Abstract
A DNA-binding protein from Xenopus laevis oocyte mitochondria which has been found associated with the D-loop also shows a strong preference for single-stranded DNA. The binding to polynucleotides is dependent on the base composition, but no sequence specificity was found. This protein, called mtSSB, binds tightly and cooperatively to single-stranded DNA. By its amino-acid composition and its binding properties it appears to be similar to the single-stranded DNA-binding proteins found in prokaryotes.
Full text
PDF


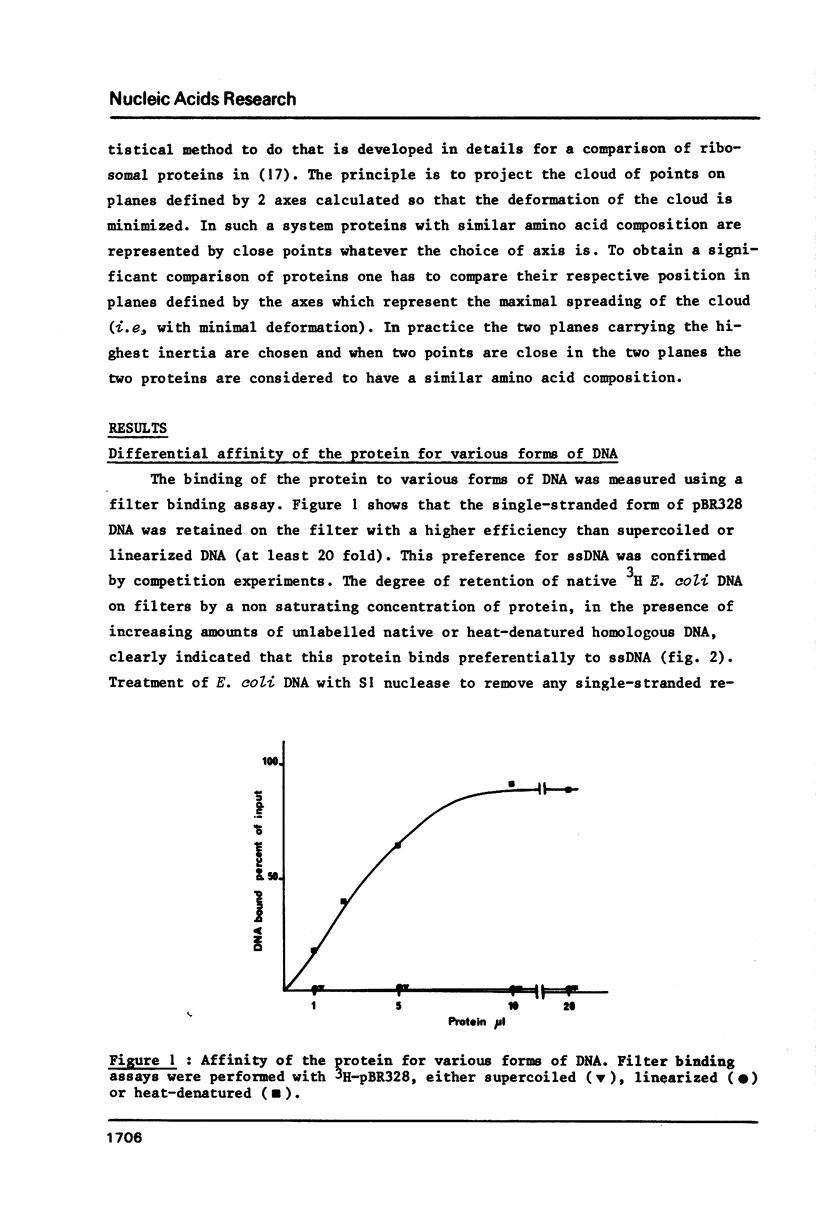
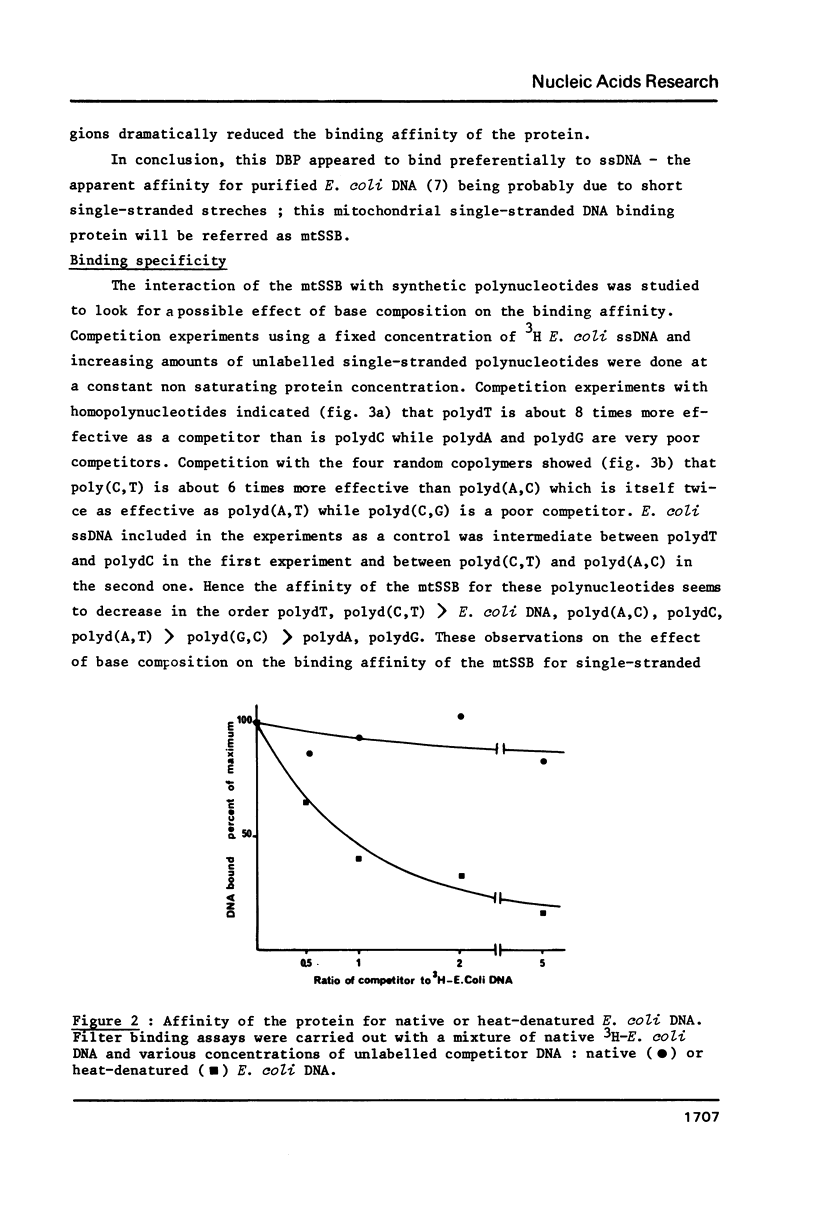
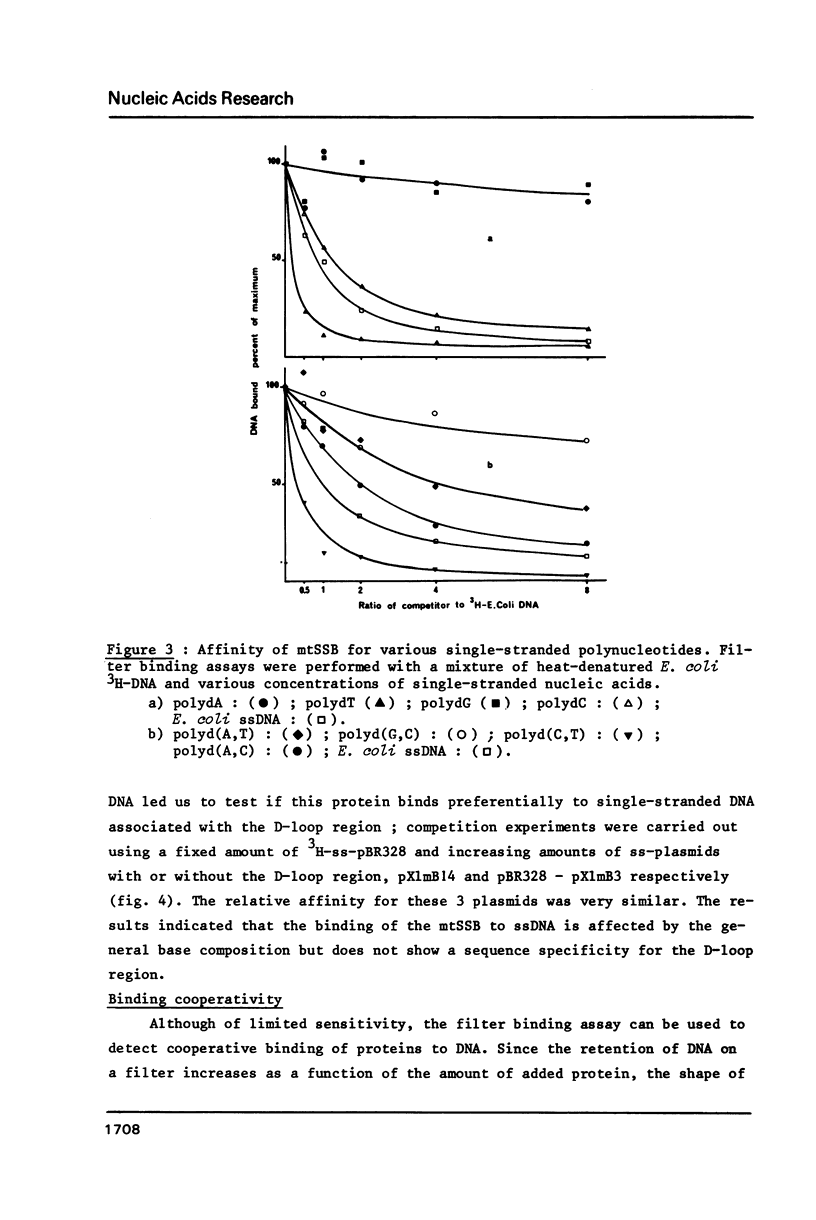
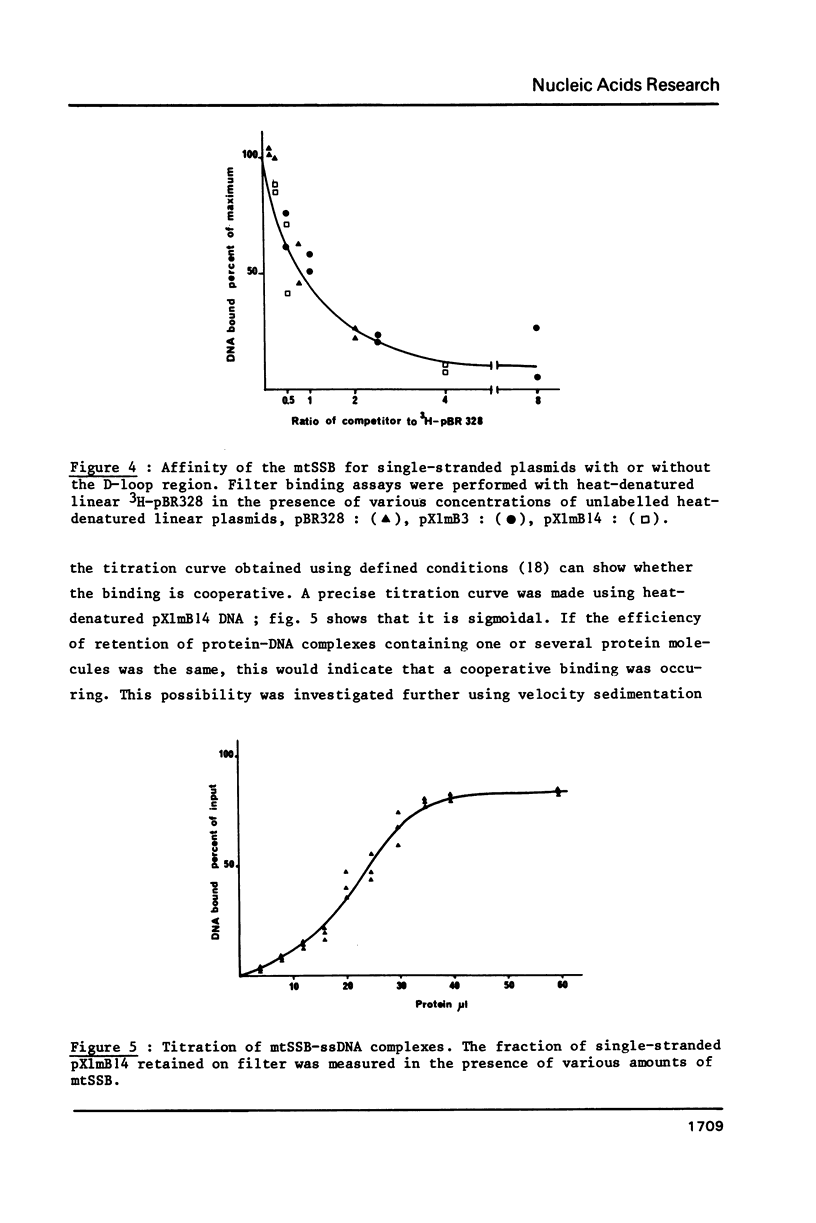
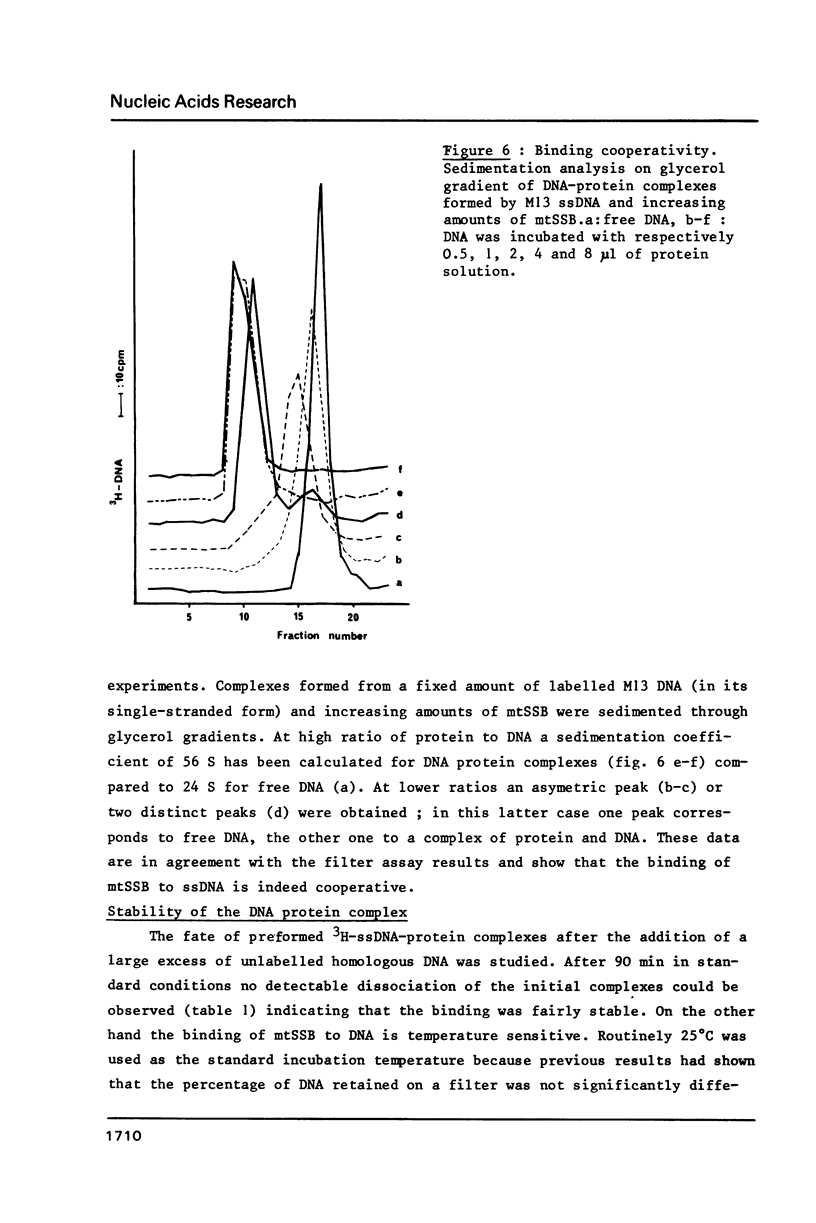
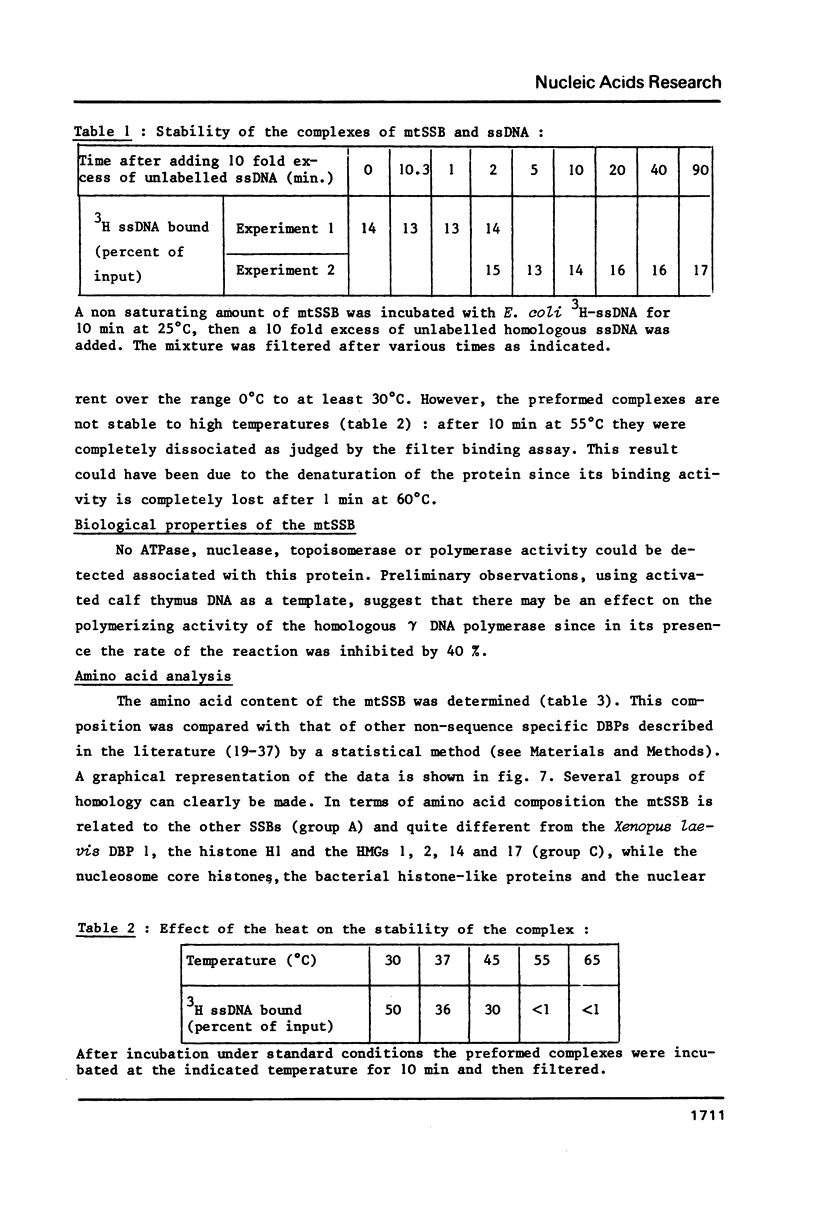

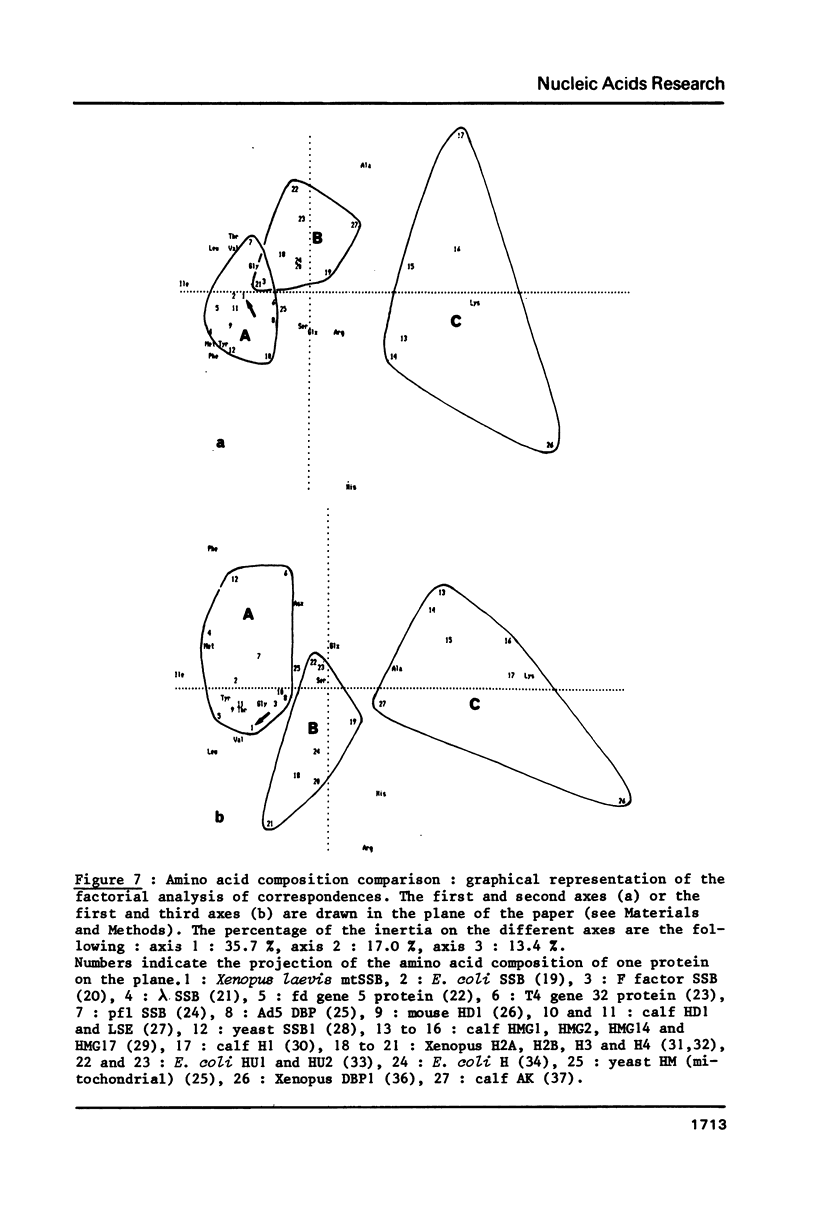



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barat M., Mignotte B. A DNA binding protein from Xenopus laevis oocyte mitochondria. Chromosoma. 1981;82(4):583–593. doi: 10.1007/BF00295014. [DOI] [PubMed] [Google Scholar]
- Benson J. R., Hare P. E. O-phthalaldehyde: fluorogenic detection of primary amines in the picomole range. Comparison with fluorescamine and ninhydrin. Proc Natl Acad Sci U S A. 1975 Feb;72(2):619–622. doi: 10.1073/pnas.72.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertazzoni U., Scovassi A. I., Brun G. M. Chick-embryo DNA polymerase gamma. Identity of gamma-polymerases purified from nuclei and mitochondria. Eur J Biochem. 1977 Dec 1;81(2):237–248. doi: 10.1111/j.1432-1033.1977.tb11945.x. [DOI] [PubMed] [Google Scholar]
- Bonne-Andrea C., Harper F., Sobczak J., De Recondo A. M. Rat liver HMG1: a physiological nucleosome assembly factor. EMBO J. 1984 May;3(5):1193–1199. doi: 10.1002/j.1460-2075.1984.tb01950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonne C., Sautiere P., Duguet M., de Recondo A. M. Identification of a single-stranded DNA binding protein from rat liver with high mobility group protein 1. J Biol Chem. 1982 Mar 25;257(6):2722–2725. [PubMed] [Google Scholar]
- Brun G., Vannier P., Scovassi I., Callen J. C. DNA topoisomerase I from mitochondria of Xenopus laevis oocytes. Eur J Biochem. 1981 Aug;118(2):407–415. doi: 10.1111/j.1432-1033.1981.tb06417.x. [DOI] [PubMed] [Google Scholar]
- Callen J. C., Tourte M., Dennebouy N., Mounolou J. C. Changes in D-loop frequency and superhelicity among the mitochondrial DNA molecules in relation to organelle biogenesis in oocytes of Xenopus laevis. Exp Cell Res. 1983 Jan;143(1):115–125. doi: 10.1016/0014-4827(83)90114-3. [DOI] [PubMed] [Google Scholar]
- Caron F., Jacq C., Rouvière-Yaniv J. Characterization of a histone-like protein extracted from yeast mitochondria. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4265–4269. doi: 10.1073/pnas.76.9.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrara G., Gattoni S., Mercanti D., Tocchini-Valentini G. P. Purification of a DNA-binding protein from Xenopus laevis unfertilized eggs. Nucleic Acids Res. 1977 Aug;4(8):2855–2870. doi: 10.1093/nar/4.8.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne A. M., Dennebouy N., Julien J. F., Lehegarat J. C., Mounolou J. C. Co-linear organization of Xenopus laevis and mouse mitochondrial genomes. Biochem Biophys Res Commun. 1984 Aug 16;122(3):918–924. doi: 10.1016/0006-291x(84)91178-1. [DOI] [PubMed] [Google Scholar]
- Chase J. W., Merrill B. M., Williams K. R. F sex factor encodes a single-stranded DNA binding protein (SSB) with extensive sequence homology to Escherichia coli SSB. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5480–5484. doi: 10.1073/pnas.80.18.5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. A. Replication of animal mitochondrial DNA. Cell. 1982 Apr;28(4):693–705. doi: 10.1016/0092-8674(82)90049-6. [DOI] [PubMed] [Google Scholar]
- Clayton D. A. Transcription of the mammalian mitochondrial genome. Annu Rev Biochem. 1984;53:573–594. doi: 10.1146/annurev.bi.53.070184.003041. [DOI] [PubMed] [Google Scholar]
- Faye G., Sor F., Glatigny A., Lederer F., Lesquoy E. Comparison of amino acid compositions of mitochondrial and cytoplasmic ribosomal proteins of Saccharomyces cerevisiae. Mol Gen Genet. 1979 Mar 27;171(3):335–341. doi: 10.1007/BF00267589. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B. Nucleic acid synthesis in embryos and its bearing on cell differentiation. Essays Biochem. 1968;4:25–68. [PubMed] [Google Scholar]
- Herrick G., Alberts B. Purification and physical characterization of nucleic acid helix-unwinding proteins from calf thymus. J Biol Chem. 1976 Apr 10;251(7):2124–2132. [PubMed] [Google Scholar]
- Hübscher U., Lutz H., Kornberg A. Novel histone H2A-like protein of escherichia coli. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5097–5101. doi: 10.1073/pnas.77.9.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenson J. C., Chin-Lin P., Gerber-Jenson B., Litman G. W. Structurally unique basic protein coextracted with histones from calf thymus chromatin. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1389–1393. doi: 10.1073/pnas.77.3.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns E. W. The isolation and purification of histones. Methods Cell Biol. 1977;16:183–203. doi: 10.1016/s0091-679x(08)60100-4. [DOI] [PubMed] [Google Scholar]
- Kruijer W., Van Schaik F. M., Sussenbach J. S. Nucleotide sequence of the gene encoding adenovirus type 2 DNA binding protein. Nucleic Acids Res. 1982 Aug 11;10(15):4493–4500. doi: 10.1093/nar/10.15.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBonne S. G., Dumas L. B. Isolation of a yeast single-strand deoxyribonucleic acid binding protein that specifically stimulates yeast DNA polymerase I. Biochemistry. 1983 Jun 21;22(13):3214–3219. doi: 10.1021/bi00282a027. [DOI] [PubMed] [Google Scholar]
- Laine B., Kmiecik D., Sautiere P., Biserte G., Cohen-Solal M. Complete amino-acid sequences of DNA-binding proteins HU-1 and HU-2 from Escherichia coli. Eur J Biochem. 1980 Feb;103(3):447–461. doi: 10.1111/j.1432-1033.1980.tb05968.x. [DOI] [PubMed] [Google Scholar]
- Litman R. M. A deoxyribonucleic acid polymerase from Micrococcus luteus (Micrococcus lysodeikticus) isolated on deoxyribonucleic acid-cellulose. J Biol Chem. 1968 Dec 10;243(23):6222–6233. [PubMed] [Google Scholar]
- Maeda K., Kneale G. G., Tsugita A., Short N. J., Perham R. N., Hill D. F., Petersen G. B. The DNA-binding protein of Pf1 filamentous bacteriophage: amino-acid sequence and structure of the gene. EMBO J. 1982;1(2):255–261. doi: 10.1002/j.1460-2075.1982.tb01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignotte B., Barat M., Marsault J., Mounolou J. C. Mitochondrial DNA-binding proteins that bind preferentially to supercoiled molecules containing the D-loop region of Xenopus laevis mtDNA. Biochem Biophys Res Commun. 1983 Nov 30;117(1):99–107. doi: 10.1016/0006-291x(83)91546-2. [DOI] [PubMed] [Google Scholar]
- Moorman A. F., De Boer P. A., De Laaf R. T., Destrée O. H. Primary structure of the histone H2A and H2B genes and their flanking sequences in a minor histone gene cluster of Xenopus laevis. FEBS Lett. 1982 Aug 2;144(2):235–241. doi: 10.1016/0014-5793(82)80645-5. [DOI] [PubMed] [Google Scholar]
- Moorman A. F., de Boer P. A., de Laaf R. T., van Dongen W. M., Destrée O. H. Primary structure of the histone H3 and H4 genes and their flanking sequences in a minor histone gene cluster of Xenopus laevis. FEBS Lett. 1981 Dec 21;136(1):45–52. doi: 10.1016/0014-5793(81)81211-2. [DOI] [PubMed] [Google Scholar]
- Nakashima Y., Dunker A. K., Marvin D. A., Konigsberg W. The amino acid sequence of a DNA binding protein, the gene 5 product of fd filamentous bacteriophage. FEBS Lett. 1974 Apr 1;40(2):290–292. doi: 10.1016/0014-5793(74)80246-2. [DOI] [PubMed] [Google Scholar]
- Planck S. R., Wilson S. H. Studies on the structure of mouse helix-destabilizing protein-1. DNA binding and controlled proteolysis with trypsin. J Biol Chem. 1980 Dec 10;255(23):11547–11556. [PubMed] [Google Scholar]
- Pörschke D., Rauh H. Cooperative, excluded-site binding and its dynamics for the interaction of gene 5 protein with polynucleotides. Biochemistry. 1983 Sep 27;22(20):4737–4745. doi: 10.1021/bi00289a019. [DOI] [PubMed] [Google Scholar]
- Sancar A., Williams K. R., Chase J. W., Rupp W. D. Sequences of the ssb gene and protein. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4274–4278. doi: 10.1073/pnas.78.7.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. A method for the fractionation of the high-mobility-group non-histome chromosomal proteins. Biochem Biophys Res Commun. 1977 Oct 10;78(3):1034–1042. doi: 10.1016/0006-291x(77)90525-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Hong G. F., Hill D. F., Petersen G. B. Nucleotide sequence of bacteriophage lambda DNA. J Mol Biol. 1982 Dec 25;162(4):729–773. doi: 10.1016/0022-2836(82)90546-0. [DOI] [PubMed] [Google Scholar]
- Van Tuyle G. C., Pavco P. A. Characterization of a rat liver mitochondrial DNA-protein complex. Replicative intermediates are protected against branch migrational loss. J Biol Chem. 1981 Dec 25;256(24):12772–12779. [PubMed] [Google Scholar]
- Williams K. R., LoPresti M. B., Setoguchi M., Konigsberg W. H. Amino acid sequence of the T4 DNA helix-destabilizing protein. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4614–4617. doi: 10.1073/pnas.77.8.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J. F., Ma D. P., Wilson R. K., Roe B. A. DNA sequence of the Xenopus laevis mitochondrial heavy and light strand replication origins and flanking tRNA genes. Nucleic Acids Res. 1983 Jul 25;11(14):4977–4995. doi: 10.1093/nar/11.14.4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury C. P., Jr, von Hippel P. H. On the determination of deoxyribonucleic acid-protein interaction parameters using the nitrocellulose filter-binding assay. Biochemistry. 1983 Sep 27;22(20):4730–4737. doi: 10.1021/bi00289a018. [DOI] [PubMed] [Google Scholar]



