Background: Complex II is not considered a significant contributor to mitochondrial ROS production.
Results: Complex II generates ROS in both the forward reaction, from succinate, and the reverse reaction, from the reduced ubiquinone pool.
Conclusion: Occupancy and reduction state of the flavin dictate its ROS producing behavior.
Significance: Based on the maximum rates observed, complex II may be a contributor to physiological ROS production.
Keywords: Bioenergetics/Electron Transfer Complex, Electron Transport System (ETS), Mitochondria, Reactive Oxygen Species (ROS), Superoxide Ion, atpenin A5, Malonate, Succinate Dehydrogenase, Succinate:Quinone Oxidoreductase
Abstract
Respiratory complex II oxidizes succinate to fumarate as part of the Krebs cycle and reduces ubiquinone in the electron transport chain. Previous experimental evidence suggested that complex II is not a significant contributor to the production of reactive oxygen species (ROS) in isolated mitochondria or intact cells unless mutated. However, we find that when complex I and complex III are inhibited and succinate concentration is low, complex II in rat skeletal muscle mitochondria can generate superoxide or H2O2 at high rates. These rates approach or exceed the maximum rates achieved by complex I or complex III. Complex II generates these ROS in both the forward reaction, with electrons supplied by succinate, and the reverse reaction, with electrons supplied from the reduced ubiquinone pool. ROS production in the reverse reaction is prevented by inhibition of complex II at either the ubiquinone-binding site (by atpenin A5) or the flavin (by malonate), whereas ROS production in the forward reaction is prevented by malonate but not by atpenin A5, showing that the ROS from complex II arises only from the flavin site (site IIF). We propose a mechanism for ROS production by complex II that relies upon the occupancy of the substrate oxidation site and the reduction state of the enzyme. We suggest that complex II may be an important contributor to physiological and pathological ROS production.
Introduction
Several potential sites of superoxide and H2O2 production have been identified in the Krebs cycle and the electron transport chain of mammalian mitochondria. They include the dihydrolipoate moieties of α-ketoglutarate dehydrogenase and pyruvate dehydrogenase (1–3), glycerol-3-phosphate dehydrogenase (GPDH),3 the electron transferring flavoprotein:Q oxidoreductase (ETFQOR) of fatty acid β-oxidation, the flavin site of complex I (site IF); the ubiquinone-reducing site in complex I (site IQ), and the ubiquinol-oxidizing site in complex III (site IIIQo) (4–8). Of these, site IF in complex I and site IIIQo in complex III (9, 10, 12,13) have been investigated in greatest detail because of their ability to generate superoxide at high rates when they are fully or partially reduced. Sites IIIQo, IQ, and IF have high maximum rates, and sites IF and IIIQo dominate native superoxide production during oxidation of NAD-linked substrates in vitro.4 Wild-type complex II makes little contribution to superoxide or hydrogen peroxide production by isolated mammalian mitochondria under normal incubation conditions (8, 14). However, mutations in the enzyme can lead it to generate reactive oxygen species (ROS) at high rates and cause pathology (15).
Mitochondrial complex II (succinate:quinone oxidoreductase; succinate dehydrogenase; SDH) oxidizes succinate to fumarate in the Krebs cycle and reduces ubiquinone (Q) in the respiratory chain. Complex II is ubiquitous in diverse aerobic species. It consists of four subunits: SDHA, the flavoprotein subunit, which contains FAD bound covalently in the active site; SDHB, the iron-sulfur protein subunit, which contains a chain of three iron-sulfur clusters, [2Fe-2S], [4Fe-4S], and [3Fe-4S]; and SDHC and SDHD, the two transmembrane cytochrome b heme subunits, which contain a heme moiety (16). The reaction mechanism of complex II has been explored in depth (17–20). During succinate oxidation, two electrons are transferred to the flavin at site IIF, then passed singly through the Fe-S clusters to reduce ubiquinone to ubiquinol at site IIQ. It is not a requirement for electrons to pass through the b heme because the 7.1 Å edge-to-edge distance between the [3Fe-4S] center and Q facilitates electron tunneling (16). Also, the very low midpoint potential of the heme (−185 mV) (21) implies that its steady-state reduction level will be negligible.
In Escherichia coli, fumarate reductase is a structural homologue of succinate dehydrogenase that catalyzes the reverse reaction under anaerobic conditions. E. coli and Ascaris suum fumarate reductases generate superoxide and hydrogen peroxide at high rates when run in reverse as succinate dehydrogenases in the presence of oxygen (22, 23). Succinate dehydrogenases from E. coli and Saccharomyces cerevisiae also generate superoxide, although at lower rates (24–27). The specific site of superoxide production has been proposed to be the flavin at site IIF, based on the autoxidation properties of flavins (28) and evidence from the E. coli studies (24, 29). However, site IIQ was proposed in the yeast studies (25, 27). The recent study on A. suum suggested that both sites were responsible for significant ROS production (23).
Succinate dehydrogenase purified from bovine heart has also been reported to generate superoxide under some conditions (30). Furthermore, mutations in the flavoprotein subunit of complex II lead to the progressive neurodegenerative disease Leigh syndrome (15). Germline mutations in the subunits SDHB, SDHC, and SDHD have been observed in patients with hereditary paragangliomas, a rare type of tumor that arises from neuroendocrine tissue in the head and neck (31). Mutations in the SDHC subunit of embryonic fibroblasts result in increased ROS production and enhanced tumorigenesis (32). Similarly, mutations in SDHB, SDHC, and SDHD have been shown to increase ROS production and lead to the stabilization of hypoxia-inducible factor 1α (HIF-1α) and to subsequent cell proliferation (33). Two mechanisms have been proposed for this tumor formation. First, succinate may act as a signaling molecule when it accumulates following loss-of-function mutations in succinate dehydrogenase; and second, ROS generated by mutated succinate dehydrogenase may cause oxidative stress or act as a signal. These mechanisms may not be mutually exclusive (31).
In the present study, we show that the flavin site of mammalian complex II (site IIF) can make superoxide or hydrogen peroxide at high rates in isolated mitochondria. The maximal rates are similar to those of the classic sites in complexes I and III. Based on the conditions required to generate ROS from this site, we suggest that complex II may be a significant source of ROS in vivo.
EXPERIMENTAL PROCEDURES
Animals and Mitochondria
Mitochondria were isolated from hind limb skeletal muscle of female Wistar rats aged 5–8 weeks, by differential centrifugation (34, 35). The animal protocol was approved by the Buck Institute Animal Care and Use Committee, in accordance with IACUC standards.
Superoxide and H2O2 Production by Isolated Mitochondria
Superoxide and hydrogen peroxide production rates were measured (without discriminating between them) as H2O2 production rate in the presence of endogenous and exogenous superoxide dismutase. H2O2 production rates were determined using Amplex UltraRed (Invitrogen, Carlsbad, CA) in a Cary Varian spectrofluorometer at the wavelength couple Ex = 560 nm, Em = 590 nm. Horseradish peroxidase was added to catalyze reaction of extramitochondrial H2O2 with Amplex UltraRed to form the fluorescent product resorufin. Mitochondria (0.3 mg protein/ml) were incubated at 37 °C in standard assay medium containing 120 mm KCl, 5 mm HEPES, 1 mm EGTA (pH 7.2 at 20 °C), and 0.3% (w/v) bovine serum albumin. All assays contained 50 μm Amplex UltraRed, 5 units horseradish peroxidase/ml, and 12 units superoxide dismutase/ml. Fluorescence emission data were calibrated using H2O2 standard curves obtained under identical conditions (14). Linear rates of change were measured for 30–60 s for each condition. All values are the empirical rates of H2O2 production (in nmol·min−1·mg mitochondrial protein−1), except for the data in Fig. 6, which were corrected for H2O2 consumption by matrix peroxidases as described previously using Equation 1 (13).
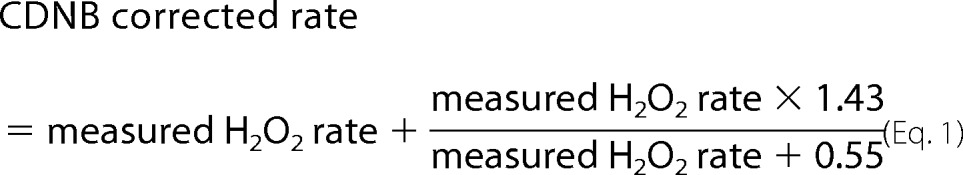 |
Superoxide released to the intermembrane space side of the inner membrane was measured directly by the acetylated cytochrome c assay (36).
FIGURE 6.

Maximum rates of superoxide/H2O2 production from different mitochondrial sites. Data were corrected for H2O2 consumption by matrix peroxidases using Equation 1. Data for the first three bars (sites IF, IQ, and IIIQo) are from footnote 4. In all cases measurements were made under conditions that we have found to maximize superoxide production rates from these sites. The final bar represents the CDNB-corrected rate of H2O2 production from site IIF (corrected data from Fig. 2). Data are means ± S.E. (n ≥ 3).
Succinate Dehydrogenase Activation State
Mitochondria (1 mg protein/ml) were incubated for 5 min with inhibitors and different succinate concentrations under conditions identical to those above, in standard assay medium (120 mm KCl, 5 mm Hepes, 1 mm EGTA) at 37 °C. To assay the activation state of succinate dehydrogenase in each condition, the mitochondria were lysed in assay medium maintained at 15 °C containing 0.1% (v/v) Triton X-100. This follows the protocol in Ref. 17 and is based on observations that below 20 °C, succinate added in the activity assay cannot compete off inhibitory oxaloacetate, and change the activation state of succinate dehydrogenase. Therefore, the activation state observed in this activity assay directly reflects the activation state during the 37 °C incubation, and during the parallel ROS assays. Succinate dehydrogenase activity was determined spectrophotometrically at 15 °C in an Olis dual beam spectrophotometer in split beam mode (λ = 600 nm, slits = 5 nm) as the rate of phenazine methosulfate (PMS) (1 mm) linked reduction of dichlorophenolindophenol (DCPIP) (50 μm) instigated by addition of 10 mm succinate. The reference cuvette contained identical components except that instead of succinate, 10 mm malonate was added. 1 mm sodium cyanide was present in all assays. Data were converted to molar units using the DCPIP extinction coefficient 21 mm−1·cm−1 (37).
Respiration Assays
Mitochondrial oxygen consumption was measured in a Clark type oxygen electrode in 120 mm KCl, 5 mm Hepes, 1 mm EGTA in the presence of either 27 mm α/β-glycerol 3-phosphate or 5 mm succinate, 4 μm rotenone, and 1 μm trifluorocarbonylcyanide phenylhydrazone (FCCP) (33). In the glycerol 3-phosphate experiments, 200 nm free Ca2+ was also present to increase affinity of glycerol-3-phosphate dehydrogenase for its substrate. Atpenin A5 was tested at the concentrations described in Fig. 3C.
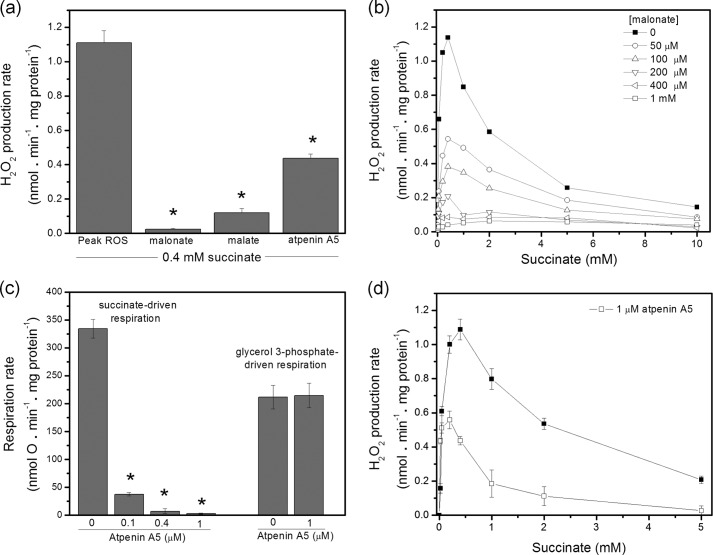
FIGURE 3.
Effect of inhibitors of complex II on H2O2 production in the presence of rotenone and myxothiazol and on respiration rate. a, rates of H2O2 production caused by addition of 400 μm succinate in the presence of 4 μm rotenone and 2 μm myxothiazol (first bar), and in the additional presence of 500 μm malonate (second bar), 1 mm malate (third bar), or 1 μm atpenin A5 (fourth bar) (n ≥ 4). Higher concentrations of each inhibitor did not have a greater inhibitory effect. b, effect of different concentrations of malonate on the titration shown in Fig. 2 (mean values shown, n = 3). c, effect of atpenin A5 on uncoupled respiration on α/β glycerol 3-phosphate (27 mm) and on succinate (5 mm). 1 μm FCCP and 4 μm rotenone were present in all experiments. d, effect of 1 μm atpenin A5 on the titration shown in Fig. 2 (n = 4). Higher concentrations of atpenin A5 did not have a greater inhibitory effect. Data are means ± S.E., except in b where only means are shown. *, significantly different from rate without inhibitor (p < 0.05). Closed symbols: uninhibited complex II, open symbols: malonate- or atpenin A5-inhibited complex II.
Data Analysis
Data are presented as mean ± S.E. Differences between substrate conditions were analyzed by t test, with p < 0.05 considered significant. Where multiple means were compared ANOVA was performed with post hoc Bonferroni used to determine significance (p < 0.05) between individual means. Normalization of the rates of H2O2 production to succinate dehydrogenase activity in Fig. 4 was performed on the mean ± S.E. for three experiments. The error was appropriately propagated to give the normalized standard error.
FIGURE 4.

Correction for the effect of atpenin A5 on the activation state of complex II. a, effect of succinate concentration and atpenin A5 on the activation state of complex II. Intact mitochondria were incubated at 37 °C for 5 min in the presence of rotenone (4 μm), myxothiazol (2 μm), and a range of succinate concentrations as indicated, in the absence (closed symbols) or presence (open symbols) of 1 μm atpenin A5. Following incubation, the mitochondria were lysed at 15 °C and the rate of PMS-linked reduction of DCPIP induced by 10 mm succinate was measured to report the activation state achieved in the prior incubation (see “Experimental Procedures”). b, data of Fig. 3d replotted after normalization to the activation state of complex II derived from a. Normalized H2O2 production = nmol H2O2·min−1·mg mitochondrial protein−1 in the initial incubation/nmol DCPIP reduced min−1·mg mitochondrial protein−1 in the subsequent activity assay. Data are means ± S.E. (n = 3); standard errors were propagated to give the normalized standard error. A Student's t test indicated that there was no significant difference between the normalized maximum rates of H2O2 production.
RESULTS
Succinate Oxidation Results in High Rates of H2O2 Production from Multiple Sites
Succinate is a useful substrate for isolated mitochondria because of its simple and rapid metabolism: it enters the mitochondrial matrix on the dicarboxylate carrier; succinate dehydrogenase oxidizes it to fumarate and passes two electrons to ubiquinone and down the electron transport chain; fumarate is converted to malate by fumarase, and malate exits on the dicarboxylate carrier in exchange for incoming succinate. The Km values of the dicarboxylate carrier and succinate dehydrogenase for succinate are around 1 mm (18, 38), so succinate is typically added at saturating concentrations (≥ 5 mm) to support rapid respiration.
In most mitochondria, succinate oxidation is kinetically and thermodynamically able to feed electrons rapidly into the Q pool and generate a high protonmotive force, making it a good substrate for superoxide or H2O2 generation. The first bar in Fig. 1a recapitulates many earlier observations that mitochondria isolated from rat skeletal muscle generate H2O2 at a high rate when incubated with 5 mm succinate in the absence of inhibitors of electron transport (39–41). Fig. 1b shows eight sites that might contribute to H2O2 production under these conditions, and the sites of action of inhibitors used in the present report to dissect out which sites run in different conditions. The second bar in Fig. 1a confirms earlier observations that addition of rotenone, an inhibitor of the Q binding site of complex I, greatly inhibits H2O2 formation, identifying the major source of H2O2 production during succinate oxidation as site IQ, driven by reverse electron transport from QH2 into complex I (13, 14, 40, 41).
FIGURE 1.
Rates of H2O2 production from multiple sites during succinate oxidation. a, rat skeletal muscle mitochondria were incubated at 37 °C in standard assay medium containing 5 mm succinate in the absence of inhibitors, and H2O2 production was measured using Amplex UltraRed (first bar). A large proportion of this signal was eliminated by the subsequent addition of the complex I inhibitor rotenone (4 μm) (second bar), showing that it arose from complex I by reverse electron transport. Subsequent addition of myxothiazol (2 μm) eliminated signal emanating from site IIIQo (third bar). Data are means ± S.E. (n ≥ 3), *, significantly different from the rate with succinate alone (p < 0.05). b, candidate sites of superoxide and H2O2 production during succinate oxidation. Succinate is oxidized to fumarate by succinate dehydrogenase, reducing the flavin of complex II (site IIF). Electrons flow to the Q-binding site of complex II (site IIQ) and into the ubiquinone pool in the mitochondrial inner membrane (QH2/Q pool). From there they can reduce complex III at the outer Q-binding site (site IIIQo) or be driven by the protonmotive force by reverse electron transport into the Q-binding site of complex I (site IQ) and on to the flavin of complex I (site IF). In principle, they may also be able to reduce glycerol-3-phosphate dehydrogenase, ETF:Q oxidoreductase (ETF:QOR), or any other enzymes that respond to QH2/Q. In addition, fumarase may convert the fumarate produced by succinate oxidation into malate, which may be converted to oxaloacetate by malate dehydrogenase. This oxaloacetate may inhibit succinate dehydrogenase. The electrons from malate dehydrogenase reduce NAD+, which can reduce sites IF and IQ and the Q pool through complex I, and in principle may be able to reduce the dihydrolipoate moieties of α-ketoglutarate dehydrogenase and pyruvate dehydrogenase. The sites of inhibition by rotenone (IF), myxothiazol (IIIQo), malonate and oxaloacetate (IIF), and atpenin A5 (IIQ) are indicated. The orange-shaded area indicates the sites that may operate when succinate or glycerol 3-phosphate are added following inhibition by rotenone and myxothiazol, which inhibit centers in the blue-shaded areas. ETF: electron transferring flavoprotein; G3P, glycerol 3-phosphate; DHAP, dihydroxyacetone phosphate.
The remaining H2O2 production in the presence of rotenone is usually attributed to site IIIQo in complex III, although a significant proportion may come from site IF following build-up of malate and NADH (42)4 during succinate oxidation. Addition of myxothiazol, an inhibitor at site IIIQo, will remove the contribution of site IIIQo (and of any other site that is downstream or depends on protonmotive force). However, the third bar in Fig. 1a shows that addition of myxothiazol did not abolish the residual H2O2 production. As discussed elsewhere,4 the smallness of the change is likely due to compensatory increases at other sites as the Q pool became reduced.
In the experiments presented below we examine the source of the H2O2 production in this condition: the presence of succinate, rotenone and myxothiazol. Fig. 1b identifies the remaining six candidate sites under these conditions: site IF (and also the dihydrolipoate moieties of α-ketoglutarate dehydrogenase and pyruvate dehydrogenase, connected through malate, malate dehydrogenase, and NADH), the flavin site (site IIF) and ubiquinone-binding site (site IIQ) of complex II, and any other redox center that is able to accept electrons from the QH2 pool, including glycerol-3-phosphate dehydrogenase, ETFQOR, and any other enzymes.
Dependence of the Observed H2O2 Production in the Presence of Rotenone and Myxothiazol on Succinate Concentration
Before establishing the sites of ROS production, we examined the characteristics of the observed H2O2 production following addition of succinate in the presence of rotenone and myxothiazol. Remarkably, we found that the observed rate of H2O2 production was greatly increased at low concentrations of succinate (Fig. 2). The peak observed rate at the optimum succinate concentration of 400 μm was 1.1 ± 0.06 nmol H2O2·min−1·mg mitochondrial protein−1. Below 400 μm succinate, rates of H2O2 production were presumably restricted by supply of electrons from succinate. Above 400 μm succinate, rates were inhibited by the presence of succinate itself, see the “Discussion.”
FIGURE 2.

Dependence of H2O2 production rate in the presence of rotenone and myxothiazol on succinate concentration. Mitochondria were incubated with rotenone (4 μm) and myxothiazol (2 μm). Succinate was then added at concentrations ranging from 25 μm to 5 mm and steady-state rates of H2O2 production were measured. Data are means ± S.E. (n = 4).
In this protocol, unlike the experiments in Fig. 1a, the inhibitors were added before succinate. Under these conditions products of succinate oxidation did not accumulate and the NAD(P)H pool was consistently maintained at less than a few percent reduced at any concentration of succinate (not shown). Since superoxide production from site IF (and other NADH-linked sites) requires significant reduction of NAD(P)H (13),4 these sites were not significant contributors to the H2O2 production observed in Fig. 2 or subsequent experiments. The remaining five candidate sites are highlighted in orange in Fig. 1b.
Effect of Inhibitors of Complex II on the Observed H2O2 Production in the Presence of Rotenone and Myxothiazol following Addition of 400 μm Succinate
Oxidation of succinate at site IIF is inhibited by substrate analogues such as malonate, oxaloacetate, and malate (17, 18, 43). Reduction of Q at site IIQ is inhibited by the drugs of the carboxin family, thenoyltrifluoroacetone (TTFA) and atpenins (44, 45). We tested the effectiveness of three site-specific inhibitors at preventing H2O2 production in the presence of rotenone and myxothiazol following addition of 400 μm succinate (Fig. 3a).
As expected, malonate fully prevented H2O2 production. To check that the apparent inhibition by malonate was not explained simply by a shift in the concentration of succinate required to give peak H2O2 production, we titrated H2O2 production with succinate at different concentrations of malonate. H2O2 production was inhibited at all succinate concentrations, with no major shift in the concentration required for peak rates (Fig. 3b). We conclude that malonate fully prevented H2O2 production by inhibiting electron supply from succinate to complex II, confirming that H2O2 production was from one or more of the five sites highlighted in orange in Fig. 1b.
We also incubated the mitochondria with 400 μm malate, which will be oxidized to oxaloacetate by malate dehydrogenase, or directly oxidized to the enol form of oxaloacetate by succinate dehydrogenase itself (43). Oxaloacetate is a potent inactivator of succinate dehydrogenase (17). The presence of malate eliminated much of the observed H2O2 production (Fig. 3a); the residual rate probably came from site IF through oxidation of NADH generated by malate dehydrogenase.
The most telling result came from the use of the site IIQ inhibitor, atpenin A5. At 1 μm, atpenin A5 fully inhibited uncoupled respiration with succinate as substrate and had no effect on uncoupled respiration with glycerol 3-phosphate as substrate (Fig. 3c), consistent with its known properties as a specific inhibitor of site IIQ (44). However, atpenin A5 did not fully inhibit H2O2 production. In the presence of 1 μm atpenin A5 about 40–50% of H2O2 production remained (Fig. 3a), showing that a substantial proportion of the observed signal was from sites upstream of the atpenin A5 inhibition site (and downstream of the malonate inhibition site), i.e. from within complex II. Fig. 3d shows a succinate titration of H2O2 production in the presence of 1 μm atpenin A5 to test whether the effect of atpenin A5 was to shift the position of the peak without inhibiting the maximum rate of H2O2 production. In the presence of atpenin A5 there was a slight left-shift in the peak, indicating that a lower succinate concentration was required to generate the peak rates. The rate of H2O2 production at the optimal succinate concentration in the presence of atpenin A5 was >50% of the rate at the peak in the absence of inhibitor, showing that >50% of the signal was from complex II. Furthermore, since atpenin A5 inhibits site IIQ, this superoxide or H2O2 must have been produced at site IIF.
Analysis of the Effect of Atpenin A5 on the Observed H2O2 Production in the Presence of Rotenone and Myxothiazol following Addition of Succinate
The observation that atpenin A5 did partially inhibit H2O2 production suggests that not all the observed H2O2 arose from site IIF of complex II, but up to 50% may have been generated by site IIQ or any of the other three downstream sites highlighted in orange in Fig. 1b. However, the oxaloacetate occupancy and succinate dehydrogenase activity of complex II are known to be very dependent upon the incubation conditions (18), so an alternative possibility is that binding of atpenin A5 at site IIQ decreased the enzymatic activity at site IIF, indirectly decreasing H2O2 production from site IIF.
To test this possibility, we measured the succinate-DCPIP oxidoreductase activity of site IIF of succinate dehydrogenase in the absence and presence of atpenin A5 (Fig. 4a). We took advantage of a unique property of the enzyme: at 15 °C, succinate added at 10 mm in the activity assay does not compete off inhibitory oxaloacetate and change the activation state of succinate dehydrogenase set up in the original 37 °C incubation at different succinate concentrations (17) (see “Experimental Procedures”). Fig. 4a indicates that higher concentrations of succinate in the 37 °C incubation led to higher activation states in the subsequent 15 °C activity assays, reflecting lower occupancy of site IIF by oxaloacetate or a succinate-mediated release of a kinetic limitation. The behavior was the same when atpenin A5 was present in the 37 °C incubation, except that the succinate dehydrogenase activity was decreased by ∼25–45% at each succinate concentration. Similar decreases in succinate dehydrogenase activity have been observed in mitochondria incubated with carboxins and TTFA (46). When the rates of H2O2 production in Fig. 3d were recalculated by normalizing to the activation state of succinate dehydrogenase in the absence and presence of atpenin A5, the peak rates of H2O2 production were not different (Fig. 4b). This result indicates that essentially none of the observed H2O2 production came from sites downstream of the atpenin A5 inhibition site, and site IIF was the only source of H2O2 production during oxidation of succinate in the presence of rotenone and myxothiazol.
H2O2 Production by Complex II during the Reverse Reaction in the Presence of Rotenone, with and without Myxothiazol, following Addition of Glycerol 3-Phosphate
Complex II is known to be fully catalytically reversible depending on the experimental conditions (47). A second test of the ability of complex II to generate superoxide or H2O2 is to reduce the Q pool by another pathway in the presence of rotenone and myxothiazol and measure whether H2O2 production during the reverse reaction is sensitive to inhibitors of complex II. Under these conditions, if the observed H2O2 is generated by any of the three sites other than complex II indicated in orange in Fig. 1b it will be insensitive to malonate and atpenin A5; if it comes from site IIQ it will be inhibited by atpenin A5 but not by malonate, and if it comes from site IIF it will be fully inhibited by both malonate and atpenin A5. Therefore, we next explored the ability of complex II to generate H2O2 with electrons flowing in the reverse direction, from QH2.
Electrons were fed into the Q pool through glycerol-3-phosphate dehydrogenase in the presence of rotenone and myxothiazol to prevent superoxide and H2O2 production from sites IQ, IF and IIIQo. Oxidation of glycerol-3-phosphate under these conditions is thought to generate superoxide or H2O2 primarily from glycerol-3-phosphate dehydrogenase (48–50). However, Fig. 5a shows that the observed rate of H2O2 production during oxidation of glycerol 3-phosphate was inhibited ∼70% by atpenin A5 (Fig. 5a). Since atpenin A5 does not inhibit respiration with glycerol 3-phosphate as substrate but is a specific inhibitor of complex II (Fig. 3c), this shows that complex II generated H2O2 at a substantial rate during the reverse reaction, when electrons entered complex II from the Q pool.
FIGURE 5.

Effects of malonate and atpenin A5 on rates of H2O2 production by mitochondria oxidizing glycerol 3-phosphate. a, α/β glycerol 3-phosphate was added at different concentrations in the presence of 4 μm rotenone, 2 μm myxothiazol, and 2 μm antimycin A. Where indicated, malonate was present at 500 μm or atpenin A5 was present at 1 μm. Data are means ± S.E. (n = 3) b, rate of H2O2 production during oxidation of 27 mm α/β glycerol 3-phosphate in the presence of 4 μm rotenone, in the absence and presence of 500 μm malonate or 1 μm atpenin A5. Neither malonate nor atpenin A5 change the rate of respiration or the reduction state of the NAD(P)H or cytochrome b pools indicating that these inhibitors do not affect substrate oxidation or the reduction state of other relevant ROS producing redox centers, as described in Ref. 12 (data not shown). Data are means ± S.E. (n = 5–6) c, normalized rates of H2O2 production in the forward reaction at 400 μm succinate are shown in the first three bars (data from Fig. 4b). 500 μm malonate or 1 μm atpenin A5 were present where indicated. The final three bars are the normalized rates of H2O2 production in the reverse reaction. Data from a at 27 mm glycerol 3-phosphate (G3P) were normalized to the activity of the enzyme incubated under identical conditions (as described for Fig. 4). The rate in the presence of 500 μm malonate was made zero by definition. Data are means ± S.E. (n = 3). *, significantly different from rate without malonate or atpenin A5 (p < 0.02).
Fig. 5a shows that malonate was as effective as atpenin A5 at inhibiting H2O2 production under these conditions. Since site IIQ was uninhibited in the presence of malonate, this result shows that ROS production by complex II during the reverse reaction was entirely from site IIF.
We have observed a similar atpenin A5 and malonate sensitivity of glycerol 3-phosphate-driven H2O2 production in rat brown adipose tissue mitochondria in the presence of rotenone and myxothiazol, suggesting that H2O2 can also be produced by complex II in mitochondria isolated from other tissues.5
The experiments above show that complex II can generate H2O2 at relatively high rates in the presence of multiple electron transport chain inhibitors and in the absence of protonmotive force. Importantly, it can also do so in the absence of such constraints. During normal, uninhibited glycerol 3-phosphate oxidation (with rotenone present to prevent reverse electron transport through complex I), a significant portion of the observed H2O2 production was sensitive to malonate and atpenin A5 (Fig. 5b). These data indicate that H2O2 production by complex II is not contingent upon extreme unphysiological constraints or the presence of complex III inhibitors.
Fig. 5c summarizes the results from Fig. 4 and Fig. 5a. During the forward reaction, H2O2 production from complex II was inhibited by malonate but not by atpenin A5. During the reverse reaction it was inhibited by both compounds. These results show that superoxide or H2O2 from complex II is produced entirely from site IIF and not from site IIQ.
Maximum Rates of H2O2 Production from Complex II
It is of note that complex II can produce superoxide or H2O2 at rates similar to those that we associate with the major mitochondrial producers, complex I and complex III. Fig. 6 shows the maximum attainable rates from sites IF, IQ, IIF, and IIIQo after correction for matrix peroxidase activity (using the CNDB correction described under “Experimental Procedures”). For this correction, we assumed that 100% of the superoxide or H2O2 from complex II was produced to the matrix. This assumption was based on observations that the rate sensitive to exogenous superoxide dismutase was small and that no superoxide was detected externally in the acetylated cytochrome c reduction assay (36) (data not shown). The maximum rate of superoxide or H2O2 production by site IIF was ∼3 times higher than the maximum rate of superoxide production from site IF. It was similar to the maximum rate from site IQ and about half of the maximum rate of superoxide production from site IIIQo.
DISCUSSION
The results presented above show that complex II in isolated mitochondria can generate superoxide or H2O2 at high rates when ubiquinone reoxidation through complex I and complex III is prevented and succinate concentration is low (Fig. 2). These rates approach or exceed the maximum rates achieved by complex I or complex III (Fig. 6). Complex II generates superoxide or H2O2 from site IIF but not site IIQ. It can do so in both the forward reaction from succinate and the reverse reaction from QH2 (Fig. 5c), even in the absence of complex III inhibitors (Fig. 5b).
Figs. 2 and 3 show that the observed rates of H2O2 production from complex II first rise to a maximum and then fall as succinate concentration is increased. The rise in H2O2 production is readily explained as the result of increasing reduction of the flavin species that reacts with oxygen to form superoxide or H2O2. There are at least three possible explanations of the fall in observed H2O2 production as succinate concentration is increased further: (a) the species that reacts with oxygen is the flavin semiquinone, which decreases in concentration as succinate concentration exceeds a threshold and over-reduces it to FADH2; (b) at higher succinate concentrations binding of succinate to the active site alters the midpoint potential of the species that reacts with oxygen, making it a poorer reductant; and (c) at higher succinate concentrations succinate occupancy of the active site hinders access of oxygen. Explanation (a) has not been tested, but is not considered likely.5 Explanation (b) is supported by evidence that the midpoint potential of the FAD in succinate dehydrogenase is negative (∼ −120 to −40 mV) at low succinate concentrations and becomes increasingly positive as the concentration of succinate increases (51). However, the complete inhibition of H2O2 production by the substrate analog malonate does not strongly support this hypothesis unless malonate can be said to change the potential of the FAD, which remains possible. Inactivation of succinate dehydrogenase by oxaloacetate is at least partially explained by the observation that oxaloacetate lowers the midpoint potential of the FAD to below that of the succinate/fumarate couple (11). In electron paramagnetic resonance experiments, the binding of either fumarate or malonate to the empty enzyme increases the observable semi-reduced flavin. In those experiments, it was assumed that the binding of substrate analogues induced a change in the protein that allowed a redistribution of electrons onto the FAD, possibly through an increase in the midpoint potential (51). However, this change in midpoint potential may not be sufficient to describe the strong inhibition of H2O2 production by substrate analogues. Explanation (c) is favored by other authors (22–24, 29). It is supported by the strong inhibition of the forward and reverse reactions by malonate (Fig. 5b), and the inhibition by malate (directly or through formation of oxaloacetate) (Fig. 3a). These hypotheses are not mutually exclusive.
A simple model for superoxide or H2O2 production by complex II is proposed in Fig. 7. In this model, superoxide is only produced when the flavin is either semi-reduced (FADH·) or fully reduced (FADH2) and the substrate binding site is empty. Electrons can reduce the flavin from the QH2 pool as shown starting bottom left, or from succinate oxidation and exit to the Q pool as shown in the central cycle clockwise from top left. This model does not differentiate between the hypotheses described above. In essence, it presents the primary conclusions of this work, complex II generates ROS when site IIF is reduced but not occupied. The model also allows for the possibility that mammalian complex II makes H2O2 by the two-electron reduction of oxygen (center). Since these experiments were performed in intact mitochondria, we cannot eliminate the possibility that some H2O2 is produced; however, it was not found to be generated in the E. coli succinate dehydrogenase (24). The authors of that study proposed that this was due to the electron accepting potential of the nearest [2Fe-2S] cluster. A comparison of enzymes with autoxidizable flavins (xanthine oxidase, aspartate oxidase, and fumarate reductase) suggested that the presence and potential of a nearby electron accepting [Fe-S] species determined whether flavin oxidation was monovalent or divalent (24). As the potential of the [2Fe-2S] in succinate dehydrogenase is ∼0 mV (16), it should provide a good acceptor for electrons from the FADH2 and limit the time it spends in contact with oxygen and minimize H2O2 production.
FIGURE 7.
Model of superoxide and H2O2 production by complex II. Ovals and stars represent the states of the flavin in site IIF. The central five states represent the normal catalytic cycle (black arrows), with succinate entering the site and being converted to fumarate, with the reduced FADH2 then reoxidized in two steps by the FeS center and ultimately the Q pool. The fully reduced flavin (FADH2) or semi-reduced flavin (FADH·) are assumed to be the relevant ROS producers (star shapes), producing H2O2 or superoxide during two-electron or one-electron oxidation, respectively (gray arrows). Electrons can enter complex II from succinate oxidation (top) and exit to the Q-pool (bottom), or enter from the Q-pool and reduce the FAD (bottom). ROS production is only possible when the site is reduced but not occupied with substrate or substrate analog (i.e. succinate (suc), fumarate (fum), oxaloacetate (oxa), malonate (mal)). When substrate or substrate analogues are in the substrate binding site, the non-ROS-producing species “FAD-suc”, “FADH2-fum”, or generically “FAD-X” or “FADH2-X” are created. Presumably, the semireduced species (FADH·) can also bind substrate or substrate analogues, but that is not represented graphically.
In summary, when complex III is inhibited and rapid reoxidation of the Q pool is prevented, mitochondrial complex II generates superoxide (or H2O2) at site IIF in both the forward and reverse reactions. It is capable of high rates of production comparable to other mitochondrial sites. We also observe significant rates of H2O2 production in the reverse reaction in the absence of complex III inhibitors. This observation suggests that complex II may be a relevant producer in vivo when multiple substrates feed electrons into the Q pool, but there is limited energetic demand (e.g. resting muscle). We also hypothesize that low substrate occupancy and relatively high reduction state may occur under other in vivo conditions such as hypoxia, and that complex II may emerge as a physiologically relevant mitochondrial ROS producer.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 AG033542, P01 AG025901, PL1 AG032118, and TL1 AG032116 and by The Ellison Medical Foundation, Grant AG-SS-2288-09.
C. L. Quinlan, J. R. Treberg, I. V. Perevoshchikova, A. L. Orr, and M. D. Brand, submitted manuscript.
A. L. Orr, C. L. Quinlan, I. V. Perevoshchikova, and M. D. Brand, submitted manuscript.
- GPDH
- glycerol-3-phosphate dehydrogenase
- DCPIP
- dichlorophenolindophenol
- ETFQOR
- electron transferring flavoprotein:Q oxidoreductase
- PMS
- phenazine methosulfate
- Q
- ubiquinone
- ROS
- reactive oxygen species
- site IF
- flavin site of complex I
- site IQ
- ubiquinone-binding site of complex I
- site IIF
- flavin site of complex II
- site IIQ
- ubiquinone-binding site of complex II
- site IIIQo
- outer ubiquinone-binding site of complex III
- TTFA
- thenoyltrifluoroacetone.
REFERENCES
- 1. Bunik V. I., Sievers C. (2002) Inactivation of the 2-oxo acid dehydrogenase complexes upon generation of intrinsic radical species. Eur. J. Biochem. 269, 5004–5015 [DOI] [PubMed] [Google Scholar]
- 2. Tretter L., Adam-Vizi V. (2004) Generation of reactive oxygen species in the reaction catalyzed by α-ketoglutarate dehydrogenase. J. Neurosci. 24, 7771–7778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grivennikova V. G., Kareyeva A. V., Vinogradov A. D. (2010) What are the sources of hydrogen peroxide production by heart mitochondria? Biochim. Biophys. Acta 1797, 939–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adam-Vizi V. (2005) Production of reactive oxygen species in brain mitochondria: contribution by electron transport chain and non-electron transport chain sources. Antioxid. Redox. Signal. 7, 1140–1149 [DOI] [PubMed] [Google Scholar]
- 5. Starkov A. A., Fiskum G. (2003) Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J. Neurochem. 86, 1101–1107 [DOI] [PubMed] [Google Scholar]
- 6. Murphy M. P. (2009) How mitochondria produce reactive oxygen species. Biochem. J. 417, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brand M. D. (2010) The sites and topology of mitochondrial superoxide production. Exp. Gerontol. 45, 466–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Quinlan C. L., Treberg J. R., Brand M. D. (2011) Mechanisms of free radical production and their relationship to the aging process, The Handbook of the Biology of Aging, pp. 45–59, Academic Press [Google Scholar]
- 9. Dröse S., Brandt U. (2008) The mechanism of mitochondrial superoxide production by the cytochrome bc1 complex. J. Biol. Chem. 283, 21649–21654 [DOI] [PubMed] [Google Scholar]
- 10. Kussmaul L., Hirst J. (2006) The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc. Natl. Acad. Sci. U.S.A. 103, 7607–7612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ackrell B. A., Kearney E. B., Edmondson D. (1975) Mechanism of the reductive activation of succinate dehydrogenase. J. Biol. Chem. 250, 7114–7119 [PubMed] [Google Scholar]
- 12. Quinlan C. L., Gerencser A. A., Treberg J. R., Brand M. D. (2011) The mechanism of superoxide production by the antimycin-inhibited mitochondrial Q-cycle. J. Biol. Chem. 286, 31361–31372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Treberg J. R., Quinlan C. L., Brand M. D. (2010) Hydrogen peroxide efflux from muscle mitochondria underestimates matrix superoxide production - a correction using glutathione depletion. FEBS J. 277, 2766–2778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. St-Pierre J., Buckingham J. A., Roebuck S. J., Brand M. D. (2002) Topology of superoxide production from different sites in the mitochondrial electron transport chain. J. Biol. Chem. 277, 44784–44790 [DOI] [PubMed] [Google Scholar]
- 15. Ackrell B. A. (2002) Cytopathies involving mitochondrial complex II. Mol. Aspects Med., 23, 369–384 [DOI] [PubMed] [Google Scholar]
- 16. Sun F., Huo X., Zhai Y., Wang A., Xu J., Su D., Bartlam M., Rao Z. (2005) Crystal structure of mitochondrial respiratory membrane protein complex II. Cell 121, 1043–1057 [DOI] [PubMed] [Google Scholar]
- 17. Gutman M., Kearney E. B., Singer T. P. (1971) Control of succinate dehydrogenase in mitochondria. Biochemistry 10, 4763–4770 [DOI] [PubMed] [Google Scholar]
- 18. Ackrell B. A., Kearney E. B., Singer T. P. (1978) Mammalian succinate dehydrogenase. Methods Enzymol. 53, 466–483 [DOI] [PubMed] [Google Scholar]
- 19. Merli A., Capaldi R. A., Ackrell B. A., Kearney E. B. (1979) Arrangement of complex II (succinate-ubiquinone reductase) in the mitochondrial inner membrane. Biochemistry 18, 1393–1400 [DOI] [PubMed] [Google Scholar]
- 20. Cecchini G. (2003) Function and structure of complex II of the respiratory chain. Annu. Rev. Biochem. 72, 77–109 [DOI] [PubMed] [Google Scholar]
- 21. Yu L., Xu J. X., Haley P. E., Yu C. A. (1987) Properties of bovine heart mitochondrial cytochrome b560. J. Biol. Chem. 262, 1137–1143 [PubMed] [Google Scholar]
- 22. Imlay J. A. (1995) A metabolic enzyme that rapidly produces superoxide, fumarate reductase of Escherichia coli. J. Biol. Chem. 270, 19767–19777 [PubMed] [Google Scholar]
- 23. Paranagama M. P., Sakamoto K., Amino H., Awano M., Miyoshi H., Kita K. (2010) Contribution of the FAD and quinone binding sites to the production of reactive oxygen species from Ascaris suum mitochondrial complex II. Mitochondrion 10, 158–165 [DOI] [PubMed] [Google Scholar]
- 24. Messner K. R., Imlay J. A. (2002) Mechanism of superoxide and hydrogen peroxide formation by fumarate reductase, succinate dehydrogenase, and aspartate oxidase. J. Biol. Chem. 277, 42563–42571 [DOI] [PubMed] [Google Scholar]
- 25. Guo J., Lemire B. D. (2003) The ubiquinone-binding site of the Saccharomyces cerevisiae succinate-ubiquinone oxidoreductase is a source of superoxide. J. Biol. Chem. 278, 47629–47635 [DOI] [PubMed] [Google Scholar]
- 26. Zhao Z., Rothery R. A., Weiner J. H. (2006) Effects of site-directed mutations in Escherichia coli succinate dehydrogenase on the enzyme activity and production of superoxide radicals. Biochem. Cell Biol. 84, 1013–1021 [DOI] [PubMed] [Google Scholar]
- 27. Szeto S. S., Reinke S. N., Sykes B. D., Lemire B. D. (2007) Ubiquinone-binding site mutations in the Saccharomyces cerevisiae succinate dehydrogenase generate superoxide and lead to the accumulation of succinate. J. Biol. Chem. 282, 27518–27526 [DOI] [PubMed] [Google Scholar]
- 28. Messner K. R., Imlay J. A. (1999) The identification of primary sites of superoxide and hydrogen peroxide formation in the aerobic respiratory chain and sulfite reductase complex of Escherichia coli. J. Biol. Chem. 274, 10119–10128 [DOI] [PubMed] [Google Scholar]
- 29. Yankovskaya V., Horsefield R., Törnroth S., Luna-Chavez C., Miyoshi H., Léger C., Byrne B., Cecchini G., Iwata S. (2003) Architecture of succinate dehydrogenase and reactive oxygen species generation. Science 299, 700–704 [DOI] [PubMed] [Google Scholar]
- 30. Zhang L., Yu L., Yu C. A. (1998) Generation of superoxide anion by succinate-cytochrome c reductase from bovine heart mitochondria. J. Biol. Chem. 273, 33972–33976 [DOI] [PubMed] [Google Scholar]
- 31. Bardella C., Pollard P. J., Tomlinson I. (2011) SDH mutations in cancer. Biochim. Biophys. Acta 1807, 1432–1443 [DOI] [PubMed] [Google Scholar]
- 32. Ishii T., Yasuda K., Akatsuka A., Hino O., Hartman P. S., Ishii N. (2005) A mutation in the SDHC gene of complex II increases oxidative stress, resulting in apoptosis and tumorigenesis. Cancer Res. 65, 203–209 [PubMed] [Google Scholar]
- 33. Guzy R. D., Sharma B., Bell E., Chandel N. S., Schumacker P. T. (2008) Loss of the SdhB, but not the SdhA, subunit of complex II triggers reactive oxygen species-dependent hypoxia-inducible factor activation and tumorigenesis. Mol. Cell Biol. 28, 718–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Affourtit C., Quinlan C. L., Brand M. D. (2012) Measurement of proton leak and electron leak in isolated mitochondria. Methods Mol. Biol. 810, 165–182 [DOI] [PubMed] [Google Scholar]
- 35. Chappell J. B., Perry S. V. (1954) Biochemical and osmotic properties of skeletal muscle mitochondria. Nature 173, 1094–1095 [DOI] [PubMed] [Google Scholar]
- 36. Azzi A., Montecucco C., Richter C. (1975) The use of acetylated ferricytochrome c for the detection of superoxide radicals produced in biological membranes. Biochem. Biophys. Res. Commun. 65, 597–603 [DOI] [PubMed] [Google Scholar]
- 37. Hatefi Y., Stiggall D. L. (1978) Preparation and properties of succinate: ubiquinone oxidoreductase (complex II). Methods Enzymol. 53, 21–27 [DOI] [PubMed] [Google Scholar]
- 38. Palmieri F., Prezioso G., Quagliariello E., Klingenberg M. (1971) Kinetic study of the dicarboxylate carrier in rat liver mitochondria. Eur. J. Biochem. 22, 66–74 [DOI] [PubMed] [Google Scholar]
- 39. Boveris A., Oshino N., Chance B. (1972) The cellular production of hydrogen peroxide. Biochem. J. 128, 617–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hansford R. G., Hogue B. A., Mildaziene V. (1997) Dependence of H2O2 formation by rat heart mitochondria on substrate availability and donor age. J. Bioenerg. Biomembr. 29, 89–95 [DOI] [PubMed] [Google Scholar]
- 41. Lambert A. J., Brand M. D. (2004) Superoxide production by NADH:ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane. Biochem. J. 382, 511–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Treberg J. R., Quinlan C. L., Brand M. D. (2011) Evidence for two sites of superoxide production by mitochondrial NADH-ubiquinone oxidoreductase (complex I). J. Biol. Chem. 286, 27103–27110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Belikova Y. O., Kotlyar A. B., Vinogradov A. D. (1988) Oxidation of malate by the mitochondrial succinate-ubiquinone reductase. Biochim. Biophys. Acta, 936, 1–9 [DOI] [PubMed] [Google Scholar]
- 44. Miyadera H., Shiomi K., Ui H., Yamaguchi Y., Masuma R., Tomoda H., Miyoshi H., Osanai A., Kita K., Omura S. (2003) Atpenins, potent and specific inhibitors of mitochondrial complex II (succinate-ubiquinone oxidoreductase). Proc. Natl. Acad. Sci. U.S.A. 100, 473–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ramsay R. R., Ackrell B. A., Coles C. J., Singer T. P., White G. A., Thorn G. D. (1981) Reaction site of carboxanilides and of thenoyltrifluoroacetone in complex II. Proc. Natl. Acad. Sci. U.S.A. 78, 825–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mowery P. C., Steenkamp D. J., Ackrell A. C., Singer T. P., White G. A. (1977) Inhibition of mammalian succinate dehydrogenase by carboxins. Arch. Biochem. Biophys. 178, 495–506 [DOI] [PubMed] [Google Scholar]
- 47. Hirst J., Sucheta A., Ackrell B. A., Armstrong F. A. (1996) Electrocatalytic voltammetry of succinate dehydrogenase: direct quantification of the catalytic properties of a complex electron-transport enzyme. J. Am. Chem. Soc. 118, 5031–5038 [Google Scholar]
- 48. Drahota Z., Chowdhury S. K., Floryk D., Mrácek T., Wilhelm J., Rauchová H., Lenaz G., Houstek J. (2002) Glycerophosphate-dependent hydrogen peroxide production by brown adipose tissue mitochondria and its activation by ferricyanide. J. Bioenerg. Biomembr. 34, 105–113 [DOI] [PubMed] [Google Scholar]
- 49. Miwa S., St-Pierre J., Partridge L., Brand M. D. (2003) Superoxide and hydrogen peroxide production by Drosophila mitochondria. Free Radic. Biol. Med. 35, 938–948 [DOI] [PubMed] [Google Scholar]
- 50. Miwa S., Brand M. D. (2005) The topology of superoxide production by complex III and glycerol-3-phosphate dehydrogenase in Drosophila mitochondria. Biochim. Biophys. Acta 1709, 214–219 [DOI] [PubMed] [Google Scholar]
- 51. Bonomi F., Pagani S., Cerletti P., Giori C. (1983) Modification of the thermodynamic properties of the electron-transferring groups in mitochondrial succinate dehydrogenase upon binding of succinate. Eur. J. Biochem. 134, 439–445 [DOI] [PubMed] [Google Scholar]