Abstract
Objective: To determine the association between tea consumption and the risk of stroke. Methods: We searched the PubMed database from January 1966 to March 2012 and reviewed reference lists of retrieved articles to identify relevant studies. Studies were included if they reported relative risks (RRs) and corresponding 95% confidence intervals (CIs) of stroke with respect to three or more categories of tea consumption. A random-effects model was used to combine the study-specific risk estimates. Results: Fourteen studies, consisting of 513 804 participants with a median follow-up of 11.5 years, were included in this meta-analysis. We observed a modest but statistically significant inverse association between tea consumption and risk of stroke. An increase of three cups/d in tea consumption was associated with a 13% decreased risk of stroke (RR 0.87; 95% CI, 0.81–0.94). The decreased risk of stroke with tea consumption was consistent among most subgroups. Based on the three studies that provided results for stroke subtypes, tea consumption was also inversely associated with the risk of ischemic stroke (RR 0.76; 95% CI, 0.69–0.84), but not cerebral hemorrhage (RR 0.96; 95% CI, 0.82–1.11) or subarachnoid hemorrhage (RR 0.81; 95% CI, 0.57–1.16). Conclusions: Tea consumption is associated with a decreased risk of stroke, particularly ischemic stroke. More well-designed, rigorously conducted studies are needed in order to make confident conclusions about the association between tea consumption and stroke subtypes.
Keywords: Tea, Stroke, Prospective studies, Dose-response meta-analysis
1. Introduction
Stroke is the second leading cause of death both in developed and developing countries (WHO, 2011). In the United States, every 40 s, one person suffers from a stroke, and every 4 min, one person dies of stroke (Roger et al., 2012). In addition, stroke is a major cause of serious long-term disability worldwide, which consumes large amounts of health resources and leads to significant economic burden (Goldstein et al., 2011). Primary prevention of stroke is, therefore, a major public health priority.
Tea is the second most consumed beverage in the world, after water (Cheng, 2006). Given its popularity, even small health benefits of tea could have considerable public health implications. Tea is generally consumed in the forms of black, green, and oolong, all of which originate from the leaves of the plant Camellia sinensis (Graham, 1992). While different processing methods produce different types of tea: black tea is fully oxidized, green tea is non-oxidized, and oolong tea is partially oxidized (Babu and Liu, 2008).
There is a great deal of evidence that tea has been associated with a reduced risk of hypertension (Yang et al., 2004; Hodgson et al., 2012), diabetes (Huxley et al., 2009; Jing et al., 2009), and atherosclerosis (Geleijnse et al., 1999; Debette et al., 2008), all of which are major risk factors of stroke (Goldstein et al., 2011). However, the results based on epidemiological studies regarding the association of tea consumption with stroke risk are quite inconsistent (Keli et al., 1996; Sesso et al., 2003a). Although an inverse association between tea consumption and stroke risk has been reported in a previous meta-analysis (Arab et al., 2009), the reported results may be susceptible to bias, as two of the included studies used the same study population (the α-tocopherol, β-carotene cancer prevention (ATBC) study) (Hirvonen et al., 2000; Larsson et al., 2008), and three studies were overlooked after a comprehensive literature search (Iwai et al., 2002; Sesso et al., 2003b; Bidel et al., 2006). In addition, five cohort studies with inconsistent results have been published since then (Tanabe et al., 2008; Lopez-Garcia et al., 2009; de Koning Gans et al., 2010; Leurs et al., 2010; Mineharu et al., 2011). Therefore, we undertook a meta-analysis of prospective cohort studies to update and quantitatively assess the association between tea consumption and the risk of stroke, overall and by subtypes.
2. Methods
2.1. Literature search and selection
We followed standard criteria for conducting meta-analysis and reporting the results (Moher et al., 2009). A search strategy (Table 1) was developed to identify studies that provided effect estimates for the potential association between tea consumption and risk of stroke. We performed an electronic search in PubMed from January 1966 to March 2012 without language restrictions. Furthermore, we reviewed reference lists of the obtained articles to search for additional studies. Only those conducted in humans were considered. When necessary, we contacted authors of original studies for additional data.
Table 1.
Search strategy and search terms used to identify studies on the association between tea consumption and stroke risk
| No. | Search terms |
| 1 | “stroke” |
| 2 | “ischemic” |
| 3 | “ischaemic” |
| 4 | “hemorrhagic” |
| 5 | “haemorrhagic” |
| 6 | 2 OR 3 OR 4 OR 5* |
| 7 | 1 AND 6 |
| 8 | “cerebrovascular accident” |
| 9 | “cerebrovascular disorders” |
| 10 | “cardiovascular disease” |
| 11 | “cerebral infarction” |
| 12 | “cerebral hemorrhage” |
| 13 | “cerebral haemorrhage” |
| 14 | “apoplexy” |
| 15 | “subarachnoid hemorrhage” |
| 16 | “subarachnoid haemorrhage” |
| 17 | “brain ischemia” |
| 18 | “brain ischaemia” |
| 19 | “intracranial hemorrhages” |
| 20 | “intracranial haemorrhages” |
| 21 | 1 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18 OR 19 OR 20 |
| 22 | “tea” |
| 23 | “black tea” |
| 24 | “green tea” |
| 25 | “oolong tea” |
| 26 | “tea polyphenol” |
| 27 | “EGCG” |
| 28 | “Flavonoids” |
| 29 | “Catechin” |
| 30 | “Flavan-3-ols” |
| 31 | “Flavonol” |
| 32 | “thearubigin” |
| 33 | “theaflavin” |
| 34 | “Camelia sinensis” |
| 35 | “specific beverage” |
| 36 | “theophylline” |
| 37 | “coffea” |
| 38 | “coffee” |
| 39 | “caffeine” |
| 40 | 22 OR 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29 OR 30 OR 31 OR 32 OR 33 OR 34 OR 35 OR 36 OR 37 OR 38 OR 39 |
| 41 | “cohort study” |
| 42 | “longitudinal study” |
| 43 | “follow-up study” |
| 44 | “prospective study” |
| 45 | “prospective cohort study” |
| 46 | 41 OR 42 OR 43 OR 44 OR 45 |
| 47 | 21 AND 40 AND 46 |
The number represents the corresponding search terms
Studies were included in this meta-analysis if they fulfilled the following criteria: (1) a prospective cohort design; (2) the exposure of interest was tea consumption; (3) the outcome of interest was total stroke and/or stroke subtypes, i.e., cerebral infarction, cerebral hemorrhage, and subarachnoid hemorrhage; and (4) the relative risks or hazard ratios with their corresponding 95% confidence intervals (CIs) of stroke relating to three or more categories of tea consumption were reported. If multiple publications from the same study population were available, the most recent and detailed study was eligible for inclusion in this meta-analysis.
2.2. Data extraction
Data were extracted independently by two investigators using a predefined data collection form, with disagreements being resolved by consensus. Relevant data included first author’s surname, publication year, country of origin, cohort name, cohort size, participants’ age, participants’ gender, duration of follow-up, tea type, outcomes (number of events), assessment of exposure and outcome, tea consumption categories, most fully adjusted relative risks and corresponding 95% CIs for every category of tea consumption, and covariates adjusted in the multivariable analysis.
For exposure values, in cups per day, we assigned the median or mean tea consumption per category as the average intake to the corresponding relative risk. When the median or mean tea consumption for each category was not available, the category midrange was applied. And if the highest category was open-ended and included no more than 20% of the study subjects, we assigned the category a value equal to 1.2 times its lower boundary; otherwise, we assigned 1.4 times its lower boundary for the expected right skewed distribution (Peters et al., 2001). If the lowest category was open-ended, we set the lower boundary to zero.
2.3. Statistical analysis
Study-specific risk estimates were extracted from each study to measure the association between tea consumption and risks of total stroke and subtypes of stroke. If the result on total stroke was not provided, we used data from fatal stroke or ischemic stroke as a surrogate for total stroke (Pan et al., 2011). To normalize the variation between studies in different exposure categories, we calculated a risk estimate for an increase of 3 cups/d in tea consumption for each study based on the method proposed by Greenland and Longnecker (1992) and Orsini et al. (2006). We obtained the summary relative risks by pooling the study-specific slopes using the inverse of the corresponding variances as weights on the basis of DerSimonian and Laird random-effects model (DerSimonian and Laird, 1986), which considers both within- and between-study variabilities. For studies which reported separate relative risks for different tea types (e.g., green tea, black tea, or oolong tea), we pooled the relative risk estimates for the different tea types, weighted by inverse of the variance within the study (Gao et al., 2005).
In addition, subgroup analyses were performed to assess the influences of participant and study characteristics on study results, according to sex (male/female), tea type (green/black), outcome (stroke incidence/mortality), duration of follow-up (>10 years/≤10 years), geographic region (Japan/USA/Europe), whether controlling hypertension and diabetes in models (yes/no), whether controlling physical activity in models (yes/no), and whether controlling fruits and vegetables intake in models (yes/no). Sensitivity analyses, by excluding each study one by one and recalculating the pooled estimates on remaining studies, were conducted to investigate whether the overall risk estimate could have been affected significantly by an individual study.
Statistical heterogeneity was assessed using the Q and I 2 statistics (Higgins et al., 2003) at a P<0.10 level of significance. Publication bias was evaluated using the Egger test (Egger et al., 1997). P<0.05 was considered representative of statistically significant publication bias. Statistical analyses were done with STATA 11.0 (StataCorp, College Station, TX, USA). All statistical tests were two-sided.
3. Results
3.1. Study characteristics
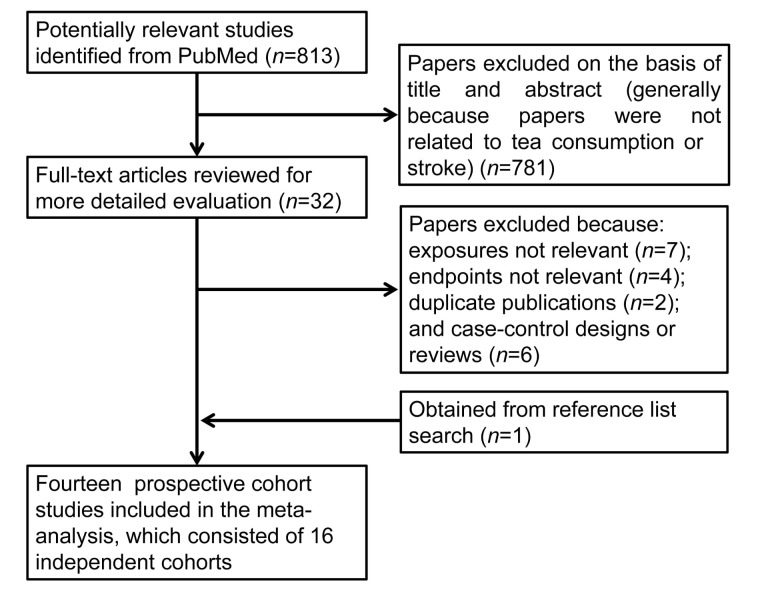
The search strategy identified 813 potentially relevant publications, of which 781 articles were excluded on the basis of title and abstract (Fig. 1). Thirty-two full-text articles were assessed. One study was obtained from reference list search. Eleven studies were excluded either because the outcomes assessed were not stroke events or because the exposure of interest was not tea. The study by Hirvonen et al. (2000) was excluded because it used the same study population as the study reported by Larsson et al. (2008), and the latter provided information with a longer duration of follow-up. The study by Iwai et al. (2002) was also excluded because data from this cohort were reported in another publication (Mineharu et al., 2011) with a larger sample size. We further excluded a case-control study and five review articles. Additionally, four primary studies (Sato et al., 1989; Klatsky et al., 1993; Yochum et al., 1999; Sesso et al., 2003a) with insufficient information for risk estimates were included by extracting relevant data from the publication by Peters et al. (2001). Thus, there were 14 prospective cohort studies (Table 2) included in this meta-analysis. In addition, three studies (Kuriyama et al., 2006; Larsson et al., 2008; Tanabe et al., 2008) provided results for stroke subtypes.
Fig. 1.
Flowchart of study assessment and selection by searching PubMed
Table 2.
Characteristics of prospective cohort studies of tea consumption and risk of stroke
| Study and country (cohort) | Population | Follow-up | Exposure and assessment | Outcome (n) and assessment | Adjusted variables |
| Sato et al., 1989 ; Japan | 9 510 women aged ≥40 years | 4 years (1984–1988) | Green tea; questionnaire, self-administered | Stroke mortality (174); death confirmed by local health authorities | Age, sex, geographic area, smoking, alcohol, and salt intake |
| Klatsky et al., 1993; USA | 12 893 adults, age NA | 8 years (1978–1988) | Black tea; questionnaire | Stroke mortality (275); the California Automated Mortality Linkage System | Age, sex, race, BMI, smoking, alcohol, education, and marital status |
| Keli et al., 1996; the Netherlands (the Zutphen study) | 552 men aged 50–69 years | 15 years (1970–1985) | Black tea; dietary history method, interviewer administered | Stroke incidence (42); all events confirmed by a neurologist or internist | Age, smoking, systolic blood pressure, serum cholesterol, alcohol, energy, and fish intake |
| Yochum et al., 1999; USA (Iowa Women’s Health study) | 34 492 women aged 55–69 years | 10 years (1986–1995) | Black tea; validated FFQ, self-administered | Stroke mortality (131); linkage with the national death index | Age, waist-to-hip ratio, BMI, smoking, high blood pressure, diabetes, estrogen replacement therapy, education, physical activity, marital status, intake of alcohol, cholesterol, vitamin E, saturated fat, total energy, dietary fiber, and whole grains |
| Sesso et al., 2003a; USA (Women’s Health study) | 37 902 women aged ≥45 years | 6.9 years (1992–NA) | Black tea; validated FFQ, self-administered | Stroke incidence (256); death certificates and medical records | Age, smoking, alcohol, exercise, BMI, diabetes, hypertension, high cholesterol, parental history of MI at age <60 years, postmenopausal hormone use, randomized aspirin, vitamin E, β-carotene treatment, intake of fruit and vegetable, fiber, folate, saturated fat, and total energy |
| Sesso et al., 2003b; USA (College Alumni Health study) | 17 228 adults (95.6% male) mean aged 59.5 years | 15 years (1977–1995) | Black tea; questionnaire, self-administered | Stroke incidence (757); self-reported and death certificates | Age, sex, smoking, alcohol, BMI, physical activity, diabetes, hypertension, and early parental death |
| Kuriyama et al., 2006; Japan (the Ohsaki study) | 40 530 adults (19 060 men and 21 470 women) aged 40–79 years | 7 years (1995–2001) | Green tea; validated FFQ, self-administered | Stroke mortality including CI, ICH, and SAH (472); death certificates | Age, sex, job status, education, BMI, physical activity, history of hypertension, diabetes, and gastric ulcer, smoking, alcohol, total energy intake, consumption of miso soup, soybean products, rice, total meat, fish, vegetables, fruits, dairy products, oolong tea, black tea, and coffee |
| Bidel et al., 2006; Finland | 3 837 type 2 diabetes patients aged 25–74 years | 20.8 years (1972–2003) | Black tea; questionnaires, self-administered | Stroke mortality (210); linkage to the National Death Registry | Age, sex, smoking, alcohol, education, BMI, systolic blood pressure, total cholesterol, and coffee consumption |
| Larsson et al., 2008; Finland (the ATBC study) | 26 556 male smokers aged 50–69 years | 13.6 years (1985–2004) | Black tea; validated FFQ, self-administered, but checked and completed with a nurse | Incidence of CI (2702), ICH (383), and SAH (196); linkage with the National Hospital Discharge Register and the National Register of Causes of Death | Age,supplementation group, smoking, alcohol, systolic and diastolic blood pressure, serum total and HDL cholesterol, BMI, physical activity, histories of diabetes, and coronary heart disease, and coffee consumption |
| Tanabe et al., 2008; Japan (Tokamachi-Nakasato cohort study) | 6 207 adults (2 029 men and 4 178 women) aged 40–89 years | 5 years (1998–2003) | Green tea; lifestyle questionnaire, self-administered | Stroke incidence including CI and ICH (101); medical chart review. Stroke subtype determined by CT or MRI | Age, sex, smoking, alcohol, personal history, intake of grain products, fruits, vegetables, salted vegetables, soybean, paste soup, milk, balance of meat and fish, time walking, roasted, black or oolong tea, and coffee |
| Lopez-Garcia et al., 2009; USA (Nurses’ Health study) | 83 076 women aged 30–55 years | 24 years (1980–2004) | Black tea; validated FFQ, self-administered | Stroke incidence (2 280); medical records reviewed by a physician, stroke was confirmed according to criteria of the US National Survey of Stroke | Age, smoking, alcohol, physical activity, aspirin use, menopausal status, hormone replacement therapy, BMI, intake of calcium, potassium, sodium, folate, glycemic load, whole grain, fish, fruits, vegetables, caffeinated coffee, and total caloric |
| de Koning Gans et al., 2010; the Netherlands (EPIC-NL cohort) | 37 514 adults aged 20–69 years | 13 years (1993–2006) | Black tea; validated FFQ, self-administered | Stroke incidence (598) and mortality (70); National Medical Registry (incidence) and Statistics Netherlands (death) | Sex, age, alcohol, smoking, cohort (strata), physical activity, waist circumference, education, menopausal status, intake of total energy, saturated fat, fiber, vitamin C, tea, and coffee |
| Leurs et al., 2010; the Netherlands (the Netherlands cohort study) | 58 279 men and 62 573 women aged 55–69 years | 10 years (1986–1996) | Black tea; validated FFQ, self-administered | Stroke mortality (708); Central Bureau of Genealogy linkage | Age, current smoking, number of cigarettes smoked, years of active smoking, and total energy intake (kcal) |
| Mineharu et al., 2011; Japan (JACC study) | 34 345 men and 48 310 women aged 40–79 years | 13.1 years (1988–2003) | Green, black, and oolong tea; validated dietary questionnaire, self-administered | Stroke mortality (1 486); death certificates | BMI, history of hypertension and diabetes, smoking, alcohol, walking hours, hours of sports participation, education, perceived mental stress, vitamin E supplement use, multivitamin use, consumption of total fruits, vegetables, beans, meat, fish, seaweeds, and total energy intake |
n: number of events; NA: not available; BMI: body mass index; FFQ: food frequency queationnaire; MI: myocardial infarction; CI: cerebral infarction; ICH: intracerebral hemorrhage; SAH: subarachnoid hemorrhage; ATBC: α-tocopherol; β-carotene cancer prevention; EPIC-NL: European prospective investigation into cancer and nutrition; JACC: the Japan collaborative cohort study for evaluation of cancer risk; CT: computed tomography; MRI: magnetic resonance imaging; HDL: high-density lipoprotein
The studies included a total of 513 804 participants with 10 192 stroke cases. Participants were free of cardiovascular disease or stroke at baseline among 12 out of the 14 included studies, and the remaining 2 studies (Sato et al., 1989; Klatsky et al., 1993) were not clearly defined. The median duration of follow-up was 11.5 years (interquartile range (IQR) 7-15 years). Five studies were conducted in the USA, 4 in Japan, 3 in the Netherlands, and 2 in Finland. Mortality from stroke among these studies ranged from 186 per 100 000 in the Netherlands to 5 473 per 100 000 in Finnish patients with type 2 diabetes. Incidence of stroke ranged from 675 per 100 000 female US health professionals to 10 174 per 100 000 male Finnish smokers. Tea consumption was only measured at baseline in most studies, whereas 3 studies (Keli et al., 1996; Sesso et al., 2003b; Lopez-Garcia et al., 2009) accounted for the changes of tea consumption over time. Stroke was assessed by death certificates, medical records, or confirmation by health authorities in the majority of the studies, whereas one study (Keli et al., 1996) was on the basis of a neurologist or internist's confirmation. Most studies provided relative risk estimates that were adjusted for age (13 studies), smoking (all 14 studies), alcohol intake (13 studies), body mass index (BMI) (9 studies), physical activity (9 studies), history of hypertension or measured blood pressure (7 studies), history of diabetes (6 studies), total energy intake (8 studies), fruits and vegetables intake (6 studies), coffee consumption (6 studies), and other dietary factors (9 studies).
3.2. Main analysis
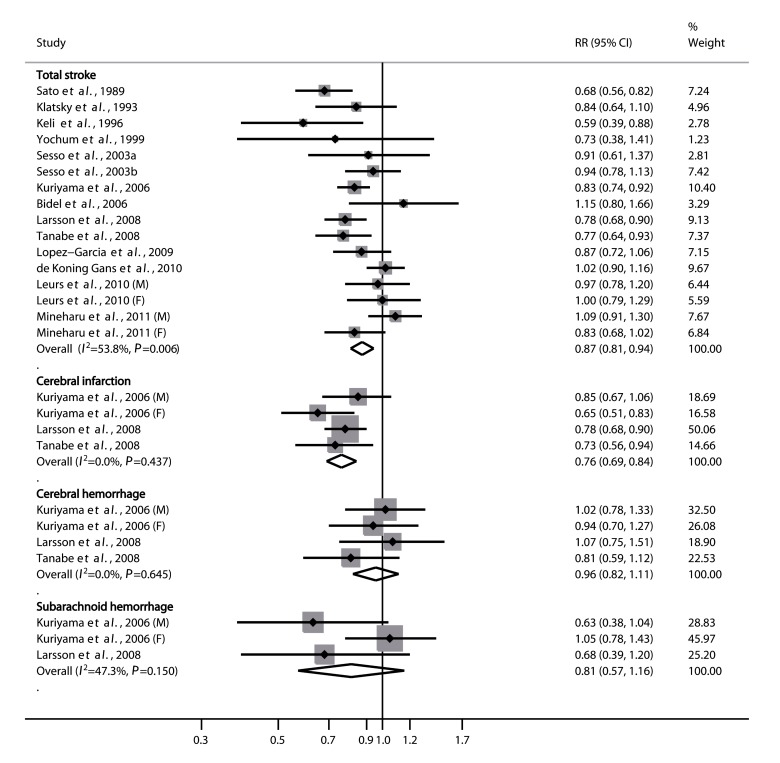
The multivariable-adjusted relative risks (RRs) of total stroke and stroke subtypes for an increase of 3 cups/d in tea consumption for the individual studies and the corresponding combined risk estimates are shown in Fig. 2. The pooled analyses indicated that increased tea consumption was significantly associated with a reduced risk of total stroke, especially cerebral infarction, but not of cerebral or subarachnoid hemorrhage. The combined RR of total stroke for every 3 cups/d increment in tea consumption was 0.87 (95% CI, 0.81–0.94), with evidence of moderate heterogeneity across studies (P=0.006, I2=53.8%). The summary risk estimates were 0.76 (95% CI, 0.69–0.84; P=0.437, I2=0%) for cerebral infarction, 0.96 (95% CI, 0.82–1.11; P=0.645, I2=0%) for cerebral hemorrhage, and 0.81 (95% CI, 0.57–1.16; P=0.15, I2=47.3%) for subarachnoid hemorrhage.
Fig. 2.
Summary relative risks (RRs) of total stroke and stroke subtypes for an increment of 3 cups/d in tea consumption
Squares represent study-specific RR estimates (size of square reflects the study’s statistical weight), horizontal lines represent 95% CIs, and the diamond represents the summary RR estimate with its corresponding 95% CI. Study-specific risk estimates were combined by using the DerSimonian and Laird random-effects model
3.3. Subgroup and sensitivity analyses
The results of subgroup analyses stratified by various study and participant characteristics are showed in Table 3. Overall, every 3 cups/d increment in tea consumption had a protective effect against total stroke in most subgroups. Notably, the observed protective effect was more pronounced in several strata of study characteristics: female participants, among drinkers of green tea, having a relatively short follow-up period (≤10 years), and among Japanese studies. Diabetes and hypertension may be potential confounders of the relationship between tea consumption and risk of stroke; however, the results were not markedly altered when we excluded the studies that did not adjust for both hypertension and diabetes (the pooled RR 0.88; 95% CI, 0.79–0.96). Likewise, the result was persistent after excluding the studies by Larsson et al. (2008) and Bidel et al. (2006), in which participants were male smokers and type 2 diabetics, respectively (the pooled RR 0.87; 95% CI, 0.81–0.95).
Table 3.
Stratified analyses relating per 3 cups of tea consumption to stroke risk by study and participant characteristics
| Group | No. of cohorts | RR (95% CI) | Q statistic | P-value | I 2-value (%) |
| Sex | |||||
| Men | 7 | 0.85 (0.75–0.97) | 18.5 | <0.01 | 67.6 |
| Women | 8 | 0.83 (0.76–0.90) | 7.9 | 0.34 | 11.3 |
| Tea type | |||||
| Green tea | 5 | 0.83 (0.72–0.96) | 13.4 | <0.01 | 70.2 |
| Black tea | 13 | 0.91 (0.83–0.98) | 16.4 | 0.17 | 26.8 |
| Duration of follow-up | |||||
| ≤10 years | 8 | 0.83 (0.76–0.91) | 9.4 | 0.23 | 25.5 |
| >10 years | 8 | 0.91 (0.81–1.02) | 19.0 | <0.01 | 63.1 |
| Geographic area | |||||
| USA | 5 | 0.89 (0.79–1.00) | 0.9 | 0.92 | 0 |
| Japan | 5 | 0.83 (0.72–0.96) | 13.8 | <0.01 | 71.0 |
| Europe | 6 | 0.91 (0.78–1.06) | 14.8 | 0.01 | 66.3 |
| Outcome | |||||
| Stroke incidence | 7 | 0.85 (0.76–0.96) | 14.5 | 0.02 | 58.7 |
| Stroke mortality | 10 | 0.89 (0.80–0.99) | 18.3 | 0.03 | 50.8 |
| Controlling hypertension and diabetes in models | |||||
| Yes | 7 | 0.88 (0.79–0.96) | 10.4 | 0.11 | 42.0 |
| No | 8 | 0.85 (0.75–0.96) | 19.8 | <0.01 | 64.7 |
| Controlling physical activity in models | |||||
| Yes | 10 | 0.88 (0.81–0.96) | 17.8 | 0.04 | 49.4 |
| No | 6 | 0.85 (0.71–1.02) | 14.1 | 0.02 | 64.6 |
| Controlling fruits and vegetables intake in models | |||||
| Yes | 6 | 0.87 (0.79–0.96) | 8.7 | 0.12 | 42.7 |
| No | 10 | 0.87 (0.78–0.98) | 23.6 | <0.01 | 61.8 |
To investigate the robustness of our findings, we performed sensitivity analyses yielding a narrow range of RRs for total stroke from a low of 0.86 (95% CI, 0.80–0.92) to a high of 0.89 (95% CI, 0.83–0.96) after exclusion of the study by Mineharu et al. (2011) and the study by Sato et al. (1989), respectively (data not shown).
3.4. Publication bias
There was little evidence of significant publication bias, as indicated by the Egger linear regression test (P=0.853).
4. Discussion
The current updated meta-analysis of 14 prospective cohort studies demonstrates a significant inverse association between tea consumption and risk of total stroke, especially ischemic stroke, in a dose-response manner. An increase of 3 cups/d in tea consumption was associated with 13% and 24% decreased risks of total stroke and ischemic stroke, respectively. Tea intake was non-significantly inversely associated with hemorrhagic stroke. The relationship persisted and remained statistically significant among most subgroups stratified by characteristics of study designs and populations.
Of note, both black tea and green tea had beneficial attributes in lowering the risk of stroke, and the observed protective effects tended to be more remarkable for green tea than for black tea. This may be attributed to the presence of polyphenolic compounds, particularly catechins, in green and black tea. Catechins, a major category of polyphenols in tea, exert a wide spectrum of beneficial effects against cardiovascular disease, including anti-oxidative, anti-inflammatory, anti-endothelial dysfunction, anti-hypertensive, and lipid lowering effects (Babu and Liu, 2008). Dry materials of green tea leaves are comprised of 30%–42% catechins, while dried black tea leaves, which undergo oxidation during manufacturing, contain 3%–10% catechins (Graham, 1992). Therefore, if catechins were the most important contributor to the beneficial effect of tea on stroke risk, the difference in protective strengths between green and black tea is reasonable.
Our results were consistent with a previously conducted meta-analysis (Arab et al., 2009). The present meta-analysis, with two times more cases and subjects, allowed us to improve the precision of risk estimates and conduct subgroup analyses to investigate potential sources of heterogeneity, thereby enhancing the clinical relevance of our findings. In addition, the presence of a dose-response relationship provided an accurate quantitative assessment. Furthermore, a prospective cohort design was used in all included studies, which should reduce selection and recall biases. Given the absence of large population-based, long-term, randomized intervention trials, our current study is a powerful approach to assess the long-term effects of tea drinking on stroke risk.
The beneficial effects of tea on stroke have a strong biological basis. High blood pressure is the most predominant risk factor for all stroke types (Goldstein et al., 2011; Roger et al., 2012). Evidence from human studies suggests that the risk of developing hypertension could be significantly reduced by regularly consuming green or oolong tea (Yang et al., 2004). Similarly, a recent randomized trial has documented that long-term consumption of black tea was significantly associated with the reduction of blood pressure (Hodgson et al., 2012). Moreover, tea consumption could favorably modulate plasma lipid profile (Inami et al., 2007; Zheng et al., 2011; Bahorun et al., 2012), and decrease plasma glucose and hemoglobin A1c levels (Fukino et al., 2008), further reducing the risk of atherosclerosis formation (Geleijnse et al., 1999; Debette et al., 2008) and diabetes (Huxley et al., 2009; Jing et al., 2009), which are major risk factors of stroke. In addition, tea ingestion has been found to decrease body weight (Basu et al., 2010) and enhance endothelial function (Ras et al., 2011), all of which might reduce the risk of stroke.
Several potential limitations of this meta-analysis should be acknowledged. First, because of the observational design, we cannot completely rule out the possibility that the observed associations were due to residual confounding. For instance, individuals with high consumption of tea are more likely to adopt a healthy lifestyle (Sesso et al., 2003b; Kuriyama et al., 2006), such as high levels of physical activity (Lee et al., 2003) and eating more fruits and vegetables (He et al., 2006), which have been shown to reduce the risk of stroke. However, in our subgroup analyses which controlled for physical activity as well as fruits and vegetables intake, the inverse association between tea intake and stroke risk persisted and remained statistically significant. Moreover, a wide range of potential confounders, including stroke risk factors, and other lifestyle and dietary factors, were adjusted for in the original studies; thus potential bias due to these factors should be reduced. Second, potential biases due to the misclassification of tea consumption might influence the findings, since measurement of tea consumption was only made at baseline in most studies, which failed to reflect long-term exposure and consider changes over time. Tea exposure was assessed using number of cups of tea consumed daily or weekly in the included studies, however, while differences in cup size and brew strength might arise from different populations. Daily or weekly amounts of dry tea leaves used for brewing may permit better quantification of tea consumption. Third, there was an insufficient number of studies which investigated the association between tea consumption and hemorrhagic stroke, and hence the observed non-significant inverse association between tea and hemorrhagic stroke may be due to type II errors and should be interpreted with caution. Fourth, substantial heterogeneity was observed across studies, which was expected considering differences in types of tea (e.g., green vs. black tea), methods of tea preparation (e.g., amounts of tea leaves, cup size, brewing time, water temperatures, and addition of milk or sugar), participants’ characteristics (e.g., gender, geographic region, genetic background, and gene-environment interactions), stroke measures, and analysis strategies. In our subgroup analyses, although moderate heterogeneity still remained in many subgroups, the summary estimates showed consistent inverse relationships. Finally, publication bias remains a concern in the meta-analysis; however, no significant evidence of publication bias was detected.
5. Conclusions
In summary, the present meta-analysis of prospective cohort studies provided strong support of a significant inverse association between tea consumption and risk of stroke, in a dose-response relationship. Further studies are warranted to address whether tea consumption is related to stroke subtypes and to explore the underlying mechanisms.
Footnotes
Project (No. 2007C13058) supported by the Science and Technology Key Project of Zhejiang Province, China
References
- 1.Arab L, Liu W, Elashoff D. Green and black tea consumption and risk of stroke: a meta-analysis. Stroke. 2009;40(5):1786–1792. doi: 10.1161/STROKEAHA.108.538470. [DOI] [PubMed] [Google Scholar]
- 2.Babu PV, Liu D. Green tea catechins and cardiovascular health: an update. Curr Med Chem. 2008;15(18):1840–1850. doi: 10.2174/092986708785132979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bahorun T, Luximon-Ramma A, Neergheen-Bhujun VS, Gunness TK, Googoolye K, Auger C, Crozier A, Aruoma OI. The effect of black tea on risk factors of cardiovascular disease in a normal population. Prev Med. 2012;54(Suppl.):S98–S102. doi: 10.1016/j.ypmed.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Basu A, Sanchez K, Leyva MJ, Wu M, Betts NM, Aston CE, Lyons TJ. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J Am Coll Nutr. 2010;29(1):31–40. doi: 10.1080/07315724.2010.10719814. [DOI] [PubMed] [Google Scholar]
- 5.Bidel S, Hu G, Qiao Q, Jousilahti P, Antikainen R, Tuomilehto J. Coffee consumption and risk of total and cardiovascular mortality among patients with type 2 diabetes. Diabetologia. 2006;49(11):2618–2626. doi: 10.1007/s00125-006-0435-9. [DOI] [PubMed] [Google Scholar]
- 6.Cheng TO. All teas are not created equal: the Chinese green tea and cardiovascular health. Int J Cardiol. 2006;108(3):301–308. doi: 10.1016/j.ijcard.2005.05.038. [DOI] [PubMed] [Google Scholar]
- 7.de Koning Gans JM, Uiterwaal CS, van der Schouw YT, Boer JM, Grobbee DE, Verschuren WM, Beulens JW. Tea and coffee consumption and cardiovascular morbidity and mortality. Arterioscler Thromb Vasc Biol. 2010;30(8):1665–1671. doi: 10.1161/ATVBAHA.109.201939. [DOI] [PubMed] [Google Scholar]
- 8.Debette S, Courbon D, Leone N, Gariépy J, Tzourio C, Dartigues JF, Barberger-Gateau P, Ritchie K, Alpérovitch A, Amouyel P, et al. Tea consumption is inversely associated with carotid plaques in women. Arterioscler Thromb Vasc Biol. 2008;28(2):353–359. doi: 10.1161/ATVBAHA.107.151928. [DOI] [PubMed] [Google Scholar]
- 9.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 10.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukino Y, Ikeda A, Maruyama K, Aoki N, Okubo T, Iso H. Randomized controlled trial for an effect of green tea-extract powder supplementation on glucose abnormalities. Eur J Clin Nutr. 2008;62(8):953–960. doi: 10.1038/sj.ejcn.1602806. [DOI] [PubMed] [Google Scholar]
- 12.Gao X, LaValley MP, Tucker KL. Prospective studies of dairy product and calcium intakes and prostate cancer risk: a meta-analysis. J Natl Cancer Inst. 2005;97(23):1768–1777. doi: 10.1093/jnci/dji402. [DOI] [PubMed] [Google Scholar]
- 13.Geleijnse JM, Launer LJ, Hofman A, Pols HA, Witteman JC. Tea flavonoids may protect against atherosclerosis: the Rotterdam Study. Arch Intern Med. 1999;159(18):2170–2174. doi: 10.1001/archinte.159.18.2170. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, Creager MA, Culebras A, Eckel RH, Hart RG, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(2):517–584. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]
- 15.Graham HN. Green tea composition, consumption, and polyphenol chemistry. Prev Med. 1992;21(3):334–350. doi: 10.1016/0091-7435(92)90041-f. [DOI] [PubMed] [Google Scholar]
- 16.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 17.He FJ, Nowson CA, MacGregor GA. Fruit and vegetable consumption and stroke: meta-analysis of cohort studies. Lancet. 2006;367(9507):320–326. doi: 10.1016/S0140-6736(06)68069-0. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirvonen T, Virtamo J, Korhonen P, Albanes D, Pietinen P. Intake of flavonoids, carotenoids, vitamins C and E, and risk of stroke in male smokers. Stroke. 2000;31(10):2301–2306. doi: 10.1161/01.str.31.10.2301. [DOI] [PubMed] [Google Scholar]
- 20.Hodgson JM, Puddey IB, Woodman RJ, Mulder TP, Fuchs D, Scott K, Croft KD. Effects of black tea on blood pressure: a randomized controlled trial. Arch Intern Med. 2012;172(2):186–188. doi: 10.1001/archinte.172.2.186. [DOI] [PubMed] [Google Scholar]
- 21.Huxley R, Lee CM, Barzi F, Timmermeister L, Czernichow S, Perkovic V, Grobbee DE, Batty D, Woodward M. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: a systematic review with meta-analysis. Arch Intern Med. 2009;169(22):2053–2063. doi: 10.1001/archinternmed.2009.439. [DOI] [PubMed] [Google Scholar]
- 22.Inami S, Takano M, Yamamoto M, Murakami D, Tajika K, Yodogawa K, Yokoyama S, Ohno N, Ohba T, Sano J, et al. Tea catechin consumption reduces circulating oxidized low-density lipoprotein. Int Heart J. 2007;48(6):725–732. doi: 10.1536/ihj.48.725. [DOI] [PubMed] [Google Scholar]
- 23.Iwai N, Ohshiro H, Kurozawa Y, Hosoda T, Morita H, Funakawa K, Okamoto M, Nose T. Relationship between coffee and green tea consumption and all-cause mortality in a cohort of a rural Japanese population. J Epidemiol. 2002;12(3):191–198. doi: 10.2188/jea.12.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jing Y, Han G, Hu Y, Bi Y, Li L, Zhu D. Tea consumption and risk of type 2 diabetes: a meta-analysis of cohort studies. J Gen Intern Med. 2009;24(5):557–562. doi: 10.1007/s11606-009-0929-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keli SO, Hertog MG, Feskens EJ, Kromhout D. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: the Zutphen study. Arch Intern Med. 1996;156(6):637–642. [PubMed] [Google Scholar]
- 26.Klatsky AL, Armstrong MA, Friedman GD. Coffee, tea, and mortality. Ann Epidemiol. 1993;3(4):375–381. doi: 10.1016/1047-2797(93)90064-b. [DOI] [PubMed] [Google Scholar]
- 27.Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N, Nishino Y, Tsubono Y, Tsuji I. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. JAMA. 2006;296(10):1255–1265. doi: 10.1001/jama.296.10.1255. [DOI] [PubMed] [Google Scholar]
- 28.Larsson SC, Mannisto S, Virtanen MJ, Kontto J, Albanes D, Virtamo J. Coffee and tea consumption and risk of stroke subtypes in male smokers. Stroke. 2008;39(6):1681–1687. doi: 10.1161/STROKEAHA.107.504183. [DOI] [PubMed] [Google Scholar]
- 29.Lee CD, Folsom AR, Blair SN. Physical activity and stroke risk: a meta-analysis. Stroke. 2003;34(10):2475–2481. doi: 10.1161/01.STR.0000091843.02517.9D. [DOI] [PubMed] [Google Scholar]
- 30.Leurs LJ, Schouten LJ, Goldbohm RA, van den Brandt PA. Total fluid and specific beverage intake and mortality due to IHD and stroke in the Netherlands Cohort Study. Br J Nutr. 2010;104(8):1212–1221. doi: 10.1017/S0007114510001923. [DOI] [PubMed] [Google Scholar]
- 31.Lopez-Garcia E, Rodriguez-Artalejo F, Rexrode KM, Logroscino G, Hu FB, van Dam RM. Coffee consumption and risk of stroke in women. Circulation. 2009;119(8):1116–1123. doi: 10.1161/CIRCULATIONAHA.108.826164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mineharu Y, Koizumi A, Wada Y, Iso H, Watanabe Y, Date C, Yamamoto A, Kikuchi S, Inaba Y, Toyoshima H, et al. Coffee, green tea, black tea and oolong tea consumption and risk of mortality from cardiovascular disease in Japanese men and women. J Epidemiol Community Health. 2011;65(3):230–240. doi: 10.1136/jech.2009.097311. [DOI] [PubMed] [Google Scholar]
- 33.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J. 2006;6(1):40–57. [Google Scholar]
- 35.Pan A, Sun Q, Okereke OI, Rexrode KM, Hu FB. Depression and risk of stroke morbidity and mortality: a meta-analysis and systematic review. JAMA. 2011;306(11):1241–1249. doi: 10.1001/jama.2011.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peters U, Poole C, Arab L. Does tea affect cardiovascular disease A meta-analysis? A meta-analysis. Am J Epidemiol. 2001;154(6):495–503. doi: 10.1093/aje/154.6.495. [DOI] [PubMed] [Google Scholar]
- 37.Ras RT, Zock PL, Draijer R. Tea consumption enhances endothelial-dependent vasodilation; a meta-analysis. PLoS One. 2011;6(3):e16974. doi: 10.1371/journal.pone.0016974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato Y, Nakatsuka H, Watanabe T, Hisamichi S, Shimizu H, Fujisaku S, Ichinowatari Y, Ida Y, Suda S, Kato K, et al. Possible contribution of green tea drinking habits to the prevention of stroke. Tohoku J Exp Med. 1989;157(4):337–343. doi: 10.1620/tjem.157.337. [DOI] [PubMed] [Google Scholar]
- 40.Sesso HD, Gaziano JM, Liu S, Buring JE. Flavonoid intake and the risk of cardiovascular disease in women. Am J Clin Nutr. 2003;77(6):1400–1408. doi: 10.1093/ajcn/77.6.1400. [DOI] [PubMed] [Google Scholar]
- 41.Sesso HD, Paffenbarger RS, Jr, Oguma Y, Lee IM. Lack of association between tea and cardiovascular disease in college alumni. Int J Epidemiol. 2003;32(4):527–533. doi: 10.1093/ije/dyg103. [DOI] [PubMed] [Google Scholar]
- 42.Tanabe N, Suzuki H, Aizawa Y, Seki N. Consumption of green and roasted teas and the risk of stroke incidence: results from the Tokamachi-Nakasato cohort study in Japan. Int J Epidemiol. 2008;37(5):1030–1040. doi: 10.1093/ije/dyn211. [DOI] [PubMed] [Google Scholar]
- 43.WHO (World Health Organization) Ten leading causes of deaths in 2008. 2011. Available from http://gamapserver.who.int/gho/interactive_charts/mbd/cod_2008/graph.html. [accessed on Apr. 8, 2012]
- 44.Yang YC, Lu FH, Wu JS, Wu CH, Chang CJ. The protective effect of habitual tea consumption on hypertension. Arch Intern Med. 2004;164(14):1534–1540. doi: 10.1001/archinte.164.14.1534. [DOI] [PubMed] [Google Scholar]
- 45.Yochum L, Kushi LH, Meyer K, Folsom AR. Dietary flavonoid intake and risk of cardiovascular disease in postmenopausal women. Am J Epidemiol. 1999;149(10):943–949. doi: 10.1093/oxfordjournals.aje.a009738. [DOI] [PubMed] [Google Scholar]
- 46.Zheng XX, Xu YL, Li SH, Liu XX, Hui R, Huang XH. Green tea intake lowers fasting serum total and LDL cholesterol in adults: a meta-analysis of 14 randomized controlled trials. Am J Clin Nutr. 2011;94(2):601–610. doi: 10.3945/ajcn.110.010926. [DOI] [PubMed] [Google Scholar]