Abstract
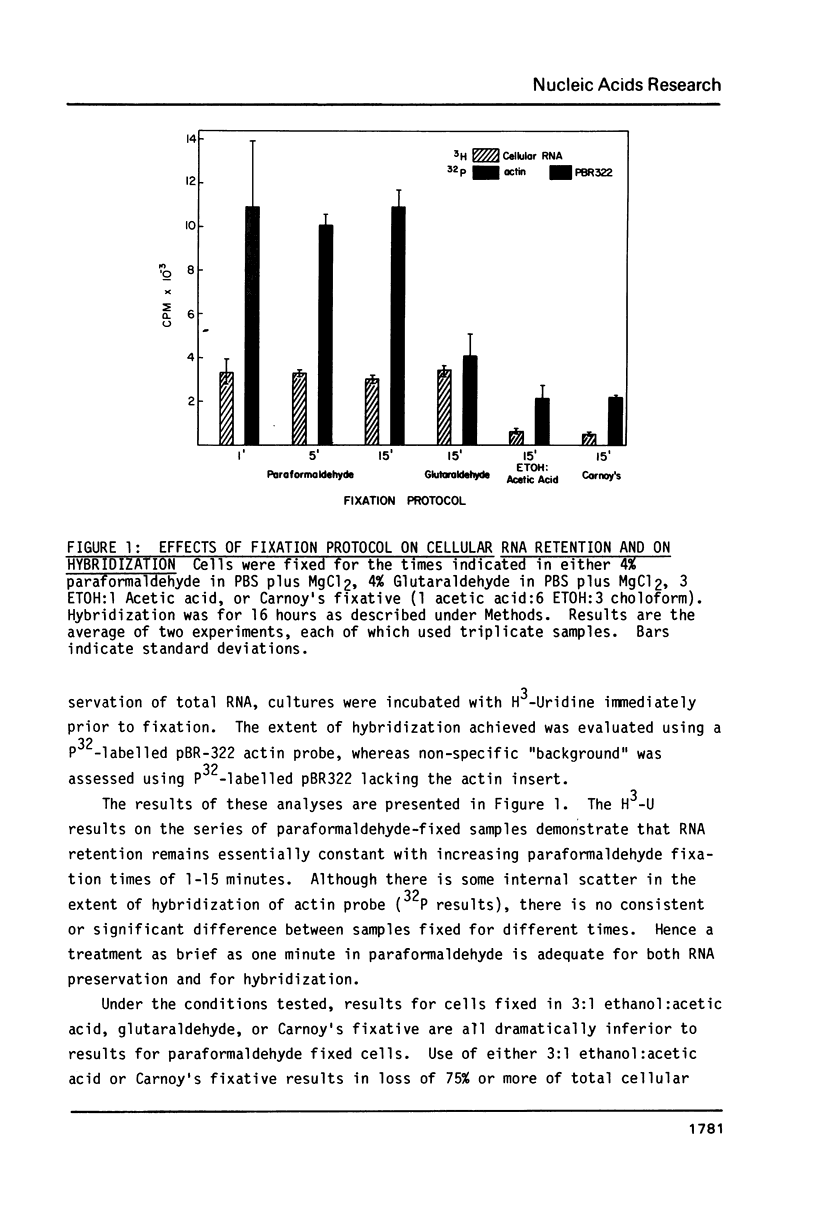
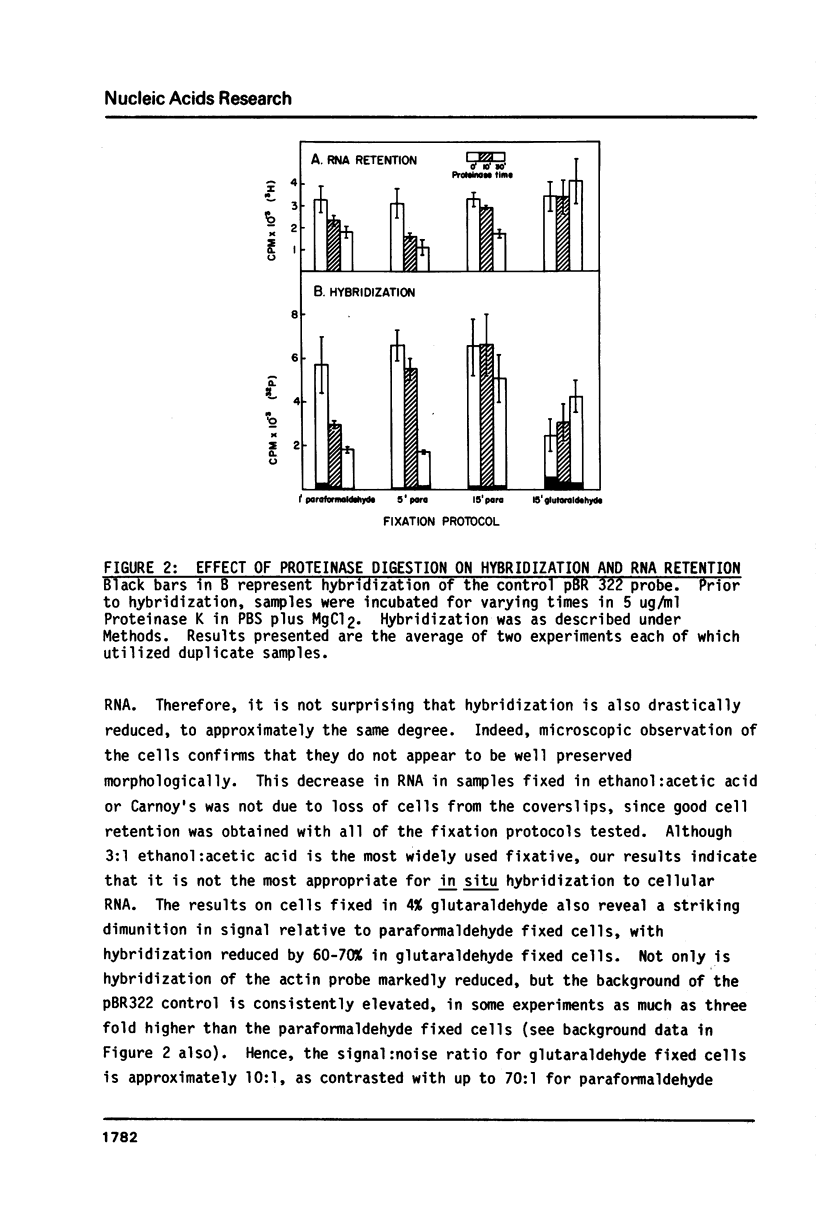
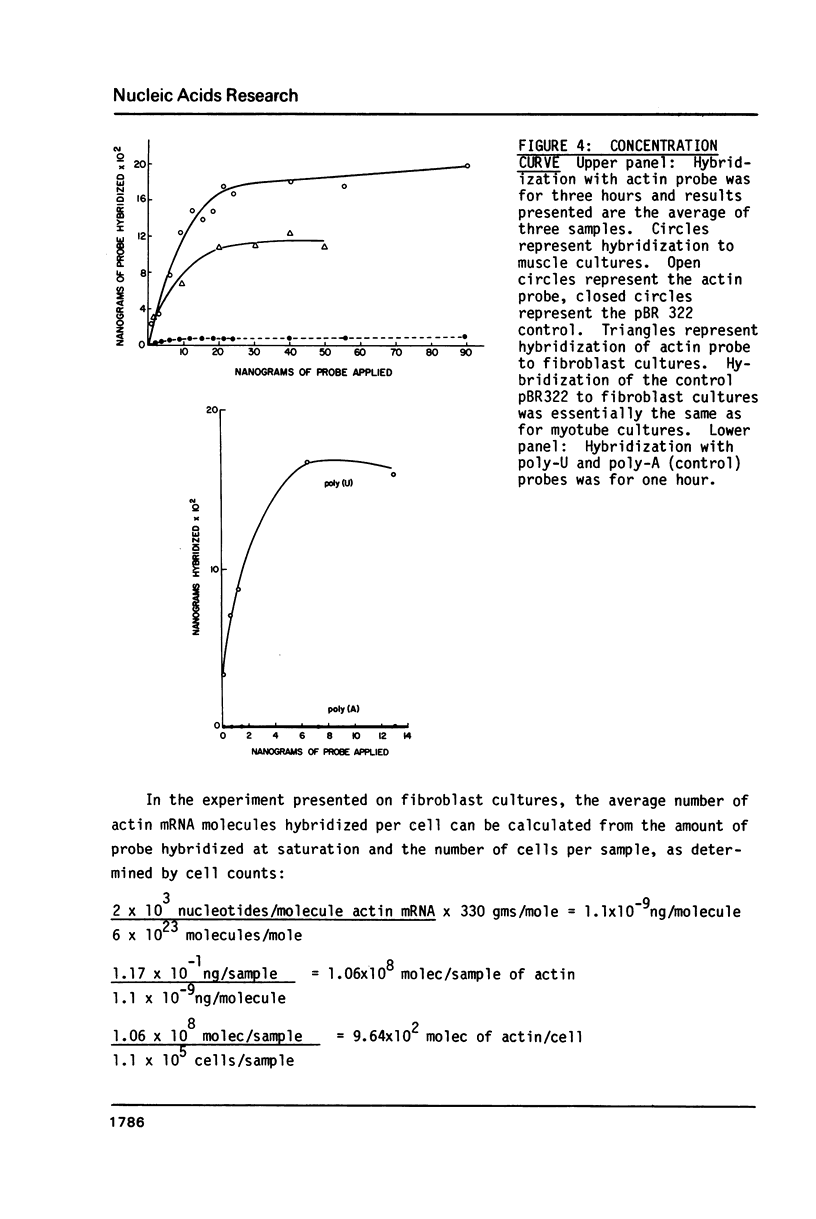
We have implemented an efficient, quantitative approach for the optimization of in situ hybridization using double-stranded recombinant DNA probes. The model system studied was actin mRNA expression in chicken embryonic muscle cultures. Actin and control (pBR322) probes were nick-translated with p32 labeled nucleotides, hybridized to cells grown on coverslips, and quantitated in a scintillation counter. Cellular RNA retention was monitored via the incorporation of H3-Uridine into RNA prior to cell fixation. Over a thousand samples were analyzed, and among the technical variables examined were the fixation protocol, proteolytic cell pretreatment, the time course of hybridization, saturation kinetics, hybridization efficiency, and effect of probe size on hybridization and network formation. Results have allowed us to develop a reproducible in situ hybridization methodology which is simpler and less destructive to cellular RNA and morphology than other protocols. Moreover, this technique is highly sensitive and efficient in detection of cellular RNAs. Lastly, the rapid quantitative approach used for this analysis is valuable in itself as a potential alternative to filter or solution hybridizations.
Full text
PDF



















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. S., Noonan D., Jeffery W. R. A model for the organization of the poly(A) . protein complex in messenger ribonucleoprotein. FEBS Lett. 1980 May 19;114(1):115–118. doi: 10.1016/0014-5793(80)80872-6. [DOI] [PubMed] [Google Scholar]
- Affara N. A., Robert B., Jacquet M., Buckingham M. E., Gros F. Changes in gene expression during myogenic differentiation. I. Regulation of messenger RNA sequences expressed during myotube formation. J Mol Biol. 1980 Jul 15;140(4):441–458. doi: 10.1016/0022-2836(80)90264-8. [DOI] [PubMed] [Google Scholar]
- Angerer L. M., Angerer R. C. Detection of poly A+ RNA in sea urchin eggs and embryos by quantitative in situ hybridization. Nucleic Acids Res. 1981 Jun 25;9(12):2819–2840. doi: 10.1093/nar/9.12.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S. L., Birkenmeier C. S. Inhibition of intractable nucleases with ribonucleoside--vanadyl complexes: isolation of messenger ribonucleic acid from resting lymphocytes. Biochemistry. 1979 Nov 13;18(23):5143–5149. doi: 10.1021/bi00590a018. [DOI] [PubMed] [Google Scholar]
- Bowman L. H., Emerson C. P., Jr Formation and stability of cytoplasmic mRNAs during myoblast differentiation: pulse-chase and density labeling analyses. Dev Biol. 1980 Nov;80(1):146–166. doi: 10.1016/0012-1606(80)90505-9. [DOI] [PubMed] [Google Scholar]
- Brahic M., Haase A. T. Detection of viral sequences of low reiteration frequency by in situ hybridization. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6125–6129. doi: 10.1073/pnas.75.12.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigati D. J., Myerson D., Leary J. J., Spalholz B., Travis S. Z., Fong C. K., Hsiung G. D., Ward D. C. Detection of viral genomes in cultured cells and paraffin-embedded tissue sections using biotin-labeled hybridization probes. Virology. 1983 Apr 15;126(1):32–50. doi: 10.1016/0042-6822(83)90460-9. [DOI] [PubMed] [Google Scholar]
- Capco D. G., Jeffery W. R. Differential distribution of poly(A)-containing RNA in the embryonic cells of Oncopeltus fasciatus. Analysis by in situ hybridization with a [3H]poly(U) probe. Dev Biol. 1978 Nov;67(1):137–151. doi: 10.1016/0012-1606(78)90305-6. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Cox K. H., DeLeon D. V., Angerer L. M., Angerer R. C. Detection of mrnas in sea urchin embryos by in situ hybridization using asymmetric RNA probes. Dev Biol. 1984 Feb;101(2):485–502. doi: 10.1016/0012-1606(84)90162-3. [DOI] [PubMed] [Google Scholar]
- Edwards M. K., Wood W. B. Location of specific messenger RNAs in Caenorhabditis elegans by cytological hybridization. Dev Biol. 1983 Jun;97(2):375–390. doi: 10.1016/0012-1606(83)90094-5. [DOI] [PubMed] [Google Scholar]
- Favre A., Morel C., Scherrer K. The secondary structure and poly(A) content of globin messenger RNA as a pure RNA and in polyribosome-derived ribonucleoprotein complexes. Eur J Biochem. 1975 Sep 1;57(1):147–157. doi: 10.1111/j.1432-1033.1975.tb02285.x. [DOI] [PubMed] [Google Scholar]
- Gall J. G., Pardue M. L. Formation and detection of RNA-DNA hybrid molecules in cytological preparations. Proc Natl Acad Sci U S A. 1969 Jun;63(2):378–383. doi: 10.1073/pnas.63.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee C. E., Roberts J. L. In situ hybridization histochemistry: a technique for the study of gene expression in single cells. DNA. 1983;2(2):157–163. doi: 10.1089/dna.1983.2.157. [DOI] [PubMed] [Google Scholar]
- Gerhard D. S., Kawasaki E. S., Bancroft F. C., Szabo P. Localization of a unique gene by direct hybridization in situ. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3755–3759. doi: 10.1073/pnas.78.6.3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godard C. M. Improved method for detection of cellular transcripts by in situ hybridization. Detection of virus-specific RNA in rous sarcoma virus-infected cells by in situ hybridization to cDNA. Histochemistry. 1983;77(1):123–131. doi: 10.1007/BF00496643. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Stowring L., Harris J. D., Traynor B., Ventura P., Peluso R., Brahic M. Visna DNA synthesis and the tempo of infection in vitro. Virology. 1982 Jun;119(2):399–410. doi: 10.1016/0042-6822(82)90099-x. [DOI] [PubMed] [Google Scholar]
- Hafen E., Levine M., Garber R. L., Gehring W. J. An improved in situ hybridization method for the detection of cellular RNAs in Drosophila tissue sections and its application for localizing transcripts of the homeotic Antennapedia gene complex. EMBO J. 1983;2(4):617–623. doi: 10.1002/j.1460-2075.1983.tb01472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S., Gillam I. C., Delaney A. D., Tener G. M. Acetylation of chromosome squashes of Drosophila melanogaster decreases the background in autoradiographs from hybridization with [125I]-labeled RNA. J Histochem Cytochem. 1978 Aug;26(8):677–679. doi: 10.1177/26.8.99471. [DOI] [PubMed] [Google Scholar]
- Jeffery W. R. Messenger RNA in the cytoskeletal framework: analysis by in situ hybridization. J Cell Biol. 1982 Oct;95(1):1–7. doi: 10.1083/jcb.95.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John H. A., Patrinou-Georgoulas M., Jones K. W. Detection of myosin heavy chain mRNA during myogenesis in tissue culture by in vitro and in situ hybridization. Cell. 1977 Oct;12(2):501–508. doi: 10.1016/0092-8674(77)90126-x. [DOI] [PubMed] [Google Scholar]
- Langer-Safer P. R., Levine M., Ward D. C. Immunological method for mapping genes on Drosophila polytene chromosomes. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4381–4385. doi: 10.1073/pnas.79.14.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister L. B., Scheller R. H., Kandel E. R., Axel R. In situ hybridization to study the origin and fate of identified neurons. Science. 1983 Nov 18;222(4625):800–808. doi: 10.1126/science.6356362. [DOI] [PubMed] [Google Scholar]
- Moon R. T., Nicosia R. F., Olsen C., Hille M. B., Jeffery W. R. The cytoskeletal framework of sea urchin eggs and embryos: developmental changes in the association of messenger RNA. Dev Biol. 1983 Feb;95(2):447–458. doi: 10.1016/0012-1606(83)90046-5. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Saidapet C. R., Munro H. N., Valgeirsdóttir K., Sarkar S. Quantitation of muscle-specific mRNAs by using cDNA probes during chicken embryonic muscle development in ovo. Proc Natl Acad Sci U S A. 1982 May;79(10):3087–3091. doi: 10.1073/pnas.79.10.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. J., Rothblum K. N. Gene switching in myogenesis: differential expression of the chicken actin multigene family. Biochemistry. 1981 Jul 7;20(14):4122–4129. doi: 10.1021/bi00517a027. [DOI] [PubMed] [Google Scholar]
- Singer R. H., Kessler-Icekson G. Stability of polyadenylated RNA in differentiating myogenic cells. Eur J Biochem. 1978 Aug 1;88(2):395–402. doi: 10.1111/j.1432-1033.1978.tb12461.x. [DOI] [PubMed] [Google Scholar]
- Singer R. H., Ward D. C. Actin gene expression visualized in chicken muscle tissue culture by using in situ hybridization with a biotinated nucleotide analog. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7331–7335. doi: 10.1073/pnas.79.23.7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venezky D. L., Angerer L. M., Angerer R. C. Accumulation of histone repeat transcripts in the sea urchin egg pronucleus. Cell. 1981 May;24(2):385–391. doi: 10.1016/0092-8674(81)90328-7. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman J. L., Petri W., Meselson M. Accumulation of a specific subset of D. melanogaster heat shock mRNAs in normal development without heat shock. Cell. 1983 Apr;32(4):1161–1170. doi: 10.1016/0092-8674(83)90299-4. [DOI] [PubMed] [Google Scholar]




