Abstract
This paper describes the current status of percutaneous coronary intervention (PCI) for totally occluded coronary arteries. Chronic total occlusion is associated with 10%–20% of all PCI procedures. Results show that opening an occluded vessel, especially one supplying a considerable area of myocardium, may be beneficial for a patient’s angina relief and heart function. We describe the devices used currently in re-canalization such as new wires, microcatheters (including Tonus and Cosair) and intravascular ultrasound guidance. Different techniques to improve the success rate and reduce complications are discussed in detail.
Keywords: Percutaneous coronary intervention, Chronic total occlusion, Retrograde approach
1. Introduction
According to the consensus from the EuroCTO Club, the definition of a chronic total occlusion (CTO) is the presence of thrombolysis in myocardial infarction (TIMI) 0 flow within the occluded segment with an estimated occlusion duration of ≥3 months (Stone et al., 2005a; 2005b). It is the reason for 10%–20% of all percutaneous coronary intervention (PCI) procedures and poses a management dilemma for the interventional cardiologist. Although remarkable progress in PCI has been achieved over the last decade, it is often stated that successful recanalization of CTOs represents the “last frontier” of PCI. CTOs represent the most technically challenging lesion subset that interventional cardiologists face, with procedural success rates considerably lower than those achieved with non-occluded coronary vessels or acutely occluded arteries.
The clinical presentation of a CTO can be very variable. Most patients present with stable angina, a change in anginal status, silent ischaemia or heart failure of ischaemic origin rather than acute myocardial infarction (MI). Well-developed collaterals may provide flow equivalent to a 90%–95% stenosis, which helps maintain myocardial viability and prevents resting myocardial ischemia (Kinoshita et al., 1995). Overall contractile function may be normal, or a regional wall-motion abnormality may be present due to hibernating myocardium or non-Q-wave MI. However, we also see patients with new-onset angina or undergoing primary PCI due to acute occlusion in a different culprit vessel, and in whom the CTO is discovered incidentally. Stress-induced ischemia measured by single-photon emission CT (SPECT) can typically be elicited in asymptomatic patients with CTO, especially in the absence of a history of prior MI.
Pathologically, the major constituent of a CTO is fibrocalcific plaque. The concentration of collagen-rich fibrous tissue is particularly dense at the proximal and distal ends of the lesion, contributing to a column-like lesion of calcified, resistant fibrous tissue surrounding a softer core of organized thrombus and lipids. The density of the proximal fibrous cap is higher than that of the distal cap. These obstructions are thus more likely to deflect guidewires into the subintimal area, creating dissection planes. Hard plaques are more prevalent with increasing CTO age (>1 year old). The extent and severity of calcification increase with occlusion duration. The age-related increase in the calcium and collagen content of CTOs in part underlies the progressive difficulty in crossing older occlusions during PCI. Another hallmark of CTOs is extensive neovascularization. Capillary density and angiogenesis increase with increasing occlusion age (Kinoshita et al., 1995).
Although collaterals maintain myocardial viability under resting conditions, they often fail to provide sufficient blood flow during periods of increased oxygen demand, resulting in lifestyle-limited angina. Successful revascularization improves anginal status, increases exercise capacity, improves left ventricular function, and reduces the need for late bypass surgery. In addition, there are data from large retrospective studies showing that patients with a successful recanalisation of a CTO have a lower mortality and need for coronary artery bypass graft (CABG) treatment than patients with unsuccessful procedures (Stone et al., 2005c). Patients with untreated CTOs face a threefold increase in cardiac mortality or complications in the case of future acute events.
However, PCI of CTOs is associated with lower success rates, higher equipment costs, increased radiation exposure for both patients and doctors, and more restenosis compared to PCI of non-total occlusions. Consideration of the best treatment modalities for CTOs should include a careful review of clinical history, the results of sensitive provocative tests, coronary anatomy, and personal experience. The indications of PCI for CTOs include (di Mario et al., 2007): medically refractory angina, a large area of ischemia shown by non-invasive studies, and favorable angiographic appearance. Many CTOs are present for which PCI is never attempted, representing one of the most common causes of referral for bypass surgery rather than PCI. The decision to attempt PCI of a CTO (versus continued medical therapy or surgical revascularization) requires an individualized risk/benefit analysis encompassing clinical, angiographic, and technical considerations. Clinically, the patient’s age, symptom severity, associated comorbidities (e.g., diabetes mellitus or chronic renal insufficiency), and overall functional status are major determinants of treatment strategy. Angiographically, the extent and complexity of coronary artery disease (e.g., single-vessel versus multivessel disease, single versus multiple total occlusions, likelihood for complete revascularization), left ventricular function, and the presence and degree of valvular heart disease should be considered. The technical probability of achieving successful recanalization of the PCI without complications must also be strongly factored into the decision-making process. With average recanalisation success rates of >70% in experienced hands using contemporary CTO techniques, the presence of a CTO should not be sufficient reason to switch from a percutaneous towards a surgical approach in multivessel disease (Stone et al., 2005b).
In general, when the CTO represents the only significant lesion in the coronary tree, PCI is warranted when the following three conditions are all present: (1) the occluded vessel is responsible for the patient’s symptoms (PCI may also be considered in selected cases of silent ischemia if it can be demonstrated that a large myocardial territory is at risk); (2) the myocardial territory supplanted by the occluded artery is viable; and (3) the likelihood of success is moderate to high (>60%), with an expected major complication rate of death of <1% and myocardial infarction of <5%. In patients with multivessel disease and one or more CTOs, the relative risks and benefits of bypass surgery compared with interventional management should be considered. The presence of any of the following may favor surgical revascularization: (1) left main artery disease; (2) complex triple-vessel disease, especially in patients with insulin-requiring diabetes, severe left ventricular dysfunction, or chronic renal insufficiency; (3) an occluded proximal left anterior descending artery (supplying a viable anterior wall), which is not favorable for PCI; and (4) multiple CTOs with a relatively low expected success rate (Stone et al., 2005b).
Compared with non-total occlusions, revascularization rates for CTOs remain disappointingly low. The overall success rate since the 1990s in different centers is about 70% (range 48%–92%). The most common reasons for procedural failure include the inability to cross the occlusion with a guidewire (80%), failure to cross the occlusion with a balloon (15%), and failure to dilate the stenosis (5%). Case selection remains one of the most important predictors of PCI success. Depending on the presence of clinical and angiographic variables, recanalization rates range from 18% to 87% (Freed and Safian, 2001):
Complete vs. functional occlusion. Functional occlusions (99% stenosis with delayed incomplete opacification of the distal vessel segment) are more often recanalized than complete occlusions (76% vs. 67%). It is essential to differentiate the true lumen of a functional occlusion from the perivascular channel of a bridging collateral; the former is a predictor of PCI success, while the latter predicts PCI failure. This distinction can usually be made by obtaining multiple angiographic projections of the occlusion.
Duration of occlusion. The duration of occlusion is estimated as the time interval between a major ischemic event (MI, new onset angina, abrupt worsening in anginal status) and PCI. Successful revascularization is highest for occlusions of <1 week, intermediate for occlusions of 2–12 weeks, and lowest for occlusions of >3 months. Occlusion duration alone should not preclude revascularization since procedural success for occlusions of >6 months may be as high as 50%–75%, and even higher in recent years.
Length of occlusion. The length of an occlusion sometimes cannot be seen and may be difficult to estimate. Contralateral angiography and multi-slice computed tomography (MSCT) are helpful for estimating the length. While it is generally felt that occlusion lengths of >15 mm are associated with lower success rates, this characteristic alone should not preclude PCI.
Side branch at point of occlusion. This occlusion characteristic is associated with reduced success due to the tendency of the guidewire to pass into the side branch.
Presence of a tapered stump. Funnel-shaped or tapered occlusions are associated with higher recanalization rates than occlusions with abrupt cutoffs. The success rate is 73%–88% vs. 60%. Tapered occlusions frequently contain small recanalized channels that escape detection by angiography but provide a potential route for successful guidewire passage.
Intracoronary bridging collaterals. Most angioplasty series suggest that the presence of bridging collaterals is the important determinant of failed CTO angioplasty. When faint bridging collaterals coexist with one or more favorable characteristics (e.g., tapered stump, short segment of occlusion), success rates may exceed 50%. However, occlusions that are associated with extensive bridging collaterals (“caput medusa”) are generally considered unsuitable for PCI due to extremely low success rates (<20%).
Other factors. Other factors variably associated with lower success rates include lesion calcification, proximal vessel tortuosity, distal location, right coronary or circumflex occlusion, diffuse proximal disease, multivessel disease, and unstable angina.
A predominance of favorable characteristics can lead highly experienced CTO-operators to a success rate of >90% while this may drop to <60%–70% in the presence of one or more unfavorable predictive factors.
2. Equipment selection
PCI of CTO lesions is associated with more procedural time, more radiation exposure time for the patient and operator, more use of contrast, and increased cost due to use of more guidewire, guiding catheters, balloons, and other equipments (such as Tornus or Corsair). The difficulty in treating CTO lesions is reflected by the success rate of PCI which is still lower for CTO PCI than for subtotal occlusions (70% vs. 98% respectively). This common and complex coronary lesion is the strongest predictor for patients referred for coronary artery bypass surgery. Improvement in equipment has improved the success rate and reduced complications, particularly with the emergence of Tornus and Corsair catheters and the availability of hydrophilic guidewires.
2.1. Guiding catheter
It is essential to obtain better back-up and coaxial alignment with a suitable guiding catheter. For CTO lesions in the left native coronary artery, a left Amplatz, XB, EBU or geometric guiding catheter will provide excellent support. For right native CTO lesions with superior take-off, a left Amplatz, El Gamal, Hockey Stick, IMA or geometric guiding catheter may be suitable. For right native CTOs with marked inferior take-off, a multipurpose or Amplatz catheter is better (Abhaichand et al., 2001). A JR4, Hockey Stick or right Amplatz catheter is more suitable for the horizontal take-off right coronary native CTO. If a Judkins or multipurpose catheter is selected, the “deep-seating” maneuver can be used to provide better support. If deep-seating is chosen first for a right coronary native CTO, a Judkins catheter with a side-hole sometimes is necessary. Another catheter is needed for bilateral angiography to show the collateral vessels of the occluded artery to facilitate recanalization (Takahashi et al., 2004).
If a CTO lesion is in a distal segment of a coronary artery and no stenosis is found in proximal segments, operators prefer 5 F (1 F≈0.33 mm) in 6 F guiding catheters to provide extra backup (Nakamura et al., 2006). The Terumo “five in six” system involves insertion of an extra length, 5 F Terumo guide catheter (Heartrail, Terumo) into a standard 6 F (or 7 F) guide catheter, such that the tip protrudes beyond the 6 F guide. It includes a 5 F guiding catheter and a 6 F or 7 F guiding catheter. If a 6 F catheter is selected, its inner diameter must be no less than 0.071″. The 5 F guiding catheter is 120 cm long, 20 cm longer than a 6 F or 7 F guiding catheter. The tip of the catheter is flexible so as not to damage a coronary artery. The support is related to the distance by which the 5 F extends beyond the 6 F guiding catheter.
2.2. Guidewires
2.2.1. Classification of guidewires for CTO PCI
Commonly, the classfication of guidewires for CTO PCI is based on coatings, tip shape, and tip stiffness: (1) Hydrophilic coating guidewire, polymer coating guidewire, and non-coating guidewire. Hydrophilic coating guidewire crosses CTO lesions easily if there is a micro channel and improves the success rate. However, hydrophilic guidewire has the shortcomings of low sensation reflection and a tendency to move easily into a false lumen. (2) Conventional tip design guidewire and tapered tip guidewire. Tapered tip guidewires have more penetrating ability and show improved success rates. (3) Soft, intermediate, and super stiffness guidewires, according to the tip stiffness.
2.2.2. Guidewires chosen for CTO PCI
For occlusion time of <6 months, we prefer intermediate guidewires such as the ACS intermediate, Miracle 3, Crosswire NT, or CrossIT XT 100. For occlusion time longer than 6 months, if the lesion tip is like a cone, we prefer ACS standard, Crosswire NT, Pilot 150 or 200, or Miracle 3 to 4.5 guidewires. If the lesion is hard and without a cone, we prefer super stiff guidewires such as Miracle 6 or 12, or tapered guidewires such as Cross-IT XT 200-400, or Conquest serial guidewires. If side branches occur before the occluded lesion, we prefer Miracle serial guidewires or Cross-IT serial guidewires. If the occluded segment is long and tortuous, Miracle serial guidewires are recommended. If the occluded segment is short and hard, Conquest serial guidewires may be of first choice.
2.2.3. Tip shaping of guidewires for CTO PCI
The correct tip shaping is a key factor for ensuring successful CTO PCI. During CTO lesion formation, 200–300 μm micro channels are formed. Therefore, tip shapes cannot be large. Tip shapes for CTO PCI can be divided into four types: (1) A tip angle of 45° with a curve length of 1.5–2.0 mm or less. This type is frequently used in clinics. (2) When the wire runs through the false lumen and needs to be directed into the true lumen, we can make a second curve at the basis of first type with a smaller angle (15°–30°) and longer curve (4–5 mm). (3) If the lesion is hard or Miracle or Conquest serial wires are used, the tip curve should be less than 45°. (4) When the occlusion occurs at a bifurcation, the tip curve should be 75° or two curves should be used according to the anatomy of the coronary artery.
Here we emphasize that if the proximal segment of the occlusion site is severely tortuous and super stiff wire cannot reach the lesion, quick rotation of wire should be avoided in case of causing coronary artery injury. In this circumstance, we prefer to use a soft wire with a microcatheter to reach the lesion site first, and then to change to stiff wires.
2.3. Microcatheters
A microcatheter used in CTO PCI should have a low outer diameter and have little influence on wire maneuverability.
2.3.1. Progreat microcatheter
Two types of Progreat microcatheters are used for CTO PCI, Progreat 2.0 F and Progreat 2.2 F. The diameter of the catheter decreases gradually and has hydrophilic coating on the outside within 60 cm of the proximal segment. The tip of the catheter is soft and is better able to maintain tip shape than that of Transit or Excelsior microcatheters. The impedance of the wire in the catheter is higher than that in the Rapid Transit catheter but lower than that in the Excelsior catheter.
2.3.2. Finecross microcatheter
The properties of the Finecross microcatheter make it one of the best at present. Its tip has a tapered shape. The diameter of the Finecross catheter decreases from 2.6 F at the bottom to 1.8 F at the tip with hydrophilic coating outside and polytetrafluoroethylene (PTFE) coating inside. It has the best ability to cross the CTO lesion and the lowest impedance of the wire.
2.3.3. Ichiba-Yari microcatheter
Ichiba-Yari includes three types of microcatheter: 380-1T, 380-2T, and 380-3T. Of those, the 380-3T has the smallest tip diameter (0.017″) and has better flexibility and traceability. It is used mainly for CTO PCI, especially for retrograde wire techniques.
With the use of this microcatheter, we can control the wire direction easily, adjust the stiffness of wire tip, and change different types of wire for selective angiography. However, the EuroCTO club does not recommend its use in angiography for identifying the true lumen. Note that the wire tip stiffness is related to the distance the tip extends beyond the end hole of the microcatheter.
2.4. Balloon dilation catheters
Although a variety of low profile and monorail balloons are available, an over-the-wire (OTW) balloon is preferred for CTO PCI. It has the advantages of increasing guiding catheter support, making it easy to change wires, increasing wire tip stiffness, and enhancing traceability.
Note that wire tip stiffness varies according to the distance that the wire extends beyond the end hole to the balloon or microcatheter. A 3-g wire can perform similar to a 12-g wire if it is used less than 5 mm from the end hole of the balloon. A 12-g wire will perform with resistance of 30 to 40 g if used within 5 mm of the end hole of a balloon or microcatheter.
2.5. Management of CTO lesion failure of balloon cross-over
The most common reason for failure of CTO PCI is failure of the wire to cross over the lesion. However, about 2%–10% of these failures occur because of failure of the balloon to pass through. The reason is related to fibrocalculous of the CTO lesion or lack of enough back up from the guiding catheter.
There are several methods to deal with this situation, including (1) changing the guiding catheter to provide better support, (2) deep sitting of the guiding catheter, (3) using a 5 F in 6 F catheter or 5 F in 7 F catheter, (4) buddy wire technique (implanting another wire into the CTO vessel), (5) anchoring technique, (6) multi-wire plaque crushing technique (the final succeeding wire is kept in the distal true lumen of the CTO lesion, then another wire (the crushing wire) is slowly inserted and manipulated to cross the occluded segment alongside the original wire. The crushing wire is usually supported by a monorail balloon catheter or microcatheter, and is used to crush the plaque and make the channel inside the occluded segment large enough to allow a balloon catheter to pass. A balloon catheter with a lower profile is then pushed while the other wire is withdrawn to facilitate passage of the balloon by counter-movement between the wire and the balloon), (7) high rotational atherectomy, (8) Tornus or Corsair catheters.
2.5.1. Tornus
The Tornus (Asahi Intecc, Co., Ltd., Japan) is a novel, OTW, very flexible, tapered metallic exchange catheter designed for complex and tight coronary lesions (Kirtane and Stone, 2007; Ge et al., 2008a). It is 135 cm long and is available either as a 2.6 F or a more flexible 2.1 F version. The catheter shaft is a coreless stainless steel coil that consists of eight stranded wires. To cross through the CTO lesion, it should be rotated counter-clockwise, manually. To pull out the Tornus, rotate it in a clockwise direction. To prevent the wire rotating when using Tornus, torquer can be used to fix the wire. It is recommended to release the torque energy after 20 rotations for the 2.6 F and 40 rotations for the 2.1 F catheter to avoid the risk of wire fractures that may cause severe problems. However, when retrieving Tornus, the Nato method cannot be used. Two other methods can be used: the first uses extension wire and the second uses balloon dilation to fix the wire so that Tornus can be retrieved. After penetration and passage of the lesion, the Tornus may serve as an exchange catheter. Through the mechanical effect of the Tornus, a smooth passage is created for a low profile balloon to pass by.
2.5.2. High frequency rotational atherectomy
This strategy should be considered early, especially in the treatment of older occlusions and severely calcified lesions.
2.5.3. Corsair
Asahi’s Corsair catheter is a dedicated CTO device. There are two types of Corsair now available. One is 135 cm long, and is used mainly for antegrade intervention. The other is 150 cm long, and is used mainly for retrograde intervention. The Corsair is a hybrid channel dilator that combines the characteristics of the Tornus device and a microcatheter. It has 5 mm soft tape with tungsten powder, and a 0.8 mm platinum marker. Corsair consists of eight thin wires (0.07 mm) wound with two larger wires (0.12 mm) from the tape to 20 cm. It has a tapered soft tip from 0.87 mm to 0.42 mm, and a hydrophilic polymer coating from the tip to 60 cm. The minimum guiding catheter compatible with the catheter is 5 F.
The manipulation of the Corsair catheter as a channel dilator is similar to that of the Tornus catheter, i.e., simultaneous counter-clockwise rotation and forward motion of the catheter. The torque device should be secured onto the guidewire and held at all times during rotation of the catheter to avoid simultaneous rotation of the guidewire. The dilation achieved by the Corsair channel dilator is similar to that obtained with a 1.25 mm diameter balloon catheter. Once the Corsair catheter crosses the septal channel, it can be used as a microcatheter or support catheter for wire exchange or manipulation. It can also be used for contrast injection through the catheter. This is useful for confirmation that the wire is in the true lumen after lesion crossing in CTO, as well as for the selection of appropriate septal channels for a retrograde approach. The wire must always be advanced ahead of the Corsair during manipulation. The Corsair tip must be constantly checked using fluoroscopy to avoid the tip becoming trapped by the lesion. Unlike the Tornus, the Corsair can be removed following the same steps as for a microcatheter by the Nato method.
2.6. Development of interventional instruments
Although the success rate of CTO PCI has improved with developments in interventional instruments and operator experience, there are still 10%–25% CTO lesion failures from PCI. Doctors and companies are still making progress in developing new instruments for CTO PCI. Two have recently been approved by the U.S. Food and Drug Administration (FDA) for sale and usage.
2.6.1. Optical coherence reflectometry (OCR) Safe-Cross RF system
The Safe-Cross RF system (intraLuminal Therapeutics, Inc., Carlsbad, CA) controls the forward movement of the wire using OCR in CTO lesions. The wire used in this system is an intermediate guidewire. Its tip can emit a radio frequency causing formation of channels in the CTO lesion facilitating the passage of interventional instruments. The tip also can emit a near infrared ray. If the tip is close to the wall (less than 1 mm), the monitor will turn red and show a warning. The radio frequency will not be transmitted. The operator can adjust the tip and if the direction is correct, the monitor will return to green. The GREAT study showed that the success rate was 55.7%. The coronary perforation rate related to use of the instrument was 0.9%.
2.6.2. FrontRunner system
The FrontRunner system (Lumend, Inc., Redwood city, CA) uses controlled, blunt micro-dissection to gently separate atherosclerotic plaque, creating a passage through the CTO. It has a distal tip size of 0.039″. The success rate of the X-39 model now available is 50.0%–61.7% in CTO PCI. The perforation rate was from 0.9% to 1.9%. It is better suited to treating stent CTO lesions than tortuous right coronary artery CTO lesions.
3. Antegrade wire techniques in PCI of CTO
3.1. Selection of guidewires
Up to 90% of aborted attempts to open a CTO are due to inability to pass the wire. Thus, guidewire selection is a critical feature of CTO PCI. Recently, some physicians have recommended the use of a special guidewire combining a hydrophilic tip and a slightly stiffer body to improve tracking in the CTO lesion. The CTO guidewire itself has to penetrate the proximal edge to advance into the core of the CTO lesion, avoiding subintimal tracking, and finally has to be localized in the distal true lumen. The guidewire should have “stiffness, steerability and slipperiness” inside the CTO lesion. However, as there is no perfect CTO guidewire, it is most important to select an appropriate wire for each CTO condition (Nakamura and Bae, 2008).
Hydrophilic wires are rarely used in cases of CTO intervention as these wires can penetrate easily into the subintimal space without tactile feedback. The only exception is a hydrophilic soft wire such as the Whisper and Fielder wire in cases of CTO lesions with a straight micro-channel (functional total occlusion). Recently, some physicians have recommended the use of hydrophilic wires to trace the very small channel inside the CTOs. Non-coated coil wires tend to advance straight with more resistance inside the CTO (especially the Miracle Brothers and Conquest guidewires) and have exceptional torque response even within a fibro-calcified CTO. A non-lubricated wire is important, especially when attempting to penetrate the distal fibrous cap of a CTO. Thus, lower-force wires are generally used initially and if highly calcified lesions are encountered, they should be substituted with more powerful, stiffer guidewires that are suitable for each situation of a CTO lesion (Nakamura and Bae, 2008).
3.2. Stabilization of the guide catheter
It is very important to select an appropriate guide catheter (a strong back-up catheter is better) not only for crossing the CTO lesions by the guidewire but also for delivering multiple devices after recanalization of the lesions. However, occasionally the guide catheter backs out as the wires or devices are advanced, and the guide has inadequate back up. Thus, it is sometimes necessary to stabilize the guide catheter by another method. At first, the guidewire has to be repositioned by deep seating or the use of a larger guide catheter. If additional back up of the guide catheter is required, the use of the anchor wire technique and anchor balloon technique (Fujita et al., 2003; Hamood et al., 2006) is needed to resolve situations, using side branches with a wire alone or balloon inflation (Surmely and Cook, 2007).
At first, to enable maximal guidewire torquability and pushability, a micro-tube catheter should be regularly used. An appropriate guidewire for each CTO condition is then selected; however, the selection process is very difficult and there is no straightforward selection procedure. Generally, torquability is proportional to the hardness of the wire tip of the guidewire. However, with a harder wire, less resistance is felt at the tip by the operator, and there is a higher risk of creating a false lumen and less ability to negotiate bending in the CTO lesion. After selection of the first guidewire, the wire has to penetrate the CTO lesion and advance to its core, while avoiding progress in a subintimal pathway, and then to cross to the distal edge of the lesion. If there are difficulties during this procedure, it may be necessary to change the guidewires and to use several special techniques to resolve the situation (Nakamura and Bae, 2008).
3.3. Parallel wire technique
When a guidewire is repeatedly advanced into the subintimal space, it can be left for use as a landmark to avoid the new wire protruding in the same space, which can easily cause collapse of the distal true lumen. Then, a second wire is advanced through a micro-tube catheter to avoid twisting the two wires (Han et al., 2008). A modification of this technique called the “seesaw wiring method” involves the alternate use of two guidewires to double the chance to place the wires in the distal true lumen. The effectiveness of the parallel wire technique is as follows: (1) The first wire can occlude the entry site of the false lumen and can be used as a landmark (with the potential to reduce the contrast). (2) The first wire can modify the arterial geometry, resulting in a reduction of the resistance for passage of the second wire. (3) The second wire can find the true lumen easier than the first wire, using the first wire as a landmark. In this situation, the tapered-tip wires are considered more adequately for the second wire than conventional wires as they can create a channel different from that created by the first wire, owing to their stiff and tapered tips (Nakamura and Bae, 2008).
3.4. See-saw wiring technique
The see-saw wiring technique is also in essence a parallel wiring technique. The difference is that the see-saw technique uses two microcatheters or OTW balloons. When the first wire fails, the second wire with the microcatheter or OTW balloon is inserted. However, there is a risk with the see-saw technique that the false lumen may enlarge and the procedure fail. Operators at the Kurashiki Central Hospital in Kurashiki, Japan, have demonstrated their ability to improve wire-crossing success over time (from 62% to 85%) when they have used these techniques (Nakamura and Bae, 2008).
3.5. Side-branch technique
If there is side branch at the segment of the occlusion and the wire has difficulty entering the true lumen, we can attempt to send the wire into the side branch and dilate it with a small balloon (Hamood et al., 2006). Then, the wire may be sent to the true lumen of the coronary artery. However, dissection caused by dilation may be large such that the wire cannot reach the occlusion. If this technique is to be tried, (1) the angle between side branch and main vessel must be less than 90°; (2) the diameter of the side branch must be no less than 1.5 mm.
3.6. Intravascular ultrasound (IVUS)-guided wiring technique
The current use of IVUS, specific to CTO intervention, employs two basic techniques: (1) IVUS identification and differentiation of the true vessel lumen from the false lumen, thereby assisting re-entry into the true lumen with a guidewire; and (2) facilitating true lumen entry of a CTO when a side branch arises just proximal to the CTO.
Sometimes, despite multiple view angiography, it is still difficult to access the exact entry point of the proximal edge of the CTO. This is typical of any flush occlusion at a bifurcating branch. The IVUS-guided wiring technique is a useful strategy, especially for the detection of the entry site of the CTO. For these cases, and if the branch is large enough to advance an IVUS catheter, the IVUS catheter can pinpoint the central area of the main lumen at the beginning of the CTO lesion. Furthermore, IVUS can provide information such as the best position for the wire entry point and can check the location of the wire in the CTO lesion. The IVUS will be less useful in cases of heavy calcification in the vessel wall at the CTO ostium or side branch. A similar technique can be used, placing the IVUS in a false lumen and identifying the parallel true lumen and placing the crossing wire in the spot on the IVUS. However, this carries the risks of increasing the size of the false lumen and of perforation.
3.7. Subintimal tracking and re-entry (STAR) technique
This technique, which has been associated with a re-intervention rate of 52.4%, is used when attempts to re-canalize the true lumen have failed. It is similar to the technique used for treating peripheral artery occlusions, and aims to create a subintimal dissection with distal re-entry. A 0.014″ hydrophilic wire with a J-configuration is used for this purpose. The hydrophilic wire is pushed through the subintimal dissection plane. When pushed distal to the occlusion, the J-tip is directed towards the true lumen, in an attempt to re-enter. In a report of 31 CTO cases, most of which had had a previous failed attempt, re-canalization with this technique was successful in 21 patients. However, it carries a higher potential for perforation than most other techniques (Nakamura and Bae, 2008).
3.8. Way to assess whether the wire in the true lumen
Prior to performing angioplasty or other desired treatments at the occlusion, the distal tip of the guidewire should be confirmed to be in the true lumen distal to the occlusion. If the guidewire has taken a path within the vessel wall, or completely external to the vessel, there is a risk of cardiac tamponade. This risk is relatively low when only the guidewire has perforated the wall. However, if angioplasty is performed, the perforation itself becomes dilated, resulting in a large leak path for arterial blood. Therefore, the practitioner should confirm that the guidewire has actually crossed the occlusion and entered the distal true vessel prior to performing angioplasty. Confirmation may be achieved by manipulating the guidewire by torquing and/or axial movement, observed during fluoroscopy. Free manipulation of the tip of the guidewire indicates that it is in the distal vessel. Angiography using one or more views can also indicate whether the guidewire tip is in the distal vessel. If the guidewire has a “j” tip on its end, the tip position may be confirmed by rotation. If the tip is in the lumen distal to the occlusion, the tip will easily rotate.
There are several methods that can be used to confirm luminal distal wire placement. First, the contralateral injection technique can be used to guide further wire manipulation into the distal vessel. This method requires the presence of sufficient collaterals to adequately opacify the occluded vessel. Second, injection of contrast distally through an OTW balloon or a transport catheter is also useful. IVUS can also confirm luminal entry versus entry into a dissection plane. The above methods, however, risk extension of a potential dissection plane with distal contrast injection or the passage of an IVUS catheter.
4. Retrograde wire techniques in PCI of CTO
The retrograde approach means that the occlusion site is approached retrogradely through a collateral channel from any other of the patient’s coronary arteries. At the present time, it is considered that the basis of a PCI strategy for a CTO lesion is still the antegrade approach. However, the retrograde approach for a CTO lesion is probably indicated in patients undergoing failed PCI by the antegrade approach (Ge et al., 2006). The retrograde approach requires an intercoronary channel (collateral), which can be an epicardial channel, interatrial channel, intraseptal channel (septal collateral), or a bypass graft. It is uncommon to find an epicardial intercoronary collateral with a suitable morphology allowing its use as a connecting channel. However, meticulous review of the angiogram of a CTO case will often reveal a septal channel between the left and right coronary arteries.
4.1. Selection of retrograde route
On the basis of angiographic findings, visible connections between the donor artery and the main artery distal to the CTO lesion are possibly available as retrograde routes. Even though collateral connections are not visible on normal angiograms, selective injection of contrast medium (≈0.5 ml) and nitrate through a microcatheter is sometimes helpful to find the existence of several collateral channels. The most important criterion for selection of a retrograde route is not the diameter but the minimum tortuosity of the channel. In addition, septal branches between the left anterior descending artery and the right coronary artery are favorable retrograde access routes for procedural safety. Although guidewire-induced perforation of epicardial arteries may cause cardiac tamponade, the same accident in septal branches resulting in intra-septal hematoma is harmless for a patient’s hemodynamic state. Selection of suitable collateral routes is the key to success in the retrograde approach (Ge et al., 2006; Saito, 2008).
4.2. Crossing guidewire via retrograde route
Two 6–8 F guiding catheters are usually engaged in the right and left coronary arteries via radial or femoral arteries. Use of guiding catheters with solid back-up force is recommended for antegrade and retrograde crossing of devices over the CTO lesions. A floppy guidewire (Runthrough-NS®, Runthrough-NS Hypercoat®, Terumo, Japan) or a plastic-jacket hydrophilic guidewire (Fielder®, Fielder-FC®, Asahi Intecc, Japan; Whisper®, Abbott, USA; Choice-PT2®, Boston Scientific, USA) is first delivered to the donor epicardial artery and advanced through the intercoronary collateral using a microcatheter (Transit®-150 cm, Cordis, USA; Excelsior®-150 cm, Boston Scientific, USA; Progreat® or Finecross®-150 cm, Terumo, Japan) to protect the channel from injury, and to achieve better wire maneuverability. After delivery of the microcatheter to the collateral channel, the tip of the wire is reshaped and shortly bent in order to cross easily within the collateral segment. Careful attempts are made to advance the wire and microcatheter alternately. When the wire cannot pass through the collateral channel, one option is to change the wire for a new hydrophilic wire (Fielder X-treme®, Asahi Intecc, Japan) with a tip of 0.009 inches (1 inch=2.54 cm). This guidewire is superior to any other wires in its ability to transit through a collateral channel. If the microcatheter does not advance, the wire can be exchanged for a low profile OTW balloon catheter by using a corresponding extension wire and balloon dilatation for the entire collateral channel (e.g., septal branch) at a low pressure (≤4 atm) (1 atm=101.325 kPa). Recently, a channel dilator catheter (Asahi Intecc, Japan) has been developed to ease retrograde wiring. After the wire reaches the epicardial artery distal to the target CTO lesion, the devices such as the wire and microcatheter or channel dilator are inserted as far as possible, because movement of the beating heart can otherwise frequently pull them out.
4.3. Retrogradely penetrating the CTO lesion
Retrograde penetration with a guidewire from the distal end (fibrous cap) of a CTO lesion is first attempted with the support of a microcatheter, OTW balloon catheter or channel dilator. When the distal fibrous cap is too hard to penetrate, pushing a reversed hydrophilic wire (e.g., Fielder X-treme®), the so-called “knuckle wire technique”, often leads it into the CTO lesion and creates a subintimal space. Otherwise, we recommend gradually changing the wire for a stiffer one (Miracle® 3, 6, 12; Conquest-Pro® or Conquest-Pro® 12, Asahi Intecc, Japan). Torque of the retrograde wire is often uncontrollable due to cardiac beating and the severe tortuosity of long-distance routes. Therefore, the stiffness of the wire tip should be raised step by step, and careful manipulation of the retrograde wire is required. Penetrating antegrade wire into the proximal fibrous cap becomes a landmark of advance of the retrograde wire and reduces the volume of contrast mediums during the procedure.
4.4. Different strategies after crossing a guidewire
After the retrograde guidewire has successfully been exchanged for a stiff guidewire, a penetration is attempted via the distal cap of the CTO lesion, and the guidewire is advanced retrogradely to the most proximal part possible. While maintaining the retrograde approach, the antegrade approach is commenced. After successful penetration of the tip of the retrograde guidewires into the CTO lesions, one or a combination of the following six different strategies is used (Saito, 2008).
4.4.1. Marker wire technique
After crossing the collateral, the retrograde wire is advanced up to the distal cap of the occlusion and is used solely as a marker for antegrade guidewire manipulation, without attempting to penetrate the occlusion retrogradely. This ‘marker wire technique’ is the simplest embodiment of retrograde techniques and does not require the crossing of the collaterals with a balloon and subsequent dilatation. Due to its relative simplicity, it is suggested as the technique of choice when embarking on a retrograde approach. This approach can reduce the use of contrast significantly.
4.4.2. Kissing wire technique
This procedure is the same as the marker wire technique, up to the point of reaching the distal cap. If the CTO lesion is relatively soft, the retrograde wire can advance relatively easily to the proximal part within the lesion. If the tip of the retrograde guidewire approaches the proximal cap of the CTO with its tip in line with the antegrade guidewire, both the antegrade and retrograde guidewires will meet in the CTO lesion.
4.4.3. Controlled antegrade and retrograde subintimal tracking (CART) technique
As described by Surmely and Cook (2007), the basic concept of the CART technique is to create a subintimal dissection with limited extension only at the site of the CTO. First, a wire is advanced antegradely from the proximal true lumen into the CTO, then into the subintimal space at the CTO site. Next, another wire is advanced through the intercoronary collateral. This wire is placed at the distal end of the CTO, then penetrates retrogradely from the distal true lumen into the CTO, and finally into the subintimal space at the CTO site. After advancing a small balloon (1.5–2.0 mm) over the retrograde wire into the subintima, the balloon is dilated in order to make the target space for antegrade guidewire access. This space is connected to the distal true lumen, so that the antegrade guidewire can easily reach the distal true lumen. To keep this subintimal space open, the deflated balloon should be left in place. Then the antegrade wire is advanced further along the deflated retrograde balloon that lies from the subintimal space to the distal true lumen. This technique allows limited subintimal tracking only in the portion of the CTO lesion and avoids the difficulty of re-entering the distal true lumen. After successful recanalization, dilatation and stent implantation are performed.
4.4.4. Reverse CART technique
The CART technique creates a connection between the subintimal space of the CTO lesion and the distal true lumen by retrograde ballooning from the distal true lumen to the subintimal space of the CTO lesion for antegrade passage of the wire. In contrast, the balloon antegradely inserted into the subintimal space within the CTO lesion can be dilated in order to make a target space for retrograde guidewire penetration into the proximal true lumen. This reverse method is called the Reverse CART technique. Compared with the CART technique, the Reverse CART technique allows the use of IVUS to guide passage of the retrograde wire into the proximal true lumen and limits the risk of creating long distal dissection (Matsumi and Saito, 2008). However, the drawback of the Reverse CART technique is the fact that manipulation of the retrograde guidewire is more difficult due to the long course and its many angulations.
4.4.5. Knuckle technique
This is a variation of the CART technique, where the dissection of the subintimal space is created by the formation of a loop in the retrograde wire which is then advanced to the occluded segment. The principle is the same as the STAR technique from the antegrade approach with the benefit that there is no flow to propagate the dissection distally. Soft hydrophilic wires are preferable for this technique. A cleavage subintimal space is created by advancing the retrograde wire with a J-loop configuration to allow a blunt dissection between the anatomical planes of the vessel. The crossing and dilatation of the septal collaterals with a balloon is not mandatory although frequent. The antegrade wire is then manipulated to enter the subintimal dissection and advanced further along the looped retrograde wire that extends from the subintimal space to the distal true lumen.
4.4.6. Pure retrograde crossing technique
This procedure is the same as the above techniques, up to the point of reaching the distal cap. In this technique, the retrograde wire is advanced through the occlusion reaching the proximal true lumen and exiting in the ascending aorta or the antegrade guiding catheter. There it can be anchored by inflating a balloon within the antegrade catheter to facilitate crossing of the occlusion with the retrograde balloon. At this point the retrograde balloon is advanced and inflated in the occlusion, re-establishing patency and allowing easy crossing of a soft wire antegradely.
4.4.7. Wire trapping technique
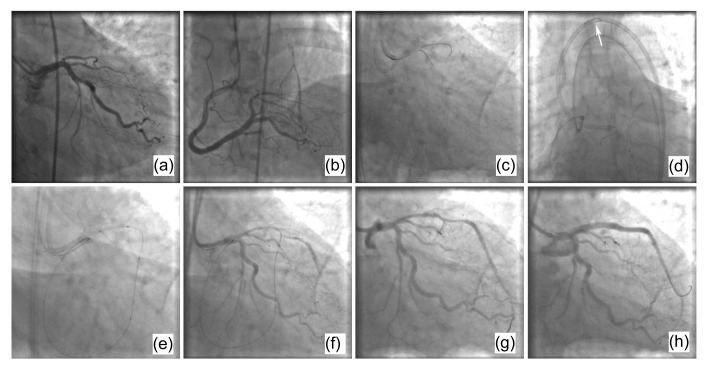
This procedure is the same as the pure retrograde crossing technique, up to the point of the wire retrogradely crossing the CTO lesion. After the retrograde wire enters the ascending aorta, a a gooseneck microsnare (Amplatz, USA) is antegradely inserted and snares the retrograde wire tip from the aorta into the antegrade guiding catheter. The microsnare is then pulled back slowly and carefully until the retrograde wire is exteriorized out of the antegrade guiding catheter. Then the balloon and stent can be antegradely delivered over the retrograde wire to re-canalize the occlusive lesion. This ‘wire trapping technique’ does not need retrograde balloon advancement and inflation in the occlusion and can avoid the potential complications associated with collateral dilatation (Ge et al., 2008b). However, it is frequently very difficult to pull the retrograde wire out of the antegrade catheter. The resistance is very strong especially when the stiff wire segment passes through tortuous collaterals. The wire tip is often broken in the antegrade catheter and eventually needs an intracatheter anchor balloon to help exteriorize it. An example of the wire trapping technique is shown in Fig. 1.
Fig. 1.
An example of the wire trapping technique
(a) A diagnostic coronary angiogram shows a chronic occlusive lesion in the ostium of the LAD. (b) The RCA provided good collaterals to the LAD. (c) A guidewire was retrogradely passed from the RCA to the LAD via a septal branch. (d) The guidewire was snared by a gooseneck microsnare (white arrow) and finally exteriorized out of the antegrade guiding catheter. (e) The antegradely delivered balloon crossed and inflated the chronic occlusive lesion. (f) The LAD restored antegrade flow after balloon inflation. (g) A second antegrade guidewire was passed through the chronic occlusive lesion. (h) The final image of the LAD with two antegradely implanted stents
4.4.8. Reverse wire trapping technique
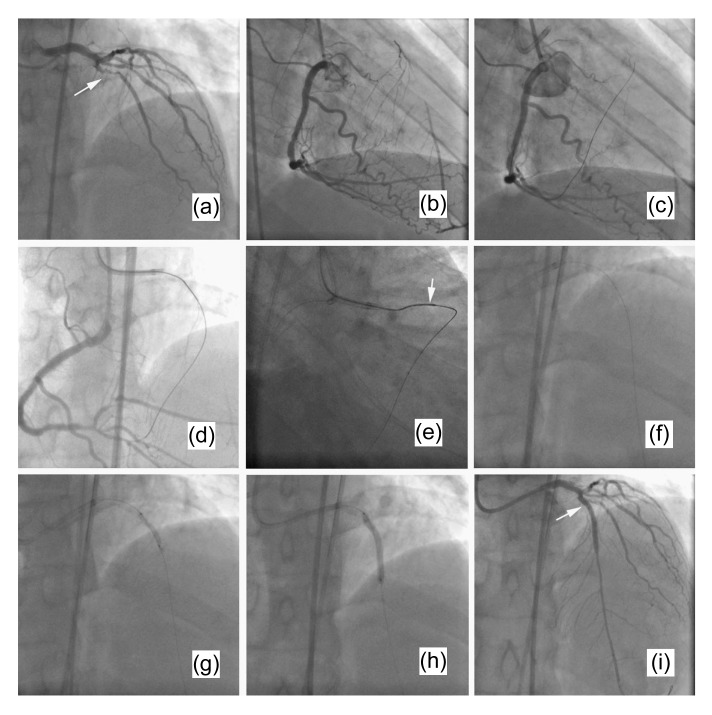
This technique is a modification of the wire trapping technique. After crossing the total occlusive lesion, entering the ascending aorta and being snared tightly by the antegrade microsnare, the retrograde wire is drawn back, dragging the microsnare tip to antegradely pass through the occlusion simultaneously. After the microsnare and microcatheter have crossed the lesion antegradely, the retrograde wire is released. Then the microsnare is drawn back and exchanged for an antegrade wire. Balloon dilation and stent implantation can then be easily performed (Ge and Zhang, 2009). An example of the reverse wire trapping technique is shown in Fig. 2.
Fig. 2.
An example of the reverse wire trapping technique
(a, b) Diagnostic coronary angiograms showing a total occlusion of the mid-LAD immediately distal to the origin of a large second diagonal branch (white arrow), with good retrograde filling from the RCA. (c) A wire was retrogradely passed from the RCA to the LAD via a septal branch. (d) The wire was guided to the antegrade guiding catheter after it crossed the chronic occlusive lesion retrogradely. (e) The wire, dragging the microsnare and microcatheter, was drawn back to the distal segment of the occlusive lesion (white arrow) after it was snared by the gooseneck microsnare. (f) The microsnare was then exchanged for an antegrade soft wire via the microcatheter which had crossed the occlusion antegradely. (g) The antegradely delivered balloon crossed and inflated the chronic occlusive lesion. (h) A stent was implanted in the occlusive lesion. (i) The final image shows an excellent result (white arrow)
4.4.9. Back-end ballooning and microcatheter reversal technique
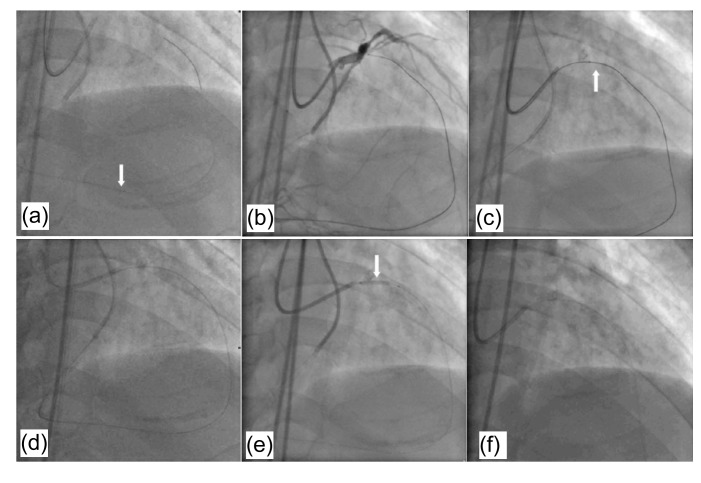
If a complex dissection is created after the retrograde balloon inflation in the previous retrograde proximal true lumen puncture strategy, no guidewire can be crossed through the lesion antegradely. In this situation, after introducing the retrograde guidewire into the antegrade guiding catheter, a microcatheter is placed over this guidewire which is exchanged for a 300 cm PCI guidewire or Rotablator® floppy guidewire. By pushing this wire to the proximal part of the antegrade guiding catheter, the distal tip of the wire finally reaches the proximal end of the antegrade guiding catheter and can be caught manually. The use of a rotablator guidewire is usually preferred because of its smaller diameter. If a standard 0.014-inch 300 cm exchange guidewire is used, it cannot be pushed to the proximal part of the antegrade guiding catheter because of significant resistance within the microcatheter. A balloon is put into the CTO lesion antegradely over the retrograde guidewire. After balloon dilation, another microcatheter is antegradely introduced over the retrograde wire and passed through the CTO lesion. The retrograde wire is then withdrawn and a floppy guidewire is introduced through the antegrade microcatheter, reaching the distal segment of the target vessel. Subsequent balloon dilatation and stent implantation are performed. An example of this technique is shown in Fig. 3.
Fig. 3.
An example of back-end ballooning and microcatheter reversal technique
(a) In a patient with a total occlusion of the proximal LAD, a wire was retrogradely passed from the RCA to the LAD via a septal branch (white arrow). (b) A Conquest guidewire crosses the chronic occlusive lesion retrogradely. (c) The retrograde wire and the microcatheter enter the antegrade guiding catheter (white arrow). (d) After exchanging for a 300 cm guidewire, this wire was pushed until its distal tip finally reached the proximal end of the antegrade guiding catheter and was caught manually. (e) The antegradely delivered balloon over the retrograde wire crossed and inflated in the chronic occlusive lesion (white arrow). (f) After balloon dilation, another microcatheter was antegradely introduced over the retrograde wire and passed through the CTO lesion. The retrograde wire was then withdrawn and a floppy guidewire was introduced through the antegrade microcatheter, reaching the distal segment of the target vessel
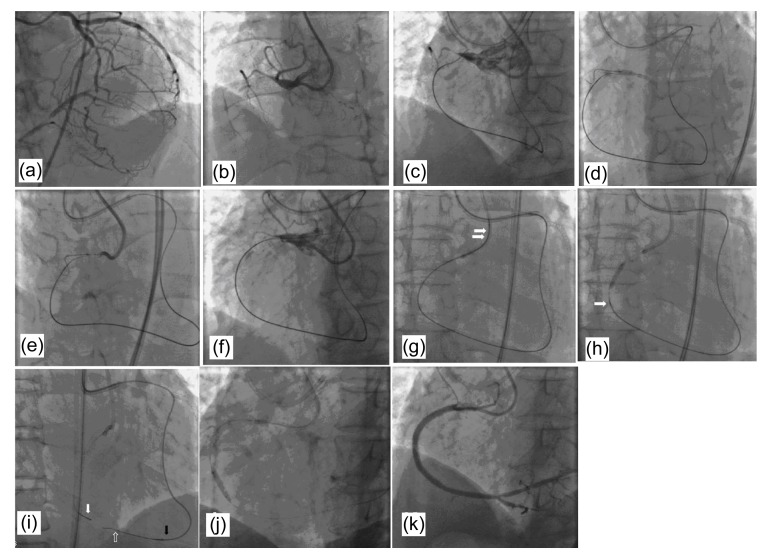
The success of PCI through the retrograde approach can be achieved by combining different antegrade and retrograde techniques. A good example is shown in Fig. 4.
Fig. 4.
A good example of success of PCI through the retrograde approach
(a, b) Diagnostic coronary angiograms show a total occlusion of the proximal LAD, with good retrograde filling from the LCA. (c–f) Reverse CART technique and IVUS-guiding technique. A retrograde wire was advanced through the septal collateral, reached the distal end of the CTO, but could not enter the proximal true lumen of the CTO lesion (c). After advancing a small balloon over the antegrade wire into the subintima, the balloon was dilated to make the target space for retrograde guidewire access (d). Then the retrograde wire was advanced from the subintimal space to the proximal true lumen (f) under IVUS guidance (e). (g) Wire trapping technique. The retrograde wire entered the aorta and was snared into the antegrade guiding catheter by a microsnare (white arrows). (h–j) After a balloon crossed and inflated the chronic occlusive lesion over the retrograde wire, a microcatheter was antegradely introduced over the retrograde wire and passed through the CTO lesion (white arrow). The retrograde wire was then withdrawn and a floppy guidewire was introduced through the antegrade microcatheter, reaching the distal segment of the target vessel. (k) Final result
5. Conclusions
Chronic total occlusion is associated with 10%–20% of all PCI procedures. Opening an occluded vessel, especially one supplying a considerable area of myocardium, may be beneficial for a patient’s angina relief and heart function.
References
- 1.Abhaichand RK, Lefèvre T, Louvard Y, Morice MC. Amplatzing a 6 Fr Judkins right guiding catheter for increased success in complex right coronary artery anatomy. Catheter Cardiovasc Interv. 2001;53(3):405–409. doi: 10.1002/ccd.1191. [DOI] [PubMed] [Google Scholar]
- 2.di Mario C, Werner GS, Sianos G, Galassi AR, Büttner J, Dudek D, Chevalier B, Lefevre T, Schofer J, Koolen J, et al. European perspective in the recanalisation of chronic total occlusions (CTO): consensus document from the EuroCTO Club. EuroIntervention. 2007;3(1):30–43. [PubMed] [Google Scholar]
- 3.Freed MS, Safian RD. Chronic Total Occlusion. In: Safian RD, Freed MS, editors. The Manual of Interventional Cardiology. 3rd Ed. Royal Oak, Mich.: Physicians’ Press; 2001. pp. 287–299. [Google Scholar]
- 4.Fujita S, Tamai H, Kyo E, Kosuga K, Hata T, Okada M, Nakamura T, Tsuji T, Takeda S, Bin Hu F, et al. New technique for superior guiding catheter support during advancement of a balloon in coronary angioplasty: the anchor technique. Catheter Cardiovasc Interv. 2003;59(4):482–488. doi: 10.1002/ccd.10551. [DOI] [PubMed] [Google Scholar]
- 5.Ge JB, Zhang F. Retrograde recanalization of chronic total coronary artery occlusion using a novel “reverse wire trapping” technique. Catheter Carciovasc Interv. 2009;74(6):855–860. doi: 10.1002/ccd.22122. [DOI] [PubMed] [Google Scholar]
- 6.Ge JB, Ge L, Qian JY, Liu XB, Wang H, Zhang F, Zhang SH. Retrograde wire technique for recanalization of a left main chronic total occlusion. Chin J Intervent Cardiol. 2006;14(1):55–56. (in Chinese) [Google Scholar]
- 7.Ge JB, Huang D, Zhang F, Qian JY, Ge L, Wang QB. Clinical experience of a novel penetration Tornus catheter in percutaneous treatment of coronary chronic total occlusion lesions. Chin J Intervent Cardiol. 2008;16(4):184–186. (in Chinese) [Google Scholar]
- 8.Ge JB, Zhang F, Ge L, Qian JY, Wang H. Wire trapping tachnoque combined with retrograde approach for recanalization of chronic total occlusion. Chin Med J (Engl) 2008;121(17):1753–1756. [PubMed] [Google Scholar]
- 9.Hamood H, Makhoul N, Grenadir E, Kusniec F, Rosenschein U. Anchor wire technique improves device deliverability during PCI of CTOs and other complex subsets. Acute Card Care. 2006;8(3):139–142. doi: 10.1080/17482940600885469. [DOI] [PubMed] [Google Scholar]
- 10.Han YL, Li Y, Wang SL, Jing QM, Ma YY, Wang G, Luan B, Wang B, Wang ZL, Wang DM. Multi-wire plaque crushing as a novel technique in treating chronic total occlusions. Chin Med J. 2008;121(6):518–521. [PubMed] [Google Scholar]
- 11.Kinoshita I, Katoh O, Nariyama J, Otsuji S, Tateyama H, Kobayashi T, Shibata N, Ishihara T, Ohsawa N. Coronary angioplasty of chronic total occlusions with bridging collateral vessels: immediate and follow-up outcome from a large single-center experience. J Am Coll Cardiol. 1995;26(2):409–415. doi: 10.1016/0735-1097(95)80015-9. [DOI] [PubMed] [Google Scholar]
- 12.Kirtane AJ, Stone GW. The anchor-Tornus technique: a novel approach to “uncrossable” chronic total occlusions. Catheter Cardiovasc Interv. 2007;70(4):554–557. doi: 10.1002/ccd.21138. [DOI] [PubMed] [Google Scholar]
- 13.Matsumi J, Saito S. Progress in the retrograde approach for chronic total coronary artery occlusion: a case with successful angioplasty using CART and reverse-anchoring techniques 3 years after failed PCI via a retrograde approach. Catheter Cardiovasc Interv. 2008;71(6):810–814. doi: 10.1002/ccd.21493. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura M, Shiba M, Wada M. A novel method for deploying a stent into a highly angulated position through use of a stent strut: application of a five-in-seven system. J Invasive Cardiol. 2006;18(3):E105–E107. [PubMed] [Google Scholar]
- 15.Nakamura S, Bae JH. Recent progress of the use of interventional therapy for chronic total occlusion. Korean Circ J. 2008;38(6):295–300. doi: 10.4070/kcj.2008.38.6.295. [DOI] [Google Scholar]
- 16.Saito S. Different strategies of retrograde approach in coronary angioplasty for chronic total occlusion. Catheter Cardiovasc Interv. 2008;71(1):8–19. doi: 10.1002/ccd.21316. [DOI] [PubMed] [Google Scholar]
- 17.Stone GW, Kandzari DE, Mehran R, Colombo A, Schwartz RS, Bailey S, Moussa I, Teirstein PS, Dangas G, Baim DS, et al. Percutaneous recanalization of chronically occluded coronary arteries: a consensus document. Part I. Circulation. 2005;112(15):2364–2372. doi: 10.1161/CIRCULATIONAHA.104.481283. [DOI] [PubMed] [Google Scholar]
- 18.Stone GW, Reifart NJ, Moussa I, Hoye A, Cox DA, Colombo A, Baim DS, Teirstein PS, Strauss BH, Selmon M, et al. Percutaneous recanalization of chronically occluded coronary arteries: a consensus document. Part II. Circulation. 2005;112(16):2530–2537. doi: 10.1161/CIRCULATIONAHA.105.583716. [DOI] [PubMed] [Google Scholar]
- 19.Stone GW, Colombo A, Teirstein PS, Moses JW, Leon MB, Reifart NJ, Mintz GS, Hoye A, Cox DA, Baim DS. Percutaneous recanalization of chronically occluded coronary arteries: procedural techniques, devices, and results. Catheter Cardiovasc Interv. 2005;66(2):217–236. doi: 10.1002/ccd.20489. [DOI] [PubMed] [Google Scholar]
- 20.Surmely JF, Cook S. Variation on the Anchor balloon technique for difficult stent delivery. Kardiovaskuläre Medizin. 2007;10:397–399. [Google Scholar]
- 21.Takahashi S, Saito S, Tanaka S, Miyashita Y, Shiono T, Arai F, Domae H, Satake S, Itoh T. New method to increase a backup support of a 6 French guiding coronary catheter. Catheter Cardiovasc Interv. 2004;63(4):452–456. doi: 10.1002/ccd.20223. [DOI] [PubMed] [Google Scholar]