Background: Coordination of the signaling cascades downstream of netrin receptors is essential in axon guidance.
Results: DSCAM collaborates with UNC5C to mediate netrin-1-induced axon growth cone collapse through the assembly of a signaling complex involving Fyn, FAK, and PAK1.
Conclusion: DSCAM functions as a repulsive netrin receptor.
Significance: Understanding the coordination of axon guidance signaling provides insight into the formation of precise neuronal circuitry during brain development.
Keywords: Axon, Neuroscience, Protein Phosphorylation, Protein-Protein Interactions, Signal Transduction, Axon Collapse, Cerebellum, Down Syndrome Cell Adhesion Molecule (DSCAM), Netrin-1, Uncoordinated-5C (UNC5C)
Abstract
In the developing nervous system, neuronal growth cones explore the extracellular environment for guidance cues, which can guide them along specific trajectories toward their targets. Netrin-1, a bifunctional guidance cue, binds to deleted in colorectal cancer (DCC) and DSCAM mediating axon attraction, and UNC5 mediating axon repulsion. Here, we show that DSCAM interacts with UNC5C and this interaction is stimulated by netrin-1 in primary cortical neurons and postnatal cerebellar granule cells. DSCAM partially co-localized with UNC5C in primary neurons and brain tissues. Netrin-1 induces axon growth cone collapse of mouse cerebellum external granule layer (EGL) cells, and the knockdown of DSCAM or UNC5C by specific shRNAs or blocking their signaling by overexpressing dominant negative mutants suppresses netrin-1-induced growth cone collapse. Similarly, the simultaneous knockdown of DSCAM and UNC5C also blocks netrin-1-induced growth cone collapse in EGL cells. Netrin-1 increases tyrosine phosphorylation of endogenous DSCAM, UNC5C, FAK, Fyn, and PAK1, and promotes complex formation of DSCAM with these signaling molecules in primary postnatal cerebellar neurons. Inhibition of Src family kinases efficiently reduces the interaction of DSCAM with UNC5C, FAK, Fyn, and PAK1 and tyrosine phosphorylation of these proteins as well as growth cone collapse of mouse EGL cells induced by netrin-1. The knockdown of DSCAM inhibits netrin-induced tyrosine phosphorylation of UNC5C and Fyn as well as the interaction of UNC5C with Fyn. The double knockdown of both receptors abolishes the induction of Fyn tyrosine phosphorylation by netrin-1. Our study reveals the first evidence that DSCAM coordinates with UNC5C in netrin-1 repulsion.
Introduction
Axon guidance cues can act as attractants or repellents and lay out a specific grid for axons and neurons to navigate in the developing nervous system (1–5). Netrins are conserved bifunctional guidance cues functioning as chemoattractants for some types of axons and repellents for others in different species ranging from Caenorhabditis elegans (C. elegans) and Drosophila, to vertebrates (6–12). The mammalian receptors of netrins are deleted in colorectal cancer (DCC)2 (13–16), neogenin/DCC-like molecule (13, 17, 18), DSCAM (19–21), and UNC5 (22–25). While DCC can mediate netrin-1 induced axon attraction, the binding of DCC and UNC5 cytoplasmic domains is sufficient to convert netrin-1 induced DCC attraction into repulsion (26–28), suggesting that the coordination of intracellular domains of these receptors plays a crucial role in netrin-1 attractive or repulsive signaling.
Drosophila Dscam is a contact-dependent homophilic cell repulsive molecule with thousands of alternatively spliced isoforms involved in axon guidance and targeting, segregation of axon branches and dendritic patterning (29–34). While the vertebrate DSCAM gene does not encode multiple isoforms, it shares functional similarities to fly Dscam. In DSCAM deficient mice, retinal ganglion cells have defects in neuronal spacing and dendritic arborization patterns exhibiting neuronal self-avoidance phenotypes (35, 36). In contrast, studies in the chicken retina have shown that DSCAM plays a role in synapse formation by promoting the targeting of retinal ganglion cell dendrites and bipolar cell axons to the same layer (37). As a conserved netrin receptor in flies and vertebrates, DSCAM collaborates with DCC involved in netrin-1 attraction (19–21). These results suggest that DSCAM may function as a bifunctional guidance receptor involved in either attractive or repulsive signaling pathways.
We report here that DSCAM functions as a repulsive receptor associating with UNC5C to mediate netrin-1-induced axon growth cone collapse, and FAK, Fyn, and PAK1 are involved in coordinating netrin-1/DSCAM and netrin-1/UNC5C repulsive signaling. These studies not only reveal the role of DSCAM in netrin-mediated repulsion, but help us better understand the complexity of netrin signaling in the developing nervous system.
EXPERIMENTAL PROCEDURES
Constructs and Reagents
Plasmids encoding the full-length human DSCAM-Flag, DSCAMΔN, DSCAMΔC, the full-length human UNC5C-HA and UNC5C truncation mutants (ΔIg1, ΔIg2, ΔIgs, ΔTSP) have been described previously (21, 38). The targeted sequences of DSCAM shRNA, control DSCAM shRNA, UNC5C shRNA, and control UNC5C shRNA are: 5′-AAAGAGTTTAGCTGAAATGCT-3′ (DSCAM shRNA), 5′-AATGCATCTCTGCAAGAGGTA-3′ (control DSCAM shRNA), 5′-AAGAACCCAAGGCTCTTCATT-3′ (UNC5C shRNA) and 5′-AACTGTACTGTGTCAGAGGAA-3′ (control UNC5C shRNA). The designed hairpin siRNA templates were inserted into the mU6pro vector between SalI and XbaI sites and verified by sequencing. The following antibodies were used: rabbit anti-Flag (Abcam, Cambridge, MA), mouse anti-myc (9E10, Upstate Biotechnology, Lake Placid, NY), rabbit anti-HA (Santa Cruz Biotechnology, Lexington, NY), rabbit anti-FAK (BD Biosciences, Franklin Lakes, NJ), rabbit anti-PAK (Cell Signaling Technology, Beverly, MA), mouse anti-Fyn (Santa Cruz Biotechnology, Lexington, NY), goat anti-UNC5C (R&D Systems, Minneapolis, MN), and the HRP-conjugated anti-rabbit, anti-mouse and anti-goat secondary antibodies (Santa Cruz Biotechnology, Lexington, NY). The purified rabbit anti-DSCAM was described previously (39). B27, Alexa Fluor 555 phalloidin and DAPI were purchased from Invitrogen (Carlsbad, CA). Netrin-1 protein and the sham-purified control were made from the conditioned media of HEK293 cells as previously described (21, 40, 41).
Immunoprecipitation (IP) and Western Blot
HEK293 cells were transfected using polyethylenimine (PEI) at 2:1 ratio with plasmids. Dissociated E15 cortical and P4 cerebellar neurons were plated on PLL-coated tissue culture dishes. Cells were lysed in mild lysis buffer (MLB: 20 mm Tris-HCl pH 7.4, 100 mm NaCl, 1% Nonidet P-40, and 0.1 mm phenylmethylsulfonyl fluoride) containing protease inhibitor mixture followed by incubation with specific antibodies for 2 h as described previously (21, 40). Protein extracts were boiled and separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE). After transferring, membranes were blocked with 5% milk and incubated in primary antibody overnight at 4 °C. Membranes were washed with PBST (1× PBS + 0.1% Tween-20) and placed in secondary antibody for 1 h. Western blots were visualized on autoradiography film (Denville Scientific, Metuchen, NJ) with the enhanced chemiluminescence kit (Thermo Fisher Scientific Inc., Waltham, MA).
Dissociated Primary Neuron Cultures and Immunostaining
The dissociated culture procedure of the E15 mouse cortex and cerebellum, and the P2–4 mouse cerebellum was conducted as described previously (21, 40, 41) with some modifications. Neurons were grown on PLL-coated glass coverslips for growth cone collapse or immunostaining assays. For immunocytochemistry of DSCAM and UNC5C in dissociated primary neurons, cells on coverslips were fixed for 10 min in 4% pre-warmed paraformaldehyde (PFA) solution (127 mm NaCl, 5 mm KCl, 1.1 mm NaH2PO4, 0.4 mm KH2PO4, 2 mm MgCl2, 5.5 mm glucose, 1 mm EGTA, 10 mm Pipes) and permeabilized with 0.1% Triton X-100 for 15 min. After being washed three times with PBS, neurons were blocked in 10% normal goat serum for 1 h and incubated with the primary antibody solution for 2 h at 37 °C (rabbit anti-DSCAM, goat anti-UNC5C). Cells were washed in PBS three times followed by incubating with the secondary antibodies (anti-rabbit-488 and anti-goat-633) at 37 °C for 1 h. Neurons on coverslips were counterstained with DAPI and mounted onto slides using Fluorogel (EMS, PA). Images were taken under a confocal microscope (Olympus IX71 Fluoview).
For studying the expression pattern of DSCAM and UNC5C in the developing nervous system, mouse embryos, and postnatal pups were collected. P2-P4 pups were perfused with 4% PFA intracardially and brain tissues transferred into cold 4% PFA overnight for further fixation. E15 mouse embryos were directly placed in cold 4% PFA overnight. Tissues were washed in PBS, transferred into sucrose-infiltration solution at 4 °C until the brain sank into the sucrose solution, and embedded in OCT. 14 μm coronal slices were cryosectioned and fixed with cold acetone on superfrost plus slides. After post-fixation with 4% PFA at room temperature for 30 min, brain slices were subjected to heat-mediated antigen retrieval using citric acid (pH 6) and blocked with 3% BSA for 1 h in 1× PBST (1× PBS with 0.1% Tween-20), and incubated with the primary antibodies overnight (rabbit anti-DSCAM and goat anti-UNC5C). After washing three times in permeabilization buffer, slices were incubated with the secondary antibody (anti-rabbit-488 and anti-goat-633) for 2 h at 37 °C. Images were taken under a confocal microscope (Olympus IX71 Fluoview).
Growth Cone Collapse
The P2-4 mouse cerebellum was dissected and sliced into 200 μm sections using a tissue chopper (Vibratome, Bannockburn, IL). Only the external granule layer (EGL) was finely dissected using a sharpened tungsten needle and trypsinized at 37 °C for 15 min (42). After dissociation, neurons (1 × 104) were nucleofected (Amaxa, Walkersville, MD) with Venus yellow fluorescent protein (Venus-YFP) only or Venus-YFP plus DSCAM shRNAs, UNC5C shRNAs or different truncation mutants of DSCAM and UNC5C (DSCAM wild type, DSCAMΔC, DSCAMΔN, UNC5C wild type, UNC5CΔC, and UNC5CΔN) using program G-013. After electroporation, cells were plated onto PLL-coated coverslips and cultured in warm media (DMEM + B27 + 20 units/ml of penicillin/streptomycin) at 37 °C with 5% CO2. After 4 h, the media was replaced with fresh media to remove toxic nucleofection solution and neurons were cultured for 14 h. Neurons were starved in DMEM + 0.1% BSA + B27 for 4 h and stimulated with HEK293 control or netrin-1 conditioned media for 30 min. Cells were fixed with 4% PFA for 15 min and stained with phalloidin and DAPI. Images of only YFP-labeled cells were taken using an epifluorescent microscope. For quantification, a single EGL cell with a clearly polarized axon was considered collapsed if it had no lamellipodia and two or fewer filopodia (43). At least 150 random growth cones were measured per group and experiments were done in triplicate. A one-way ANOVA followed by post hoc testing with Fisher's Least Significant Difference (LSD) was performed to detect statistical significance among different groups.
RESULTS
Biochemical Characterization of DSCAM and UNC5C Interaction
The protein structure of DSCAM is similar to DCC and both receptors are required for netrin signaling (19–21). UNC5 binds to DCC to switch DCC-mediated attraction into repulsion, raising the possibility that DSCAM may interact with UNC5 as well. To determine a potential interaction between DSCAM and UNC5, Flag-tagged full-length human DSCAM (DSCAM-Flag) and the full-length human UNC5C tagged with HA (UNC5C-HA) were expressed in HEK293 cells, cell lysates were immunoprecipitated with anti-Flag for DSCAM and immunoblots probed with either anti-HA for UNC5C or anti-Flag for DSCAM (Fig. 1A). UNC5C-HA coimmunoprecipitated with DSCAM (Fig. 1A). The reciprocal IP confirmed the interaction of DSCAM and UNC5C in HEK293 cells (data not shown).
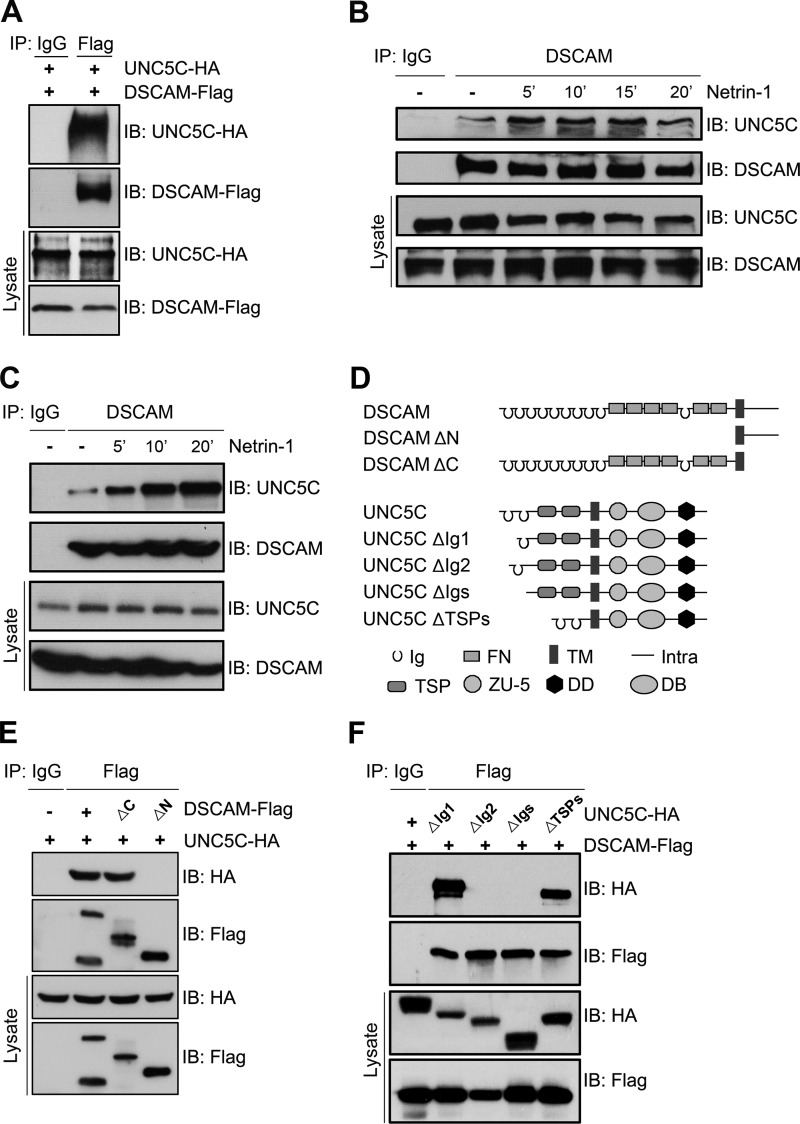
FIGURE 1.
DSCAM associates with UNC5C. A, co-immunoprecipitation of DSCAM and UNC5C in transfected HEK293 cells. Human full-length DSCAM-Flag and UNC5C-HA were co-transfected in HEK293 cells. DSCAM was immunoprecipitated with anti-Flag, and the membrane was blotted for UNC5C. B, induction of the interaction of endogenous DSCAM and UNC5C in dissociated cortical neurons by netrin-1. Cortical neurons from E15 mouse embryos were cultured 18 h and stimulated with netrin-1 or control conditioned media. DSCAM was immunoprecipitated using anti-DSCAM followed by immunoblotting with anti-UNC5C antibody. C, time-dependent induction of endogenous DSCAM and UNC5C interaction in P2 cerebellar granule cells by netrin-1. Netrin-1 persistently increased DSCAM/UNC5C interaction over 20 min. D, diagram illustrating full-length and mutant proteins of DSCAM and UNC5C. E, full-length DSCAM or DSCAMΔC, but not DSCAMΔN, interacts with UNC5C in transfected HEK293 cells. F, mapping the domain in UNC5C responsible for the interaction with DSCAM. HEK293 cells were transfected with a DSCAM construct and a construct encoding different truncated mutants of UNC5C tagged with HA.
To examine whether endogenous DSCAM interacts with UNC5C, primary cortical neurons were used because these neurons express both DSCAM and UNC5C and cortex dissection, dissociation, and culturing is fairly straightforward (21, 23). After 24 h of primary culture, dissociated neurons were serum-starved in DMEM containing B27 for 4 h and stimulated with either purified netrin-1 (250 ng/ml) or sham-purified control. DSCAM was immunoprecipitated with anti-DSCAM and blotted for UNC5C and DSCAM (Fig. 1B). We found that UNC5C was co-immunoprecipitated with DSCAM and netrin-1 stimulation increased the DSCAM/UNC5C interaction (Fig. 1B). Netrin-1 enhanced the interaction of DSCAM and UNC5C within 5 min and the enhancement was sustained up to 20 min (Fig. 1B). To study whether DSCAM interacts with UNC5C in the postnatal mouse cerebellum, P2 mouse cerebellar neurons were dissociated and stimulated with or without netrin-1, as described previously (21, 40, 41). Cell lysates were immunoprecipitated with anti-DSCAM and blotted for DSCAM and UNC5C. Netrin-1 dramatically induced DSCAM/UNC5C interaction and continued to increase this interaction over 20 min (Fig. 1C). Netrin-1-induced interaction of endogenous DSCAM and UNC5C in E15 cortical and P2 cerebellar neurons was also observed in the reciprocal co-immunoprecipitation (data not shown). These above data demonstrate a physiological interaction between DSCAM and UNC5C, and this interaction is stimulated by netrin-1.
DSCAM is a transmembrane protein belonging to the immunoglobulin (Ig) superfamily. Biochemical analysis of DSCAM truncation mutants (DSCAMΔC and DSCAMΔN) co-transfected with UNC5C in HEK293 cells revealed the DSCAM extracellular domain was essential for DSCAM interaction with UNC5C (Fig. 1, D and E and the reciprocal IP not shown). To investigate the domains of UNC5C that associate with DSCAM, UNC5C-HA deletion constructs were expressed in HEK293 cells (Fig. 1, D and F). Deletion of Ig2 or both Ig domains eliminated the interaction whereas deletion of Ig1 and two TSP domains did not alter DSCAM association, indicating the Ig2 domain of UNC5C is required for the interaction with DSCAM (Fig. 1F and the reciprocal IP not shown). Therefore, DSCAM and UNC5C interact through their extracellular domains.
Co-expression of DSCAM and UNC5C in Neurons
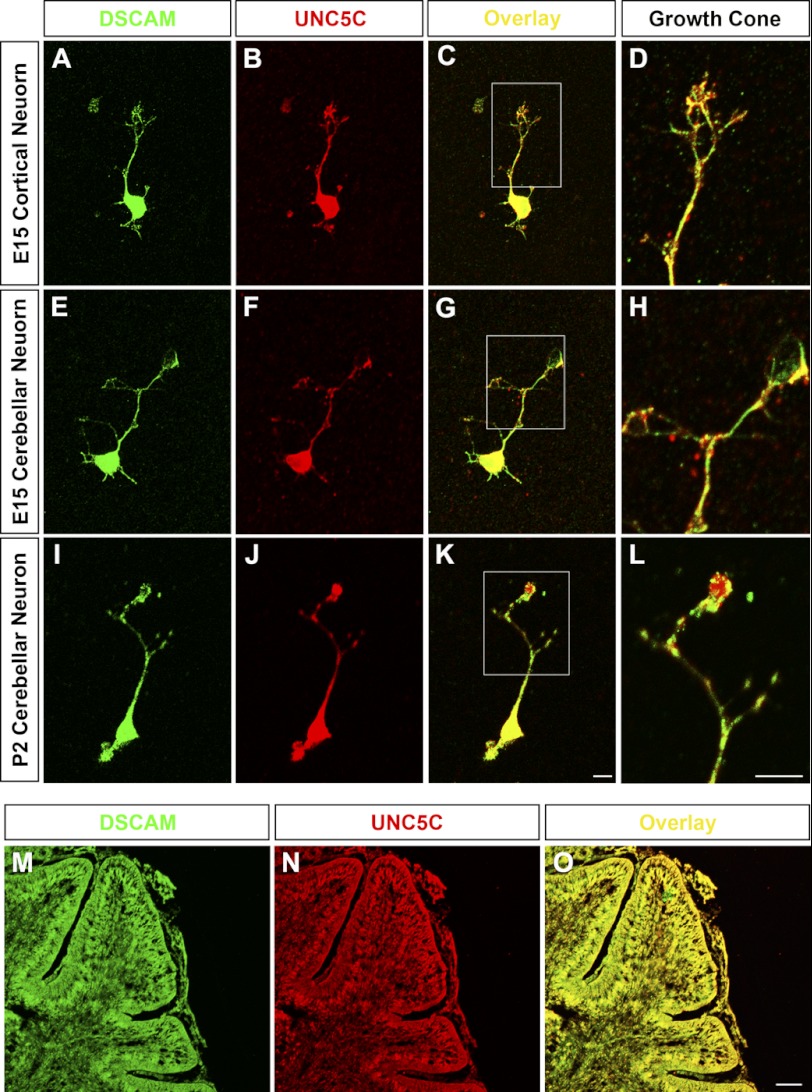
To examine whether DSCAM is co-expressed with UNC5C in primary neurons, brain tissues from the P2 cerebellum, E15 cortex and E15 cerebellum were dissociated and cultured. We used antibodies for DSCAM and UNC5C and found co-expression of DSCAM with UNC5C in these primary neurons (Fig. 2, A--L). DSCAM partially co-localized with UNC5C in the soma, dendrites, axons, axonal branches as well as growth cones (Fig. 2, C, D, G, H, K, and L). Cerebellar cryosections were also immunostained with DSCAM and UNC5C to reveal their expression patterns in different layers of P2 cerebellar (Fig. 2, M–O) and cortical slices (data not shown). In the postnatal cerebellum, both DSCAM and UNC5C were widely expressed in different neuronal layers, including EGL, PCL (the Purkinje cell layer), and IGL (the internal granule layer) (Fig. 2, M–O). DSCAM and UNC5C were also widely expressed in the embryonic and postnatal cortex (data not shown). These DSCAM and UNC5C expression patterns are consistent with in situ hybridization results in previous studies (42, 44, 45).
FIGURE 2.
Co-expression of DSCAM and UNC5C in primary culture neurons and the postnatal cerebellum. A–D, expression of DSCAM (A, C, and D) and UNC5C (B, C, and D) in a primary cortical neuron from E15 mouse. C is the merged picture of A and B. D is the higher magnification image of growth cone in C. E–L, expression of DSCAM (E, G, H, I, K, and L) and UNC5C (F, G, H, J, K, and L) in primary neurons from E15 (E–H) and P2 (I–L) cerebella. G and K are the superimposed images of E-F and I-J, respectively. H and L are higher magnification images of growth cones in G and K, respectively. Primary neurons were cultured overnight, immunostained with anti-DSCAM and anti-UNC5C and followed by incubation with secondary antibodies (antibody to rabbit-488 for DSCAM and to goat-633 for UNC5C). Scale bar, 10 μm. M–O, expression of DSCAM (M) and UNC5C (N) in the P2 mouse cerebellum. O is the superimposed image of M and N. Both DSCAM and UNC5C were widely expressed in the PCL, EGL, and IGL. Scale bar, 100 μm.
DSCAM Is Required for Netrin-1-mediated EGL Cell Growth Cone Collapse
DSCAM not only interacts with UNC5C (Fig. 1), but also partially co-localizes with UNC5C in dissociated cerebellar granule cells (Fig. 2, A--L). Both DSCAM and UNC5C are also co-expressed in postnatal cerebellar EGL cells (Fig. 2, M–O) where UNC5 mediates netrin-1 repulsion in the developing cerebellum. However, whether DSCAM plays a functional role in netrin-1 repulsion is unknown. To address this question, several short hairpin based RNA interference (RNAi) constructs (shRNAs) targeting a sequence common to human and mouse DSCAM were directly nucleofected into dissociated postnatal cerebellar granule cells. One shRNA construct significantly knocked down the level of endogenous DSCAM protein in P2 cerebellar granule neurons, whereas another did not (Fig. 6J and data not shown, respectively). In subsequent experiments, these RNAi reagents were used as DSCAM shRNA and DSCAM control shRNA, respectively.
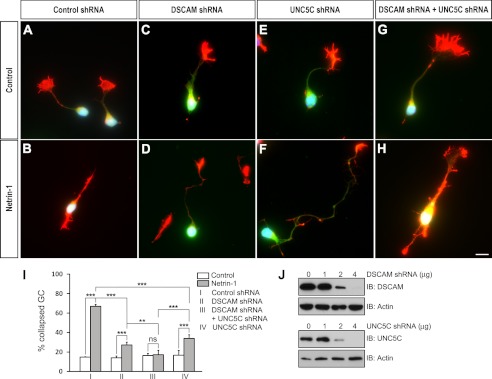
FIGURE 6.
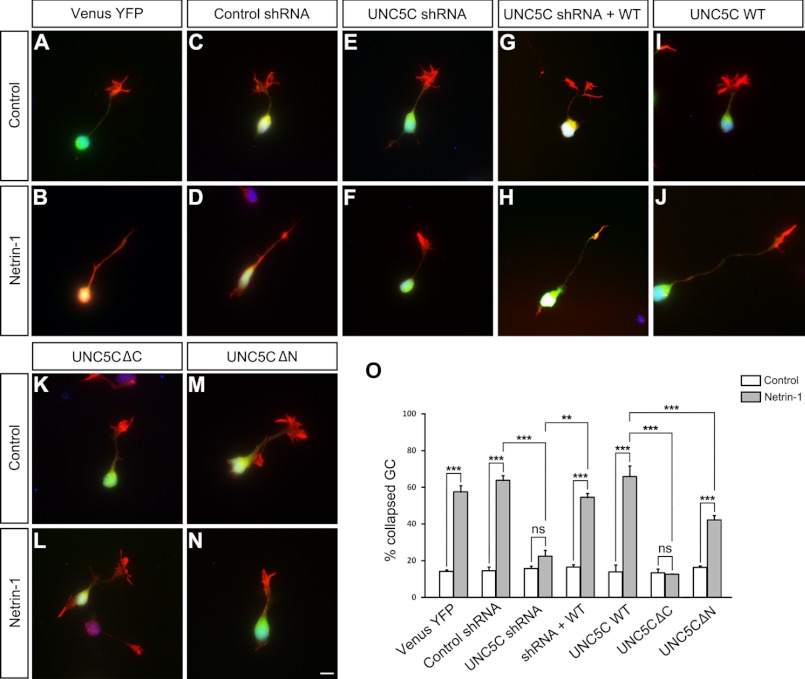
DSCAM collaborates with UNC5C in netrin-1-induced cerebellar EGL growth cone collapse. P2 cerebellar EGL cells were transfected with Venus-YFP (1 μg) plus 2 μg of either DSCAM control shRNA (A and B), DSCAM shRNA (C and D), UNC5C shRNA (E and F), or both DSCAM and UNC5C shRNAs (G and H). Neurons were stained with Alexa Fluor 555 phalloidin (red) and DAPI (blue). Scale bar, 10 μm. I. Quantification of EGL growth cone collapse. The y axis is the percentage of collapsed axon growth cones. Data represent the mean ± S.D. of three separate experiments. ***: p < 0.001; **: p < 0.01; ns: not significant (one-way Anova and Fischer LSD post-hoc comparisons). J, dose-dependent knockdown effects of DSCAM shRNA (upper first panel) and UNC5C shRNA (upper third panel) in P4 cerebellar granule cells.
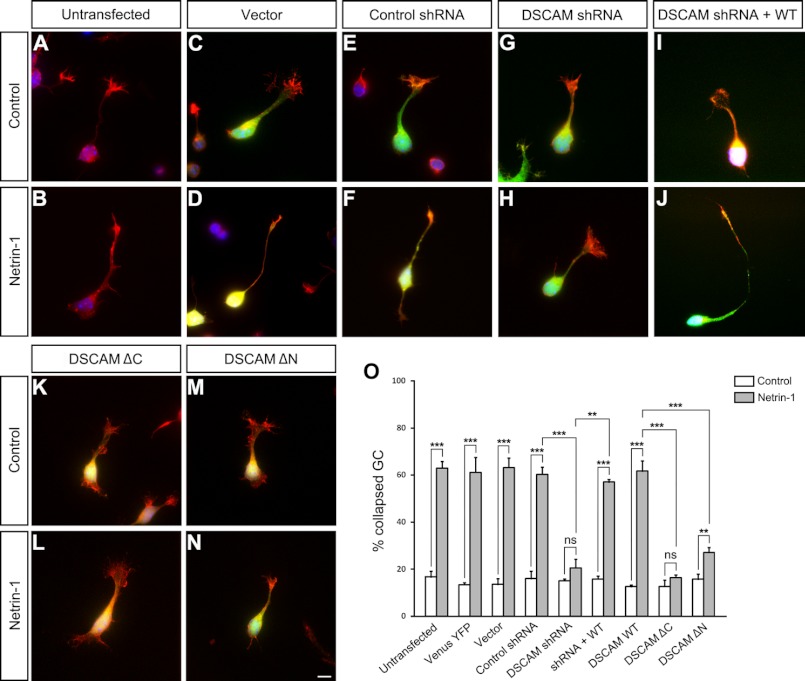
The in vitro growth cone collapse assay has been widely used to study the molecular mechanisms of axon guidance, especially in repulsive signaling. For example, UNC5A mediated growth cone collapse in netrin-1 signaling (43, 46). In this assay, dissociated cerebellar granule cells were stimulated by netrin-1 in bath incubation. Therefore, netrin-1 was distributed throughout the media (no gradient) and caused the axon growth cone to collapse (43). To examine the potential role of DSCAM in this process, we developed an in vitro growth cone collapse assay of postnatal cerebellar EGL cells induced by netrin-1 stimulation. The P2 cerebellar EGL was dissected using fine tungsten needles to extract small tissue pieces and dissociated in cold HBSS medium (42). Primary EGL cells were nucleofected with a construct expressing Venus-YFP together with control shRNA or DSCAM shRNA. After 18 h of culture and 4 h starvation, these neurons were stimulated with netrin-1 or control media and fixed. Netrin-1 stimulated axon growth cone collapse of EGL cells in both untransfected (Fig. 3, A, B, and O) and Venus-YFP-transfected neurons (images not shown and quantification in Fig. 3O). DSCAM knockdown abolished netrin-1-induced growth cone collapse (Fig. 3, G, H, and O) compared with Venus-YFP only (images not shown and quantification in Fig. 3O), the empty vector (Fig. 3, C, D, and O), and DSCAM control shRNA (Fig. 3, E, F, and O). Overexpression of wild-type DSCAM rescued the effect of DSCAM shRNA on growth cone collapse (Fig. 3, I, J, and O). In neurons transfected with the wild-type human DSCAM, netrin-1 still induced growth cone collapse with a similar percentage of collapsed EGL growth cones (images not shown and quantification in Fig. 3O). However, overexpression of DSCAMΔC, a truncation mutant without the intracellular domain of DSCAM, eliminated netrin-1-induced growth cone collapse, suggesting that the DSCAM intracellular domain is required for mediating downstream netrin-1 repulsion (Fig. 3, K, L, and O). Overexpression of DSCAMΔN, a truncated form of DSCAM without the extracellular domain, could only partially inhibit netrin-1-induced growth cone collapse (Fig. 3, M, N, and O), indicating that the DSCAM extracellular domain also plays a role in growth cone collapse. These results suggest that DSCAM is required for netrin-1 repulsion.
FIGURE 3.
DSCAM was required for netrin-1-induced cerebellar EGL neuron growth cone collapse. Cerebellar EGL cells from P2 mouse pups were dissociated and cultured. Primary neurons were either untransfected (A and B) or co-transfected Venus-YFP (1 μg) with 4 μg of either mU6 vector (C and D), control shRNA (E and F), DSCAM shRNA (G and H), DSCAM shRNA plus wild-type DSCAM (I and J), DSCAMΔC (K and L), or DSCAMΔN (M and N), and plated on coverslips coated with PLL. Neurons were labeled with YFP and filamentous actin was visualized by staining with Alexa Fluor 555 phalloidin (red). Nuclei were stained with DAPI (blue). Only the growth cones of YFP-positive neurons not in contact with other cells were measured and used in the statistical analyses. Scale bar, 10 μm. O, quantification of netrin-1-induced growth cone collapse. The y axis is the percentage of collapsed axon growth cones. Data are mean ± S.E. from three separate experiments. ***: p < 0.001; **: p < 0.01; ns: not significant (one-way Anova and Fischer LSD post-hoc comparisons).
The Role of UNC5C in Netrin-1-mediated EGL Cell Growth Cone Collapse
Netrin-1 repels both axon projection and neuronal migration from postnatal EGL explants in vitro (42). The phenotype of UNC5C knock-out mice suggests that UNC5C may also play a repulsive role in postnatal cerebellar neuronal migration in vivo (24). To determine whether UNC5C is required for netrin-1-mediated cerebellar EGL cell repulsion, several UNC5C shRNAs targeting the sequence common to human and mouse UNC5C were transfected directly into postnatal cerebellar granule cells. After 24 h, one shRNA construct efficiently reduced the UNC5C protein levels and was used in functional assays as UNC5C shRNA, whereas another could not knock down UNC5C and was used as UNC5C control shRNA (Fig. 6J and data not shown, respectively). To determine the potential role of endogenous UNC5C in netrin-1-induced EGL neuron growth cone collapse, we performed the same in vitro EGL neuron growth cone collapse assay as described above. P2 cerebellar EGL cells were transfected with either Venus-YFP only or Venus-YFP plus the control UNC5C shRNA or UNC5C shRNA followed by stimulation with netrin-1 or control media. As expected, netrin-1 induced growth cone collapse in the Venus-YFP group (Fig. 4, A, B, and O). UNC5C shRNA blocked growth cone collapse in netrin-1 bath incubation (Fig. 4, E, F, and O), whereas the expression of UNC5C control shRNA had no effect on netrin-1-induced growth cone collapse (Fig. 4, C, D, and O). The effect of UNC5C shRNA on growth cone collapse could be rescued by the overexpression of wild-type UNC5C (Fig. 4, G, H, and O). Further analysis revealed that the UNC5C intracellular domain was necessary for netrin-1-induced growth cone collapse (Fig. 4, I, L, and O). However, overexpression of UNC5CΔN partially inhibited netrin-1-induced growth cone collapse (Fig. 4, M and O). Comparing netrin-1 induced growth cone collapse from the Venus-YFP-transfected neurons (Fig. 4, A, B, and O) to UNC5CΔN-transfected neurons (Fig. 4, M, O), the difference was statistically significant (p < 0.001). Over-expression of wild type human UNC5C had no effect on netrin-1-induced growth cone collapse (Fig. 4, I, J, and O). These results indicate that UNC5C is involved in netrin-1-induced growth cone collapse.
FIGURE 4.
UNC5C-mediated netrin-1-induced cerebellar EGL growth cone collapse. P2 cerebellar EGL cells were transfected with either Venus-YFP only (1 μg, A and B) or Venus-YFP (1 μg) plus 4 μg of either UNC5C control shRNA (C and D), UNC5C shRNA (E and F), UNC5C shRNA plus wild-type UNC5C (G and H), wild-type UNC5C (I and J), UNC5CΔC (K and L), or UNC5CΔN (M and N). Neurons were stained with Alexa Fluor 555 phalloidin (red) and DAPI (blue). The growth cone area was examined as indicated in Fig. 3. Scale bar, 10 μm. O, quantification of EGL growth cone collapse. The y axis is the percentage of collapsed axon growth cones. Data are mean ± S.E. from three separate experiments. ***: p < 0.001; **: p < 0.01; ns: not significant (one-way Anova and Fischer LSD post-hoc comparisons).
Coordination of DSCAM and UNC5C in Netrin-1-induced Growth Cone Collapse
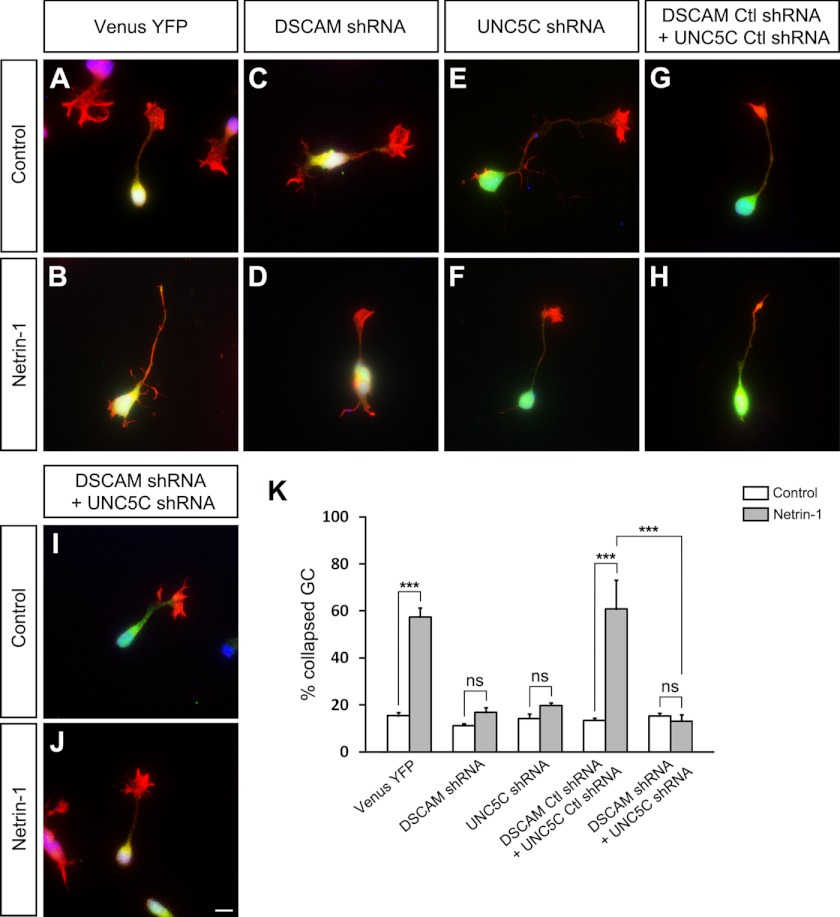
The data outlined above suggest that both DSCAM and UNC5C play a role in netrin-1-induced growth cone collapse. To further study the DSCAM/UNC5C interaction in netrin-1 repulsion, DSCAM and UNC5C were both knocked-down together. As expected, the percentage of neuronal growth cone collapse in the Venus-YFP group increased with netrin-1 versus without netrin-1 stimulation (Fig. 5, A, B, and K). In neurons transfected with DSCAM shRNA, netrin-1 could not induce growth cone collapse (Fig. 5, C, D, and K). Expression of UNC5C shRNA in cerebellar EGL cells abolished netrin-1-induced growth cone collapse as well (Fig. 5, E, F, and K). Expression of both DSCAM and UNC5C control shRNAs did not affect the effect of netrin-1 on growth cone collapse (Fig. 5, G, H, and K). However, simultaneous knockdown of DSCAM and UNC5C blocked netrin-1 collapse (Fig. 5, I–K). Comparing netrin-1-induced growth cone collapse from the control shRNAs-transfected group (Fig. 5, H and K) to the DSCAM and UNC5C shRNAs-transfected group (Fig. 5, J and K), the difference is statistically significant (p < 0.001). These results suggest that both DSCAM and UNC5C are required for netrin-1-induced growth cone collapse of postnatal EGL cells.
FIGURE 5.
Simultaneous knockdown of DSCAM and UNC5C abolished netrin-1-induced EGL cell growth cone collapse. A, B, and K, EGL neurons transfected with Venus-YFP only (1 μg). Netrin-1 treatment induced EGL growth cone collapse. C, D, and K. Neurons transfected with Venus-YFP (1 μg) and DSCAM shRNA (4 μg). DSCAM shRNA inhibited the induction of growth cone collapse by netrin-1. E, F, and K, EGL cells transfected with Venus-YFP (1 μg) and UNC5C shRNA (4 μg). Netrin-1-promoted collapse was also blocked by UNC5C shRNA. G, H, and K, neurons co-transfected with both control DSCAM (4 μg) and UNC5C (4 μg) shRNAs. Netrin-1 stimulation induced growth cone collapse. I–K, expression of both DSCAM (4 μg) and UNC5C(4 μg) shRNAs in EGL cells blocked netrin-1-induced growth cone collapse. Scale bar, 10 μm. K, quantification of axon growth cone collapse. Data are mean ± S.E. from three separate experiments. One-way Anova and Fischer LSD post-hoc comparisons were performed. ***: p < 0.001; ns: not significant.
To determine the coordination of DSCAM and UNC5C in netrin repulsive signaling, we assessed netrin-1-induced growth cone collapse after partial knockdown of either DSCAM, UNC5C, or both in P2 cerebellar EGL cells (Fig. 6). As shown in Fig. 6J, the knockdown efficiency of DSCAM or UNC5C shRNA was dose-dependent and expression of 2 μg of either one partially inhibited netrin-1-induced growth cone collapse (Fig. 6, A–F and I). Interestingly, partial knockdown of both DSCAM and UNC5C abolished the effect of netrin-1 on growth cone collapse (Fig. 6, G–I), suggesting that DSCAM collaborated with UNC5C in netrin-mediated growth cone collapse. Previous studies have shown that DCC collaborates with DSCAM and UNC5 in netrin signaling. However, blockage of DCC function by the DCC function blocking antibody could not affect netrin-1-induced growth cone collapse (data not shown). This result is consistent with previous studies, suggesting DCC is not required for netrin-mediated repulsion of postnatal EGL cells (45).
Coordination of Netrin/DSCAM and Netrin/UNC5C Repulsive Signaling via Src Family Kinases
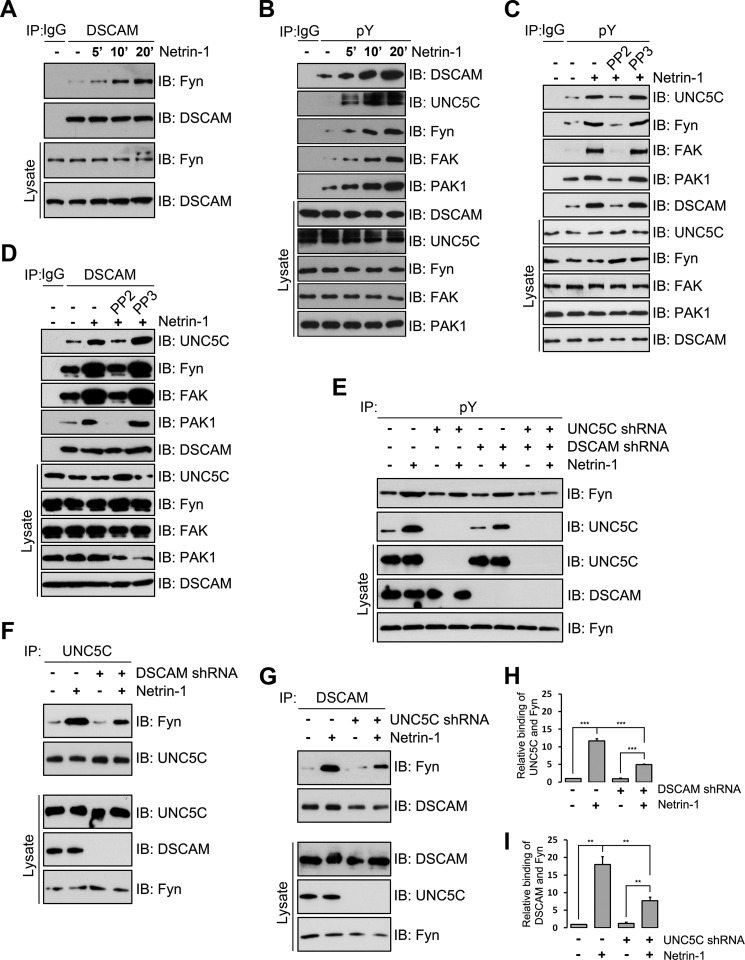
Although FAK and Src family kinases are required for netrin attractive signaling (40, 47–50), they are involved in the netrin-1-induced tyrosine phosphorylation of UNC5C (51, 52). Our previous studies have revealed that netrin-1 increases tyrosine phosphorylation of DSCAM and Fyn in HEK293 cells (21). To further examine whether DSCAM interacts with FAK and Fyn in netrin signaling, we co-transfected DSCAM with FAK or Fyn in HEK293 cells. The results from the IP experiments showed that DSCAM interacted with FAK and Fyn, and netrin-1 increased these interactions (data not shown). In primary postnatal cerebellar neurons, endogenous DSCAM also interacted with FAK and Fyn, and netrin-1 increased these interactions as well (Fig. 7, A and D and data not shown). Netrin-1 also increased tyrosine phosphorylation of endogenous DSCAM, UNC5C, Fyn, and FAK (Fig. 7, B and C and the reciprocal IP not shown). The interaction and tyrosine phosphorylation increased within 5 min and persisted up to 20 min (Fig. 7, A and B and data not shown). Interestingly, netrin-1 stimulation also increased PAK1 tyrosine phosphorylation as well as the interaction of PAK1 with DSCAM (Fig. 7, B–D). Netrin-1-induced tyrosine phosphorylation of these proteins and the interaction of DSCAM with UNC5C, Fyn, FAK, and PAK1 were inhibited by PP2, a pharmacological inhibitor of the Src family kinases, but not PP3, an inactive control for PP2 (Fig. 7, C and D), suggesting that Src family kinases may play a role in netrin/DSCAM/UNC5C signaling.
FIGURE 7.
DSCAM correlates with UNC5C via assembling Fyn, FAK, and PAK1 complexes in netrin signaling. A, interaction of endogenous DSCAM and Fyn in primary P2 cerebellar neurons. Neurons were stimulated with netrin-1 for 5 to 20 min. The anti-DSCAM antibody was used to immunoprecipitate proteins and the blot was analyzed with anti-Fyn (upper first panel) or anti-DSCAM (upper second panel). Endogenous DSCAM forms a protein-protein complex with Fyn, and netrin-1 increased these interactions. B, tyrosine phosphorylation of DSCAM, UNC5C, Fyn, FAK, and PAK1 was induced by netrin-1. C, PP2, but not PP3, blocked netrin-stimulated tyrosine phosphorylation of endogenous DSCAM, UNC5C, Fyn, FAK, and PAK1 in primary P2 cerebellar neurons. D, induction of the interaction of DSCAM with UNC5C, Fyn, FAK, and PAK1 by netrin-1 was inhibited by PP2. E, involvement of DSCAM in netrin-1 induced tyrosine phosphorylation of Fyn and UNC5C. P2 cerebellar granule cells were transfected either with 4 μg of DSCAM shRNA, UNC5C shRNA, or DSCAM shRNA plus UNC5C shRNA using nucleofection and stimulated with purified chicken netrin-1. Expression of DSCAM shRNA partially inhibited netrin-1 induced tyrosine phosphorylation of Fyn and UNC5C. Double knockdown of DSCAM and UNC5C abrogated the induction of Fyn tyrosine phosphorylation by netrin-1. F, knockdown of DSCAM inhibited netrin-1 induced interaction of UNC5C with Fyn in P2 cerebellar granule cells. DSCAM was immunoprecipitated using anti-DSCAM and the membrane was blotted for Fyn using anti-Fyn. G, expression of UNC5C shRNA inhibited netrin-1 induced interaction of DSCAM and Fyn. H and I, quantification showing relative binding of Fyn with UNC5C in F and DSCAM in G, respectively. ***, p < 0.001, **, p < 0.01 (one-way ANOVA and Fisher LSD post-hoc comparisons).
To further examine whether DSCAM is required for netrin/UNC5C signaling, postnatal cerebellar granular cells were transfected with either DSCAM shRNA or control shRNA. The co-immunoprecipitation results showed that knockdown of DSCAM partially inhibited netrin-induced tyrosine phosphorylation of UNC5C and Fyn (Fig. 7E) as well as the interaction of UNC5C with Fyn (Fig. 7, F and H). Similarly, knockdown of UNC5C by RNAi also partially inhibited netrin-induced tyrosine phosphorylation of Fyn (Fig. 7E) and the interaction of DSCAM with Fyn (Fig. 7, G and I). Double knockdown of both receptors totally abolished tyrosine phosphorylation of Fyn induced by netrin-1(Fig. 7E). These results suggest that DSCAM coordinates with UNC5C via Src family kinases such as Fyn in netrin repulsive signaling.
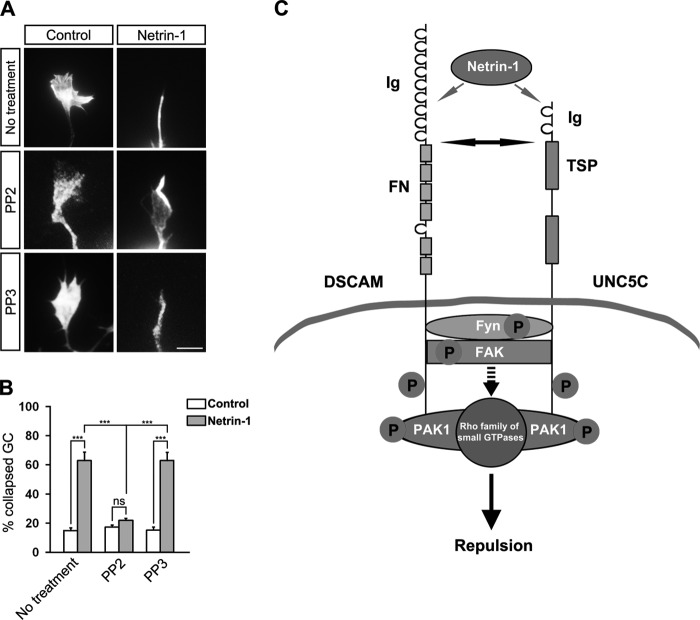
To investigate the functional role of Src family kinases in netrin-1-induced repulsion, we tested the effect of PP2 on netrin-1-induced growth cone collapse in postnatal cerebellar EGL cells as described above. As expected, netrin-1 stimulation caused EGL cell growth cone collapse in the absence of drug treatment (Fig. 8A, upper panels; quantification in 8B). However, PP2 (0.2 μm) blocked the netrin-1-promoted collapse (Fig. 8A, middle panels), resulting in a decreased percentage of collapsed axons (Fig. 8B). In contrast, axon collapse induced by netrin-1 was not inhibited by the same concentration of PP3 (Fig. 8A, lower panels, and Fig. 8B). These results indicate that Src family kinases are required for netrin-1-induced growth cone collapse.
FIGURE 8.
Inhibition of netrin-1-induced axon growth cone collapse by Src family kinase inhibitor and a functional model of netrin repulsive signaling. A, axon growth cones from dissociated postnatal cerebellar EGL cells plated on PLL in the absence (left panels) or presence (right panels) of netrin-1 without drug treatment (upper panels) or with PP2 (middle panels) or PP3 treatments (lower panels). Neurons were labeled with Alexa Fluor 555 phalloidin and DAPI. Scale bar, 5 μm. B, quantification of axon growth cone collapse. Data are mean ± S.D. from three separate experiments. A one-way Anova and Fischer LSD post-hoc comparisons were performed. ***: p < 0.001; ns: not significant. C, simple model demonstrating the coordination of the signal transduction cascades downstream of netrin/DSCAM and netrin/UNC5C in axon repulsion. Netrin-1 increases the interaction of DSCAM and UNC5C, and activates the intracellular protein kinase activities, such as, Src family kinases, FAK and PAK1, which may further regulate small GTPases activities to promote repulsion. Ig, immunoglobulin domain; FN, fibronectin type III domain; TSP, thrombospondin domain.
DISCUSSION
DSCAM and DCC are known to mediate netrin-1 attraction, whereas UNC5 mediates repulsion. UNC5 bound to DCC can switch DCC attraction to repulsion and the intracellular domain of UNC5 is crucial for transducing the repulsive signal. In this study, we demonstrated that DSCAM in association with UNC5C functions as a repulsive receptor of netrin-1 to promote the cerebellar EGL axon growth cone collapse.
In Drosophila, the homophilic binding of two identical Dscam isoforms is crucial for self-avoidance, a repulsive process that allows axonal and dendritic processes to uniformly cover their synaptic fields in the developing nervous system (53–56). While the vertebrate DSCAM gene does not encode multiple isoforms (57), it also promotes homophilic repulsion in patterning neural circuits, self-avoidance and tiling, a developmental mechanism that prevents processes from the same class of cells from occupying overlapping synaptic fields (36). However, in the chick visual system, DSCAM promotes homophilic adhesion in layer-specific targeting (37), suggesting DSCAM is involved in a variety of different wiring events in a context-dependent manner. Previous studies including ours indicate that DSCAM functions as a netrin-1 attractive receptor in commissural axons (19–21). Protein structure of DSCAM is similar to that of DCC and the interaction of DCC and UNC5 mediates netrin-1 repulsion. Our biochemical data indicated that DSCAM interacted with UNC5C in transfected HEK293 cells and primary neurons, and that netrin-1 stimulation induced the interaction of endogenous DSCAM and UNC5C (Fig. 1). These results provide a framework for a model that DSCAM may function as a repulsive receptor in netrin-1 signaling. Co-expression of DSCAM and UNC5C was found in dissociated cortical and cerebellar neurons as well as in different neuron layers of postnatal cerebellar slices (Fig. 2). Previous in situ hybridization studies have shown that netrin-1 and UNC5C are strongly expressed in postnatal cerebellar EGL neurons (42), and DSCAM mRNA is widely expressed throughout the nervous system including cerebellar EGL, PCL, and IGL neurons (44, 58). In the developing nervous system of Drosophila, Dscam1-based repulsive self-avoidance genetically counters Netrin-B/Frazzled-promoted attraction in the sensory neuron dendritic targeting (59), suggesting Dscam1-mediated repulsion is required for normal dendritic patterning in netrin signaling. Results from our functional assays suggest that DSCAM plays a crucial role in netrin-1-induced growth cone collapse of postnatal cerebellar EGL cells (Figs. 3, 5, 6, and 8) further supporting our hypothesis that DSCAM indeed functions as a netrin-1 repulsive receptor.
The intracellular domain of DSCAM is important for mediating netrin-1 signaling since netrin-1-induced collapse is blocked after overexpression of DSCAMΔC, the truncated mutant of DSCAM without the intracellular domain, in postnatal cerebellar EGL cells (Fig. 3). Surprisingly, overexpression of DSCAMΔN, the truncated mutant of DSCAM without the extracellular domain, only partially blocks netrin-1-induced growth cone collapse (Fig. 3). These results suggest that DSCAM may need to coordinate with other receptors at the extracellular level and/or through downstream signal transduction cascades to mediate netrin repulsion.
Previous studies have shown that overexpression of UNC5A in hippocampal neurons (43) and UNC5B in cortical neurons (46) causes netrin-1-mediated axon growth cone collapse. Netrin-1 repels axon projection from cerebellar EGL explants in an in vitro co-culture assay (42). These results suggest that UNC5 may be involved in netrin-1-mediated EGL repulsion. Our data indicate that netrin-1 causes EGL neuron growth cone collapse, even without overexpression of exogenous UNC5 family members in these neurons (Figs. 3–6 and 8). Chemorepulsive signaling is thought to be mediated by UNC5C in cerebellar neuronal migration in vivo (24), therefore, it is plausible to speculate that endogenous UNC5C is required for netrin-1-induced EGL repulsion. Our data indicate that UNC5C knockdown eliminates EGL growth cone collapse by netrin-1, suggesting that endogenous UNC5C indeed plays a role in netrin-1 repulsion (Fig. 4).
Knockdown of either DSCAM, UNC5C, or both in cerebellar EGL neurons results in loss of netrin-1-induced growth cone collapse (Figs. 3–5), suggesting that both DSCAM and UNC5C are interdependent in netrin-1 induced repulsion in EGL neurons. Interestingly, partial knockdown of both DSCAM and UNC5C eliminates netrin-1-induced growth cone collapse, whereas partial knockdown of either one only partially inhibits the effect of netrin-1 on growth cone collapse (Fig. 6). These results indicate that endogenous DSCAM and UNC5C coordinate with each other to mediate netrin-1-induced growth cone collapse. Further analysis reveals that both intracellular domains of DSCAM and UNC5C are required for transducing the netrin-1 repulsive signal (Figs. 3 and 4). DSCAM interacts with UNC5C through the extracellular domain (Fig. 1) and overexpression of truncation mutants of either DSCAMΔN (Fig. 3) or UNC5CΔN (Fig. 4) partially inhibits netrin-1 repulsive signaling. The binding of netrin-1 to DSCAM and UNC5C may initiate the cross talk of their downstream signals, ultimately mediating netrin-1 repulsion. Therefore, signal cascades downstream of DSCAM and UNC5C probably play a crucial role in netrin repulsive signaling.
Recent studies have shown that draxin is a repulsive guidance cue involved in commissural axon projection in the developing nervous system (60). Interestingly, draxin binds multiple netrin receptors: DCC, neurogenin, UNC5, and DSCAM, and the interaction of draxin and DCC is required for draxin-mediated neurite outgrowth and guidance (61). It remains to determine whether DSCAM and UNC5 are also involved in draxin-mediated axon repulsion and how draxin coordinates with netrins via netrin receptors to maneuver growth cone steering. UNC5A trafficking from the growth cone surface is an important mechanism for netrin-1 induced neuron growth cone collapse (43, 62). Further investigation will be necessary to understand whether a similar mechanism is involved in the coordination of netrin/DSCAM and netrin/UNC5C signaling.
The signaling mechanisms downstream of netrin/UNC5-mediated chemorepulsion are considerably less well understood than those of netrin/DCC-induced chemoattraction. Previous studies suggest that FAK and Src family kinases are required for netrin-induced mammalian UNC5 tyrosine phosphorylation (27, 51), which leads to the binding of the tyrosine phosphatase SHP2 (PTPN11) to UNC5 (63). In C. elegans, MAX-2, a PAK family member, and MAX-1, an adaptor protein, are required for netrin/UNC5 repulsion (64, 65). The identities of the intracellular signal transduction components mediating netrin/DSCAM response are less known in comparison to netrin/DCC and netrin/UNC5 signaling. Drosophila Dscam interacts with PAK1 (31) and expression of DSCAM induces PAK1 phosphorylation in mammalian cells (39). In HEK293 cells, netrin-1 induces phosphorylation of PAK1 and tyrosine phosphorylation of DSCAM and Fyn in the presence of DSCAM (21). In this study, we show that DSCAM interacts with UNC5C, PAK1, FAK, and Fyn in both HEK293 cells and primary neurons, and that netrin-1 stimulation increases these interactions. Netrin-1 also induces the tyrosine phosphorylation of endogenous DSCAM, UNC5C, FAK, Fyn, and PAK1 in primary postnatal cerebellar neurons. Blocking Src family kinases inhibits netrin-1-induced tyrosine phosphorylation of these signaling molecules and the interaction of endogenous DSCAM with UNC5C, Fyn, FAK, and PAK1 as well as EGL cell growth cone collapse. Since netrin/UNC5 and netrin/DSCAM share similar downstream signaling molecules, one appealing hypothesis is that in netrin-promoted repulsion, the signal transduction cascade downstream of DSCAM and UNC5C coordinate through regulation of multiple endogenous protein kinase activities, such as Src family kinases, FAK, and PAK1 (Fig. 8C). In this signal transduction pathway, Src family kinases form a positive feedback loop for coordinating netrin-1 repulsive signaling because PP2 treatment not only inhibited netrin-1-induced tyrosine phosphorylation of DSCAM, UNC5C, Fyn, FAK, and PAK1 (Fig. 7C) but also blocked the interaction of DSCAM with these proteins (Fig. 7D). The knockdown of DSCAM in primary postnatal cerebellar granular cells partially inhibited netrin-induced tyrosine phosphorylation of UNC5C and Fyn (Fig. 7E) as well as interaction of UNC5C with Fyn (Fig. 7, F and H). Similarly, UNC5C knockdown also partially inhibited the tyrosine phosphorylation of Fyn and interaction of DSCAM with Fyn induced by netrin-1 (Fig. 7, E, G, and I). Netrin-1 induced Fyn tyrosine phosphorylation was abolished by double knockdown of both receptors (Fig. 7E). These RNAi results further support our hypothesis that DSCAM collaborates with UNC5C to mediate netrin-1-induced axon growth cone collapse and Src family kinases is required for coordinating netrin-1/DSCAM/UNC5C repulsive signaling.
We and others have previously shown that DCC interacts with Fyn, FAK and PAK1, and that these kinases are essential for attractive signaling by netrin-1 (40, 47–50). p130CAS is a critical component in netrin signaling, functioning downstream of Fyn and FAK and upstream of the small GTPases Rac1 and Cdc42 (41, 66, 67). TRIO and DOCK180, two guanine nucleotide exchange factors (GEFs), are required for netrin attractive signaling by activating Rac1 (68–71). Inhibition of RhoA is thought to be involved in netrin-induced attraction (72). Also, PAK1 is a downstream effector of Cdc42 and Rac1 (73). Our data indicate that FAK, Fyn and PAK1, the same signaling components in netrin-mediated attractive signaling, play a role in netrin-mediated repulsion, suggesting that DSCAM may coordinate with UNC5C through regulation of different small GTPase activities in netrin repulsive signaling (Fig. 8C). Further investigation is necessary to untangle the role of small GTPases underlying the coordination of DSCAM and UNC5C in netrin-mediated repulsive signaling.
In summary, our data suggest that DSCAM functions as a repulsive netrin receptor, collaborating with UNC5C to mediate axon growth cone collapse, and Fyn/FAK/PAK1 is the downstream signaling complex required for the coordination of netrin/DSCAM and netrin/UNC5C downstream signaling.
Acknowledgments
We thank Dr. William T Gunning III for help on brain slice preparation and Dr. Song-Tao Liu for help with imaging. We thank Dr. Richard Komuniecki for comments on the manuscript.
This work was supported, in whole or in part, by the National Institutes of Health (to G. L. and K. G.) and the Whitehall Foundation (to G. L.).
- DCC
- deleted in colorectal cancer
- DSCAM
- Down syndrome cell adhesion molecule
- UNC5
- uncoordinated-5
- EGL
- external granule layer
- PCL
- Purkinje cell layer
- IGL
- internal granule layer
- FAK
- focal adhesion kinase
- PAK1
- p21-activated kinase 1
- CAS
- Crk-associated substrate
- GEF
- guanine nucleotide exchange factor.
REFERENCES
- 1. Tessier-Lavigne M., Goodman C. S. (1996) The molecular biology of axon guidance. Science 274, 1123–1133 [DOI] [PubMed] [Google Scholar]
- 2. Dodd J., Jessell T. M. (1988) Axon guidance and the patterning of neuronal projections in vertebrates. Science 242, 692–699 [DOI] [PubMed] [Google Scholar]
- 3. Guan K. L., Rao Y. (2003) Signaling mechanisms mediating neuronal responses to guidance cues. Nat. Rev. Neurosci. 4, 941–956 [DOI] [PubMed] [Google Scholar]
- 4. Lai Wing, Sun K., Correia J. P., Kennedy T. E. (2011) Netrins: versatile extracellular cues with diverse functions. Development 138, 2153–2169 [DOI] [PubMed] [Google Scholar]
- 5. Zou Y., Lyuksyutova A. I. (2007) Morphogens as conserved axon guidance cues. Curr. Opin. Neurobiol. 17, 22–28 [DOI] [PubMed] [Google Scholar]
- 6. Hedgecock E. M., Culotti J. G., Hall D. H. (1990) The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron 4, 61–85 [DOI] [PubMed] [Google Scholar]
- 7. Tessier-Lavigne M., Placzek M., Lumsden A. G., Dodd J., Jessell T. M. (1988) Chemotropic guidance of developing axons in the mammalian central nervous system. Nature 336, 775–778 [DOI] [PubMed] [Google Scholar]
- 8. Ishii N., Wadsworth W. G., Stern B. D., Culotti J. G., Hedgecock E. M. (1992) UNC-6, a laminin-related protein, guides cell and pioneer axon migrations in C. elegans. Neuron 9, 873–881 [DOI] [PubMed] [Google Scholar]
- 9. Kennedy T. E., Serafini T., de la Torre J. R., Tessier-Lavigne M. (1994) Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell 78, 425–435 [DOI] [PubMed] [Google Scholar]
- 10. Kolodziej P. A., Timpe L. C., Mitchell K. J., Fried S. R., Goodman C. S., Jan L. Y., Jan Y. N. (1996) frazzled encodes a Drosophila member of the DCC immunoglobulin subfamily and is required for CNS and motor axon guidance. Cell 87, 197–204 [DOI] [PubMed] [Google Scholar]
- 11. Mitchell K. J., Doyle J. L., Serafini T., Kennedy T. E., Tessier-Lavigne M., Goodman C. S., Dickson B. J. (1996) Genetic analysis of Netrin genes in Drosophila: Netrins guide CNS commissural axons and peripheral motor axons. Neuron 17, 203–215 [DOI] [PubMed] [Google Scholar]
- 12. Serafini T., Kennedy T. E., Galko M. J., Mirzayan C., Jessell T. M., Tessier-Lavigne M. (1994) The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell 78, 409–424 [DOI] [PubMed] [Google Scholar]
- 13. Keino-Masu K., Masu M., Hinck L., Leonardo E. D., Chan S. S., Culotti J. G., Tessier-Lavigne M. (1996) Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell 87, 175–185 [DOI] [PubMed] [Google Scholar]
- 14. Fearon E. R., Cho K. R., Nigro J. M., Kern S. E., Simons J. W., Ruppert J. M., Hamilton S. R., Preisinger A. C., Thomas G., Kinzler K. W. (1990) Identification of a chromosome 18q gene that is altered in colorectal cancers. Science 247, 49–56 [DOI] [PubMed] [Google Scholar]
- 15. Cooper H. M., Armes P., Britto J., Gad J., Wilks A. F. (1995) Cloning of the mouse homologue of the deleted in colorectal cancer gene (mDCC) and its expression in the developing mouse embryo. Oncogene 11, 2243–2254 [PubMed] [Google Scholar]
- 16. Fazeli A., Dickinson S. L., Hermiston M. L., Tighe R. V., Steen R. G., Small C. G., Stoeckli E. T., Keino-Masu K., Masu M., Rayburn H., Simons J., Bronson R. T., Gordon J. I., Tessier-Lavigne M., Weinberg R. A. (1997) Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature 386, 796–804 [DOI] [PubMed] [Google Scholar]
- 17. Keeling S. L., Gad J. M., Cooper H. M. (1997) Mouse Neogenin, a DCC-like molecule, has four splice variants and is expressed widely in the adult mouse and during embryogenesis. Oncogene 15, 691–700 [DOI] [PubMed] [Google Scholar]
- 18. Meyerhardt J. A., Look A. T., Bigner S. H., Fearon E. R. (1997) Identification and characterization of neogenin, a DCC-related gene. Oncogene 14, 1129–1136 [DOI] [PubMed] [Google Scholar]
- 19. Andrews G. L., Tanglao S., Farmer W. T., Morin S., Brotman S., Berberoglu M. A., Price H., Fernandez G. C., Mastick G. S., Charron F., Kidd T. (2008) Dscam guides embryonic axons by Netrin-dependent and -independent functions. Development 135, 3839–3848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ly A., Nikolaev A., Suresh G., Zheng Y., Tessier-Lavigne M., Stein E. (2008) DSCAM is a netrin receptor that collaborates with DCC in mediating turning responses to netrin-1. Cell 133, 1241–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu G., Li W., Wang L., Kar A., Guan K. L., Rao Y., Wu J. Y. (2009) DSCAM functions as a netrin receptor in commissural axon pathfinding. Proc. Natl. Acad. Sci. U.S.A. 106, 2951–2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ackerman S. L., Kozak L. P., Przyborski S. A., Rund L. A., Boyer B. B., Knowles B. B. (1997) The mouse rostral cerebellar malformation gene encodes an UNC-5-like protein. Nature 386, 838–842 [DOI] [PubMed] [Google Scholar]
- 23. Leonardo E. D., Hinck L., Masu M., Keino-Masu K., Ackerman S. L., Tessier-Lavigne M. (1997) Vertebrate homologues of C. elegans UNC-5 are candidate netrin receptors. Nature 386, 833–838 [DOI] [PubMed] [Google Scholar]
- 24. Przyborski S. A., Knowles B. B., Ackerman S. L. (1998) Embryonic phenotype of Unc5h3 mutant mice suggests chemorepulsion during the formation of the rostral cerebellar boundary. Development 125, 41–50 [DOI] [PubMed] [Google Scholar]
- 25. Engelkamp D. (2002) Cloning of three mouse Unc5 genes and their expression patterns at mid-gestation. Mech. Dev. 118, 191–197 [DOI] [PubMed] [Google Scholar]
- 26. Hong K., Hinck L., Nishiyama M., Poo M. M., Tessier-Lavigne M., Stein E. (1999) A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell 97, 927–941 [DOI] [PubMed] [Google Scholar]
- 27. Killeen M., Tong J., Krizus A., Steven R., Scott I., Pawson T., Culotti J. (2002) UNC-5 function requires phosphorylation of cytoplasmic tyrosine 482, but its UNC-40-independent functions also require a region between the ZU-5 and death domains. Dev. Biol. 251, 348–366 [DOI] [PubMed] [Google Scholar]
- 28. Keleman K., Dickson B. J. (2001) Short- and long-range repulsion by the Drosophila Unc5 netrin receptor. Neuron 32, 605–617 [DOI] [PubMed] [Google Scholar]
- 29. Chen B. E., Kondo M., Garnier A., Watson F. L., Püettmann-Holgado R., Lamar D. R., Schmucker D. (2006) The molecular diversity of Dscam is functionally required for neuronal wiring specificity in Drosophila. Cell 125, 607–620 [DOI] [PubMed] [Google Scholar]
- 30. Hummel T., Vasconcelos M. L., Clemens J. C., Fishilevich Y., Vosshall L. B., Zipursky S. L. (2003) Axonal targeting of olfactory receptor neurons in Drosophila is controlled by Dscam. Neuron 37, 221–231 [DOI] [PubMed] [Google Scholar]
- 31. Schmucker D., Clemens J. C., Shu H., Worby C. A., Xiao J., Muda M., Dixon J. E., Zipursky S. L. (2000) Drosophila Dscam is an axon guidance receptor exhibiting extraordinary molecular diversity. Cell 101, 671–684 [DOI] [PubMed] [Google Scholar]
- 32. Wang J., Zugates C. T., Liang I. H., Lee C. H., Lee T. (2002) Drosophila Dscam is required for divergent segregation of sister branches and suppresses ectopic bifurcation of axons. Neuron 33, 559–571 [DOI] [PubMed] [Google Scholar]
- 33. Zhu H., Hummel T., Clemens J. C., Berdnik D., Zipursky S. L., Luo L. (2006) Dendritic patterning by Dscam and synaptic partner matching in the Drosophila antennal lobe. Nat. Neurosci. 9, 349–355 [DOI] [PubMed] [Google Scholar]
- 34. Zhan X. L., Clemens J. C., Neves G., Hattori D., Flanagan J. J., Hummel T., Vasconcelos M. L., Chess A., Zipursky S. L. (2004) Analysis of Dscam diversity in regulating axon guidance in Drosophila mushroom bodies. Neuron 43, 673–686 [DOI] [PubMed] [Google Scholar]
- 35. Fuerst P. G., Bruce F., Tian M., Wei W., Elstrott J., Feller M. B., Erskine L., Singer J. H., Burgess R. W. (2009) DSCAM and DSCAML1 function in self-avoidance in multiple cell types in the developing mouse retina. Neuron 64, 484–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fuerst P. G., Koizumi A., Masland R. H., Burgess R. W. (2008) Neurite arborization and mosaic spacing in the mouse retina require DSCAM. Nature 451, 470–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yamagata M., Sanes J. R. (2008) Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature 451, 465–469 [DOI] [PubMed] [Google Scholar]
- 38. Kruger R. P., Lee J., Li W., Guan K. L. (2004) Mapping netrin receptor binding reveals domains of Unc5 regulating its tyrosine phosphorylation. J. Neurosci. 24, 10826–10834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li W., Guan K. L. (2004) The Down syndrome cell adhesion molecule (DSCAM) interacts with and activates Pak. J. Biol. Chem. 279, 32824–32831 [DOI] [PubMed] [Google Scholar]
- 40. Liu G., Beggs H., Jürgensen C., Park H. T., Tang H., Gorski J., Jones K. R., Reichardt L. F., Wu J., Rao Y. (2004) Netrin requires focal adhesion kinase and Src family kinases for axon outgrowth and attraction. Nat. Neurosci. 7, 1222–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu G., Li W., Gao X., Li X., Jürgensen C., Park H. T., Shin N. Y., Yu J., He M. L., Hanks S. K., Wu J. Y., Guan K. L., Rao Y. (2007) p130CAS is required for netrin signaling and commissural axon guidance. J. Neurosci. 27, 957–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alcántara S., Ruiz M., De Castro F., Soriano E., Sotelo C. (2000) Netrin 1 acts as an attractive or as a repulsive cue for distinct migrating neurons during the development of the cerebellar system. Development 127, 1359–1372 [DOI] [PubMed] [Google Scholar]
- 43. Bartoe J. L., McKenna W. L., Quan T. K., Stafford B. K., Moore J. A., Xia J., Takamiya K., Huganir R. L., Hinck L. (2006) Protein interacting with C-kinase 1/protein kinase Calpha-mediated endocytosis converts netrin-1-mediated repulsion to attraction. J. Neurosci. 26, 3192–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Barlow G. M., Micales B., Chen X. N., Lyons G. E., Korenberg J. R. (2002) Mammalian DSCAMs: roles in the development of the spinal cord, cortex, and cerebellum? Biochem. Biophys. Res. Commun. 293, 881–891 [DOI] [PubMed] [Google Scholar]
- 45. Guijarro P., Simó S., Pascual M., Abasolo I., Del Rio J. A., Soriano E. (2006) Netrin1 exerts a chemorepulsive effect on migrating cerebellar interneurons in a Dcc-independent way. Mol. Cell Neurosci. 33, 389–400 [DOI] [PubMed] [Google Scholar]
- 46. Hata K., Kaibuchi K., Inagaki S., Yamashita T. (2009) Unc5B associates with LARG to mediate the action of repulsive guidance molecule. J. Cell Biol. 184, 737–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ren X. R., Ming G. L., Xie Y., Hong Y., Sun D. M., Zhao Z. Q., Feng Z., Wang Q., Shim S., Chen Z. F., Song H. J., Mei L., Xiong W. C. (2004) Focal adhesion kinase in netrin-1 signaling. Nat. Neurosci. 7, 1204–1212 [DOI] [PubMed] [Google Scholar]
- 48. Li W., Lee J., Vikis H. G., Lee S. H., Liu G., Aurandt J., Shen T. L., Fearon E. R., Guan J. L., Han M., Rao Y., Hong K., Guan K. L. (2004) Activation of FAK and Src are receptor-proximal events required for netrin signaling. Nat. Neurosci. 7, 1213–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Meriane M., Tcherkezian J., Webber C. A., Danek E. I., Triki I., McFarlane S., Bloch-Gallego E., Lamarche-Vane N. (2004) Phosphorylation of DCC by Fyn mediates Netrin-1 signaling in growth cone guidance. J. Cell Biol. 167, 687–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shekarabi M., Moore S. W., Tritsch N. X., Morris S. J., Bouchard J. F., Kennedy T. E. (2005) Deleted in colorectal cancer binding netrin-1 mediates cell substrate adhesion and recruits Cdc42, Rac1, Pak1, and N-WASP into an intracellular signaling complex that promotes growth cone expansion. J. Neurosci. 25, 3132–3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li W., Aurandt J., Jürgensen C., Rao Y., Guan K. L. (2006) FAK and Src kinases are required for netrin-induced tyrosine phosphorylation of UNC5. J. Cell Sci. 119, 47–55 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52. Tang X., Jang S. W., Okada M., Chan C. B., Feng Y., Liu Y., Luo S. W., Hong Y., Rama N., Xiong W. C., Mehlen P., Ye K. (2008) Netrin-1 mediates neuronal survival through PIKE-L interaction with the dependence receptor UNC5B. Nat. Cell Biol. 10, 698–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Matthews B. J., Kim M. E., Flanagan J. J., Hattori D., Clemens J. C., Zipursky S. L., Grueber W. B. (2007) Dendrite self-avoidance is controlled by Dscam. Cell 129, 593–604 [DOI] [PubMed] [Google Scholar]
- 54. Hughes M. E., Bortnick R., Tsubouchi A., Bäumer P., Kondo M., Uemura T., Schmucker D. (2007) Homophilic Dscam interactions control complex dendrite morphogenesis. Neuron 54, 417–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Soba P., Zhu S., Emoto K., Younger S., Yang S. J., Yu H. H., Lee T., Jan L. Y., Jan Y. N. (2007) Drosophila sensory neurons require Dscam for dendritic self-avoidance and proper dendritic field organization. Neuron 54, 403–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hattori D., Millard S. S., Wojtowicz W. M., Zipursky S. L. (2008) Dscam-mediated cell recognition regulates neural circuit formation. Annu. Rev. Cell Dev. Biol. 24, 597–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Agarwala K. L., Nakamura S., Tsutsumi Y., Yamakawa K. (2000) Down syndrome cell adhesion molecule DSCAM mediates homophilic intercellular adhesion. Brain Res. Mol. Brain Res. 79, 118–126 [DOI] [PubMed] [Google Scholar]
- 58. Yamakawa K., Huot Y. K., Haendelt M. A., Hubert R., Chen X. N., Lyons G. E., Korenberg J. R. (1998) DSCAM: a novel member of the immunoglobulin superfamily maps in a Down syndrome region and is involved in the development of the nervous system. Hum. Mol. Genet 7, 227–237 [DOI] [PubMed] [Google Scholar]
- 59. Matthews B. J., Grueber W. B. (2011) Dscam1-mediated self-avoidance counters netrin-dependent targeting of dendrites in Drosophila. Curr. Biol. 21, 1480–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Islam S. M., Shinmyo Y., Okafuji T., Su Y., Naser I. B., Ahmed G., Zhang S., Chen S., Ohta K., Kiyonari H., Abe T., Tanaka S., Nishinakamura R., Terashima T., Kitamura T., Tanaka H. (2009) Draxin, a repulsive guidance protein for spinal cord and forebrain commissures. Science 323, 388–393 [DOI] [PubMed] [Google Scholar]
- 61. Ahmed G., Shinmyo Y., Ohta K., Islam S. M., Hossain M., Naser I. B., Riyadh M. A., Su Y., Zhang S., Tessier-Lavigne M., Tanaka H. (2011) Draxin inhibits axonal outgrowth through the netrin receptor DCC. J. Neurosci. 31, 14018–14023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Williams M. E., Wu S. C., McKenna W. L., Hinck L. (2003) Surface expression of the netrin receptor UNC5H1 is regulated through a protein kinase C-interacting protein/protein kinase-dependent mechanism. J. Neurosci. 23, 11279–11288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tong J., Killeen M., Steven R., Binns K. L., Culotti J., Pawson T. (2001) Netrin stimulates tyrosine phosphorylation of the UNC-5 family of netrin receptors and induces Shp2 binding to the RCM cytodomain. J. Biol. Chem. 276, 40917–40925 [DOI] [PubMed] [Google Scholar]
- 64. Lucanic M., Kiley M., Ashcroft N., L'Etoile N., Cheng H. J. (2006) The Caenorhabditis elegans P21-activated kinases are differentially required for UNC-6/netrin-mediated commissural motor axon guidance. Development 133, 4549–4559 [DOI] [PubMed] [Google Scholar]
- 65. Huang X., Cheng H. J., Tessier-Lavigne M., Jin Y. (2002) MAX-1, a novel PH/MyTH4/FERM domain cytoplasmic protein implicated in netrin-mediated axon repulsion. Neuron 34, 563–576 [DOI] [PubMed] [Google Scholar]
- 66. Li X., Saint-Cyr-Proulx E., Aktories K., Lamarche-Vane N. (2002) Rac1 and Cdc42 but not RhoA or Rho kinase activities are required for neurite outgrowth induced by the Netrin-1 receptor DCC (deleted in colorectal cancer) in N1E-115 neuroblastoma cells. J. Biol. Chem. 277, 15207–15214 [DOI] [PubMed] [Google Scholar]
- 67. Shekarabi M., Kennedy T. E. (2002) The netrin-1 receptor DCC promotes filopodia formation and cell spreading by activating Cdc42 and Rac1. Mol. Cell Neurosci. 19, 1–17 [DOI] [PubMed] [Google Scholar]
- 68. Li X., Gao X., Liu G., Xiong W., Wu J., Rao Y. (2008) Netrin signal transduction and the guanine nucleotide exchange factor DOCK180 in attractive signaling. Nat. Neurosci. 11, 28–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Briançon-Marjollet A., Ghogha A., Nawabi H., Triki I., Auziol C., Fromont S., Piché C., Enslen H., Chebli K., Cloutier J. F., Castellani V., Debant A., Lamarche-Vane N. (2008) Trio mediates netrin-1-induced Rac1 activation in axon outgrowth and guidance. Mol. Cell. Biol. 28, 2314–2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Forsthoefel D. J., Liebl E. C., Kolodziej P. A., Seeger M. A. (2005) The Abelson tyrosine kinase, the Trio GEF and Enabled interact with the Netrin receptor Frazzled in Drosophila. Development 132, 1983–1994 [DOI] [PubMed] [Google Scholar]
- 71. Watari-Goshima N., Ogura K., Wolf F. W., Goshima Y., Garriga G. (2007) C. elegans VAB-8 and UNC-73 regulate the SAX-3 receptor to direct cell and growth-cone migrations. Nat. Neurosci. 10, 169–176 [DOI] [PubMed] [Google Scholar]
- 72. Moore S. W., Correia J. P., Lai Wing Sun K., Pool M., Fournier A. E., Kennedy T. E. (2008) Rho inhibition recruits DCC to the neuronal plasma membrane and enhances axon chemoattraction to netrin 1. Development 135, 2855–2864 [DOI] [PubMed] [Google Scholar]
- 73. Bagrodia S., Cerione R. A. (1999) Pak to the future. Trends Cell Biol. 9, 350–355 [DOI] [PubMed] [Google Scholar]