation at 4 °C.
FIGURE 4.
Time course of temperature-mediated TRPA1 action in transfected and native HEK cells. Intracellular Ca2+ was determined with the cell-permeable form of Fluo-4 fluorescence indicator upon stimulation with cold. A, cold stimulation was followed by significant increase of intracellular calcium in HEK cells expressing the TRPA1 Glu-179 channel (p < 0.05); in contrast, no significant increase was observed in untransfected native cells or cells expressing the Lys-179 variant. B, cold stimulation of native cells or cells expressing TRPA1 Glu-179 pretreated with 15 μm selective TRPA1 inhibitor HC030031. Although cold stimulation after 2 min was not inhibited, no increase was observed after 5 min or longer. All values (relative fluorescence units, RFU) represent the difference to calcium fluorescence determined at 37 °C, indicating the change upon temperature stimuli.
Furthermore, we performed experiments with TRPA1-specific inhibitor HC030031 (15 μm) to prove that cold-initiated increase of the calcium fluorescence (in cells expressing Glu-179) is predominantly mediated by Glu-179 channel action. As shown in Fig. 4B, the presence of HC030031 in cell culture medium during cold stimulation allowed the initial calcium increase (2 min of stimulation), however, completely abolished further calcium increase. We also tested whether the Glu-179 genetic variant at position 179 of TRPA1 results in aberrant sensitivity to heat. Incubation at a temperature of 49 °C failed to activate either Glu-179 or Lys-179 TRPA1 (data not shown), indicating that neither TRPA1 variant is activated by heat.
TRPA1 Involvement in Formation of High Molecular Weight Protein Complexes
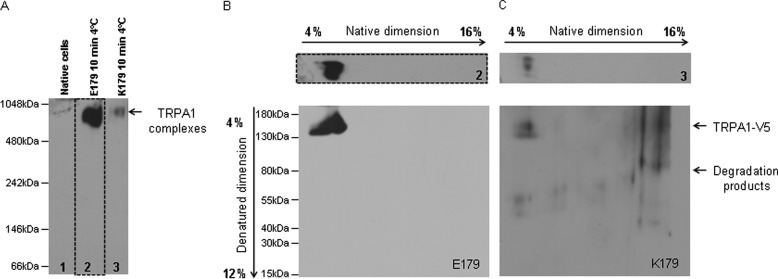
We were questioning the possible underlying mechanism why variant Lys-179 channel failed to be activated by cold temperature. For this purpose, blue native page was performed. Protein complexes were isolated from untransfected cells, cells expressing either Glu-179 or Lys-179 were stimulated for 10 min with 4 °C, and membrane-associated protein complexes were isolated under native conditions. First, protein complexes were separated during native PAGE in presence of Coomassie dye G-250 according to their size. Subsequently, the gel was blotted, and TRPA1 complexes were detected with use of antibody raised against V5 epitope (Fig. 5A). The analysis revealed strong differences in amount of TRPA1 complexes between cell expressing Glu-179 and Lys-179. We observed that Glu-179 TRPA1 (Fig. 5A, lane 2) forms high molecular protein complexes upon cold stimulation, whereas by variant Lys-179 (Fig. 5A, lane 3) this formation was strongly diminished. The size of complexes was in both cases identical (molecular range between 1048 and 480). The difficulties to detect TRPA1 monomers on blot represent a limitation of blue native gel electrophoresis. The reason might be the improper folding of artificial monomeric protein containing His tag and V5 epitope, thus preventing the recognition by anti-V5 antibody. Another known reason is interference of high concentrated Coomassie day with antibody binding. Prior to the loading of samples into the blue native gel electrophoresis, the amounts of TRPA1 variants were estimated in Western blot. Thus equal loading was quarantined. Moreover, as expected, we did not detect any TRPA1 complexes in untransfected cells (Fig. 5A, lane 1).
FIGURE 5.
Identification of TRPA1-containing protein complexes. The two-dimensional blue native PAGE was used to identify TRPA1-protein complexes. A, TRPA1 complexes separated in first native dimension were detected in immunoblot with mouse anti-V5 antibody. Untransfected cell lysate was used as a negative control (lane 1). We observed an intensive V5-specific band with a size between 1048 and 480 kDa in samples isolated from cells expressing wild type Glu-179 TRPA1 (lane 2), whereas a weak V5 signal appeared in the lysate of cells expressing the variant Lys-179 TRPA1 (lane 3). TRPA1 Lys-179 exhibits diminished ability to form complexes. As additional proof of TRPA1 in complexes detected in native PAGE, we denatured lanes 2 and 3 and separated the single components of each of them in the second SDS-PAGE. B, a strong V5-specific signal was found in the blot of the second dimension of lane 2. Here, a single TRPA1 molecule was detected in the location related to the previously detected native TRPA1 complexes. C, a weak, partially degraded signal appeared on blot of lane 3. Equal amounts of TRPA1 variants were loaded into the blue native gel.
The second SDS dimension of each lane followed the blue native PAGE. Prior to separation in an SDS gel, lanes were maintained in denaturing buffer to destroy any kind of protein interactions in TRPA1 complexes. Second, the SDS dimension was also analyzed by immunoblotting with anti-V5 antibody. Fig. 5B shows that, the complex from lane 2 (originated from cells expressing Glu-179 TRPA1) in fact contains TRPA1 monomers. As expected, low amount of complexes in sample from cells expressing Lys-179 TRPA1 led to negligible detection of monomers (Fig. 5C). Here, also some degradation products of TRPA1 monomers were found.
DISCUSSION
TRP channels are fundamental molecules that sense noxious stimuli and integrate the generated action potentials of somatosensory nociceptors into the cause-and-effect chain of pain perception. The complexity of sensory signaling, including molecules such as TRP channels, challenges previously published works (21). Our work highlights some functional consequences of naturally occurring genetic TRPA1 variants. Some evidence for modulation of human pain behavior by genetic variants in the TRPA1 gene has been published previously (5, 11, 12, 22). Recently, we observed that the change of a glutamate residue to lysine at TRP peptide position 179 (E179K) diminished occurrence of PHS in patients suffering from neuropathic pain (12). The effect was independent of pain syndrome etiology, gender, or somatosensory profile. Subsequently, designed homology models of TRPA1 ankyrins facilitated the prediction of the possible three-dimensional effects of the E179K variant. Because the glutamate side chain is exposed to solvent in our model, it appears unlikely that a lysine substitution would cause significant changes in protein folding or stability. Although residue 179 is not on the concave face most commonly taking part in ligand interactions, it may participate in interactions with a regulatory protein or other ligand or perhaps with another region of the TRPA1 protein. Substitution of a glutamate to a lysine could therefore affect the affinity for such a ligand by affecting the electrostatic properties of the ankyrin repeats.
Additionally, rare PHS occurrence in subjects carrying Lys-179 TRPA1 suggests the aberrant sensitivity to painful cold. It is worth further discussion if the genetic variant of the cold receptor TRPA1 exhibits loss of functionality or if diminished expression could be the reason for PHS absence. The role of TRPA1 in perception of noxious cold has been a controversial debate. On the one hand, some research groups have shown that when TRPA1 is expressed in heterologous system, HEK293 cells, it became activated at temperatures below 17 °C, also temperature defined as noxious by humans (10, 23). In contrast, other laboratories (24, 25) observed that exogenously expressed channels were not activated by noxious cold. Additionally, the indirect activation of TRPA1 by cold-induced release of intracellular calcium was suggested (5). These conflicting findings may depend on the following: (i) differences in the preparation of HEK293 cell expression model, (ii) the stimulation conditions, and finally, (iii) on the genetic profile of TRPA1. The experiments presented here were designed under conditions similar to Sawada et al. (23), who suggested that long term expression (>24 h) could result in loss of TRPA1 cold sensitivity. Furthermore, they observed that the TRPA1-mediated inward currents reached maximum at temperature ∼5 °C. Moreover, the short term stimulation at 4 °C has been described as sufficient to increase the expression of TRPA1 in rat dorsal root ganglion neurons in injury or inflammation (9). We have performed transient transfection of HEK293T17 cells followed by the stimulation with 4 °C at selected time points, 20 h after transfection. Additionally, there is evidence that temperature, simulating the noxious heat shock ∼46–54 °C, triggers stress kinase-orchestrated cellular response accompanied by increased expression of heat-sensitive TRP channels (26). Following the established experimental setting, we observed differential regulation of TRPA1 variants by cold and heat stimuli. The inherent differences in expression pattern were observed after 10 min of stimulation. Although Glu-179 TRPA1 expression was up-regulated on protein levels, Lys-179 protein only was increased moderately by cold. Noxious temperatures probably increase Glu-179 protein stability. Endocytosis trafficking and degradation in the proteasome are the crucial points to be studied. However, the HEK293 heterologously expressing TRPA1 channels represent an artificial in vitro cell system. The TRPA1 cDNA inserted in pcDNA3.1-V5/His vector under control of the CMV promoter consisted only of the coding sequence and not the regulatory sequences naturally affecting TRPA1 gene expression. This fact is a limitation of our study. Moreover, it is unknown whether the CMV promoter in pcDNA3.1-V5/His vector is sensitive to cold or hot temperatures.
There is mounting evidence for the involvement of stress kinases in the regulation of the cellular responses related to temperature stimulation. The extracellular signal-regulated protein kinase 1/2 (ERK1/2) and another type of mitogen-activated protein kinase, p38 MAPK, have been postulated to be involved in TRP-associated responses to noxious temperatures in sensory neurons (13, 27, 28). The impact of kinases such as p38 and ERK1/2 on the regulation of TRPA1 synthesis and/or activity upon cold and heat needs to be further investigated.
Subsequently, we questioned whether variant Lys-179 protein is able to respond to cold comparably with the wild type Glu-179 TRPA1. The proper localization of the channel close to the plasma membrane is a requirement for its action. It was proposed previously that TRPA1 translocation to the membrane (exocytosis) represents the control mechanism of TRPA1 action (29). Immunofluorescence labeling of TRPA1 confirmed the intact surface trafficking under conditions defined in our experimental settings. Both Glu-179 and Lys-179 TRPA1 channels were localized in the cytoplasm, and they underwent trafficking into the plasma membrane upon cold stimulation. Interestingly, TRPA1 variants exhibited stronger heat-induced translocation into the plasma membrane than that observed after cold stimulus. However, overall immunostaining of both variants was similar and did not give any explanation for the different protein expression of both variants observed in the Western blot after temperature stimuli.
Furthermore, functionality of TRPA1 E179K determined through measurement of intracellular calcium in dependence on temperature revealed no increase of intracellular calcium in cells expressing variant Lys-179 channel upon cold stimulation. Moreover, there is evidence that other cold-sensitive channels operate in HEK293 cells, thus leading to TRPA1 activation indirectly via increased intracellular Ca2+ (5). In our experimental settings, we observed that the short maintenance of untransfected cells and cells expressing variant Lys-179 TRPA1 at 4 °C caused moderate increase in intracellular calcium. In contrast, calcium-related fluorescence detected in cells HEK293T17 expressing Glu-179 TRPA1 was increased significantly in comparison with untransfected cells. It is likely that the TRPA1 activation by cold consists of two distinct molecular mechanisms, direct activation (30) and indirect activation via increased intracellular calcium (5). Additionally, we showed that only short (2 min) cold activation of Glu-179 TRPA1 is insensitive to treatment with the TRPA1 inhibitor HC030031. It is likely that only activated channel can bind HC030031 followed by channel inactivation and 2 min of incubation with inhibitor may be not sufficient. However, the inhibitor experiments gave convincing evidence that cold-triggered calcium increase is mediated specifically via Glu-179 activation.
Initially, we did not expect any TRPA1 response to heat. Despite of heat-triggered up-regulation of the wild type TRPA1 protein and its proper localization close to plasma membrane, heat did not activate TRPA1 channel. Although there is evidence of a cross-talk between TRPA1 and the heat receptor TRPV1 (10), in our experimental model, TRPV1 was not expressed, and obviously heat did not trigger significant calcium influx into native HEK293T17 cells under our experimental setting. It has been accepted that TRPA1 mediates response to noxious cold, but nothing is known about its role in keeping homeostasis upon heat in cells fighting for survival.
Because the residue at position 179 in ANK4 of TRPA1 appears to be important for protein-protein interactions of channel, we hypothesized that the loss of cold sensitivity by variant Lys-179 TRPA1 could be caused by its disability to form larger protein complexes. The data that we obtained in two-dimensional blue native gel electrophoresis analysis supported our hypothesis. Indeed, variant Lys-179 TRPA1 protein showed diminished ability to form protein complexes upon cold stimulation. Interestingly, it was suggested that active TRPA1 channels form tetramers (31). We have observed complexes with molecular weight, suggesting the coexistence of even larger protein complexes. The identification of single components included in TRPA1 complexes is yet to be investigated.
In conclusion, our in vitro results support our previously published clinical association between the TRPA1 genotype and occurrence of PHS. The enhanced TRPA1 expression in wild type carriers could cause cold hypersensitivity of nociceptive fibers in neuropathic pain subjects. Additionally, we defined only the wild type TRPA1 to be activated by cold in our experimental settings. In contrast, the variant TRPA1 Lys-179 channel was only negligibly activated by cold. The inability of Lys-179 TRPA1 to respond to cold associates with the loss of channel ability to form oligomeric forms.
Acknowledgment
We thank Dr. Rachelle Gaudet (Harvard University) for the preparation of structural model of human TRPA1 variants as well as helpful discussions while preparing the manuscript.
This work was supported by a grant from the Medical Faculty (University of Kiel).

This article contains supplemental Fig. 1.
- PHS
- paradoxical heat sensation.
REFERENCES
- 1. Woolf C. J. (2011) Central sensitization: Implications for the diagnosis and treatment of pain. Pain 152, S2–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baron R., Binder A., Wasner G. (2010) Neuropathic pain: Diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 9, 807–819 [DOI] [PubMed] [Google Scholar]
- 3. Levine J. D., Alessandri-Haber N. (2007) TRP channels: Targets for the relief of pain. Biochim. Biophys. Acta 1772, 989–1003 [DOI] [PubMed] [Google Scholar]
- 4. Story G. M. (2006) The emerging role of TRP channels in mechanisms of temperature and pain sensation. Curr. Neuropharmacol. 4, 183–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zurborg S., Yurgionas B., Jira J. A., Caspani O., Heppenstall P. A. (2007) Direct activation of the ion channel TRPA1 by Ca2+. Nat. Neurosci. 10, 277–279 [DOI] [PubMed] [Google Scholar]
- 6. Karashima Y., Talavera K., Everaerts W., Janssens A., Kwan K. Y., Vennekens R., Nilius B., Voets T. (2009) TRPA1 acts as a cold sensor in vitro and in vivo. Proc. Natl. Acad. Sci. U.S.A. 106, 1273–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ji G., Zhou S., Carlton S. M. (2008) Intact Aδ-fibers up-regulate transient receptor potential A1 and contribute to cold hypersensitivity in neuropathic rats. Neuroscience 154, 1054–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Katsura H., Obata K., Mizushima T., Yamanaka H., Kobayashi K., Dai Y., Fukuoka T., Tokunaga A., Sakagami M., Noguchi K. (2006) Antisense knockdown of TRPA1, but not TRPM8, alleviates cold hyperalgesia after spinal nerve ligation in rats. Exp. Neurol. 200, 112–123 [DOI] [PubMed] [Google Scholar]
- 9. Obata K., Katsura H., Mizushima T., Yamanaka H., Kobayashi K., Dai Y., Fukuoka T., Tokunaga A., Tominaga M., Noguchi K. (2005) TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J. Clin. Invest. 115, 2393–2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Story G. M., Peier A. M., Reeve A. J., Eid S. R., Mosbacher J., Hricik T. R., Earley T. J., Hergarden A. C., Andersson D. A., Hwang S. W., McIntyre P., Jegla T., Bevan S., Patapoutian A. (2003) ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112, 819–829 [DOI] [PubMed] [Google Scholar]
- 11. Kremeyer B., Lopera F., Cox J. J., Momin A., Rugiero F., Marsh S., Woods C. G., Jones N. G., Paterson K. J., Fricker F. R., Villegas A., Acosta N., Pineda-Trujillo N. G., Ramírez J. D., Zea J., Burley M. W., Bedoya G., Bennett D. L., Wood J. N., Ruiz-Linares A. (2010) A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron 66, 671–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Binder A., May D., Baron R., Maier C., Tölle T. R., Treede R. D., Berthele A., Faltraco F., Flor H., Gierthmühlen J., Haenisch S., Huge V., Magerl W., Maihöfner C., Richter H., Rolke R., Scherens A., Uçeyler N., Ufer M., Wasner G., Zhu J., Cascorbi I. (2011) Transient receptor potential channel polymorphisms are associated with the somatosensory function in neuropathic pain patients. PLoS One 6, e17387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wittig I., Braun H. P., Schägger H. (2006) Blue native PAGE. Nature Protoc. 1, 418–428 [DOI] [PubMed] [Google Scholar]
- 14. Donnerer J., Liebmann I. (2011) A fluorescence-immunohistochemical study on phosphorylation of ERK1/2, p38, and STAT3 in rat dorsal root ganglia following noxious stimulation of hind paw sensory neurons. Tissue Cell. 43, 178–189 [DOI] [PubMed] [Google Scholar]
- 15. Doerner J. F., Gisselmann G., Hatt H., Wetzel C. H. (2007) Transient receptor potential channel A1 is directly gated by calcium ions. J. Biol. Chem. 282, 13180–13189 [DOI] [PubMed] [Google Scholar]
- 16. Macpherson L. J., Dubin A. E., Evans M. J., Marr F., Schultz P. G., Cravatt B. F., Patapoutian A. (2007) Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 445, 541–545 [DOI] [PubMed] [Google Scholar]
- 17. Hinman A., Chuang H. H., Bautista D. M., Julius D. (2006) TRP channel activation by reversible covalent modification. Proc. Natl. Acad. Sci. U.S.A. 103, 19564–19568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gaudet R. (2008) A primer on ankyrin repeat function in TRP channels and beyond. Mol. Biosyst. 4, 372–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Michaely P., Tomchick D. R., Machius M., Anderson R. G. (2002) Crystal structure of a 12-ANK repeat stack from human ankyrinR. EMBO J. 21, 6387–6396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shaw G., Morse S., Ararat M., Graham F. L. (2002) Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells. FASEB J. 16, 869–871 [DOI] [PubMed] [Google Scholar]
- 21. Stucky C. L., Dubin A. E., Jeske N. A., Malin S. A., McKemy D. D., Story G. M. (2009) Roles of transient receptor potential channels in pain. Brain Res. Rev. 60, 2–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim H., Mittal D. P., Iadarola M. J., Dionne R. A. (2006) Genetic predictors for acute experimental cold and heat pain sensitivity in humans. J. Med. Genet. 43, e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sawada Y., Hosokawa H., Hori A., Matsumura K., Kobayashi S. (2007) Cold sensitivity of recombinant TRPA1 channels. Brain Res. 1160, 39–46 [DOI] [PubMed] [Google Scholar]
- 24. Bautista D. M., Jordt S. E., Nikai T., Tsuruda P. R., Read A. J., Poblete J., Yamoah E. N., Basbaum A. I., Julius D. (2006) TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124, 1269–1282 [DOI] [PubMed] [Google Scholar]
- 25. Jordt S. E., Bautista D. M., Chuang H. H., McKemy D. D., Zygmunt P. M., Högestätt E. D., Meng I. D., Julius D. (2004) Mustard oils and cannabinoids excite sensory nerve fibers through the TRP channel ANKTM1. Nature 427, 260–265 [DOI] [PubMed] [Google Scholar]
- 26. Mizushima T., Obata K., Yamanaka H., Dai Y., Fukuoka T., Tokunaga A., Mashimo T., Noguchi K. (2005) Activation of p38 MAPK in primary afferent neurons by noxious stimulation and its involvement in the development of thermal hyperalgesia. Pain 113, 51–60 [DOI] [PubMed] [Google Scholar]
- 27. Liu M. G., Wang R. R., Chen X. F., Zhang F. K., Cui X. Y., Chen J. (2011) Differential roles of ERK, JNK, and p38 MAPK in pain-related spatial and temporal enhancement of synaptic responses in the hippocampal formation of rats: Multielectrode array recordings. Brain Res. 1382, 57–69 [DOI] [PubMed] [Google Scholar]
- 28. Mizushima T., Obata K., Katsura H., Yamanaka H., Kobayashi K., Dai Y., Fukuoka T., Tokunaga A., Mashimo T., Noguchi K. (2006) Noxious cold stimulation induces mitogen-activated protein kinase activation in transient receptor potential (TRP) channels TRPA1- and TRPM8-containing small sensory neurons. Neuroscience 140, 1337–1348 [DOI] [PubMed] [Google Scholar]
- 29. Schmidt M., Dubin A. E., Petrus M. J., Earley T. J., Patapoutian A. (2009) Nociceptive signals induce trafficking of TRPA1 to the plasma membrane. Neuron 64, 498–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang Y. Y., Chang R. B., Waters H. N., McKemy D. D., Liman E. R. (2008) The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J. Biol. Chem. 283, 32691–32703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cvetkov T. L., Huynh K. W., Cohen M. R., Moiseenkova-Bell V. Y. (2011) Molecular architecture and subunit organization of TRPA1 ion channel revealed by electron microscopy. J. Biol. Chem. 286, 38168–38176 [DOI] [PMC free article] [PubMed] [Google Scholar]