Abstract
The vascular endothelial growth factor (VEGF) and its receptor (VEGFR) have been shown to play major roles not only in physiological but also in most pathological angiogenesis, such as cancer. VEGF belongs to the PDGF supergene family characterized by 8 conserved cysteines and functions as a homodimer structure. VEGF-A regulates angiogenesis and vascular permeability by activating 2 receptors, VEGFR-1 (Flt-1) and VEGFR-2 (KDR/Flk1 in mice). On the other hand, VEGF-C/VEGF-D and their receptor, VEGFR-3 (Flt-4), mainly regulate lymphangiogenesis. The VEGF family includes other interesting variants, one of which is the virally encoded VEGF-E and another is specifically expressed in the venom of the habu snake (Trimeresurus flavoviridis). VEGFRs are distantly related to the PDGFR family; however, they are unique with respect to their structure and signaling system. Unlike members of the PDGFR family that strongly stimulate the PI3K-Akt pathway toward cell proliferation, VEGFR-2, the major signal transducer for angiogenesis, preferentially utilizes the PLCγ-PKC-MAPK pathway for signaling. The VEGF-VEGFR system is an important target for anti-angiogenic therapy in cancer and is also an attractive system for pro-angiogenic therapy in the treatment of neuronal degeneration and ischemic diseases.
Keywords: VEGF, VEGF receptor, tumor angiogenesis, anti-angiogenic therapy, neuronal degeneration, pro-angiogenic therapy
Introduction
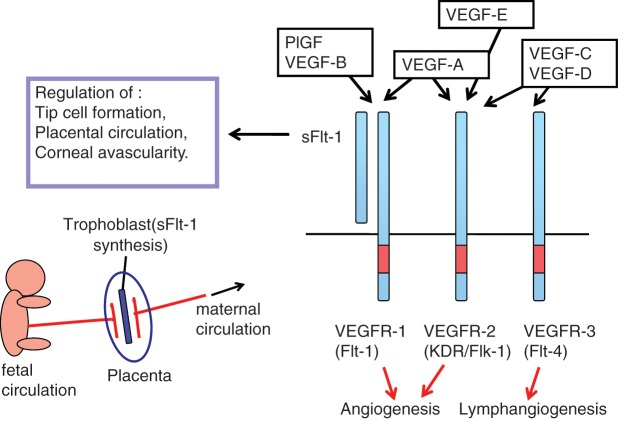
Angiogenesis, the formation and maintenance of blood vessel structures, is essential for the physiological functions of tissues and is important for the progression of diseases such as cancer and inflammation.1,2 In recent decades, a variety of signaling molecules, such as VEGF-VEGFRs, ephrin-Eph receptors, angiopoietin-Tie, and the Delta-Notch system, have been identified as playing important roles in angiogenesis. Among these, vascular endothelial growth factors (VEGFs) and receptors (VEGFRs) regulate both vasculogenesis, the development of blood vessels from precursor cells during early embryogenesis, and angiogenesis, the formation of blood vessels from pre-existing vessels at a later stage3 (Fig. 1). The VEGF family of genes contains at least 7 members, including the viral genome–derived VEGF-E, whereas the VEGFR family of genes has 3 to 4 members depending on the vertebrate species.4,5 VEGF-A and its receptors VEGFR-1 and VEGFR-2 play major roles in physiological as well as pathological angiogenesis, including tumor angiogenesis. VEGF-C/D and their receptor VEGFR-3 can regulate angiogenesis at early embryogenesis but mostly function as critical regulators of lymphangiogenesis.6
Figure 1.
The VEGF and VEGFR system. VEGF-A and its receptors, VEGFR-1 and VEGFR-2, play a major role in vasculogenesis and angiogenesis. In addition, sFlt-1, a soluble form of VEGFR-1, is expressed in various cells such as trophoblasts and negatively regulates angiogenesis.
VEGF-A has a variety of functions, including pro-angiogenic activity, vascular permeability activity, and the stimulation of cell migration in macrophage lineage and endothelial cells. Recently, anti–VEGF-VEGFR drugs such as an anti–VEGF-A neutralizing antibody and multikinase inhibitors have been developed and widely used for the treatment of major solid tumors.7,8 The clinical efficacy of these medicines has been well evaluated; however, none of them provide a complete cure for cancer patients. The molecular basis of the refractoriness in some tumors and the acquisition of resistance to these medicines should be extensively studied to develop more efficient anti-angiogenic therapies.
On the other hand, VEGFs have pro-angiogenic potential for the maintenance of various tissues at physiological levels and for the formation of new blood vessels to overcome ischemic diseases. The utility of VEGF family members in pro-angiogenic medicine, together with the possible side effects, should be characterized in more detail for clinical applications.
Structure and Function of the VEGF Family
VEGF, also known as VEGF-A, is a protein with vascular permeability activity that was originally purified from a fluid secreted by a tumor.9 A few years later, a protein with angiogenic activity was independently purified and named VEGF.10 Molecular cloning, however, revealed that these 2 proteins were identical and encoded by a single gene.3 The VEGF family includes VEGF-A, VEGF-B, VEGF-C, VEGF-D, PlGF (placental growth factor), VEGF-E (Orf-VEGF), and Trimeresurus flavoviridis svVEGF. With the exception of the latter 2 members, 5 genes of the VEGF family exist in mammalian genomes, including humans. Essentially, all the VEGFs have 8 conserved cysteine residues at fixed positions, which are very similar to the PDGF family such as M-CSF (CSF-1), SCF (stem cell factor), and Flt3L (Flt3 ligand). Among the 8 cysteines, 6 residues form 3 S-S intramolecular bonds and generate 3 loop structures.11 The remaining 2 cysteines form 2 S-S intermolecular bonds, contributing to the stable homodimer structure of VEGF. We have shown that a structure combined with loop 1 and loop 3 in VEGF-A and VEGF-E is essential for the binding and activation of VEGFR-2.12
VEGF-A
Through alternative splicing, the VEGF-A protein contains subtypes, such as peptides of 121, 165, 189, and 206 amino acids in humans.3 Except for VEGF-A121, the other peptides have a basic stretch near the carboxyl terminus. The basic stretch of VEGF-A165 has a weak affinity for acidic materials such as heparin/heparan sulfate and to neuropilin-1, a membrane protein involved in neuronal cell regulation and a coreceptor for VEGF-A. The basic stretch of VEGF-A189 has a strong binding affinity to heparin/heparan sulfate, and thus, most of the VEGF-A189 molecules appear to be localized on the cell surface or in the extracellular matrix.
The VEGF-A gene is unique in terms of its haploid insufficiency: even if only a single copy of the VEGF-A gene is deficient (heterozygotic VEGF-A gene knockout mice; VEGF-A+/– mice), the mutant embryo dies at early embryogenesis (E10-E11) due to immature formation and dysfunction of the circulatory system.3 This indicates that the local concentration of VEGF-A in tissue is tightly regulated in embryogenesis, and half the level of the VEGF-A protein is insufficient to complete the formation of the closed circulatory system in the body.
Among subtypes of VEGF-A, VEGF-A165 is most important both quantitatively and qualitatively. Maes et al. reported that VEGF-A165 is essential and sufficient for angiogenesis because VEGF-A164 transgenic mice in a VEGF-A–null genetic background are alive and essentially healthy.13 More recently, another subtype of VEGF-A, VEGF-Axxxb, was reported in humans.14 VEGF-Axxxb activates the receptor much more weakly than the normal VEGF-A, suggesting that VEGF-Axxxb could be a physiological competitor against VEGF-A.
VEGF-A binds to and activates both VEGFR-1 and VEGFR-2, promoting angiogenesis, vascular permeability, cell migration, and gene expression.5 In addition, Lee et al. showed that an autocrine loop of VEGF-A and its receptor system exist within vascular endothelial cells, contributing to endothelial functions.15
PlGF and VEGF-B
These molecules bind to and activate only VEGFR-1. As will be described later, VEGFR-1 has the ability to bind tightly to its ligands but has a weak tyrosine kinase activity, generating signals weaker than VEGFR-2. Both PlGF–/– and VEGF-B–/– mice are alive at birth with no significant defects related to angiogenesis, suggesting that these genes are dispensable at embryogenesis. However, under pathological conditions, synergism between PlGF and VEGF-A has been shown to contribute to angiogenesis.16 VEGF-B–/– mice have the phenotype of an atrial conduction defect.17 In addition, VEGF-B was recently reported to protect against the degeneration of sensory neurons.18 These results indicate that, although PlGF and VEGF-B are not essential at embryogenesis, they have a variety of functions under pathological or stressed conditions.
VEGF-C and VEGF-D
These 2 members of the VEGF family are produced as premature forms and are cleaved by proteases such as furin in both the amino- and carboxyl-terminal portions.19 After processing, these molecules develop a higher affinity for VEGFR-3, which is expressed on lymphatic endothelial cells and stimulates the receptor for lymphangiogenesis. In addition, these proteins have a weak affinity for VEGFR-2, activating angiogenesis to some extent. VEGF-C is expressed during embryogenesis, whereas VEGF-D is expressed after birth during adult stages. This difference in gene expression is thought to be a major cause for lethality in VEGF-C–/– mice but not in VEGF-D–/– mice. VEGF-C–/– mice show severe accumulation of fluid in tissues due to poor development of lymph vessels.6
VEGF-E, an Angiogenic Protein Encoded in the Pro-Angiogenic Orf Virus Genome
The Orf virus, a parapoxvirus infecting sheep, goats, and sometimes humans, is known to induce angiogenesis at sites of infection on the skin. In 1994, Lyttle et al. found that a gene in the viral genome encodes a protein distantly related to VEGF/PDGF.20 The amino acid identity of this gene product in the NZ7 strain of the Orf virus shows a limited (25%) degree of identity to human VEGF-A and 19% to human PDGF. This low homology suggested that NZ7-derived proteins bind weakly to VEGFR and/or PDGFR. However, to our surprise, we found that this protein (designated VEGF-ENZ7) tightly binds to and activates VEGFR-2, but not other VEGFRs (VEGFR-1, VEGFR-3) nor PDGFR21 (Fig. 2). Therefore, VEGF-E is unique in terms of its specificity to VEGFR-2. None of the angiogenic factors encoded in the human genome has been reported to exhibit such VEGFR-2 specificity. The products of other Orf virus clones, such as NZ2 and D1701 strains, also show essentially the same specificity.22,23
Figure 2.
Unique activation of VEGFRs by VEGF-E and Trimeresurus flavoviridis svVEGF. VEGF-E encoded in the Orf viral genome binds to and activates only VEGFR-2, inducing well-organized blood vessels. T. flavoviridis svVEGF stimulates vascular permeability by activation of VEGFRs in a specific manner.
Because the human genome does not contain the original VEGF-E gene, and because the genomic structure of VEGF-E in the Orf virus genome suggests an insertion of a DNA sequence carrying the VEGF-E gene into the viral sequence, the original VEGF-E might be present in the genome of some vertebrates other than mammals. Infection of an animal by the precursor Orf virus might have led to the incorporation of the VEGF-E gene into the viral genome, maintaining the gene as a pro-angiogenic factor to facilitate viral production in the infected dermal tissues. Such a capture of a biologically active gene into a viral genome is well known to have occurred in the cases of viral oncogene–containing retroviruses such as Rous sarcoma virus.24
T. flavoviridis svVEGF, a VEGF-Like Molecule Secreted in Snake Venom
Snake venom contains a variety of molecules that attack target animals both directly (as toxins) and indirectly (as toxin-promoting materials). From T. flavoviridis (habu) snake venom, Takahashi et al. purified a protein bearing weak angiogenic activity and strong vascular permeability activity.25 Interestingly, this protein, named T. flavoviridis svVEGF, binds tightly to VEGFR-1 and weakly to VEGFR-2 (Fig. 2). Furthermore, the T. flavoviridis svVEGF protein is synthesized only in the venom tissue and secreted into the venom fluid, which appears to be the first case of a VEGF family protein being secreted from tissues of the body. This may suggest that the biological function of this protein is not for increasing the vascular permeability within the snake body itself but rather promoting vascular permeability in the local tissues of targeted animals. Because T. flavoviridis svVEGF itself is not toxic to cultured mammalian cells, the purpose of this vascular permeability activity is considered to be linked to the circulation of real toxins into the target animals and promotion of their efficacy.
Structure of VEGFRs
VEGFRs are typical tyrosine kinase receptors (TKRs) carrying an extracellular domain for ligand binding, a transmembrane domain, and a cytoplasmic domain, including a tyrosine kinase domain4 (Fig. 1). The overall structure of VEGFRs is similar to that of the PDGFR family members; however, these 2 receptor families have clear differences: the PDGFR extracellular domain contains 5 immunoglobulin (Ig)–like domains, whereas VEGFRs bear 7 Ig-like domains. Both TKRs share a tyrosine kinase domain with a long kinase insert (KI) of 60 to 70 amino acids; however, the amino acid sequences in the KI of these 2 TKRs are very different from each other. The KIs in PDGFR family members contain 1 or 2 Tyr(Y)-x-x-Met(M) motifs as autophosphorylation sites, and these motifs have been shown to be strong binding sites for the SH2 domain of the p85 subunit in the PI3-kinase complex and to activate the PI3K pathway. These autophosphorylation sites were demonstrated to be crucial for the cell growth signal mediated by PDGFR and for the cell transformation signal mediated by v-Fms, an activated form of M-CSFR (a member of the PDGFR family). However, none of the VEGFRs contain this Y-x-x-M motif in their KI region or in the carboxyl-terminal region, indicating that the downstream signaling from VEGFRs may be different from that of the PDGFR family.5
Signaling of VEGFRs
VEGF-A binds to and activates VEGFR-1 (Flt-1) and VEGFR-2 (KDR/Flk-1 in mice). VEGFR-1 has a high affinity for VEGF-A (Kd = 1~10 pM), which is one order higher than that of VEGFR-2, whereas its tyrosine kinase activity is approximately 10-fold weaker than that of VEGFR-2.26 The major pro-angiogenic signal is generated from the ligand-activated VEGFR-2. Within the KI or carboxyl-terminal region, TKRs have tyrosine autophosphorylation sites, which are important for the downstream signal. Unlike most of the TKRs that activate the Ras pathway or PI3K pathway, we found that the PLCγ-PKC-MAPK pathway is highly activated in VEGF-bound VEGFR-2 and used as a crucial signal for endothelial proliferation. An SH2 domain of PLCγ specifically binds to the 1175-PY site of VEGFR-2 (1173-PY in mice) and further activates PKC, particularly the PKCβ pathway.27,28 An 1175-phenylalanine (F) mutant of VEGFR-2 significantly decreases the MAPK pathway under stimulation with VEGF and cannot efficiently activate the endothelial proliferation signal. Furthermore, mice with an amino acid knock-in at the 1173 site from Y to F (VEGFR-2/flk-1 1173F/F mutant mice) are embryonic lethal at about E9.0 with no formation of blood vessels similar to the flk-1–/– mice.29 On the other hand, another knock-in mouse (VEGFR-2/flk-1 1212F/F) was essentially healthy.
Taken together, these results strongly suggest that the PLCγ-PKC-MAPK pathway initiated from the VEGFR-2 1175-PY site plays a pivotal role in pro-angiogenic signaling from VEGFR-2. A spontaneous mutant of zebrafish carrying the lethal circulatory system abnormality was shown to have a mutation in the fish PLCγ1 gene, indicating that the PLCγ-PKC pathway is also important for angiogenesis in fish.30 VEGFR-2 stimulates not only angiogenic signals but also the secretion of various proteins such as the von Willebrand factor (vWF) from endothelial cells. vWF secretion was recently reported to be dependent on 1175-PY in VEGFR-2.31 In addition, Sase et al. indicated that the signaling from the 1175-PY site on VEGFR-2 to PLCγ is essential for endothelial specification of VEGFR-2–positive vascular progenitor cells in embryonic stem (ES) cell culture.32 On the other hand, the 951-PY on VEGFR-2 is important for cell migration signals.33
VEGFR-1 has a much weaker kinase activity than VEGFR-2, and the signaling cascade is not fully understood. The 1169-Y on VEGFR-1 corresponding to 1175-Y on VEGFR-2 is a PLCγ activation site from VEGFR-1. However, we found that 1169-PY is not a major autophosphorylation site on VEGFR-1. Consistent with this finding, direct pro-angiogenic activity from VEGFR-1 is usually weak or undetectable.34
In addition to vascular endothelial cells, VEGFR-1 is expressed on macrophage lineage cells and facilitates migration of these cells. Recently, we have shown that a scaffold protein RACK1 is involved in this migration signal and that the VEGFR-1-RACK1-PI3K-Akt pathway appears to be important for this signal.35
The biological functions of VEGFR-1 have been the topic of several studies. Fong et al. demonstrated that flt-1–null mutant mice (flt-1–/– mice) die at approximately E8.5 due to an overgrowth of vascular endothelial cells and disorganization of blood vessels.36 This suggests a negative regulatory role of VEGFR-1 (Flt-1) in angiogenesis at early embryogenesis. To examine whether the tyrosine kinase of VEGFR-1 is essential to the negative role of this receptor, we generated a mutant mouse strain that lacks the tyrosine kinase (TK) domain of VEGFR-1 (flt-1 TK–/– mice). To our surprise, the mutant mice were essentially healthy with an almost normal circulatory system, indicating that the negative role of VEGFR-1 is independent of its tyrosine kinase activity but dependent on the ligand-binding domain.37 This flt-1 TK–/– mouse is useful for clarifying the role of the VEGFR-1 signal and to see whether it is important for the progression of diseases such as cancer. Using the mutant mice, we have found, along with others, that flt-1 TK–/– mice show a slower tumor growth, a lower level of metastasis (particularly lung metastasis in the carcinogenesis model), and a milder inflammation reaction in the rheumatoid arthritis model compared with those in wild-type mice.38-41 Furthermore, we and others have found that the wild-type mice carrying the flt-1 TK–/– bone marrow show slower tumor growth similar to that in the flt-1 TK–/– mice.38,39 Using an anti–VEGFR-1 neutralizing antibody, Kaplan et al. indicated that VEGFR-1–positive bone marrow precursor cells play a significant role in the formation of a premetastatic niche, which promotes tumor metastasis.42 These results indicate that VEGFR-1 signaling is important for the progression of tumors in vivo mostly via bone marrow–derived VEGFR-1–positive cells. In addition, Wu et al. reported that some human tumors, such as breast carcinomas, express VEGFR-1 and utilize its signaling directly for tumor growth.43
VEGFR-3 has a typical tyrosine kinase like other VEGFRs, and upon stimulation with VEGF-C, the PKC pathway and Ras pathway were reported to be activated for lymphangiogenesis. However, it remains to be clarified which autophosphorylation site(s) on the tyrosine residues in VEGFR-3 is responsible for these pathways and is critical for lymphangiogenesis.
Unique Characteristics of sFlt-1
The VEGFR-1 (Flt-1) gene expresses 2 mRNAs: one is a long form of approximately 8 kb, and the other is a short form of 2.5 to 3.0 kb.44 The short mRNA is highly expressed in normal placenta, encoding a soluble form of Flt-1 known as sFlt-1.44,45 The sFlt-1 contains 6 Ig-like domains with a short, 31 amino acid–long tail derived from the 5′ region of intron 13 and exhibits a strong binding ability to VEGF-A, PlGF, and VEGF-B.45,46
Within the placenta, trophoblasts located between the fetal and maternal blood vessel systems preferentially express sFlt-1 (Fig. 1). Thus, an interesting possibility is that sFlt-1 functions as a biochemical barrier between fetal and maternal circulation in the placenta by suppressing excess angiogenesis and abnormal vascular permeability. From this model, the level of sFlt-1 should be controlled at an appropriate physiological range because overtrapping of VEGF may cause severe problems in the circulatory system of the placenta. Interestingly enough, abnormal overexpression of sFlt-1 in the placenta was observed in a major disease in the field of obstetrics. In 2003, Maynard et al. and Koga et al. reported that patients with preeclampsia have abnormally high levels of sFlt-1 in serum and plasma.47,48 The trophoblasts were the major cell types producing large amounts of sFlt-1 in patients with this disease. Furthermore, Levine et al. demonstrated an intimate relationship between the serum levels of sFlt-1 and the degree of preeclampsia.49 This strongly suggests that abnormal suppression of VEGF-A by sFlt-1 causes hypertension and proteinuria in the patients. Supporting this idea, Maynard et al. reported that an artificial expression of sFlt-1 with a vector system in pregnant rats induces symptoms such as hypertension and proteinuria, indicating that sFlt-1 is, at least partly, the cause of the preeclamptic syndromes.47
A podocyte-specific knockout of the VEGF-A gene in mice demonstrated damage to the glomerular microvasculature and an induction of proteinuria. Thus, a severe block and decrease in the level of VEGF-A in the kidney by overexpressed sFlt-1 in preeclampsia may result in glomerular dysfunction and proteinuria. Interestingly, similar symptoms (hypertension and proteinuria) were observed under anti–VEGF-VEGFR therapy in cancer patients (see below).
sFlt-1 was also found to be expressed in corneal epithelial cells.50 This strongly suggests that sFlt-1 suppresses angiogenesis near the lens and maintains the transparency of the eye.
Anti–VEGF/VEGFR Therapy and Anticancer Therapy
The VEGF-VEGFR system is unique in that it consists of a very limited number of molecules that play a central role in angiogenesis. The major ligand (VEGF-A) is a single gene product, and it utilizes only 2 TKRs (VEGFR-1 and VEGFR-2), although neuropilin-1 is used as a coreceptor. Other ligands, such as PlGF, VEGF-C, and VEGF-D, and the receptor VEGFR-3 appear to be partly involved in pathological angiogenesis, such as tumor vasculature. On the other hand, tumors metastatic to lymph nodes express higher levels of VEGF-C/D, suggesting that the VEGF-C/D and VEGFR-3 system plays an important role in lymph vessel–dependent tumor cell migration into lymph nodes. The angiopoietin-Tie system is also involved in pathological angiogenesis, but the details of its role in the process of carcinogenesis are not fully understood.
On the basis of these results, anti–VEGF-VEGFR drugs such as anti–VEGF-A neutralizing antibody and tyrosine kinase inhibitors have been developed, and bevacizumab (anti–VEGF-A humanized monoclonal antibody) has been approved for the treatment of colorectal, breast, lung (non–small cell type), and renal cancers as well as for glioblastoma patients.3,51 Multikinase inhibitors such as sorafenib and sunitinib are now approved for renal and hepatic cancer patients.
In addition to these medicines, others that target the VEGF-VEGFR system, including VEGF-Trap (a fusion protein of VEGFR-1 and VEGFR-2 ligand-binding domains), anti–VEGFR-1 or anti–VEGFR- 2 neutralizing antibody, soluble VEGFR-3, VEGFR-1 or VEGFR-2 peptide vaccine therapy,52 and anti-PlGF antibody,53,54 have been developed and are undergoing preclinical and clinical trials.
The Molecular Basis of Anti-Angiogenic Therapy
In 1993, Kim et al. demonstrated that antihuman VEGF-A neutralizing antibody efficiently suppressed human tumor growth in immune-deficient mice.55 In this case, the antibody only suppressed tumor-derived human VEGF-A, but not the host-derived mouse VEGF-A, which is secreted from mouse bone marrow–derived cells as well as tumor-associated fibroblasts. Furthermore, tumor growth was suppressed by the antibody treatment alone, without combination with chemotherapy. This indicates that the major effect of the anti–VEGF-A antibody was to block the formation of blood vessels in tumor tissues as well as to suppress pre-existing tumor vasculature by inducing apoptotic death of endothelial cells by blocking tumor-derived VEGF-A.
However, in clinical trials, treatment of cancer patients with an anti–VEGF-A antibody alone did not produce significant suppression of tumor growth, except for renal cancer. In contrast to murine tumor transplantation models, the growth rate of tumors in patients is usually slower, and tumor angiogenesis may develop more slowly compared with the murine tumor models. In clinics, the vasculature in tumors might be more stable than that in the murine system and less sensitive to VEGF-A blockade alone.
Another hypothesis regarding the efficacy of the anti-VEGF antibody and anti-VEGFR tyrosine kinase inhibitor on tumor growth in patients is termed “vascular normalization,” whereby the absorption of VEGF-A induces a transiently normalized vascular structure, more stabilized and well covered with pericytes with lower vascular permeability.56 These conditions may result in a lower tissue pressure within tumors, having a better diffusion of anticancer drugs. It is probable that both vascular normalization and suppression of new tumor angiogenesis can occur in parallel within the tumors in patients treated with anti–VEGF-VEGFR drugs (Fig. 3).
Figure 3.
A possible response of tumor cells to anti-angiogenic therapy: a model. A direct suppression of tumor angiogenesis and “vascular normalization” results in the suppression of tumor growth. However, after long-term therapy, tumor cells under hypoxia and low nutrition double stress acquire a resistant phenotype.
Side Effects and Refractoriness to Anti-Angiogenic Therapy
A variety of side effects, such as hypertension, renal dysfunction, proteinuria, thrombosis, bleeding, and arrhythmia, have been reported in patients under anti–VEGF-VEGFR therapy.7,8 Among these, the frequency of hypertension and proteinuria is higher than that of others, suggesting a direct relationship with the blockage of VEGF-A in tissues. A decrease in the level of VEGF-A in the kidney could induce damage to vascular endothelial cells in glomeruli, and such a dysfunction of glomerular microvasculature may cause proteinuria. However, the molecular basis of hypertension under VEGF-VEGFR blockage remains to be clarified.
Whether tumor cells acquire refractoriness or resistance to anti-angiogenic therapy after long-term treatment is an important question. In clinical trials, the efficacy of anti–VEGF-VEGFR therapy on the increase in survival time seems sometimes inconsistent. Survival time did not increase stably during the course of treatment, and in some trials after a long period, the efficacy appears to decrease, suggesting a resistance or refractoriness of tumors to this treatment. In addition, in some preclinical and clinical trials, glioblastoma showed an enhanced invasiveness after anti-angiogenic therapy57 (Fig. 3).
Many experimental models could be introduced and studied to understand this resistance. Casanovas et al. reported that gene expression of angiogenic factors such as FGF other than VEGF is a cause of resistance against anti-VEGF therapy in mice.58 We hypothesized that, under a long-term anti-angiogenic therapy, tumor cells may receive at least 2 stresses, hypoxia and low nutrition conditions. We developed a simple in vitro model system in which tumor cells were cultured under these double stresses (double deprivation stress [DDS]). After approximately 10 cycles of DDS culture, tumor cells showed up-regulation of phospho-Akt, a higher survival rate, and increased invasiveness.59 Thus, DDS under anti-angiogenic therapy might induce, to some extent, a malignant phenotype of tumors. A strategy should be developed to overcome this possible malignant phenotype after anti-angiogenic therapy.
Pro-Angiogenic Therapy
Ischemic heart failure and cerebral attacks with thrombosis or bleeding are major diseases in humans. Furthermore, recent studies strongly suggest that some degenerative diseases, such as neuronal degeneration, are due to lower circulation as well as lower VEGF-VEGFR signaling in neuronal cells.60,61
For the treatment of ischemic heart and brain diseases, pro-angiogenic therapy could be useful because these diseases are essentially due to poor circulatory conditions. Among the VEGF family, VEGF-A plays a crucial role in blood vessel formation in embryogenesis and the earlier stages after birth. However, in adult stages, VEGF-A stimulates not only VEGFR-2 but also VEGFR-1 (Flt-1), which enhances the migration of inflammatory cells such as macrophages, resulting in inflammation and hypervascular permeability. Several articles have previously reported that K14 promoter–driven VEGF-A transgenic mice exhibit severe inflammation with edema in dermal tissues with angiogenesis, this being a model of psoriasis vulgaris, which is a chronic inflammatory skin disease.62 On the other hand, we have shown that the VEGFR-2–specific ligand VEGF-E induces well-organized blood vessels with pericyte coverage and maintains normal vascular permeability. No clear inflammatory reaction was observed in VEGF-E transgenic mice. K14-PlGF only showed a very limited angiogenic response.63,64
In the case of motoneuron degeneration, such as the amyotrophic lateral sclerosis (ALS) rat model, Storkebaum et al. injected VEGF-A protein intracerebroventricularly and found that the treatment significantly prolonged the survival of SOD1G93A mutant rats, which have ALS-like phenotypes.61 Oosthuyse et al. previously showed that VEGF-A stimulates motoneuron functions via 2 mechanisms: the increase in circulation to supply sufficient amounts of oxygen and nutrition, and the activation of VEGFR-2, which is expressed at lower levels in motoneuronal cells60 (Fig. 4). For ALS, VEGF-E could also be useful because it activates VEGFR-2, similar to VEGF-A without the support of the coreceptor, neuropilin-1.
Figure 4.
A pro-angiogenic therapy using the VEGF-VEGFR system. Recent studies suggest that sensory neurons express VEGFR-1 and motoneurons express VEGFR-2. These receptors are biologically functional, and therefore, an appropriate ligand, such as VEGF-E, can be used for pro-angiogenic therapy as well as for neuron protection therapy.
Taken together, stimulation of VEGFR-2 and VEGFR-1 with VEGF-A or VEGFR-2 alone with VEGF-E is a possible pro-angiogenic therapy. VEGF-E is a particularly attractive molecule because it does not enhance an inflammatory reaction, whereas VEGF-A might through the activation of VEGFR-1. Although the VEGF-E gene is not present in the human genome, we have humanized VEGF-E by replacing approximately half of the sequence with the human-derived sequence PlGF to avoid immunogenicity.
Conclusions
Phylogenetically, VEGF-A is highly conserved from fish to mammals. Furthermore, 3 VEGFR systems with a soluble form of VEGFR-1 are maintained from amphibians to mammals. These findings suggest that VEGF-VEGFR is not only used for the formation of blood vessels but also for the maintenance of various tissues such as neuronal tissue. The VEGF-VEGFR system is closely linked to other angiogenesis regulatory systems such as angiopoietin-Tie and Delta (Dll4)–Notch systems for tight regulation of angiogenesis. More extensive studies on the VEGF-VEGFR system and its relationship with other regulators are necessary and should open new fields toward developing better strategies to treat cancer and other diseases.
Footnotes
Declaration of Conflicting Interests: The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was supported by a Grant-in-aid Special Project Research on Cancer-Bioscience [grant number 17014020] from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1. Risau W. Mechanism of angiogenesis. Nature. 1997;386:671-4 [DOI] [PubMed] [Google Scholar]
- 2. Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353-64 [DOI] [PubMed] [Google Scholar]
- 3. Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967-74 [DOI] [PubMed] [Google Scholar]
- 4. Shibuya M. Role of VEGF-Flt receptor system in normal and tumor angiogenesis. Adv Cancer Res. 1995;67:281-316 [DOI] [PubMed] [Google Scholar]
- 5. Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312:549-60 [DOI] [PubMed] [Google Scholar]
- 6. Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell. 2002;1:219-27 [DOI] [PubMed] [Google Scholar]
- 7. Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-42 [DOI] [PubMed] [Google Scholar]
- 8. Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542-50 [DOI] [PubMed] [Google Scholar]
- 9. Senger DR, Galli SJ, Dvorak AM, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983-5 [DOI] [PubMed] [Google Scholar]
- 10. Leung DW, Cachianes G, Kuang W-J, et al. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306-9 [DOI] [PubMed] [Google Scholar]
- 11. Muller YA, Li B, Christinger HW, Wells JA, Cunningham BC, de Vos AM. Vascular endothelial growth factor: crystal structure and functional mapping of the kinase domain receptor binding site. Proc Natl Acad Sci U S A. 1997; 94:7192-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kiba A, Yabana N, Shibuya M. A set of loop-1 and -3 structures in the novel VEGF family member, VEGF-ENZ-7, is essential for the activation of VEGFR-2 signaling. J Biol Chem. 2003;278:13453-61 [DOI] [PubMed] [Google Scholar]
- 13. Maes C, Carmeliet P, Moermans K, et al. Impaired angiogenesis and endochondral bone formation in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Mech Dev. 2002;111:61-73 [DOI] [PubMed] [Google Scholar]
- 14. Pritchard-Jones RO, Dunn DB, Qiu Y, et al. Expression of VEGF(xxx)b, the inhibitory isoforms of VEGF, in malignant melanoma. Br J Cancer. 2007;97:223-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee S, Chen TT, Barber CL, et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691-703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carmeliet P, Moons L, Luttun A, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575-83 [DOI] [PubMed] [Google Scholar]
- 17. Aase K, von Euler G, Li X, et al. Vascular endothelial growth factor-B-deficient mice display an atrial conduction defect. Circulation. 2001;104:358-64 [DOI] [PubMed] [Google Scholar]
- 18. Dhondt J, Peeraer E, Verheyen A, et al. Neuronal FLT1 receptor and its selective ligand VEGF-B protect against retrograde degeneration of sensory neurons. FASEB J. Epub 2011. Jan 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McColl BK, Paavonen K, Karnezis T, et al. Proprotein convertases promote processing of VEGF-D, a critical step for binding the angiogenic receptor VEGFR-2. FASEB J. 2007;21:1088-98 [DOI] [PubMed] [Google Scholar]
- 20. Lyttle DJ, Fraser KM, Fleming SB, Mercer AA, Robinson AJ. Homologs of vascular endothelial growth factor are encoded by the poxvirus orf virus. J Virol. 1994;68:84-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ogawa S, Oku A, Sawano A, Yamaguchi S, Yazaki Y, Shibuya M. A novel type of vascular endothelial growth factor: VEGF-E (NZ-7 VEGF) preferentially utilizes KDR/Flk-1 receptor and carries a potent mitotic activity without heparin-binding domain. J Biol Chem. 1998;273:31273-82 [DOI] [PubMed] [Google Scholar]
- 22. Meyer M, Clauss M, Lepple-Wienhues A, et al. A novel vascular endothelial growth factor encoded by Orf virus, VEGF-E, mediates angiogenesis via signalling through VEGFR-2 (KDR) but not VEGFR-1 (Flt-1) receptor tyrosine kinases. EMBO J. 1999;18:363-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wise LM, Veikkola T, Mercer AA, et al. Vascular endothelial growth factor (VEGF)-like protein from orf virus NZ2 binds to VEGFR2 and neuropilin-1. Proc Natl Acad Sci U S A. 1999;96:3071-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jove R, Hanafusa H. Cell transformation by the viral src oncogene. Annu Rev Cell Biol. 1987;3:31-56 [DOI] [PubMed] [Google Scholar]
- 25. Takahashi H, Hattori S, Iwamatsu A, Takizawa H, Shibuya M. A novel snake venom vascular endothelial growth factor (VEGF) predominantly induces vascular permeability through preferential signaling via VEGF receptor-1. J Biol Chem. 2004;279:46304-14 [DOI] [PubMed] [Google Scholar]
- 26. Sawano A, Takahashi T, Yamaguchi S, Aonuma T, Shibuya M. Flt-1 but not KDR/Flk-1 tyrosine kinase is a receptor for placenta growth factor (PlGF), which is related to vascular endothelial growth factor (VEGF). Cell Growth Diff. 1996;7:213-21 [PubMed] [Google Scholar]
- 27. Takahashi T, Ueno H, Shibuya M. VEGF activates protein kinase C-dependent, but Ras-independent Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene. 1999;18:2221-30 [DOI] [PubMed] [Google Scholar]
- 28. Takahashi T, Yamaguchi S, Chida K, Shibuya M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-γ and DNA synthesis in vascular endothelial cells. EMBO J. 2001;20:2768-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sakurai Y, Ohgimoto K, Kataoka Y, Yoshida N, Shibuya M. Essential role of Flk-1 (vascular endothelial growth factor receptor-2) tyrosine residue-1173 in vasculogenesis in mice. Proc Natl Acad Sci U S A. 2005;102:1076-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lawson ND, Mugford JW, Diamond BA, Weinstein BM. Phospholipase C gamma-1 is required downstream of vascular endothelial growth factor during arterial development. Genes Dev. 2003;7:1346-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xiong Y, Huo Y, Chen C, et al. Vascular endothelial growth factor (VEGF) receptor-2 tyrosine 1175 signaling controls VEGF-induced von Willebrand factor release from endothelial cells via phospholipase C-gamma 1- and protein kinase A-dependent pathways. J Biol Chem. 2009;284:23217-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sase H, Watabe T, Kawasaki K, Miyazono K, Miyazawa K. VEGFR2-PLCgamma1 axis is essential for endothelial specification of VEGFR2+ vascular progenitor cells. J Cell Sci. 2009;122:3303-11 [DOI] [PubMed] [Google Scholar]
- 33. Matsumoto T, Bohman S, Dixelius J, et al. VEGF receptor-2 Y951 signaling and a role for the adapter molecule TSAd in tumor angiogenesis. EMBO J. 2005;24:2342-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sawano A, Takahashi T, Yamaguchi S, et al. The phosphorylated 1169-tyrosine containing region of Flt-1 kinase (VEGFR-1) is a major binding site for PLCγ. Biochem Biophys Res Commun. 1997;238:487-91 [DOI] [PubMed] [Google Scholar]
- 35. Wang F, Yamauchi M, Muramatsu M, Osawa T, Tsuchida R, Shibuya M. RACK1 regulates VEGF/Flt1-mediated cell migration via activation of a PI3K/Akt pathway. J Biol Chem. 2011;286:9097-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fong GH, Rossant J, Gertsentein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66-70 [DOI] [PubMed] [Google Scholar]
- 37. Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci U S A. 1998;95:9349-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kerber M, Reiss Y, Wickersheim A, et al. Flt-1 signaling in macrophages promotes glioma growth in vivo. Cancer Res. 2008;68:7342-51 [DOI] [PubMed] [Google Scholar]
- 39. Muramatsu M, Yamamoto S, Osawa T, Shibuya M. VEGF-1 signaling promotes mobilization of macrophage-lineage cells from bone marrow and stimulateolid tumor growth. Cancer Res. 2010;70:8211-21 [DOI] [PubMed] [Google Scholar]
- 40. Hiratsuka S, Nakamura K, Iwai S, et al. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung specific metastasis. Cancer Cell. 2002;2:289-300 [DOI] [PubMed] [Google Scholar]
- 41. Murakami M, Iwai S, Hiratsuka S, et al. Signaling of vascular endothelial growth factor receptor-1 tyrosine kinase promotes rheumatoid arthritis through activation of monocyte/macrophages. Blood. 2006;108:1849-56 [DOI] [PubMed] [Google Scholar]
- 42. Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu Y, Hooper AT, Zhong Z, et al. The vascular endothelial growth factor receptor (VEGFR-1) supports growth and survival of human breast carcinoma. Int J Cancer. 2006;119:1519-29 [DOI] [PubMed] [Google Scholar]
- 44. Shibuya M, Yamaguchi S, Yamane A, et al. Nucleotide sequence and expression of a novel human receptor-type tyrosine kinase gene (flt) closely related to the fms family. Oncogene. 1990;5:519-24 [PubMed] [Google Scholar]
- 45. Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A. 1993;90:10705-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kondo K, Hiratsuka S, Subbalakshmi E, Matsushime H, Shibuya M. Genomic organization of the flt-1 gene encoding for vascular endothelial growth factor (VEGF) receptor-1 suggests an intimate evolutionary relationship between the 7-Ig and the 5-Ig tyrosine kinase receptors. Gene. 1998;208:297-305 [DOI] [PubMed] [Google Scholar]
- 47. Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Koga K, Osuga Y, Yoshino O, et al. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab. 2003;88:2348-51 [DOI] [PubMed] [Google Scholar]
- 49. Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672-83 [DOI] [PubMed] [Google Scholar]
- 50. Ambati BK, Nozaki M, Singh N, et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Peak SJ, Levin VA. Role of bevacizumab therapy in the management of glioblastoma. Cancer Manag Res. 2010;2:97-104 [PMC free article] [PubMed] [Google Scholar]
- 52. Wada S, Tsunoda T, Baba T, et al. Rationale for antiangiogenic cancer therapy with vaccination using epitope peptides derived from human vascular endothelial growth factor receptor 2. Cancer Res. 2005;65:4939-46 [DOI] [PubMed] [Google Scholar]
- 53. Van de, Veire S, Stalmans I, Heindryckx F, et al. Further pharmacological and genetic evidence for the efficacy of PlGF inhibition in cancer and eye disease. Cell. 2010;141:178-90 [DOI] [PubMed] [Google Scholar]
- 54. Bais C, Wu X, Yao J, et al. PlGF blockade does not inhibit angiogenesis during primary tumor growth. Cell. 2010;141:166-77 [DOI] [PubMed] [Google Scholar]
- 55. Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841-4 [DOI] [PubMed] [Google Scholar]
- 56. Fukumura D, Jain RK. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc Res. 2007;74(2-3):72-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zuniga RM, Torcuator R, Jain R, et al. Efficacy, safety and patterns of response and recurrence in patients with recurrent high-grade gliomas treated with bevacizumab plus irinotecan. J Neurooncol. 2009;91:329-36 [DOI] [PubMed] [Google Scholar]
- 58. Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8: 299-309 [DOI] [PubMed] [Google Scholar]
- 59. Osawa T, Muramatsu M, Watanabe M, Shibuya M. Hypoxia and low nutrition double stress induces aggressiveness in a murine model of melanoma. Cancer Sci. 2009;100:844-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Oosthuyse B, Moons L, Storkebaum E, et al. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet. 2001;28:131-8 [DOI] [PubMed] [Google Scholar]
- 61. Storkebaum E, Lambrechts D, Dewerchin M, et al. Treatment of motoneuron degeneration by intracerebroventricular delivery of VEGF in a rat model of ALS. Nat Neurosci. 2005;8: 85-92 [DOI] [PubMed] [Google Scholar]
- 62. Detmar M, Brown LF, Schon MP, et al. Increased microvascular density and enhanced leukocyte rolling and adhesion in the skin of VEGF transgenic mice. J Invest Dermatol. 1998;111:1-6 [DOI] [PubMed] [Google Scholar]
- 63. Kiba A, Sagara H, Hara T, Shibuya M. VEGFR-2-specific ligand VEGF-E induces non- edematous hyper-vascularization in mice. Biochem Biophys Res Commun. 2003;301:371-7 [DOI] [PubMed] [Google Scholar]
- 64. Zheng Y, Watanabe M, Kuraishi T, Hattori S, Kai C, Shibuya M. Chimeric VEGF-ENZ7/PlGF specifically binding to VEGFR-2 accelerates skin wound healing via enhancement of neovascularization. Arterioscler Thromb Vasc Biol. 2007;27:503-11 [DOI] [PubMed] [Google Scholar]