ABSTRACT
Individuals diagnosed with impaired glucose tolerance (i.e., prediabetes) are at increased risk for developing diabetes. We proposed a clinical trial with a novel adaptive randomization designed to examine the impact of a home-based physical activity (PA) counseling intervention on metabolic risk in prediabetic elders. This manuscript details the lessons learned relative to recruitment, study design, and implementation of a 12-month randomized controlled PA counseling trial. A detailed discussion on how we responded to unforeseen challenges is provided. A total of 302 older patients with prediabetes were randomly assigned to either PA counseling or usual care. A novel adaptive design that reallocated counseling intensity based on self-report of adherence to PA was initiated but revised when rates of non-response were lower than projected. This study presents baseline participant characteristics and discusses unwelcome adaptations to a highly innovative study design to increase PA and enhance glucose metabolism when the best-laid plans went awry.
KEYWORDS: Prediabetes, Adaptive design, Randomized controlled trial, Telephone, Exercise, Aging
INTRODUCTION
Type 2 diabetes is a common, chronic, and costly disease affecting an estimated 23% of adults over the age of 60 and is the seventh leading cause of death in the USA [1]. The prevalence of diabetes is even higher (~28%) among users of Department of Veterans Affairs (VA) healthcare [2]. The annual incidence of diabetes in veterans is ~2% per year [2] and is the third most common diagnosis in the VA healthcare system [2]. Two known contributors to the increased incidence of diabetes are the increased epidemic of obesity and low levels of physical activity. Prediabetes, an intermediate category between normoglycemia and diabetes, is a clinical diagnosis characterizing those individuals who have fasting blood glucose levels higher than normal (>100 mg/dL) but not high enough to be classified as diabetes (>126 mg/dL). Recent estimates by the Department of Health and Human Services suggest that prediabetes is becoming more common, affecting nearly 79 million adults in 2010 [1].
The VA has aggressively addressed the existing obesity and diabetes epidemics with the implementation of weight reduction programs (e.g., MOVE! Weight Management Program for Veterans [3]), various diabetes education programs, and performance measures aimed at attaining optimal management of dyslipidemia, diabetes, and obesity. Despite overwhelming evidence that physical activity can favorably impact all of the medical conditions described above, staffing resources and space for physical activity are inequitable throughout the VA; this is especially true for the older Veteran. A study by Littman et al. [4], reported that 59.2% of veterans over the age of 70 do little to no activity, underscoring the magnitude of the sedentary problem among our older veterans. Furthermore, the MOVE! program is only targeted toward veterans under the age of 70.
Physical activity promotion efforts for diabetes control have typically been limited to group-based programs delivered in a controlled, classroom environment. Barriers such as travel (distance and time), transportation, time constraints, and mobility requirements may limit accessibility of such programs. Given these challenges, promoting increased physical activity via remote pathways, such as the telephone, may provide an attractive alternative to older adults. We developed a multicomponent physical activity counseling (PAC) program that successfully increased physical activity and improved rapid gait speed in older veterans [5, 6]. Given the literature suggesting improved glycemic control with moderate doses of physical activity, we proposed to examine whether our PAC, which promotes moderate physical activity tailored to older adults, would provide glycemic benefit to older overweight veterans with impaired glucose tolerance.
The Enhancing Fitness in Older Overweight Veterans with Impaired Fasting Glucose Study (Enhanced Fitness) is a 12-month randomized controlled clinical trial comparing a multi-component PAC program to usual care (UC). Recognizing that lifestyle change and chronic disease management require a dynamic approach which includes modifying treatment protocols to cope with evolving barriers and successes, the Enhanced Fitness Study proposed a highly innovative adaptive randomization design.
Adaptive design studies identify a priori intermediate, early markers of response which indicate whether the current treatment protocol is effective or whether alternative treatments are needed in order to impact a chosen outcome [7, 8]. Although adaptive designs are becoming more common in pharmaceutical trials, adaptive designs in behavioral studies are rare and, to our knowledge, have never been used to promote physical activity. Carels and colleagues [9, 10] report results of two behavioral adaptive design trials aimed at weight loss. Individuals not meeting weight loss targets at pre-determined points were re-assigned to receive higher doses of counseling which resulted in better weight loss. These studies suggest that an adaptive approach is more likely to achieve intermediate behavioral goals and have superior treatment outcomes compared to a “one size fits all” approach. The current study was designed to examine whether adopting an adaptive design approach to physical activity promotion would improve diabetes-specific health outcomes.
In this study, we proposed a two-stage adaptive design in which change in physical activity in response to our PAC intervention was assessed early in the study. Non-responders to PAC were to be reallocated to a more intense intervention which consisted of PAC plus group-based cognitive behavioral exercise counseling. It was hypothesized that cognitive behavioral exercise counseling would complement the activity counseling piece of the intervention by improving individuals’ attitudes toward physical activity and increasing activity behavior. Such improvements have been demonstrated in previous physical activity studies with older adults [11, 12]. Alternatively, responders to PAC were to be randomized to maintained or lower (less costly) doses of PAC.
This manuscript details the study design, theoretical framework, methods, baseline characteristics, and unexpected barriers during implementation of the Enhanced Fitness trial. Results of the trial will be presented in subsequent manuscripts.
PRIMARY RESEARCH GOALS
The primary hypothesis is that a multicomponent PAC intervention administered through a physician-endorsed, home-based model will have a beneficial and clinically meaningful impact on insulin metabolism and markers of the metabolic syndrome superior to UC. The primary outcome is improved insulin action as measured by fasting insulin and glucose levels using the homeostasis model assessment of insulin resistance (HOMA-IR) and hemoglobin A1C (HbA1c) as a secondary indicator of improved glycemic control. Other secondary outcomes include self-reports of physical activity, health-related quality of life, and physical function; a physical performance battery including measures of rapid and usual gait speed, chair stands, balance, and 6-min walk distance; fasting lipids, weight, waist circumference, and blood pressure.
STUDY DESIGN
Overview
Enhanced Fitness was originally designed as a four-arm adaptive randomization trial [7] to be conducted over a 3-year period in primary care clinics at the VA Medical Center (VAMC) in Durham, NC, USA, and the VA Community-Based Outpatient Clinic (CBOC) in Raleigh, NC, USA. Briefly, adaptive randomization, also known as sequential randomization, starts with an initial randomization. At some predetermined point, study participants are re-randomized based on responses to an intermediate measure—typically either an intermediate outcome or a measure of process, like compliance. Reassessment and re-randomization can occur more than once, hence the name sequential or adaptive randomization.
We chose the simplest adaptive design with only one intermediate assessment for re-randomization. Assessment, reassessment, and allocation to new therapies based on health outcomes are a hallmark of primary care. In the context of this behavioral trial, increased physical activity was reassessed at 3 months among all study participants. Individuals originally allocated to UC remained in UC. Among those allocated to PAC, individuals considered respondents, defined as completing 75% or more of their self-identified physical activity goals, were re-randomized to receive either a maintained (continued care) or less costly/less intensive intervention (reduced calls). All non-respondents initially randomized to PAC were to be offered a supplemental group-based cognitive behavioral exercise counseling class that has demonstrated success in enhancing physical activity among elders with chronic conditions [12]. This design led to a multiple-armed RCT with four “strategies” [13]: (1) enhanced fitness continued care, (2) enhanced fitness reduced calls, (3) enhanced fitness plus group-based cognitive behavioral exercise counseling, and (4) UC (see Fig. 1a).
Fig 1.
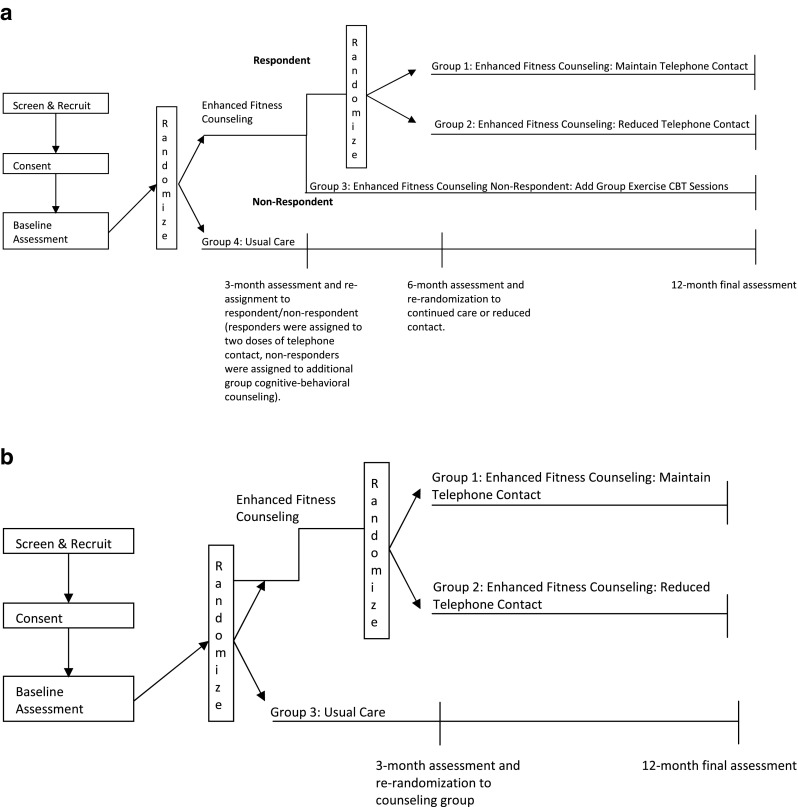
a Four-arm RCT design. b 3-arm RCT design
The purpose of this paper is to describe a series of lessons learned as we faced situations that forced us to amend the study design and eligibility criteria. Our ability to successfully navigate these challenges was due to our ongoing investigator meetings (weekly for core team and initially monthly for the entire team) in which these issues were discussed, debated, and resolved. Our first challenge arose when we determined that the rates of non-respondents were far lower than the projected 35%. During the first 6 months of study implementation, we noted that among the first 50 enrolled patients completing the 3-month assessment, none of these individuals met our criteria for non-respondents. During this time, we also examined data collected on our early drop-outs. Of six PAC participants who withdrew from the study prior to the 3-month assessment, only two would have been classified as non-responders.
Our study team (principal investigators, health counselor, statistical team) remained engaged in what would be a 6-month discussion during which we examined why we were experiencing greater drop-out in the PAC arm than anticipated and how to abate that and whether our initial determination of respondent/non-respondent was occurring too late in the intervention. Our investigation into the latter point indicated that in this study, contrary to our prior studies, there was no clear time point at which response to the intervention could be clearly determined across all individuals. These scientific discussions eventually led us to the realization that based on the numbers enrolled to date, it was logistically impractical and statistically unfeasible to retain the fourth arm of the trial. Thus, we were forced to alter the study design and eliminated the Group Exercise Counseling arm of the study. As such, Enhanced Fitness became a three-armed RCT: UC, enhanced fitness counseling continued care and enhanced fitness counseling reduced care groups (see Fig. 1b for modified study design). The latter two groups thus constituted the PAC group. This amended study design had no impact on the primary and secondary outcomes which included comparisons between PAC and UC, and we further note that the initial high early drop-out in the PAC group diminished over the course of the trial.
Theoretical framework for the enhanced fitness intervention
The core components of the Enhanced Fitness intervention were based upon the theoretical constructs developed for the Activity Counseling Trial, the Physician-Based Assessment and Counseling for Exercise Program, and other successful physical activity promotion trials [6, 14]. The primary theoretical basis of this intervention relies heavily on Social Cognitive Theory (SCT; [15, 16]), in which behavior influences, and is influenced by, within-person factors and factors in the social and physical environments. The primary intervention strategy is designed to enhance self-efficacy for physical activity, an important predictor of physical activity in older adults [17]. Elements of SCT integrated into this intervention include modeling, self-monitoring, goal setting, reinforcement, and cognitive reframing. We also used the transtheoretical model “stage of change” concept as a guide to select appropriate intervention materials for individuals based on their motivation and readiness to change behavior [18].
Figure 2 depicts how the theoretical framework for the Enhanced Fitness intervention was integrated into each component. Although the amendment to the design eliminating the group cognitive behavioral exercise counseling for the non-responders will not allow us to examine its impact, the overall integration of the theoretical framework for this study was not affected by this design change.
Fig 2.
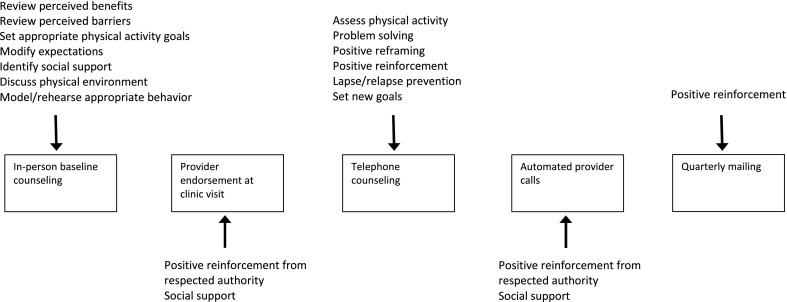
Theoretical framework of the Enhanced Fitness counseling intervention
Study participants
The principles guiding the selection of the inclusion and exclusion criteria for Enhanced Fitness were to ensure the enrollment of participants who satisfy four major criteria: (1) free of frank diabetes, but at high risk for developing diabetes; (2) independent mobility; (3) receiving primary care at the VA; and (4) over 60 years of age (see Table 1 for a complete list of eligibility criteria). Initially, our study was limited to veterans ages 65 and over. The unexpectedly high number of seniors already diagnosed with diabetes, 3,584 of 10,221 medical records screened, required us to lower our eligibility age to 60. We also originally intended to limit our study to BMI <40, thinking that morbidly obese individuals would be unable to complete the prescribed exercise regimen. However, early on in our recruitment efforts, we met and excluded several individuals during their in-person enrollment appointment who satisfied all other inclusion criteria save the BMI upper limit and who seemingly would benefit from our study without ill effects. As such, we amended the study to increase the upper BMI limit to 45. We excluded individuals who were already engaging in regular, vigorous physical activity and those who had medical contraindications to unsupervised physical activity. Individuals with documented substance abuse or psychiatric conditions (e.g., schizophrenia) that make them less likely to respond to the proposed intervention were also excluded. Eligibility was established through a multi-step process involving telephone and in-person screening.
Table 1.
Enhanced fitness inclusion and exclusion criteria
| Inclusion criteria | |
|---|---|
| Demographics | Adults 60 years of age or older |
| Patient status | Followed in Primary Care, Women’s Health, or Geriatric Clinics at Durham VAMC or Raleigh Outpatient Clinic (CBOC) with an assigned PCP, and have had at least one visit to the hospital or outpatient clinic in the previous 12 months |
| Fasting blood glucose | 100 mg/dL ≤ FBG ≤ 125 mg/dL, following an overnight fast |
| Glycated hemoglobin | HbA1c < 7% |
| BMI | 25 kg/m2 ≤ BMI ≤ 45 kg/m2 |
| Mobility | Able to walk 30 ft without human assistance (assistive devices were acceptable) |
| Exclusion criteria | |
| Diabetes | Clinical history of diabetes or newly diagnosed diabetes at screening |
| Recent history of CVD | Clinical history of cardiovascular disease (CVD) occurring within the past 6 months, including unstable angina, ventricular tachycardia, COPD with two or more hospitalizations within the past year and/or on oxygen, and stroke with moderate to severe aphasia |
| Hypertension | Uncontrolled hypertension (diastolic BP > 110 mmHg or systolic BP >200 mmHg) |
| Physical activity | Patients who had been regularly and vigorously physically active for 6 months or longer, or patients for whom physical activity would be unsafe or contraindicated |
| Medications | Patients taking insulin or other medication used to control sugar levels (i.e. Metformin). |
| Impairments | Hearing loss severe enough to interfere with the ability to receive telephone counseling, and visual impairment severe enough to interfere with the ability to review written materials |
| Other | Diagnosis of unstable mental or behavioral disorder, diagnosis of severe dementia, patients whose physician declined approval for another reason |
| Other chronic conditions | Diagnosis of chronic pain (interferes with patient’s ability to exercise) or a terminal diagnosis |
Recruitment
Recruitment of study participants began in the spring of 2008 and concluded in March of 2010. Initially, the medical records of veterans followed in Primary Care, Women’s Health, or Geriatric Clinics at Durham VAMC or Raleigh CBOC were screened. The names of eligible patients were sent to their primary care provider (PCP) for final approval. In an attempt to minimize patient travel burden, we tried to link the enrollment visit with another scheduled medical appointment at the hospital. Within 1 month of the patient’s scheduled medical appointment, the patient was mailed a letter of invitation describing the study, which included a copy of the consent form, a return postcard to indicate interest, and a cover letter of introduction from the patient’s PCP. Two weeks after the recruitment packet was mailed, a follow-up telephone call was made to invite formal patient enrollment. During that call, verbal consent was obtained. Prospective participants were also screened for cognitive, hearing, or visual impairments and completed an exercise history. Potentially eligible veterans were given an appointment for an in-person screening and enrollment session. After written consent was obtained during the enrollment visit, blood pressure, weight, height, waist circumference, pain score, and fasting glucose level as measured by a finger stick were recorded. If deemed eligible up to this point, individuals were sent to the phlebotomy laboratory of the Durham VA for additional blood work. They then returned to the study office to complete a baseline survey and performance assessment. With the exception of the study coordinator and the health counselor, all research personnel were blinded to individual group assignment for the duration of the study.
Randomization
After the baseline assessment was completed, patients were assigned to the Enhanced Fitness Counseling group or the Usual Care group using random allocation generated by the statistician. Re-randomization in adaptive design studies requires oversampling within the study; thus, allocation between the PAC and UC arms was not 1:1. We chose the simplest adaptive design (i.e., only one re-randomization point), which required us to oversample in the PAC arm so that subsequent secondary analyses pertaining to higher or lower doses of telephone contact would be adequately powered for comparisons against UC. As UC is presumably comprised of responders and non-responders, for comparative analyses, groups in the PAC arm also needed to be comprised of responders and non-responders. Thus, optimum sample size allocations (based upon the original four-arm study design) were calculated as 180 patients to PAC and 120 patients to UC.
Re-assignment to maintained telephone contact, reduced telephone contact, or maintained telephone contact + cognitive behavioral exercise counseling in the PAC arm was to occur at 3 months post-baseline. Following the amendment to eliminate the supplemental group exercise counseling, only re-randomization to maintained telephone contact or reduced telephone contact was retained. Block randomization ensured that PAC patients had a 50/50 chance of being assigned to either a higher or lower dose of contact.
Intervention delivery and materials
The Enhanced Fitness Counseling intervention included five primary elements: (1) in-person baseline counseling, (2) PCP endorsement at the next clinic visit, (3) telephone counseling for 1 year, (4) monthly automated telephone encouragement by the patient’s PCP starting at the second month, and (5) individualized quarterly feedback reports mailed to the participant summarizing progress in time spent on both walking and leg strengthening exercises compared to the desired goals. Previous research has demonstrated therapeutic benefits of both endurance and resistance exercise among individuals with insulin resistance [19]. Thus, consistent with recommendations from the American Diabetes Association [19], the American College of Sports Medicine, the American Heart Association [20], and the US Physical Activity Guidelines [21], the long-term goals of this study were for participants to accomplish: [1] 30 or more minutes of lower extremity aerobic exercise, preferably walking, on five or more days of the week, and [2] 15 min of exercises to increase lower extremity strength on three non-consecutive days each week, which is of particular importance to maximize mobility in older adults.
Contact schedule
The contact schedule for Enhanced Fitness Counseling Continued Care and Enhanced Fitness Counseling Reduced Care is in the following text and summarized in Table 2.
Table 2.
Enhanced Fitness counseling intervention elements
| Continued care group | Reduced care group | |
|---|---|---|
| In-person counseling at baseline | × | × |
| PCP endorsement at next clinic visit | × | × |
| Telephone counseling three times within first 6 weeks, then every 4 weeks | × | |
| Telephone counseling three times within first 6 weeks, every 4 weeks until 6 months, then every 8 weeks | × | |
| Automated-telephone PCP endorsement | × | × |
| Quarterly tailored mailed report | × | × |
Element 1: in-person baseline counseling
All participants randomized to the Enhanced Fitness Counseling intervention received an in-person consultation with the health counselor. A single health counselor with over 20 years of exercise and lifestyle counseling experience performed all of the counseling. The baseline counseling was designed to obtain an accurate view of the patient’s functional status, which may differ from the medical record, and establish a realistic starting point for engaging in physical activity. The counselor utilized a structured protocol called “Planning the First Step” first developed for the Veterans LIFE study [5] and modified for this study to reflect glycemic control. Individuals were given a notebook containing handouts on the health benefits of exercise, tips for exercising safely, and a poster with specific exercises. They were also given elastic bands of different resistances and a pedometer. During this initial consultation, the participants practiced leg strengthening exercises, modeled walking with a non-shuffling gait, and were shown how to record physical activity.
Element 2: PCP endorsement at the next clinic visit
The PCP was notified when a patient had enrolled in our study. The day before a patient in the PAC arm of our study had a routine clinic visit, we sent the PCP a reminder to endorse physical activity and/or participation in the Enhanced Fitness study and document the endorsement in the patient’s electronic file. We did not impose a standardized method of endorsement.
Element 3: telephone counseling
The health counselor contacted each participant three times for follow-up counseling within the first 6 weeks (biweekly) and once every 4 weeks thereafter for those randomized to the Continued Care. Those randomized to the Reduced Calls group received a phone call once every 8 weeks, after the initial 6 weeks. Each phone call adhered to a standardized protocol that reinforced continued physical activity, identified strategies for overcoming barriers, and helped customize individually feasible activities. The counselor also identified individual motivators with a focus on creating a physical and social environment conducive to increasing physical activity. Finally, alternative activities and benefits of exercise that the participant may not have previously considered were discussed.
Element 4: automated telephone encouragement by PCP
The literature suggests that PCPs may play a powerful role in promoting adherence to more healthful behaviors [22]. Indeed results of our previous telephone counseling studies [6] support the role of PCPs as an important influence over patients’ motivation to adhere to behavior change recommendations. However, most PCPs experience considerable time constraints in their day-to-day dealings with patients. To preserve clinician time, we use automated telephone calls to deliver messages of encouragement to the participants directly from their own PCP. Each participating PCP recorded a generic message encouraging their patient to keep up with the exercise program created through Enhanced Fitness. These automated calls, with participant names inserted in each greeting by the computer, were made monthly starting at the second month of the intervention and were a way of providing further support and provider endorsement.
We also sent birthday cards to all study participants as a way to encourage participant retention.
Element 5: quarterly tailored mail report
To give patients tangible reinforcement and motivation, participants were mailed a customized progress report every 3 months. The report consisted of a letter with a greeting of encouragement as well as a graph showing the participant’s change over time in comparison to the long-term goals of 150 total minutes of walking or other aerobic exercise each week and 45 total minutes of leg strengthening exercises per week. The message of encouragement was tailored for the following scenarios: (a) making regular progress, (b) improving time spent in leg strengthening exercises but not in walking, (c) improving time spent in walking but not in leg strengthening exercises, (d) decreasing time spent on one activity but not the other, (e) decreasing time in both activities, and (f) meeting the goals.
Usual care
Participants randomized to the Usual Care group received the regular standard of care as provided in their usual primary, women’s health, or geriatric clinic.
MOVE! Program
During the planning of this trial, the VA National Center for Health Promotion and Disease Prevention mandated the implementation of the MOVE! program. Reviewers of this grant noted the evolving implementation of MOVE! and expressed concern as to its impact on our study procedures and outcomes. Given the reviewers’ concern over this element, for resubmission we chose to embrace the MOVE! program as a soon-to-be standard of care. At this time, it was not clear to us how the mandated MOVE! program would be operationalized, so we arranged to have MOVE! consults submitted for each of our study participants. The Durham VA MOVE! offers a series of interactive group lessons promoting strategies to favorably modify lifestyle behaviors. Although consults to MOVE! are based on patient self-referral, we did not know that would be the case at the time this study was implemented.
Measures
Assessments were performed at baseline and at 3 and 12 months post-randomization at the Durham VAMC. Psychosocial measures and functional performance assessments were completed by trained study personnel.
Demographic and clinical characteristics
The following demographic and biometric characteristics were collected at baseline: age, gender, race/ethnicity, income, education level, height, weight, blood pressure, and waist circumference. To assess the effect of comorbid conditions on function as well as the potential effect of the intervention on symptom severity, we used the Older Americans Resources & Services Comorbidity and Symptom Index [23]. The index ascertains either an affirmative or negative response to unique medical conditions and selected symptoms. For each affirmative answer, the effect of the disease or symptom on function is assessed using a four-point Likert scale ranging from “not at all” to “quite a bit.”
Fasting blood glucose, insulin, lipids
Patients were instructed to refrain from eating or drinking anything, except for water and medications, past midnight the evening before their appointment, resulting in an 8-h fast consistent with ADA guidelines. All biochemical measurements, including fasting blood glucose, HbA1c, fasting insulin, and blood lipids, were performed in the Durham VAMC’s central laboratory by technicians not affiliated with the study. Fasting glucose and insulin levels were used to calculate HOMA-IR.
Physical activity
Physical activity was assessed with a modified version of the Community Healthy Activities Model Program for Seniors (CHAMPS) questionnaire [24, 25]. The CHAMPS questionnaire provides several scores for analysis. The CHAMPS questionnaire has good construct validity and reliability and is sensitive to change [25]. We have modified the CHAMPS so that all activities are collected using minutes of reported activity as a continuous variable [6] rather than pre-specified categorical variables, enabling us to capture small changes in minutes of physical activity. The advantages of the CHAMPS questionnaire for this study are that it was specifically developed for older adults and has been tested with interventions using home-based programming.
To validate self-reported physical activity, we collected ambulatory activity recordings from a subset of participants in each group using a StepWatch Activity Monitor (SAM; Cyma Corporation). These data will be used [1] to provide preliminary insight into the effect of our multicomponent physical activity counseling intervention on actual vs. self-report activity of study participants and [2] to validate the accuracy of step counts recorded with study pedometers. To achieve these objectives, we directly monitored 1 week of ambulatory activity in 34 control participants and 36 intervention participants at each measurement interval (baseline, 3 months, 12 months).
Health-related quality of life
The SF-36 [26] is a widely used measure of self-reported general health and function that has been validated across a variety of populations. Subscale scores range from 0 to 100, with higher scores indicating better function. The SF-36 and its subscales are well validated and are reliable and sensitive to change in this population [27, 28].
Physical performance
Gait speed was derived from an 8-ft walk test. This task was repeated to produce two trials of usual and fast walking speed with the fastest value for each task recorded for scoring purposes. Other tests of physical performance included three tests of standing balance and five chair stands, as described by Guralnik and colleagues [29], which, combined with usual gait speed, are used to calculate the score for the Short Physical Performance Battery. Scoring ranges from 0 to 12, with a higher score indicating better physical performance. Grip strength was measured with the selection of the best of three trials of preferred hand grip using a hand-held dynamometer. The 6-min walk test was used as an indicator of cardiorespiratory capacity. The 6-min walk test was performed in a secluded hallway, and the distance covered by each participant is measured and recorded. Each participant was tested individually and was supervised by trained research staff blinded to intervention status.
Social cognitive measures
We utilized four brief measures based on social cognitive theory that address [1] self-efficacy for walking [30, 2] self-efficacy for strengthening exercises, [3] motivation for physical activity [6], and [4] satisfaction with physical function.
MOVE! participation
To control for participation in the MOVE! program, we tracked participation in the MOVE! activities for all study participants. We note that participation in MOVE! activities is highly variable because patients must themselves call the MOVE! office to schedule an orientation session and each subsequent group counseling session. However, tracking MOVE! utilization among our patient group will provide us an opportunity to look at how MOVE! is utilized and its impact on health behavior and health outcomes.
Data analysis
The primary hypothesis of this trial was to evaluate the mean difference between PAC and UC groups on changes from baseline in insulin action-(HOMA). As noted previously, the adaptive design analysis required oversampling within the PAC group so that we would be appropriately powered to answer primary aims contrasting PAC overall to UC as well as secondary aims contrasting maintained dose PAC to UC and lower does PAC to UC. Fortunately, our revision to the adaptive design did not affect our ability to make these comparisons although it did result in disparate sample sizes within the comparison groups.
Sample size and power
The Enhanced Fitness Study targeted the recruitment and follow-up of 300 participants (PAC = 180, UC = 120). Prior studies of exercise [31] observed a standardized difference [32] of 0.56 on fasting insulin. If a similar effect was observed in this sample, controlling for two contrasts, we would project a power of 98.6%, assuming an overall type I error rate of 0.05 (two-tailed) for the primary contrast (PAC vs. UC). This design is 80% powered to detect a standardized difference of 0.39. These estimates include allowance for a 12.5% loss to follow-up over 12 months and a single primary outcome, HOMA. Changes to the protocol did not affect the power estimates for our primary and secondary aims.
RESULTS
We recruited participants until we reached our targeted sample size (N = 302). As indicated in Fig. 3, patients were recruited from an initial medical record screening of 10,221 age-eligible veterans. Of these, 1,778 met the initial study criteria. The most common reasons for exclusion during the review of medical records was that the patient did not have impaired glucose tolerance (n = 1,960) or the patient was not free from frank diabetes (n = 3,584). Fifteen additional potential participants were deemed ineligible by their PCP, leaving 1,763 to be contacted for enrollment. Telephone contact for enrollment was attempted for 1,398 individuals who indicated interest in the study or did not return an “opt out” postcard. Potential participants were subjected to further screening during this telephone contact. Of those that remained eligible, 561 patients agreed to report for an initial enrollment visit. A total of 496 participants completed further evaluations of eligibility (e.g., fingerstick for fasting glucose, laboratory blood draw, BMI). A subset of 194 individuals was deemed ineligible after consenting to participate in the study, resulting in 302 participants randomized.
Fig 3.
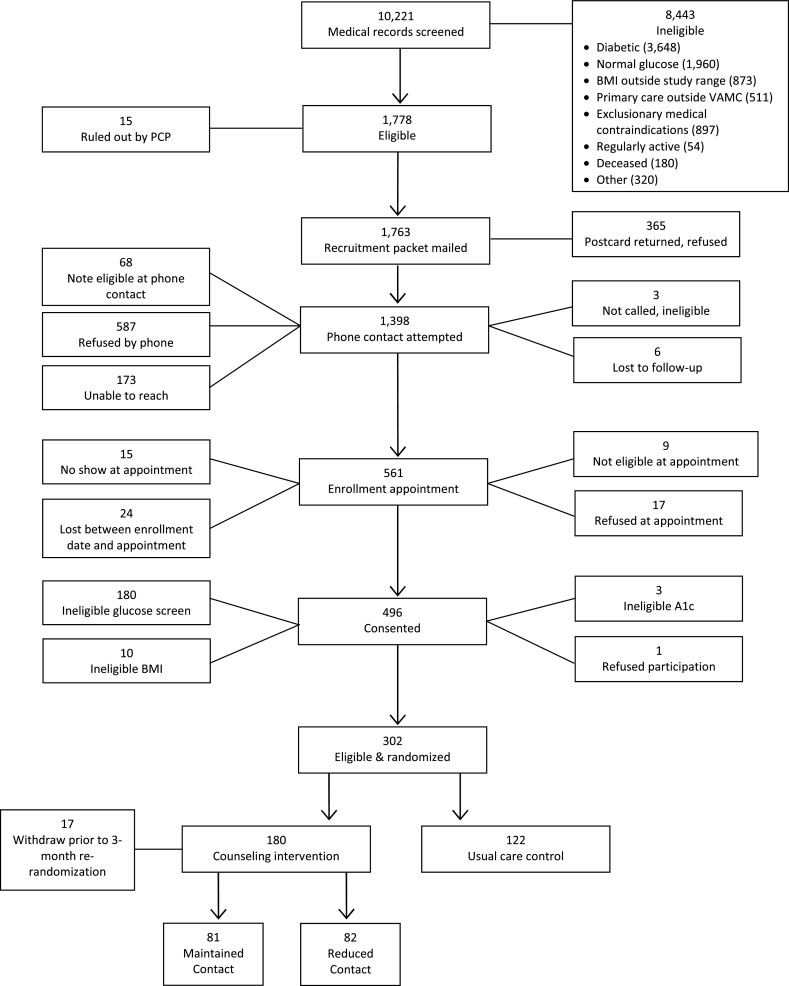
CONSORT of patients through screening, recruitment, and randomization
For this baseline article, we examined means and frequencies of the various outcomes without attention to randomization. Data are presented in Table 3. On average, study participants were 67 years old, White, and obese (mean BMI = 31.2 kg/m2, mean waist circumference = 104.1 cm). All but ten were male, which reflects the relative paucity of female veterans in this particular age group. Fifty-two percent reported some college or more advanced education, while ~10% did not graduate from high school. The participants self-reported an average of four comorbid conditions, with hypertension (72%), arthritis (52%), and heart conditions (34%) being the most prevalent. They also reported an average of two symptoms, with shortness of breath with exertion (53%) and pain (59%) being the most prevalent. In general, this sample had normal blood pressure and cholesterol levels, likely indicative of successful medication management.
Table 3.
Summary of baseline participant characteristics for the total sample and the sample stratified by age. Data presented as mean (SD) or percent
| Total sample (N = 302) | |
|---|---|
| Demographics | |
| Age (year) | 67.4 (6.2) |
| Gender (male) | 96.70% |
| Race (white) | 70.20% |
| Education (some college or more) | 52.60% |
| Biometrics | |
| Number of comorbidities | 4.1 (2.4) |
| Self-reported symptoms (no.) | 2.2 (1.6) |
| BMI (kg/m2) | 31.2 (3.6) |
| Waist circumference (cm) | 104.1 (8.8) |
| Blood pressure | |
| Systolic (mmHg) | 126.0 (15.6) |
| Diastolic (mmHg) | 72.5 (7.7) |
| Primary outcomes | |
| Fasting blood glucose (mg/dL) | 110.5 (7.0) |
| Fasting insulin (μU/ml) | 11.1 (7.9) |
| HOMA-IR | 3.0 (2.2) |
| Secondary outcomes | |
| Hemoglobin A1c (%) | 5.9 (0.4) |
| HDL (mg/dL) | 38.9 (12.0) |
| LDL (mg/dL) | 108.9 (31.0) |
| Total cholesterol (mg/dL) | 174.3 (35.1) |
| Triglycerides (mg/dL) | 137.0 (103.8) |
| Physical performance | |
| Usual gait speed (m/s) | 1.2 (0.3) |
| Rapid gait speed (m/s) | 1.9 (0.4) |
| Short physical performance battery (range 0–12) (higher score = higher function) | 10.8 (1.7) |
| Grip strength (kg) | 35.5 (8.0) |
| Six-minute walk test (yards) | 544.4 (126.4) |
| SF-36: general health (% very good or excellent) | 38.70% |
| SF-36: physical function (0–100) | 72.4 (23.5) |
| SF-36: pain (0–100) | 63.0 (25.8) |
| SF-36: vitality (0–100) | 58.4 (20.7) |
| SF-36: role-physical function (0–100) | 63.2 (39.4) |
| SF-36: role-emotional function (0–100) | 77.4 (36.7) |
| SF-36: social function (0–100) | 81.8 (24.2) |
| SF-36: mental health (0–100) | 79.4 (19.7) |
| Physical activity | |
| CHAMPS: moderate endurance exercises (min/week) | 41.0 (90.5) |
| CHAMPS: strengthening exercises (min/week) | 28.6 (82.1) |
| Psychosocial outcomes | |
| Self-efficacy: Walking for 30 min (range 0–100) (higher score = greater efficacy) | 69.9 (31.3) |
| Self-efficacy: strengthening exercises (range 0–100) (higher score = greater efficacy) | 66.1 (31.3) |
| Motivation: walking for 30 min (% very much or completely) | 72.80% |
| Motivation: leg strengthening exercises for 15 min (% very much or completely) | 62.70% |
| Satisfaction with physical function (range 1–7) (higher score = greater satisfaction) | 4.8 (1.7) |
The fasting blood glucose, fasting insulin, HbA1c, and HOMA values confirm that this is a sample of older adults with prediabetes. The physical performance scores indicate average physical functioning. The average usual and rapid walking speeds were 1.2 and 1.9 m/s, respectively, and the average total score on the SPPB was 10.8. Although only 38.7% of the sample rated their general health as very good or excellent, both self-reported physical function and satisfaction with physical function were high. From the SF-36, self-reported pain, vitality, emotional and social function, and mental health were all moderate to high, indicative of better functioning. The distance walked during the 6-min walk test is commensurate with the 20th percentile of age- and gender-matched national norms [33], which is suggestive of low cardiorespiratory fitness levels. As expected, given the eligibility criteria of our study, physical activity levels were relatively low, with participants averaging 41 min/week of moderate-intensity aerobic activities and 29 min/week of leg strengthening activities. Study participants reported being highly efficacious and highly motivated to participate in regular, sustained endurance and strengthening activities.
DISCUSSION
The Enhanced Fitness Study, as originally conceived, was designed to be one of only a few behavioral studies [9, 10, 34, 35] to employ an adaptive randomization design within the context of a lifestyle intervention. Carels and colleagues have employed a similar design for behavioral trials for weight loss. As this paper details, we experienced a number of unanticipated issues which affected the design and implementation of this study. We discuss these here, along with a brief summary of lessons learned, in hopes that other investigators might benefit from our experiences.
First and foremost was our mandated change in design. We had proposed a highly innovative, cutting edge adaptive design which we believed was highly reflective of primary care practice, e.g., offering a treatment, in this case physical activity counseling, and then adding or reducing treatment based on responses or non-responses to the treatment, i.e., physical activity. We had carefully created a measurable indicator of adherence to physical activity (self-report achievement of 75% of PA counseling goals) that, based on our previous research [36], we hoped would guide projections of respondent or non-respondent in the current trial. To our surprise, the non-response rate to physical activity in this study was extremely low, with no PAC participants being classified as non-responders, and we realized that we would simply be unable to implement group-based counseling for non-respondents. Although we were forced to amend the number of arms in this trial, we were still able to retain the most innovative aspect of this trial—the adaptive design, albeit with three arms—and did not need to compromise on the proposed primary and secondary aims.
Another lesson learned for individuals directing behavioral interventions would be to not rely on self-report of behaviors for critical aspects of study design. Here, we classified respondents and non-respondents based upon self-report achievement of 75% of their self-selected physical activity goals. We had proposed to collect accelerometry data on a sub-set of study participants as a validation of self-report but in fact we probably should have instituted accelerometry on the full sample, minimally until the critical 3-month reassessment. The difficulty of identifying early on those individuals who have not taken up a behavioral intervention and providing adjunct resources to improve the efficacy remains a challenge. Future studies which explore the feasibility and utility of adaptive design trials in the context of physical activity will inform public health efforts to provide person-centered care to improve health behaviors and associated clinical outcomes.
Along with consideration on how the decision rule for re-assignment is operationalized and measured, it is also important to examine the impact of individual characteristics on study implementation. For example, upon entry into this study, the participants indicated that they were highly motivated to participate in physical activity and were confident in their ability to do so over the course of the study. It is conceivable that these individual characteristics also contributed to the paucity of non-respondents by self-report observed in this study, though we recognize that there is a difference between claiming to be motivated to change a behavior and actually changing the behavior.
A second lesson, which is probably more generalizable to all clinical researchers, pertains to patient recruitment. We had made realistic recruitment projections for this study using data gleaned from medical record reviews relative to the number of patients in our catchment area with a diagnosis of prediabetes. We did not, however, anticipate the rapidly escalating prevalence of diabetes in our population (65+ years) which affected the total number of pre-diabetics available for the proposed study. As a result, we had to widen our net for available patients by recruiting patients from CBOCs serviced by our VA medical center and reducing the study age by 5 years (to 60+ years of age). At the same time, we recognized early on that our exclusion of morbidly obese individuals (BMI ≥ 40) due to reservations that they would be ill-equipped to handle the rigors of this activity program was unfounded. As such, we amended our protocol to extend the upper limit of BMI to 45 and thus were able to include individuals who had great potential to benefit from this lifestyle intervention.
As noted in the Consort diagram (Figure 3; Appendix), 50% of those initially deemed eligible to participate self-selected out of the study; the overwhelming majority of whom simply stated that they were “not interested.” Further consideration of this refusal rate by the study personnel and principal investigators led us to question the risk awareness among these at-risk patients. NHANES data from 2005 to 006 showed that only 7% of adults with prediabetes (determined during the medical exam portion of this survey) were ever told that they had the condition by their healthcare provider [37]. These numbers, combined with our own, may reflect a need to educate both our patients at risk for diabetes and our healthcare providers on the signs and symptoms of prediabetes and associated health risks if left untreated.
We also were successful in meeting our study sample because we rigorously tracked, on a weekly basis, the number of patients eligible, enrolled, and randomized against projected weekly estimates. If we fell below projected randomization estimates, we immediately over-enrolled in successive weeks until we met our projected estimate guidelines. Failure to recruit sufficient numbers of study participants is one of the biggest challenges faced by clinical trialists. We have developed a weekly tracking system that alerts us when recruitment efforts lag and we make a concerted effort to over-recruit and compensate for such deficits until we are once again on par with our estimates. This system is largely responsible for our success in meeting patient accrual goals within projected timelines.
A third lesson pertains to the realities of conducting a behavioral trial within a clinical setting. Although the clinical environment offers many advantages in the way of recruitment and access to patient data (particularly relevant in the VHA with the electronic medical records), it also poses some logistical and programmatic challenges. Most notably in this study was the system-wide rollout of the MOVE! weight management program by the VA National Center for Health Promotion and Disease Prevention. As the MOVE! program was still in its early stages at the point we were beginning this study, it was unclear how MOVE! would be implemented in the care setting (e.g., relevant patient groups, behaviors to be addressed etc.). As such, we adopted a broad view of its implementation and integrated referrals to MOVE! for all participants into our study design. Although this referral muddies the organic nature of both the PAC and UC arms, the study team decided that the only way to retain the “controlled” element of this trial was to standardize access to MOVE! across groups. We also recognized that this was an important opportunity to capture additional information, including how many participants actually enrolled in MOVE! and the extent of their involvement (i.e., number of classes attended). This information could later be used to examine the impact of MOVE! participation on study outcomes. Thus, what was once viewed as an obstacle quickly became a study strength.
Despite these challenges, we were able to complete enrollment for this study without altering the primary research questions. The baseline characteristics of this study sample indicate that this is an at-risk group that could benefit from a health promotion intervention. Guidelines for physical activity and exercise in diabetes were published in 2002 and summarized by Sigal and colleagues [19] in a recent review. They concluded that at least 150 min of moderate-intensity physical activity per week is recommended for glycemic control. The insufficient levels of physical activity (mean of 40 min/week spent in endurance activities, 29 min/week in leg strengthening activities) and low physical fitness observed in our sample underscore the importance of interventions to improve metabolic, behavioral, and functional outcomes among prediabetics. That this sample demonstrated average physical functioning and reported moderate/high confidence in their ability to meet the current national physical activity guidelines is promising for these individuals are not only physically capable of exercise but are also confident in their ability to do so on a regular basis; suggesting that this may be a critical juncture for interventions to attenuate the functional and behavioral declines associated with the progression of this disease.
In closing, unanticipated problems are a reality of research. Indeed unforeseen obstacles may be more the rule than the exception in behavioral work, where the focal point is the very heterogeneous state of human behavior. Clearly, thorough consideration of recruitment strategies, mode of intervention, measurement tools, implementation strategies, and data analysis strategies are a critical element of any behavioral trial. However, things do not always go as planned, and as such it is imperative to have regular investigator meetings during the planning and implementation of the study. It is also important to consider the composition of the research team when looking ahead to troubleshooting unanticipated issues while the study is in the field. The ability to navigate the inevitable unforeseen circumstances that accompany research is essential to retaining the scientific integrity of a behavioral trial that is underway. Although just a snapshot of lessons learned, our experiences here underscore the importance of flexibility, where feasible and scientifically consistent with the overall study aims, in both study design and implementation to keep a study moving forward.
Acknowledgments
This study was funded by a VA Health Services Research and Development grant IIR-06-252-3 (Morey PI) and National Institute on Aging grant AG028716. Intervention materials have been developed with prior support from VA Rehabilitation Research Service grants (RRD-E2756R, RRD-E3386R) and National Cancer Institute grant CA106919. Katherine Hall is supported by a Department of Veterans Affairs Advanced Geriatrics fellowship. Helen Lum is supported by a National Institute on Aging grant, T32 AG000029-33. Hayden Bosworth is supported by a Department of Veterans Affairs Health Services Research and Development Career Scientist award (RCS 08–027). The authors thank Dr. Kenneth Jones, Director of the VA National Center for Health Promotion and Disease Prevention, for his review of this manuscript. The views expressed by the authors do not necessarily reflect the views of the Department of Veterans Affairs. ClinicalTrials.gov identifier: NCT00594399.
APPENDIX—CONSORT CHECKLIST
Footnotes
Implications
Practice: Provider support alongside a health counselor may be efficacious for increasing physical activity levels among older adult patients. Thus, this may be a feasible practice model to sustain.
Policy: Alternative designs to the traditional two-armed trial comparing an intervention versus usual care may point to more effective ways of treating this patient population that is already using substantial healthcare dollars. However, implementation of these alternative designs into clinical care should be bolstered by evidence-based research.
Research: Sequential adaptive designs are a promising design for behavioral trials because they mimic clinical care. Careful attention to objective measures of physical activity adherence will strengthen the viability of this novel approach to promoting behavior change.
References
- 1.Centers for Disease Control and Prevention. National Diabetes Fact Sheet: National estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2011.
- 2.Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care. 2004;27(Suppl 2):10–21. doi: 10.2337/diacare.27.suppl_2.B10. [DOI] [PubMed] [Google Scholar]
- 3.Kinsinger, L. S., Jones, K. R., Kahwati, L., et al. (2009). Design and dissemination of the MOVE! weight-management program for veterans. Prevention of Chronic Disease, 6(3). [PMC free article] [PubMed]
- 4.Littman AJ, Forsberg CW, Koepsell TD. Physical activity in a national sample of veterans. Medicine and Science in Sports and Exercise. 2009;41(5):1006–1013. doi: 10.1249/MSS.0b013e3181943826. [DOI] [PubMed] [Google Scholar]
- 5.Morey MC, Peterson MJ, Pieper CF, et al. Project LIFE—Learning to Improve Fitness and Function in Elders: Methods, design, and baseline characteristics of randomized trial. Journal of Rehabilitation Research and Development. 2008;45(1):31–42. doi: 10.1682/JRRD.2007.03.0044. [DOI] [PubMed] [Google Scholar]
- 6.Morey MC, Peterson MJ, Pieper CF, et al. The Veterans Learning to Improve Fitness and Function in Elders Study: A randomized trial of primary care-based physical activity counseling for older men. Journal of American Geriatrics Society. 2009;57(7):1166–1174. doi: 10.1111/j.1532-5415.2009.02301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavori PW, Dawson R. Dynamic treatment regimes: Practical design considerations. Clinical Trials. 2004;1:9–20. doi: 10.1191/1740774S04cn002oa. [DOI] [PubMed] [Google Scholar]
- 8.Lavori PW, Dawson R. Adaptive treatment strategies in chronic disease. Annual Review of Medicine. 2008;59:443–453. doi: 10.1146/annurev.med.59.062606.122232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carels RA, Darby L, Cacciapaglia HM, et al. Using motivational interviewing as a supplement to obesity treatment: A stepped-care approach. Health Psychology. 2007;26(3):369–374. doi: 10.1037/0278-6133.26.3.369. [DOI] [PubMed] [Google Scholar]
- 10.Carels RA, Wott CB, Young KM, et al. Successful weight loss with self-help: A stepped-care approach. Journal of Behavioral Medicine. 2009;32(6):503–509. doi: 10.1007/s10865-009-9221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rejeski WJ, Focht BC. Aging and physical disability: On integrating group and individual counseling with the promotion of physical activity. Exercise and Sport Sciences Reviews. 2002;30(4):166–170. doi: 10.1097/00003677-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Rejeski WJ, Brawley LR, Ambrosius WT, et al. Older adults with chronic disease: Benefits of group-mediated counseling in the promotion of physically active lifestyles. Health Psychology. 2003;22(4):414–423. doi: 10.1037/0278-6133.22.4.414. [DOI] [PubMed] [Google Scholar]
- 13.Murphy SA. An experimental design for the development of adaptive treatment strategies. Statistics in Medicine. 2005;24:1455–1481. doi: 10.1002/sim.2022. [DOI] [PubMed] [Google Scholar]
- 14.Dubbert PA, Cooper KM, Kirchner KA, Meydrech EF, Bilbrew D. Effects of nurse counseling on walking for exercise in elderly primary care patients. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2002;57(11):733–740. doi: 10.1093/gerona/57.11.M733. [DOI] [PubMed] [Google Scholar]
- 15.Bandura A. Social foundations of thought and action: A social cognitive theory. Englewood Cliffs: Prentice Hall; 1986. [Google Scholar]
- 16.Bandura A. Self-efficacy: The exercise of control. New York: Freeman; 1997. [Google Scholar]
- 17.McAuley E, Elavsky S, Motl RW, Konopack JF, Hu L, Marquez DX. Physical activity, self-efficacy, and self-esteem: longitudinal relationships in older adults. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 2005;60(5):268–275. doi: 10.1093/geronb/60.5.P268. [DOI] [PubMed] [Google Scholar]
- 18.Prochaska J, DiClemente C. Stages of change in the modification of problem behaviors. Progress in Behavior Modification. 1992;28:183–218. [PubMed] [Google Scholar]
- 19.Sigal RJ, Wasserman DH, Kenny GP, Castaneda-Sceppa C. Physical activity/exercise and type 2 diabetes. Diabetes Care. 2004;27(10):2518–2539. doi: 10.2337/diacare.27.10.2518. [DOI] [PubMed] [Google Scholar]
- 20.Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: Recommendation from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1094–1105. doi: 10.1161/CIRCULATIONAHA.107.185650. [DOI] [PubMed] [Google Scholar]
- 21.Physical activity guidelines advisory committee report. Washington, D.C.: Department of Health and Human Services; 2008. [DOI] [PubMed] [Google Scholar]
- 22.Rodin J, Janis IL. The social power of health-care practitioners as agents of change. Journal of Social Issues. 1979;35(1):60–81. doi: 10.1111/j.1540-4560.1979.tb00789.x. [DOI] [Google Scholar]
- 23.Fillenbaum G. Multidimensional functional assessment of older adults. Hillsdale: Erlbaum; 1988. [Google Scholar]
- 24.Stewart AL, Mills KM, Sepsis PG, et al. Evaluation of CHAMPS, a physical activity promotion program for older adults. Annals of Behavioral Medicine. 1997;19(4):353–361. doi: 10.1007/BF02895154. [DOI] [PubMed] [Google Scholar]
- 25.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: Outcomes for interventions. Medicine and Science in Sports and Exercise. 2001;33(7):1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Ware JE, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36) Medical Care. 1992;30:473–483. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 27.McHorney CA, Ware JE, Raczek AE. The MOS 36-item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical Care. 1993;31(3):247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 28.McHorney CA, Ware JE, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Medical Care. 1994;32(1):40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. Journal of Gerontology. 1994;49(2):85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 30.McAuley E. Self-efficacy and the maintenance of exercise participation in older adults. Journal of Behavioral Medicine. 1993;16:103–113. doi: 10.1007/BF00844757. [DOI] [PubMed] [Google Scholar]
- 31.Kraus WE, Torgan CE, Duscha BD, et al. Studies of a targeted risk reduction intervention through defined exercise (STRRIDE) Medicine and Science in Sports and Exercise. 2001;33(10):1774–1784. doi: 10.1097/00005768-200110000-00025. [DOI] [PubMed] [Google Scholar]
- 32.Cohen J. Statistical power analysis for the behavioral sciences, revised edition. Hillsdale: Erlbaum; 1987. [Google Scholar]
- 33.Rikli RE, Jones CJ. Senior fitness test manual. Champaign: Human Kinetics; 2001. [Google Scholar]
- 34.Coulton S, Watson J, Bland M, Drummond C, Kaner E, Godfrey C, et al. The effectiveness and cost-effectiveness of opportunistic screening and stepped care interventions for older hazardous alcohol users in primary care—a randomised control trial protocol. Health Services Research. 2008;12(8):129. doi: 10.1186/1472-6963-8-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Apil, S. R., Hoencamp, E., Haffmans, P. M. S, P. (2011). A stepped care relapse prevention program for depression in older people: a randomized controlled trial. International Journal of Geriatric Psychiatry. [DOI] [PubMed]
- 36.Morey, M. C., Dubbert, P. M., Doyle, M. E., et al. (2003). From supervise to unsupervised exercise: factors associated with exercise adherence. Journal of Aging and Physical Activity, 11(351–368).
- 37.Geiss LS, James C, Gregg EW, Albright A, Williamson DF, Cowie CC. Diabetes risk reduction behaviors among U.S. adults with prediabetes. American Journal of Preventive Medicine. 2010;38(4):403–409. doi: 10.1016/j.amepre.2009.12.029. [DOI] [PubMed] [Google Scholar]


